Abstract
Synthetic glycopolymers that emulate cell-surface mucins have been used to elucidate the role of mucin overexpression in cancer. However, because they are internalized within hours, these glycopolymers could not be used to probe processes that occur on longer time scales. Here, we tested a panel of glycopolymers bearing a variety of lipids to identify those that persist on cell membranes. Strikingly, we found that cholesterylamine (CholA)-anchored glycopolymers are internalized into vesicles that serve as depots for delivery back to the cell surface, allowing for the display of cell-surface glycopolymers for at least 10 days, even while cells are dividing. As with native mucins, cell-surface display of CholA-anchored glycopolymers influenced focal adhesion distribution. Furthermore, we show that these mimetics enhance survival of nonmalignant cells in a zebrafish model of metastasis. CholA-anchored glycopolymers therefore expand applications of glycocalyx engineering in glycobiology.
Keywords: Glycopolymers, Glycoconjugates, Lipids, Cancer, Cell Adhesion
Global changes in glycosylation can accompany inflammation, microbial infection and tumorigenesis.[1] For example, heavily glycosylated mucin glycoproteins have long been known to be upregulated in epithelial carcinomas, though until recently their contribution to oncogenesis remained largely mysterious.[2] Mucins are membrane-associated glycoproteins with densely glycosylated ectodomains; their glycans often comprise >50% of the mass of the molecule.[3] It was recently shown that mucins’ biophysical bulk may actually bolster adhesion of tumor cells in minimal adhesion settings, such as the metastatic niche.[4] Additionally, broad upregulation of certain glycan motifs, such as sialic acid, can play an important role in how the tumor and the host immune system interact.[5]
These and other studies of glycobiology have benefitted from chemical approaches, such as the display of synthetic glycoconjugates on live cells via chemical glycocalyx engineering.[6] One of the most straightforward methods to control cell surface glycan display is through passive insertion of lipid-anchored glycopolymers into the plasma membrane.[5,7–11] This strategy offers many advantages over other techniques, such as covalent attachment of glycoconjugates to engineered cell-surface molecules.[11,12] Because lipid-anchored glycopolymers will insert into any plasma membrane, any naïve cell type can be used, including primary cells and those not amenable to genetic manipulation. However, previously reported lipid-conjugated glycopolymers, including those functionalized with dipalmitoyl phosphatidylethanolamine-based anchors (DPPE, 1, Fig. 1a), are constitutively internalized by cells.[8] As a consequence, their plasma membrane residence half-lives are only 5-6 hours, which limits their application to studies of biological processes occurring on short time scales.[5,13] Many events of interest, such as tumor growth and metastasis, unfold on much longer timescales. At present, the role of glycosylation in such processes cannot be addressed by lipid-insertion-based glycocalyx engineering.
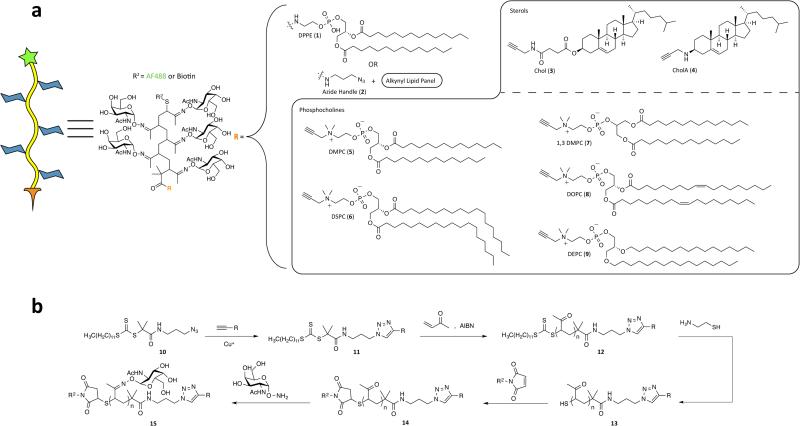
Figure 1.
Synthesis of a panel of lipid-anchored glycopolymers. a) Structure and representation of our glycopolymers with differing alkynyl lipids, R. b) Synthesis of dual-end-functionalized glycopolymers.
Here, we report the synthesis and cell trafficking behavior of a panel of lipid anchors engineered to improve the plasma membrane residence times of glycopolymers. We show that cholesterylamine (CholA), a lipid known to recycle back to the cell surface after internalization,[14,15] is capable of shuttling glycopolymers through this pathway continuously for up to 10 days, resulting in persistent display of glycopolymers on the plasma membrane. Moreover, cells incubated with CholA-anchored glycopolymers internalize some portion into a reservoir that serves as a depot for continuous cell-surface delivery, even in daughter cells derived from a labeled mother cell. Once resident on cells, the CholA-anchored glycopolymers replicate known effects of mucins on focal adhesion distribution and cell survival, both in cell culture and in a zebrafish model of tumor cell metastasis.
Although the mechanisms underlying native lipid trafficking throughout a cell are not well understood, small changes in lipid structure can have a profound effect on internalization kinetics and pathway.[16,17] With little basis for rational design, we synthesized a panel of phosphoglycerolipids that varied in length (dimyristoyl DMPC (5) and distearoyl DSPC (6)), regiochemistry (1,3 DMPC (7)), unsaturation (dioleyl, DOPC (8)), or linkage type to glycerol (e.g., diether lipid DEPC (9)) in the hope of improving cell-surface half-life relative to DPPE (Fig. 1a). To integrate these lipids into glycopolymers, we crafted the synthetic approach outlined in Fig. 1b. The lipids were outfitted with clickable alkyne groups (see Scheme S1) for conjugation to an azidefunctionalized chain-transfer agent (CTA) (10) capable of reversible addition-fragmentation chain-transfer (RAFT) polymerization.[18] In addition, we prepared the two sterol derivatives cholesteryl-succinic acid propargylamide (Chol (3))[19] and N-propargyl 3β-cholesterylamine (CholA (4)) using the procedure described in Fig. S2. Notably, Peterson and coworkers discovered that CholA and various CholA-small molecule conjugates have the unique ability to persist on cell surfaces by virtue of continuous recycling from an endocytic compartment.[14,20–22]
After clicking the alkynyl lipids to azide-CTA 10, we generated endfunctionalized glycopolymers using an approach similar to our previous work.[23] Briefly, we performed RAFT polymerization with methyl vinyl ketone (MVK) to generate lipid-anchored poly(MVK) polymers of specified lengths (~200 DP) and low polydipersity (PDIs = 1.1-1.66) (see SI for details). The terminal trithiocarbonate was cleaved, and the free thiol was alkylated with a maleimide probe (either Alexa Fluor 488 (AF488) or biotin). Finally, the ketone groups were condensed with aminooxy-N-acetylgalactosamine (GalNAc) to form hydrolytically-stable oxime-conjugated glycopolymers.[24]
Using AF488-labeled polymers, we sought to measure the efficiency with which each glycopolymer was incorporated into cell membranes. We incubated Jurkat cells (107/mL in serum-free media) for 1 h at room temperature with 10 μM lipid-conjugated glycopolymer, washed the cells, and then measured their fluorescence by flow cytometry (Figure 2a). For each lipid tested, we observed saturation kinetics, with the amount of glycopolymer displayed on the membrane increasing as a function of loading concentration (see Figure S3). The polymers with the highest levels of incorporation were the phosphocholine derivatives with shorter fatty acids (DMPC and 1,3 DMPC) and the sterols (Chol and CholA).
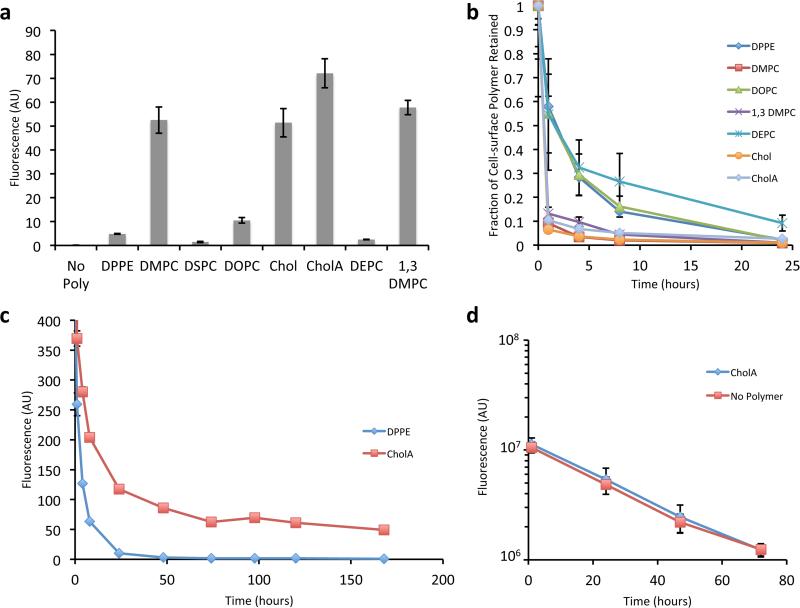
Figure 2.
Glycopolymers with different lipid anchors have different cell-surface kinetics. a) Fluorescence of Jurkat cells incubated with 10 μM of AF488-capped polymer with the given lipid anchor. b) Fractional cell-surface retention of biotin-capped polymers relative to levels immediately following polymer loading. c) Absolute cell-surface abundance of biotin-capped CholA-anchored glycopolymers on long timescales. d) Effect of CholA-anchored glycopolymers on fluorescence over time of cells labeled with a cytosolic cell-tracking dye. For a, b, c and d, shown are mean +/− standard deviation of three replicate experiments.
To quantitate the persistence time of glycopolymers on the cell surface, we used biotin-capped polymers that could be detected with a membrane-impermeant AF488-labeled anti-biotin antibody. After incubation with glycopolymers, cells were washed, and incubated in complete media at 37 °C until a given time point, when the cells were washed and cell-surface labeled for flow cytometry analysis. By comparing the mean fluorescence intensity with that obtained immediately after glycopolymer loading, the fraction of polymer remaining on the cell surface could be calculated (Figure 2b). All of the glycopolymers underwent a significant decrease in cell surface abundance during the first hour after loading.
However, analysis of later time points revealed that CholA-anchored glycopolymers exhibit double exponential kinetics rather than the single exponential function observed for other lipids. After an initially rapid decline, the surface population of CholA-anchored glycopolymers stabilized and remained steady for days, at levels orders of magnitude higher than DPPE (Figure 2c). We were able to observe the presence of glycopolymers on cell surfaces up to ten days after labeling (Figure S4). To our knowledge, these are the most persistent synthetic glycoconjugates ever displayed on a cell's surface. Because an equilibrium between solution phase and cell-surface glycopolymers could develop, we tried washing the cells every day and found that we were able to deplete the cells over time (Figure S5), thus the labeling is reversible if driven by mass action.
Labeling cells with a cytoplasmic tracking dye along with biotin-capped CholA-anchored glycopolymers allowed us to monitor the fate of glycopolymers in a population of dividing cells. We found that while cells show a marked deceleration in their loss of cell-surface polymers after day 1 (Figure 2c), they actually continue to divide up to day 3 and beyond (Figure 2d). Importantly, the polymers are passed down uniformly from mother cells to daughter cells as reflected in a stable unimodal distribution of labeling (Figure S6).
Analysis of the temperature dependence of cell-surface residence time suggested that the rapid initial loss of CholA-anchored glycopolymers is due to endocytosis (Figure S7). Microscopy analysis of our AF488-capped CholA-anchored polymers revealed that, indeed, the initially rapid internalization yields dense reservoirs of glycopolymer within the cell (Figures 3a and S8). By contrast, DPPE-anchored glycopolymers formed diffuse internal puncta. Based on Peterson's reports on the recycling capability of cholesterylamine conjugates, we speculated that CholA was mediating continuous recycling of our glycopolymers from these reservoirs to the cell surface.[14,15,25]
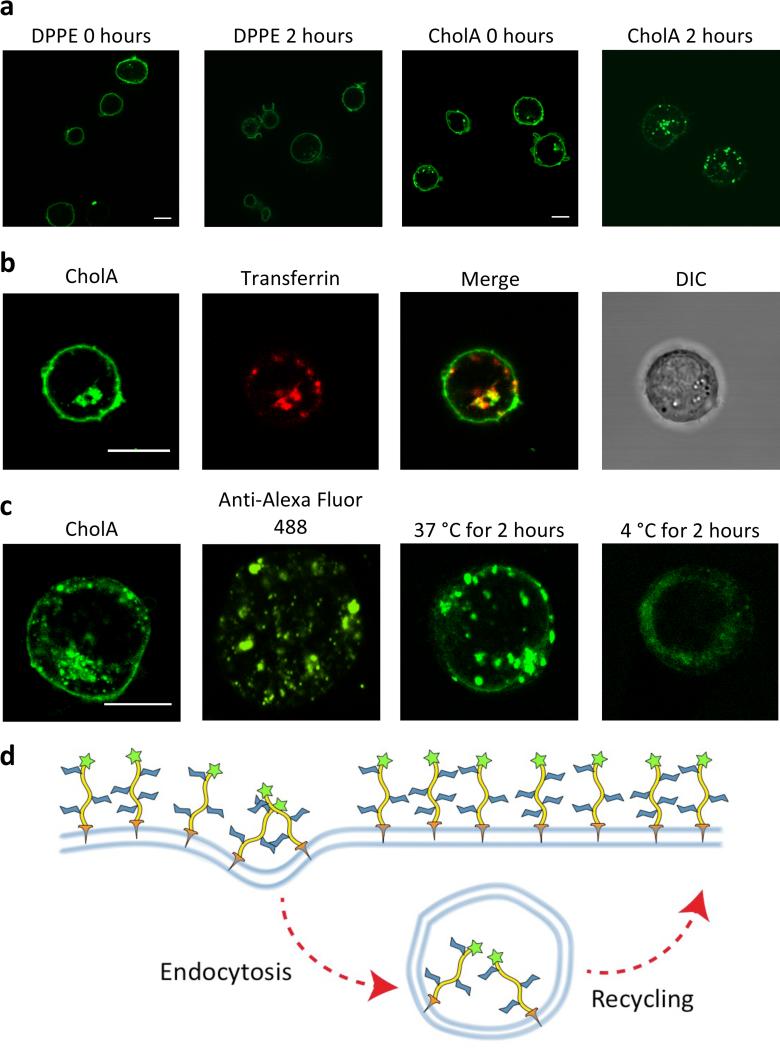
Figure 3.
CholA-anchored glycopolymers recycle from reservoirs inside the cell to the cell surface. a) Jurkats were labeled with 5 μM AF488-capped glycopolymers. b) Jurkats loaded with CholA glycopolymers and incubated with AF647-transferrin. c) Jurkats loaded with CholA-anchored glycopolymers, then treated with anti-AF488 antibody to quench cell surface fluorescence, then allowed to incubate at the indicated temperature. d) A model for the trafficking of CholA-anchored glycopolymers. Scale bars are 10 μm.
The endocytic recycling compartment (ERC) is an organelle responsible for replenishing membrane-associated proteins and lipids once they have been depleted by endocytosis.[16] The plasma protein transferrin is a known marker of the ERC.[26] We observed that Alexa Fluor 647-labeled transferrin colocalized with the reservoirs of CholA-anchored glycopolymers (Figure 3b). To visualize recycling of the glycopolymer from internal reservoirs back to the cell surface, we first quenched those AF488-capped glycopolymers present on the cell surface with an anti-AF488 antibody[27] and observed loss of cell surface labeling as expected (Figure 3c). Next, we either let these cells warm for 2 h at 37 °C or kept them on ice, then imaged them again. Only the warmed cells were able to replenish their cell surface population of AF488-capped glycopolymers, ostensibly via recycling from internal compartments (Figure 3c). We now had a model for the mechanism by which CholA-anchored glycopolymers could utilize recycling to replenish the cell surface, resulting in apparent cell-surface persistence (Figure 3d).
Having identified glycopolymers that stably populate the cell surface, we next sought to determine whether they impart biological effects on cells similar to native mucins. It was recently discovered that bulky cell-surface mucins can, counterintuitively, promote the adhesion of cells in minimal adhesion settings.[4] By obscuring the majority of binding between integrins and their ligands in the extracellular matrix, a bulky glycocalyx actually drives integrin clustering via a kinetic funnel wherein new bonds are more likely to be made near established ones (Figure 4a).[28] This clustering activates integrins and promotes signaling and cell survival, which can overcome adhesion-mediated programed cell death (i.e. anoikis) that healthy cells normally undergo.[29–31]
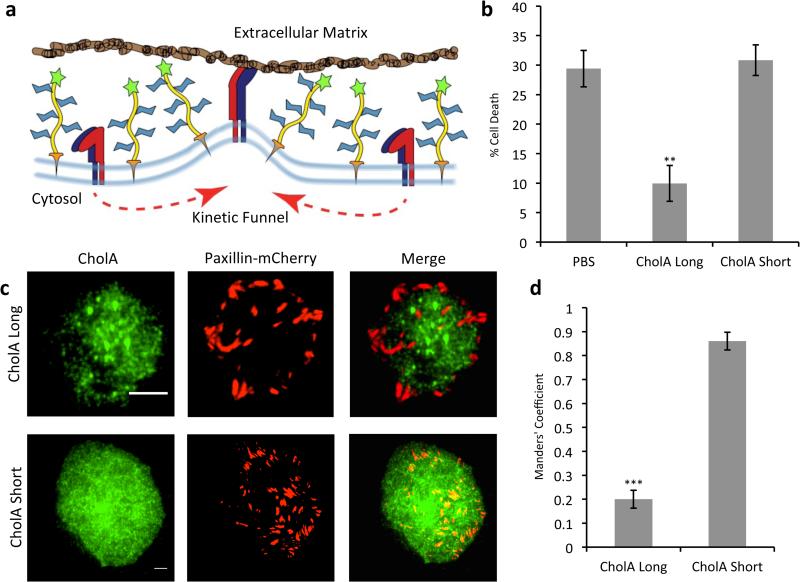
Figure 4.
CholA-anchored glycopolymers are excluded from sites of focal adhesion formation and drive a resistance to anchorage-dependent cell death. a) A kinetic funnel model for glycocalyx-driven integrin clustering. b) Effect of polymers on viability of MCF-10A cells plated on soft substrates. Error bars are SEM for at least three independent experiments. c) TIRF imaging of mCherry-paxillin expressing MCF-10A breast epithelial cells incubated with biotin-capped CholA polymers and stained with AF488-anti-biotin. Scale bars are 5 μm. d) Manders’ Colocalization Coefficients for coincidence of mCherry-paxillin with AF488-anti-biotin antibody for cells treated as in (c). Error bars are SEM for at least five cells quantified from at least two independent experiments. **p<0.01, ***p<0.001.
After confirming that our CholA-anchored glycopolymers are themselves nontoxic (Figure S9), that polymer length does not affect cell-surface persistence (Figures S10 and S11), and that our polymers are long lived on MCF-10A breast epithelial cells (Figure S12), we tested our polymers for their ability to improve the survival of nonmalignant cells in a model of the minimal adhesion setting. Soft polyacrylamide gels (140 Pa) functionalized with fibronectin offer nonideal substrates for cell growth, and roughly a third of nonmalignant breast epithelial cells (MCF-10As) plated on them die within 24 h (Figure 4b). CholA-anchored glycopolymers (DP: 719, PDI: 1.1, ~90 nm in length) much longer than the height of integrins on the cell surface (~20 nm)[32] prevented more than two thirds of this apoptosis. In control experiments, CholA-anchored glycopolymers much shorter than integrins (DP: 36, PDI: 1.20, ~3 nm) were shown to offer no significant protection from anoikis (Figure 4b).
We hypothesize that increased survival of cells painted with long glycopolymers results from clustering of integrins driven by segregation from glycopolymer rich zones, a phenomenon we sought to visualize directly by total internal reflection fluorescence (TIRF) microscopy. MCF-10A cells expressing the fluorescent-focal adhesion protein fusion mCherry-paxillin were incubated with biotin-capped CholA-anchored glycopolymers, plated on fibronectin-functionalized coverslips and allowed to adhere, then fixed and stained with AF488-labeled anti-biotin antibody. As shown in Figure 4c, TIRF microscopy analysis revealed that long CholA-anchored glycopolymers are excluded from sites of adhesion formation, while the short are not, consistent with the biophysical model for integrin clustering.
To quantify this effect, we calculated the Manders’ Colocalization Coefficients (MCC) for mCherry-paxillin in cells loaded with either long or short glycopolymers. This metric determines the fraction of red pixels that are colocalized with green pixels. In essence, it seeks to answer the question: what percentage of focal adhesion area is also occupied by glycopolymers? With short glycopolymers (3 nm), we calculated a high degree of overlap; 86% of paxillin is coincident with polymer. With long glycopolymers (90 nm), on the other hand, a dramatic segregation was seen; less than 20% of paxillin was coincident with the polymers (Figure 4d). Further image analysis showed that the average area per adhesion for cells treated with long CholA-anchored glycopolymers was almost twice that observed on cells treated with short glycopolymers (Figure S13). These metrics confirm that, like the natural mucin MUC1[4], the long CholA-anchored glycopolymers are excluded from areas of adhesion and thus likely drive integrin clustering via a kinetic funnel effect.
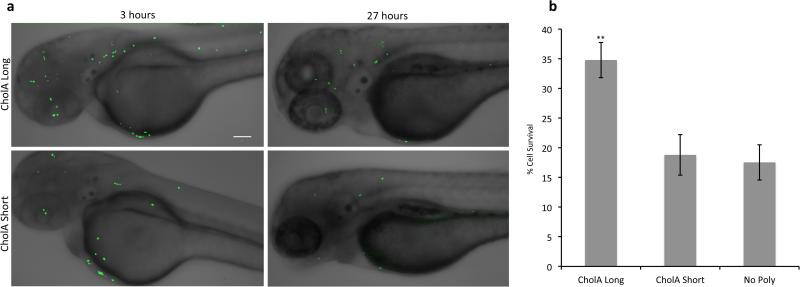
The ability to evade anoikis is a hallmark of malignancy and a prerequisite for tumor metastasis. It has been postulated that mucin overexpression promotes these processes by the mechanism outlined above. To determine whether CholA-anchored glycopolymers can similarly promote cell survival and dissemination in vivo, we turned to a zebrafish model of metastasis.[33] Zebrafish embryos 48 hours post-fertilization (hpf) were injected with MCF-10A cells constitutively expressing the green fluorescent protein-histone H2B fusion (GFP-H2B). MCF-10A cells were first incubated with either long CholA-anchored glycopolymers, short CholA-anchored glycopolymers, or PBS alone. Cells were allowed to disseminate throughout the fish for 3 h, at which point images were acquired and the number of MCF-10A cells present at day 0 was quantified by their fluorescent nuclei. Twenty-four hours later, images were taken again of the same live fish (Figures 5a and S14). The MCF-10A cells that were still viable maintained their fluorescent nuclei.[34] As in the in vitro model, cells coated with long CholA-anchored glycopolymers showed almost twice the survival rate of cells with short glycopolymers or no polymers at all (Figure 5b). Thus, CholA-anchored glycopolymers have allowed us to test the effects of a bulky glycocalyx on cell survival over a 27-h period in vivo.
Figure 5.
Long CholA-anchored glycopolymers protect previously nonmalignant cells from apoptosis in vivo. a) Zebrafish embryos, 48 hpf, were injected with GFP-H2B expressing MCF-10A cells loaded with either long (90 nm) or short (3 nm) CholA-anchored glycopolymers and imaged at the indicated time points. b) Effect of polymers on viability of cells injected as in (a) after 27 hours. Scale bars are 100 μm. Error bars are SEM of five biological replicates. **p<0.01.
In conclusion, CholA-anchored glycopolymers enable long-lived glycocalyx engineering of naïve cells and provide tools to answer questions hereto unapproachable. We also envision utilizing these reagents for translational applications, such as protection of precious or vulnerable cells (e.g. stem cells) from hostile environments (e.g. the immune system) or tailored homing of engineered cells to target tissues.
Supplementary Material
Acknowledgements
We would like to thank Matt Rubashkin, Matt Paszek, and Valerie Weaver for the gift of mCherry-paxillin expressing MCF-10A cells; Alex Hughes and Zev Gartner for their gift of GFP-H2B expressing MCF-10A cells; Brian Belardi for synthetic expertise, experimental advice, and countless helpful discussions; Kamil Godula, Jason Hudak, and Steve Canham for synthetic expertise; Jen-Yi Lee and the CRL Molecular Imaging Center, which is supported by the Gordon and Betty Moore Foundation; Alex Hughes and the EBBG for assistance with image quantitation and general tomfoolery. This work was funded by a grant from the US National Institutes of Health (R01 GM59907). E.C.W. was supported by a predoctoral fellowship from the US National Institutes of Health (F31CA200544).
References
- 1.Ohtsubo K, Marth JD. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Kufe DW. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingsworth MA, Swanson BJ. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 4.Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Hudak JE, Lakins JN, Wijekoon AC, Cassereau L, et al. Nature. 2014;511:319–25. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudak JE, Canham SM, Bertozzi CR. Nat. Chem. Biol. 2014;10:69–75. doi: 10.1038/nchembio.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxon E, Bertozzi CR. Annu. Rev. Cell Dev. Biol. 2001;17:1–23. doi: 10.1146/annurev.cellbio.17.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Medof ME, Nagarajan S, Tykocinski ML. FASEB J. 1996;10:574–586. doi: 10.1096/fasebj.10.5.8621057. [DOI] [PubMed] [Google Scholar]
- 8.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. J. Am. Chem. Soc. 2008;130:5947–5953. doi: 10.1021/ja710644g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belardi B, Odonoghue GP, Smith AW, Groves JT, Bertozzi CR. J. Am. Chem. Soc. 2012;134:9549–9552. doi: 10.1021/ja301694s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang ML, Godula K. ACS Chem. Neurosci. 2014;5:873–5. doi: 10.1021/cn500194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulsipher A, Griffin ME, Stone SE, Hsieh-Wilson LC. Angew. Chemie. 2015;127:1486–1490. doi: 10.1002/anie.201409258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemieux GA, Bertozzi CR. Trends Biotechnol. 1998;16:506–513. doi: 10.1016/s0167-7799(98)01230-x. [DOI] [PubMed] [Google Scholar]
- 13.Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. J. Am. Chem. Soc. 2014;136:6794–6797. doi: 10.1021/ja5005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonyarattanakalin S, Martin SE, Dykstra SA, Peterson BR. J. Am. Chem. Soc. 2004;126:16379–16386. doi: 10.1021/ja046663o. [DOI] [PubMed] [Google Scholar]
- 15.Hymel D, Peterson BR. Adv. Drug Deliv. Rev. 2012;64:797–810. doi: 10.1016/j.addr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxfield FR, McGraw TE. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 17.Singh RD, Liu Y, Wheatley CL, Holicky EL, Makino A, Marks DL, Kobayashi T, Subramaniam G, Bittman R, Pagano RE. J. Biol. Chem. 2006;281:30660–30668. doi: 10.1074/jbc.M606194200. [DOI] [PubMed] [Google Scholar]
- 18.Lv W, Liu L, Luo Y, Wang X, Liu Y, Colloid Interface Sci J. 2011;356:16–23. doi: 10.1016/j.jcis.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Liu D, Zhou B, Deng J, Yang W. React. Funct. Polym. 2012;72:832–838. [Google Scholar]
- 20.Boonyarattanakalin S, Athavankar S, Sun Q, Peterson BR. J. Am. Chem. Soc. 2006;128:386–387. doi: 10.1021/ja056126j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boonyarattanakalin S, Hu J, Dykstra-Rummel SA, August A, Peterson BR. J. Am. Chem. Soc. 2007;129:268–269. doi: 10.1021/ja067674f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Q, Cai S, Peterson BR. J. Am. Chem. Soc. 2008;130:10064–10065. doi: 10.1021/ja803380a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godula K, Bertozzi CR. J. Am. Chem. Soc. 2010;132:9963–9965. doi: 10.1021/ja103009d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, et al. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16101–6. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boonyarattanakalin S, Martin SE, Sun Q, Peterson BR. J. Am. Chem. Soc. 2006;128:11463–11470. doi: 10.1021/ja062377w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayor S, Presley JF, Maxfield FR, Cell Biol J. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stutzin A. FEBS Lett. 1986;197:274–280. doi: 10.1016/0014-5793(86)80341-6. [DOI] [PubMed] [Google Scholar]
- 28.Paszek MJ, Boettiger D, Weaver VM, Hammer DA. PLoS Comput. Biol. 2009:5. doi: 10.1371/journal.pcbi.1000604. DOI 10.1371/journal.pcbi.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frisch SM, Francis H, Cell Biol J. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giancotti FG, Ruoslahti E. Science (80−. ) 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 31.Boettiger D. Curr. Opin. Cell Biol. 2012;24:592–599. doi: 10.1016/j.ceb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Eng ET, Smagghe BJ, Walz T, Springer TA. J. Biol. Chem. 2011;286:35218–35226. doi: 10.1074/jbc.M111.275107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng Y, Xie X, Walker S, White DT, Mumm JS, Cowell JK. BMC Cancer. 2013;13:453. doi: 10.1186/1471-2407-13-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Nat. Rev. Drug Discov. 2011;10:221–237. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.