Abstract
C-reactive protein (CRP), an innate immune mediator, is elevated in the circulation prior to symptoms in patients with preeclampsia (PE), a severe hypertensive pregnancy disorder with high mortality and morbidity. However, the specific sources underlying increased CRP and the role of elevated CRP in PE are undefined. Here, we report that circulating CRP levels are significantly increased in a large cohort of normotensive pregnant individuals compared to nulligravid women and is further increased in PE patients. These findings led us to further discover that placental syncytiotrophoblasts are previously unrecognized cellular sources of CRP and underlie elevated CRP in normotensive pregnant women and the additional increase in PE patients. Next, we demonstrated that injection of CRP induces PE features including hypertension (157.08 mmHg CRP treated vs. 118.99 mmHg control), proteinuria (35.0 mg/μg CRP treated vs. 14.1 mg/μg control), kidney and placental damage and increased levels of sFlt-1 in pregnant mice but not nonpregnant mice. We hypothesize that phosphocholine transferase, a placental specific enzyme posttranslationally modifying neurokinin B (NKB), is essential for the pathogenic role of CRP in PE through activation of the neurokinin 3 receptor. Overall, our studies have provided significant new insight regarding the pathogenic role of CRP in PE and highlighted innovative therapeutic strategies.
Keywords: Pre-Eclampsia, Inflammation, Acute-Phase Proteins, Kinins, Placental Circulation
INTRODUCTION
Preeclampsia (PE) is a serious disease of pregnancy affecting approximately 8% of pregnant women and accounts for more than 50,000 maternal deaths worldwide per year. The key symptoms of PE are hypertension and proteinuria, and the disease is frequently associated with intrauterine growth restriction (IUGR), a condition that puts the fetus at risk for numerous cardiovascular disorders later in life. Thus, PE is a leading cause of maternal and neonatal morbidity and mortality and has both acute and long-term impact on moms and babies. Despite substantial research efforts, the etiology and pathogenesis of PE remain poorly understood and the clinical management of PE is hampered due to lack of pre-symptomatic screening, reliable diagnostic tests and effective therapy. The only effective treatment is delivery of the fetus, often resulting in serious complications of prematurity for the neonate. Thus, understanding the mechanisms involved in the pathogenesis of PE is extremely urgent and important for early detection and safe and effective therapeutic strategies for disease.
Of the prevailing hypotheses underlying PE, inflammatory mediators have been implicated in the pathophysiology of this disease. An acute innate immune mediator, C-reactive protein (CRP), is a pentamer with approximately 26 kDa monomeric subunits noncovalently bound to each other. CRP production is stimulated by the potent cytokines IL-6/8 as well as TNF-α1, 2 and is predominantly produced and secreted by liver. Although CRP is largely thought to work with complement components to assist in the removal of foreign pathogens by binding with phosphocholine on the membrane of the pathogen, a growing body of studies suggest that prolonged elevated CRP links with cardiovascular risk and likely play a detrimental role in disease development by targeting on host tissues or signaling inflammatory cells to the site of insult.3-5 For example, recent studies have demonstrated that elevated CRP is associated with renal dysfunction and atherosclerosis secondary to intimal arterial damage.6-9 Moreover, persistently elevated CRP is correlated to progressive decline of kidney function in patients with chronic kidney disease likely due to deposition of CRP in glomeruli and subsequent increase in oxidative stress. Of note, early studies showed that CRP directly induces endothelial damage by increasing foam cell formation and subsequently leading to atherosclerosis.10, 11 Notably, previous human studies report that circulating CRP is elevated in preeclamptic patients and its elevation is correlated to the clinical symptoms.12, 13 Moreover, plasma CRP is found to be elevated prior to maternal disease development in patients with PE,12, 14, 15 implicating that CRP is likely a pre-symptomatic biomarker for early detection. However, the functional role of circulating CRP in PE remains unknown.
Intriguingly, recent studies show that neurokinin B (NKB), a known pathogenic molecule of PE, is posttranslationally modified by a phosphocholine transferase (PCT; i.e. PCYT1b).16, 17 Posttranslational modification (PTM) is an essential biological mechanism to control fundamental cellular and systemic functions. Modifications of biological molecules typically serve to amplify or diminish activity of the molecule, or alter their ligand-receptor relationship. Phosphorylation, glycosylation, ubiquitination, methylation, and acetylation are common and wel-recognized PTM. However, phosphocholination modification has only recently begun to be studied and is poorly understood. At this moment, phosphocholine modification carried by PCT is only detected in two tissues, placenta and testis.18 One of the endogenous phosphocholinated molecules is NKB and phosphocholine modified NKB (PC-NKB) preferentially activates neurokinin 3 receptor (NK3R), a Gq coupled transmembrane receptor.16, 17 The NK3R has been hypothesized to be instrumental in the development of hypertension in pregnancy, however, due to the pleiotropic nature of NKB, the exact mechanism of specific activation of the NK3R has not been elucidated. In view of the facts that 1) NKB is produced predominantly from placentas and contributes to PE by activating NK3R, 2) NKB is phosphocholinated, 3) placenta is one of two tissues expressing PCT, and 4) CRP is elevated in PE and known to bind with phosphocholine, we hypothesize that elevated CRP is not just an early biomarker but likely a pathogenic factor contributing to PE by binding to phosphocholinated NKB and preferentially activating NK3R.
Results
Placenta is an additional source for increased circulating CRP in preeclamptic patients
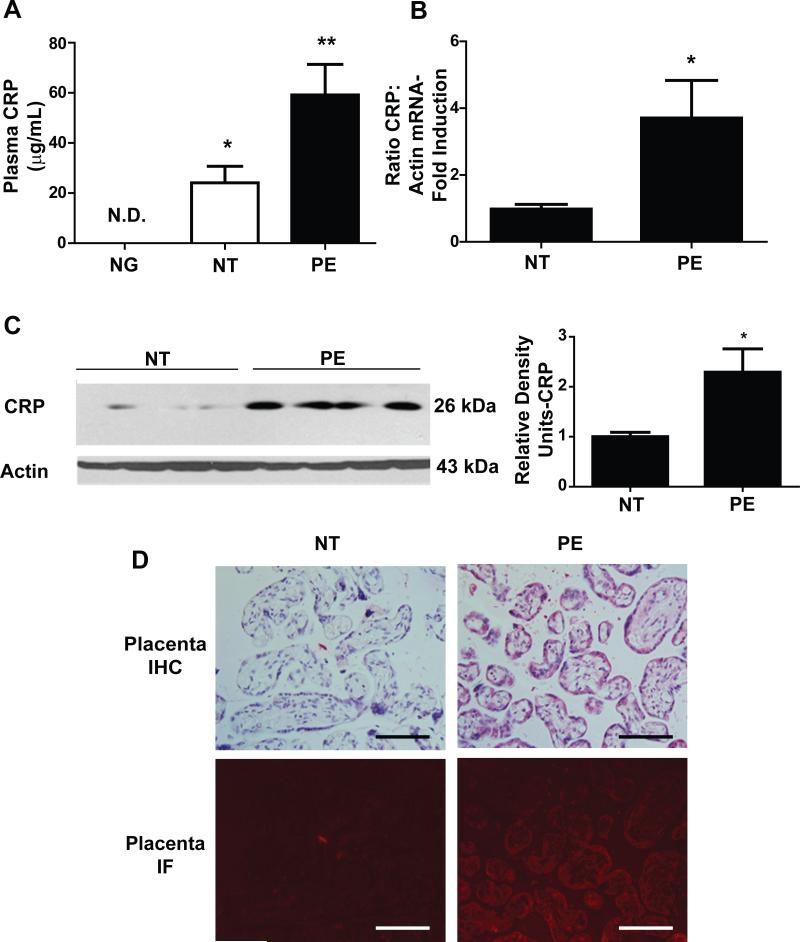
Although CRP is increased in the circulation under PE conditions, the specific cell type responsible for its elevation remains largely unknown. In an effort to determine the potential source for increased circulating CRP under PE conditions, we first measured circulating CRP in a cohort human blood samples including nulligravid women (NG=21), normotensive pregnant women (NT=15) and preeclamptic patients (PE=15) (Detail see Supplementary Table 1). Intriguingly, the CRP level in sera of the nulligravid women was below the standard detectable threshold provided by the assay. However, circulating CRP level was significantly increased in the normal pregnant women compared to nulligravid individuals and further increased in the preeclamptic women compared to NT (Figure 1A). Although CRP is predominantly produced by the liver under nonpregnant state, our studies suggest that the placenta is likely an additional source for increased circulating CRP seen in NT and further increased CRP seen in PE setting.
Figure 1. Placenta expresses CRP transcripts and syncytiotrophoblast cells are additional source for increased circulating CRP in PE patients.
(A) CRP levels in the sera of a large cohort of women including PE patient (n=15), normotensive pregnant women, NT (n=15) and nulligravids (NG, n=21). * = p < 0.05 versus NG, ** = p < 0.05 versus NT (B) qRT-PCR showed the presence of CRP mRNA in term placental tissue lysates. PE patients (n=6) have a 4-fold increase in CRP mRNA copies versus the NT cohort (n=6). * = p < 0.05 (C) Western blotting confirmed an increased presence of CRP in PE patient placental tissue. (D) Placental tissue isolated from PE patients showed significant CRP in the villus trophoblast cells of terminal placental villi. This was confirmed on immunofluorescence through a dual-staining protocol. (40x magnification; Scale bar = 100 μm).
To test this intriguing possibility, we first conducted quantitative RT-PCR to determine if CRP transcripts are expressed in the placentas. We found that CRP mRNA was present in the placentas of NT individuals and that CRP mRNA levels significantly increased around 4-5 fold in placentas from women with PE (Fig. 1B). Next, we performed western blot analysis and found that CRP protein levels were also increased in PE compared to the NT placentas (Fig. 1C). Moreover, immunohistochemistry and immunofluorescence dual-staining demonstrated that CRP was localized in the villus syncytiotrophoblast cells, around the villus border (Fig. 1D). Thus, we have shown that 1) syncytiotrophoblast cells in the placenta express CRP and they are a significant source contributing to increased circulating CRP under normal pregnancy state; 2) elevated placental CRP is an additional source responsible for substantial increased CRP under PE conditions.
CRP induces pathophysiology of PE in pregnant mice but not nonpregnant mice
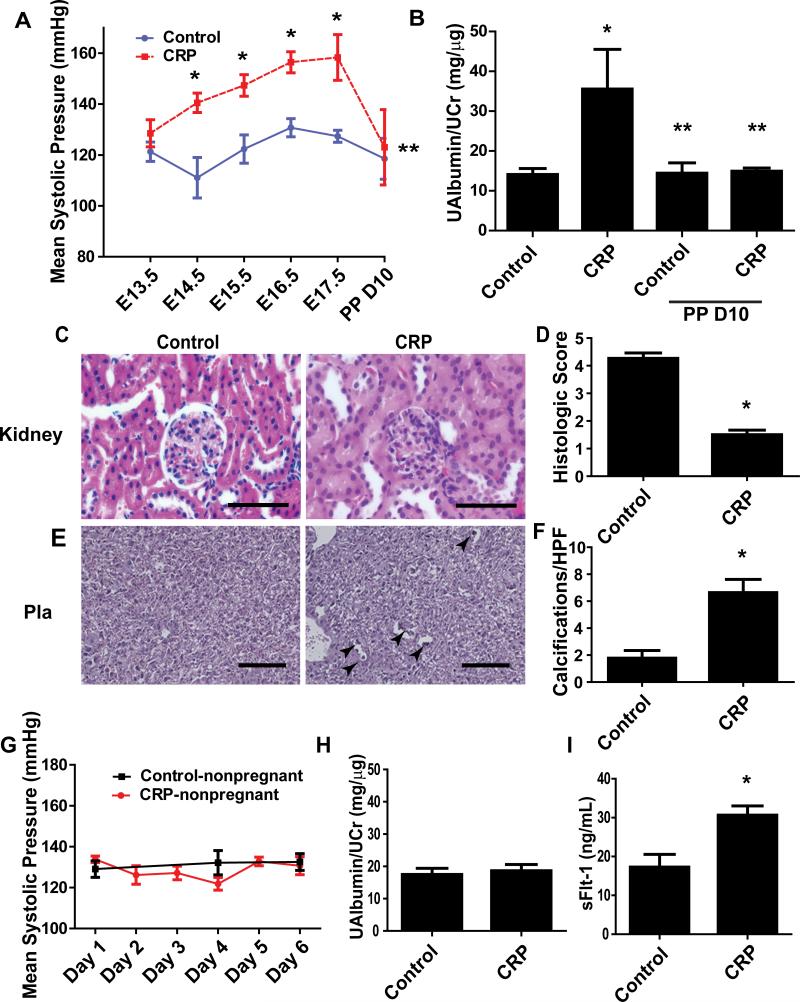
To examine the pathogenic role of CRP in PE, we infused CRP into pregnant C57BL/6 mice on E13 and E14 of their gestation period to achieve a similar concentration as PE patients. Our data indicate that a pathological concentration of CRP seen in PE patients at term (75 μg/mL; based on the upper standard deviation detectable in our circulating CRP ELISA; Fig. 1A) was sufficient to cause an elevation in mean systolic pressure in CRP-infused mice (157.08 mmHg vs. 118.99 mmHg control) (Fig. 2A). To verify the level of CRP injected into mice, sera was sampled at the endpoint of the experiment on E18 and measured by ELISA. We observed a mean level of 11.6 μg/mL of circulating CRP in our mice. The level of CRP on E18 is consistent with extrapolated predictions of two injections of 75 μg/mL on E13/14 (Supplementary Fig. 1). Proteinuria was also significantly elevated in CRP-infused mice (Fig. 2B). Similar to PE patients, both hypertension and proteinuria were significantly dropped to the basal levels postpartum (Fig. 2A-B). Additionally, histological studies demonstrated the typical glomerular damage featured with decreased capillary lumen space and narrowing Bowman's capsule space (Fig. 2C). Histologic glomerular scoring, indicating an overall decrease in the health of the glomerulus in the outer renal cortical region (Fig. 2D). Furthermore, histological studies showed that increased placental damage characterized with increased placental calcification in the CRP-infused pregnant mice (Fig. 2E-F). Altogether, we provide the first evidence that CRP infusion directly induces pathophysiology of PE in pregnant mice as seen in PE patients.
Figure 2. CRP induces features of PE—hypertension, proteinuria, renal and placental damage in pregnant mice but not nonpregnant mice.
(A) 75 ug/mL IV injections on E13/E14 induced mean systolic pressure elevation by E14.5 and dropped postpartum day 5-10. * = p < 0.05; ** = p <0.05 from CRP E17.5 (n=5-8) (B) Microalbuminuria/creatinine ratio assayed by ELISA was significantly increased in the mice injected with CRP but diminished by postpartum D10. * = p < 0.05; ** = p < 0.05 vs. CRP peri-partum (n=5-8) (C) Oil-immersion microscopy showed CRP injection resulted in impaired glomerular histological condition. (100x magnification; scale bar = 50 μm) (D) Blinded quantification of glomerular sections revealed a decrease in glomerular score of CRP injected murine renal histological sections (n=10 fields per kidney; 7 animals). * = p < 0.05 (E) Analysis of placental sections at 20x microscopy reveals significant placental damage as reflected by an increase in placental calcification and scarring in placentas of CRP injected mice (Arrows: indicate placental calcification; scale bar = 200 μm). (F) Confirmation by random high-powered field quantifications of placental sections (n=10 fields per placenta; 7 animals). * = p < 0.05. (G) Nonpregnant mice injected with equivalent CRP concentrations (n=5) exhibited no elevation in mean systolic pressure versus nonpregnant mice injected with IV saline (n=10). (H) Microalbumin/creatinine ratio was not significantly altered in CRP injected nonpregnant mice relative to saline injected controls. (n=5-10) (I) sFlt-1 level in the circulation was induced by CRP injection in pregnant mice. * = p < 0.05.
Next, to determine whether CRP-induced PE pathophysiology is dependent on pregnancy, we infused similar concentrations of CRP into nonpregnant mice. The key features of PE including hypertension and proteinuria were not observed in CRP-infused nonpregnant mice (Fig 2G-H). sFlt-1, a pathogenic factor, is predominantly produced in the placentas and known to be induced by inflammatory factors 19, 20. Thus, CRP may be a previously unrecognized factor contributing to increased sFlt-1 production in the placentas and subsequently leading to PE. Supporting this possibility, we found that sFlt-1 levels in the sera were significantly increased in the pregnant mice with infusion of CRP compared to the control pregnant mice (Fig. 2I). In contrast, sFlt-1 levels were extremely low and no difference observed in the nonpregnant mice with or without CRP infusion (data not shown). Thus, we demonstrated that CRP is a novel pathogenic factor contributing to pathophysiology of PE including hypertension, proteinuria, kidney and placental damage and increased sFlt-1 secretion.
Antagonism of NK3R or specific knockdown of NK3R attenuates CRP-induced pathophysiology of PE in pregnant mice
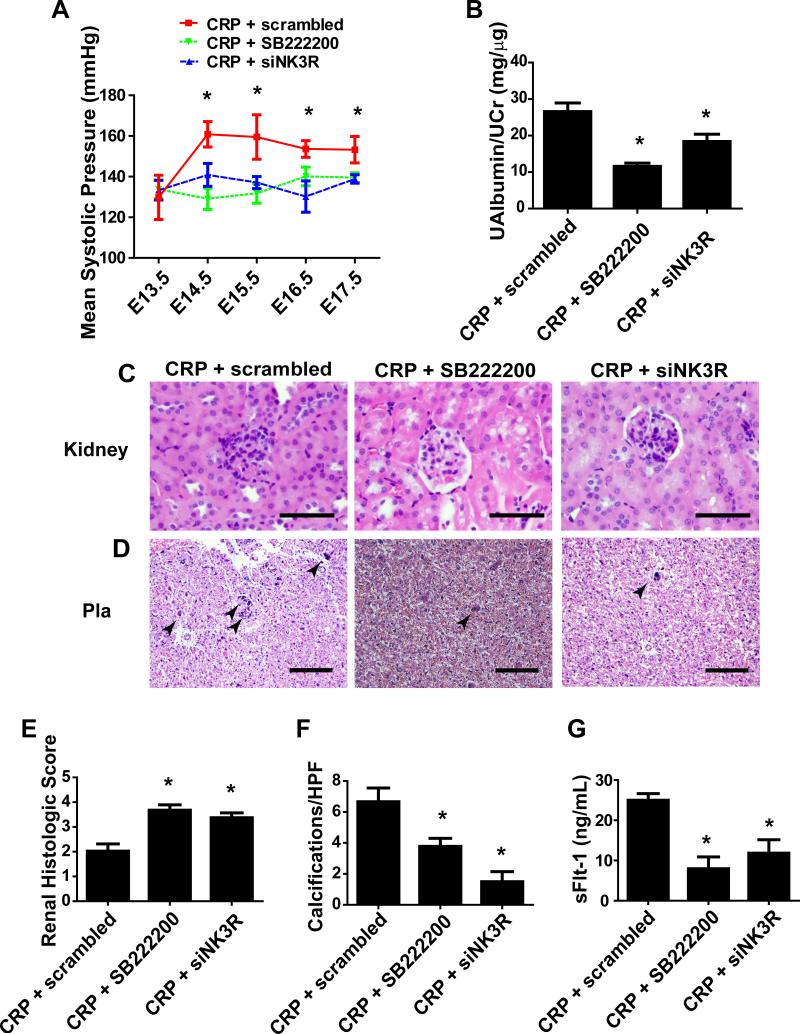
Because CRP binds with phosphocholine and phosphocholinated NKB (PC-NKB) preferentially activates NK3R, it is possible that CRP-induced pathophysiology is dependent on NK3R activation. To test this intriguing possibility, we treated CRP-infused mice with or without NK3R specific inhibitor, SB222200. We found that SB222200 treatment significantly reduced mean systolic pressure and proteinuria in CRP-infused pregnant mice (Fig 3A-B). There was a marked reduction in glomerular damage as indicated by an overall improvement tubular diameter, Bowman's capsule diameter and glomerular scoring (Fig. 3C/E). We also observed a decrease in placental calcifications of CRP-infused pregnant mice with SB222200 treatment (Fig. 3D/F). Finally, treatment with SB222200 decreased CRP-induced sFlt-1 levels in the pregnant mice (Fig. 3G).
Figure 3. Pharmacologic antagonism or in vivo siRNA knockdown of NK3R attenuates systolic pressure, proteinuria, placental and kidney damage, sFlt-1 production.
(A) Co-injection of SB222200 prevented CRP induced mean systolic pressure of pregnant mice when administered on E13/E14. Administration of nanoparticle-encapsulated siRNA with CRP on E13/14 also reduced the CRP induced mean systolic pressure of the pregnant mice. * = p < 0.05 CRP + scrambled vs. CRP + siNK3R and CRP + SB222200; (n=5-8) (B) Cotreatment with either SB222200 or NK3R siRNA reduced microalbuminuria/creatinine ratio. * = p < 0.05; (C) Glomerular damage was significantly attenuated by coadministration of SB222200 or NK3R siRNA as shown by H&E stained renal sections. (100x magnification; scale bar = 50 μm) (D) Placental damage was attenuated by cotreatment of SB222200 or NK3R siRNA, as indicated by reduction of placental calcifications and scarring shown on H&E placental sections. (20x magnification; scale bar = 200 μm) (E) Histologic scoring of glomerular damage based on double-blind scoring criteria (n=10 fields per kidney; 7 animals). (F) Quantification of placental calcifications based on blinded image analysis (Arrows: indicate placental calcification; n=10 fields per placenta; 7 animals). * = p < 0.05 (G) sFlt-1 production is significantly attenuated in pregnant mice with co-administration of SB222200 or siRNA for NK3R. * = p < 0.05
To further validate our pharmacological studies, we performed an in vivo knockdown of the NK3R via encapsulation of siRNA specific for the NK3R by a nanoparticle delivery system (Altogen). First, we demonstrated that siRNA specific for NK3R significantly reduced more than half of NK3R protein levels in the placentas compared to the scrambled siRNA in the CRP-infused pregnant mice (Supplementary Fig. 2A). In contrast, the efficiency of knockdown of NK3R in the kidneys was less evident compared to the placental tissues (Supplementary Fig. 2B). Thus, we concluded from these results that siRNA specifically for NK3R successfully reduced NK3R in the placentas but not kidneys in the CRP-infused pregnant mice. Next, we found that knockdown of NK3R more than half by specific siRNA was sufficient to attenuate mean systolic pressure and proteinuria in CRP-infused pregnant mice compared to the pregnant mice with nanoencapsulated scrambled RNA (Fig 3A). Furthermore, CRP-induced placental calcifications, kidney damage and increased circulating sFlt-1 levels were significantly attenuated by specific NK3R siRNA knockdown in pregnant mice (Fig. 3C-G). Thus, both pharmacological studies using specific NK3R antagonist and quasi-genetic studies using siRNA to specific knockdown of NK3R provide strong in vivo evidence that CRP-induced PE pathophysiology is signaling via NK3R.
Knockdown of phosphocholine transferase ameliorates CRP-induced PE features in pregnant mice
Because NKB is modified by placental phosphocholine transferase (PCT) (i.e. PCYT1b) and PCNKB preferentially activates NK3R, it is possible that CRP-mediated activation of NK3R and subsequent disease development are dependent on the placental PCT. To overcome the difficulty of lack of a potent and specific inhibitor for PCT, we performed quasi-genetic studies using nanoparticle encapsulated siRNA specifically to knockdown the synthesis of this important enzyme in CRP-infused pregnant mice. First, we confirmed that siRNA specific for PCT significantly reduced mRNA of this enzyme in the placentas of CRP-infused mice compared to the scrambled siRNA (Fig. 4A). Additionally, knockdown of PCYT1b by specific siRNA for PCT significantly attenuated mean systolic pressure and proteinuria in the CRP-infused pregnant mice versus the CRP-infused pregnant mice injected with scrambled siRNA (Fig. 4A-B). Furthermore, CRP-induced placental calcifications, kidney damage and increased circulating sFlt-1 levels were significantly attenuated by specific PCT siRNA knockdown in pregnant mice (Fig. 4C-G). Thus, quasi-genetic studies using siRNA to specifically knockdown PCT revealed that placental PCT, which is a key enzyme responsible for NKB phosphocholination, is essential for CRP-induced PE pathophysiology.
Figure 4. In vivo siRNA knockdown of PCT (PCYT1b) attenuated systolic pressure, proteinuria, placental and kidney damage, sFlt-1 production.
(A) Confirmation of knockdown is shown by qRT-PCR on placental lysates (n=5). Administration of nanoparticle-encapsulated siRNA for PCYT1b with CRP on E13/14 reduced the mean systolic pressure of the pregnant mice. * = p < 0.05 (B) Cotreatment of PCYT1b siRNA reduced microalbuminuria/creatinine ratio. * = p < 0.05 (C) Glomerular damage was significantly attenuated by coadministration of PCYT1b siRNA as shown by H&E stained renal sections. (100x magnification; scale bar = 50 μm) (D) Placental damage was attenuated by cotreatment with PCYT1b siRNA, as indicated by reduction of placental calcifications and scarring shown on H&E placental sections. (20x magnification; scale bar = 200 μm) (E) Histologic scoring of glomerular damage based on double-blinded scoring criteria (n=10 fields per kidney; 7 animals). * = p < 0.05 (F) Quantification of placental calcifications based on blinded image analysis (n=10 fields per placenta; 7 animals). * = p < 0.05 (G) CRP induced sFlt-1 production was significantly attenuated in pregnant mice with coadministration of SB222200 or siRNA for PCYT1b. * = p < 0.05
Elevated CRP and NKB are co-localized in syncytiotrophoblast cells of placentas of PE patients
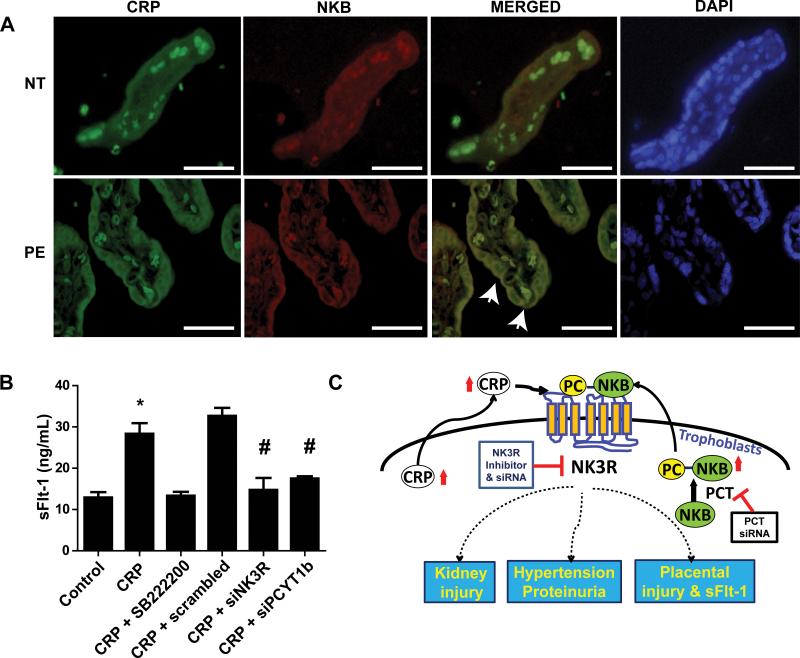
To extend our mouse findings to human, we performed coimmunofluorescence staining to determine the localization of CRP and NKB in the term placentas from NT pregnant women and PE patients. Specifically, we found that CRP and NKB were observed in the syncytiotrophoblast cells of the maternal villi and substantially increased in the placentas of PE compared to NT pregnant women (Fig. 5A). Additionally, co-localization of CRP and NKB was visualized along the cellular membrane of the villus syncytiotrophoblast cells (Fig. 5A). It is interesting to note that the CRP and NKB were extranuclear and mainly outside of the cytoplasm of the trophoblast cells. Thus, these translational human studies demonstrated that CRP and NKB are elevated and colocalized in the syncytiotrophoblast cells in human placentas from PE patients.
Figure 5. CRP and NKB are localized in the syncytiotrophoblast cells of placentas of PE patients and CRP directly induces sFlt-1 secretion from cultured term human villous explants via NK3R signaling in a PCT-dependent manner.
(A) Coimmunofluorescence revealed increased CRP and NKB in syncytiotrophoblast cells in terminal villi. On the merged image, membrane colocalization was visualized along the syncytiotrophoblast cells. Some autofluorescence can be seen intravascularly due to the presence of autofluorescent RBCs. (40x magnification; scale bar = 100 μm). (B) Placental villus explants isolated from normal patients and treated with or without CRP in the presence or absence of series of drugs or siRNA. CRP directly induced secretion of sFlt-1 from cultured human villus explants, but this increase was attenuated by treatment with SB222200 or siRNA for NK3R or PCYT1b. * = p < 0.05 difference from control; # = p < 0.05 difference from CRP + scrambled. (n = 4 wells placental villous explant culture) (C)Working model: CRP is expressed in the placentas and elevated CRP in the syncytiotrophoblast cell is additional source underlying increased circulating CRP in PE patients. Locally-synthesized CRP cross-talks with post-translationally modified phosphocholinated NKB (PC-NKB) by placental-specific enzyme, PCT. Subsequently, CRP and PC-NKB work together preferentially activates NK3R leading to increased sFlt-1 secretion. Our findings reveal novel mechanisms for pathogenesis of PE and identify innovative therapeutic possibility for prevention and treatments.
CRP signaling via NK3R directly induces sFlt-1 secretion from cultured human placental villus explants in a PCT-dependent manner
Although we showed that CRP-induced hallmark features including hypertension, proteinuria and increased circulating sFlt-1 levels in an intact animal by activating NK3R and in a PCT-dependent manner, the pathological role of CRP in humans remains unidentified. To test the significance of CRP in PE in humans, we took advantage of primary cultured human villous explants to determine if CRP signaling via NK3R contributes to PE by directly inducing sFlt-1 secretion in a PCT-dependent manner. Similar to mouse finding, we found that CRP treatment directly induced sFlt-1 secretion from cultured human villous explants from NT pregnant women at term (Fig. 5B). Moreover, SB222200, a specific inhibitor of NK3R, and siRNA specific for NK3R significantly reduced CRP-induced sFlt-1 secretion from cultured human villous explants (Fig.5B). Finally, siRNA specific for PCT also significantly reduced CRP-induced sFlt-1 secretion from cultured human villous explants (Fig. 5B). Taken together, these results translate our mouse finding to human pregnancy by showing that CRP signaling via NK3R contributes to sFlt-1 secretion in a PCT-dependent manner from cultured human villous explants.
DISCUSSION
CRP is increased in the circulation at early stage prior to PE symptoms and its elevation is correlated to the disease severity. However, nothing is known about its role in PE, the specific cell types responsible for its increased production and the molecular basis for its function. Here we have provided human evidence showing that CRP transcripts are present in syncytiotrophoblast cells of normal placentas and further increased in the PE placentas, indicating that syncytiotrophoblast cells in the placenta are a previously unrecognized additional source for increased circulating CRP seen in PE patients. Extending human studies we have further demonstrated the pathogenic nature of CRP in PE by showing that injection of CRP alone is sufficient to regenerate the key features of PE including hypertension, proteinuria, kidney damage and impaired placentas in pregnant mice. Mechanistically, we revealed CRP signaling via NK3R contributes to sFlt-1 secretion and disease development in PCT-dependent manner in intact animals and in cultured human villous explants. Overall, we have provided both human and mouse evidence that increased CRP contributes to PE pathophysiology by cross-talking with PCT and NK3R. Thus, our findings have identified detrimental role of CRP, new sources for its production and novel signaling cascade for its pathogenic effects in PE and immediately suggest novel therapeutic opportunities.
CRP is predominantly produced by hepatocytes under nonpregnant states. Prior to our studies it was unclear what specific cell type was responsible for increased circulating CRP in PE patients. Here we demonstrated for the first time that placentas contain transcripts for CRP and CRP transcript levels are significantly increased in the placentas of PE patients compared to NT pregnant individuals. However, CRP levels are virtually undetectable in nulligravid women. Using immunohistological studies, we further found that CRP is predominantly expressed in syncytiotrophoblast cells of human placentas. Thus, we provide human evidence that placental syncytiotrophoblast cell is a previously unrecognized cell type expressing CRP and it is additional source contributing to circulating CRP in PE patients. Understanding how CRP gene expression is regulated under physiological and pathological pregnancy will be important questions for us to further address.
CRP is an innate immune factor and works with complement components to kill the bacteria and virus or remove damaged tissues. Because of its early rise in the inflammatory process, CRP is often considered as an important early predictor for the immune response. Although the transient elevation of CRP in response to the presence of pathogens is beneficial, the prolonged and persistent elevation of CRP is likely harmful resulting in damage to host tissues.4, 5, 7, 10, 21, 22 Some studies have suggested that obesity and increasing BMI, correlated with late-onset PE, are key factors in increasing CRP and those cytokines instrumental in its production and release.23
However, multiple human studies have shown that circulating CRP is elevated at the early stage prior to clinical symptoms in PE patients and its level is correlated to the severity of the disease.3, 24 Moreover, several studies indicate that the elevation of circulating CRP is correlated to multiple pathogenic factors including TNF-α, IL-6, excess complement production and soluble VEGF, implicating its role in endothelial dysfunction, uterine arterial constriction, macrophage activation, and arterial plaque formation.25 However, the pathogenic role of elevated circulating CRP in PE has not been previously examined in vivo. Here we demonstrated for the first time that injection of CRP to achieve similar concentrations to those seen in PE patients induced hallmark features of PE including hypertension, proteinuria, kidney damage and placental impairment in pregnant mice. Because PE is a pregnancy related disease, the placenta has been long considered to play an important role in the disease development. To examine the role of placentas in CRP-induced hypertension and proteinuria, we injected similar amount of CRP into nonpregnant as pregnant mice. In contrast to pregnant mice, CRP failed to induce PE features in nonpregnant mice. These findings are consistent with reports of LaMarca and colleagues showing that TNF-α, IL-6, and IL-17 can only induce high blood pressure in pregnant rats, not in nonpregnant rats.26-28 We further discovered that CRP induces sFlt-1 production in pregnant mice but not nonpregnant mice, suggesting the placenta as the source of increased sFlt-1. Extending our mouse studies, we have demonstrated that CRP directly induces sFlt-1 secretion from cultured human villous explants. Elevated sFlt-1 contributes to pathophysiology of PE including abnormal placentation, hypertension and kidney injury.29 Thus, a possible explanation for CRP only inducing PE features in pregnant mice but not nonpregnant mice is that CRP only induces secretion of placental derived toxic factor, sFlt-1, during pregnancy. Taken together, our studies revealed that elevated CRP is a potent immune mediator responsible for increased sFlt-1 secretion and impaired placentas. Without interference, CRP-sFlt-1-placental damage functions as malicious cycle leading to progression of the disease and symptom development (Fig. 5C).
NKB is mainly secreted from brain and placentas. Early human studies found that circulating NKB is significantly elevated in PE patients.30-32 More recent studies showed that endogenous NKB is modified by phosphocholine transferase (PCT). The phosphocholinated NKB (PC-NKB) preferentially activates NK3R, a Gq coupled receptor. As such, elevated NKB activating NK3R induces calcium influx and subsequent vascular hypertension and kidney damage. During infection, CRP binds with phosphocholine on the membrane of bacteria and virus and works with complement systems and inflammatory cells together to eventually kill foreign invaders. Here we have provided multiple lines of evidence supporting a novel but compelling molecular basis that under PE condition in the absence of infection, increased CRP signaling via NK3R contributes to PE pathophysiology in a PCT-dependent manner. First, we have provided in vivo animal evidence that CRP-induced PE pathophysiology is significantly reduced by blocking NK3R signaling by its specific antagonist or by lowering its protein levels by its specific siRNA. Subsequently, using quasi-genetic studies to specific knockdown PCT, CRP-induced PE features in the pregnant mice are significantly ameliorated. Thus, both pharmacological and quasi-genetic studies provide strong in vivo functional evidence that PCT mediated NKB phosphocholination, and NK3R signaling underlies CRP-induced pathogenic effects in pregnant mice.
We have further validated our mouse finding and provided human evidence showing that NKB and CRP are co-localized in membrane of syncytiotrophoblast cells of villous human placentas and their levels were significantly increased in the placentas of PE patients compared to the NT. Similar to our mouse finding, we have demonstrated that CRP signaling via NK3R directly induces sFlt-1 secretion from cultured human villous explants in a PCT-dependent manner. PCT is only expressed in two organs including placentas and testis. So far, two endogenous molecules are identified to be modified by PCT, i.e., NKB and corticotropin releasing factor (CRF).16 PCT-mediated PTM of these two molecules is considered leading to PC-NKB preferentially activating NK3R and preventing PC-CRF degradation in circulation, respectively. Because placenta serves as the only source of PCT necessary for posttranslational phosphocholination of NKB, PE features were not observed in nonpregnant CRP-injected mice. Thus, our findings support a novel working model: increased CRP functionally coupled with PCT, a key enzyme carrying posttranslational modification of NKB only occurring in the placentas, preferentially activates NK3R and subsequently promote disease development (Fig. 5C). Interfering with these pathogenic molecules including placental specific PCT and NK3R are promising therapeutic possibilities (Fig.5C). Although our results clearly show that PCT and NK3R signaling are functionally required for pathogenic role of CRP in PE, we have been unable to demonstrate how PCT-mediated PC-NKBs cross-talk with CRP. In view of important role of PCT and NK3R in CRP-induced pathophysiology of PE, one of the most reasonable possibilities is that CRP and PC-NKB directly interact and then this complex preferentially activates NK3B and leads to PE features. Although our human studies have showed that CRP and NKB are co-localized on the membrane of syncytiotrophoblast cells of human placentas, our coimmunoprecipitation pull down assay has been unable to show a direct interaction of these two molecules. Our lack of success may reflect a weak interaction that does not persist through such experiments or low abundance of PC-NKB that does not allow us to pull down the complex. Nevertheless, lacking direct evidence for the interaction of CRP with PC-NKB does not prevent us to conclude that CRP is pathogenic for PE functioning through NK3R signaling in a PCT-dependent manner. Thus, the important and clear evidence of CRP functionally dependent on NK3R and PCT leads to several exciting new directions including determining the molecular mechanisms by which CRP pathogenesis requires PCT and activates NK3R signaling and developing a specific and potent PCT and NK3R inhibitors to treat PE.
In conclusion, our findings are extremely innovative since nothing was known about the role of CRP and placental PCT in PE until we revealed that placental PCT and NK3R are key factors responsible for CRP-induced pathophysiology of PE. Moreover, our discovery that placentas contain CRP transcripts and syncytiotrophoblast cells contribute to increase in circulating CRP in PE patients are also novel. Finally, our findings are clinically significant since we have determined that CRP is not only an early biomarker, but it has a pathogenic role in PE Thus, our current studies have added significant new insight to the pathogenesis of PE, have revealed early pre-symptomatic pathogenic biomarker and thereby have opened up novel therapeutic possibilities for the disease prevention and treatment.
Supplementary Material
PERSPECTIVES.
The work presented here is the first to show a pathogenic role for elevated CRP in PE via activation of NK3R. Additionally, our work also revealed a critical role for placenta-specific phosphocholine transferase (PCTY1b) in CRP-induced PE features. Finally, we have also demonstrated that placenta is additional source contributing to elevated circulating CRP in PE. Significantly, we validate our mouse studies and demonstrated that CRP activates NK3R coupled with PCTY1b contributing to increased production of the anti-angiogenic factor, sFlt-1 in cultured human placental villous explants. Thus, our findings have revealed a CRP coupled with PCTY1b contributes to features of PE in pregnant mice and increased production of sFlt-1 by human placental villous explants by activating NK3R. As such, our work has revealed NKB, PCTY1b and NK3R as potential therapeutic targets for PE.
NOVELTY AND SIGNIFICANCE.
What Is New?
An acute inflammatory mediator, CRP, is elevated in the circulation and placentas of women with PE. Thus, placenta is an additional source contributing to elevated CRP in PE
Injection of recombinant murine CRP into pregnant mice results in increased sFlt-1 production, hypertension and proteinuria via cross-talk with NKB and subsequent activation of NK3R.
A critical role for placenta-specific phosphocholine transferase (PCTY1b) in the modification of NKB was identified in CRP-induced pathophysiology of PE.
CRP-NKB cross-talks with neurokinin 3 receptor (NK3R), increasing sFlt-1 production in cultured human placenta villous explants in an PCTY1b dependent manner
What is relevant?
Our data reveal a previously unrecognized pathogenic role of CRP signaling via NK3B in the pathogenesis of PE dependent on placental specific enzyme, phosphocholine transferase, highlighting potential therapeutic possibilities.
Summary.
We report that CRP, previously thought to be a nonspecific inflammatory mediator, is elevated in circulation of normotensive pregnant women compared to nulligravid women and further elevated in women with PE. Additional experiments determined that CRP is produced by the syncytiotrophoblasts of the placenta. We demonstrated the pathogenic role of CRP by infusion of recombinant CRP into pregnant mice to show features of PE, including hypertension and proteinuria. Furthermore, by antagonism or knockdown of the NK3R using pharmacologic or siRNA methodology, we found that this receptor is essential for the CRP-induced features of PE including hypertension, proteinuria, placental and kidney pathology. Additionally, we found that the placenta-specific enzyme, phosphocholine transferase (PCTY1b), was required for the CRP-induced pathophysiology in pregnant mice. Finally, we validated our mouse studies in human and showed that CRP induced sFlt-1 secretion by isolated human placental villus explants via NK3R activation in a PCTY1b-dependent manner. Altogether, our human and mouse studies reveal CRP cross talks with NKB signaling contributes to pathophysiology of PE in a PCTY1bdepenent manner, and thereby highlight potential therapeutic possibilities.
Acknowledgments
Sources of Funding
This work was supported by National Institute of Health Grants HL119549 (to Y.X.), TL1 TR000369 (to N.F.P), RC4HD067977 and HD34130 (to Y.X and R.E.K.) and by China National Science Foundation 81228004 (to Y.X.).
Footnotes
Conflict of Interest/Disclosures
The authors have no contributable conflicts of interest and nothing to disclose.
References
- 1.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for c-reactive protein on leukocytes is fcgamma receptor ii. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derzsy Z, Prohaszka Z, Rigo J, Jr., Fust G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2010;47:1500–1506. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Boij R, Svensson J, Nilsson-Ekdahl K, Sandholm K, Lindahl TL, Palonek E, Garle M, Berg G, Ernerudh J, Jenmalm M, Matthiesen L. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol. 2012;68:258–270. doi: 10.1111/j.1600-0897.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 5.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting c-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–1221. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 6.Castellano G, Di Vittorio A, Dalfino G, Loverre A, Marrone D, Simone S, Schena FP, Pertosa G, Grandaliano G. Pentraxin 3 and complement cascade activation in the failure of arteriovenous fistula. Atherosclerosis. 2010;209:241–247. doi: 10.1016/j.atherosclerosis.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 7.Grad E, Danenberg HD. C-reactive protein and atherothrombosis: Cause or effect? Blood Rev. 2013;27:23–29. doi: 10.1016/j.blre.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Grad E, Pachino RM, FitzGerald GA, Danenberg HD. Role of thromboxane receptor in c-reactive protein-induced thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:2468–2474. doi: 10.1161/ATVBAHA.112.256073. [DOI] [PubMed] [Google Scholar]
- 9.Labarrere CA, Zaloga GP. C-reactive protein: From innocent bystander to pivotal mediator of atherosclerosis. Am J Med. 2004;117:499–507. doi: 10.1016/j.amjmed.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Guo F, Liu JT, Wang CJ, Pang XM. Pravastatin inhibits c-reactive protein generation induced by fibrinogen, fibrin and fdp in isolated rat vascular smooth muscle cells. Inflamm Res. 2012;61:127–134. doi: 10.1007/s00011-011-0396-4. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Badiwala MV, Weisel RD, Li SH, Wang CH, Fedak PW, Li RK, Mickle DA. C-reactive protein activates the nuclear factor-kappab signal transduction pathway in saphenous vein endothelial cells: Implications for atherosclerosis and restenosis. J Thorac Cardiovasc Surg. 2003;126:1886–1891. doi: 10.1016/j.jtcvs.2003.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Cetin I, Cozzi V, Papageorghiou AT, Maina V, Montanelli A, Garlanda C, Thilaganathan B. First trimester ptx3 levels in women who subsequently develop preeclampsia and fetal growth restriction. Acta Obstet Gynecol Scand. 2009;88:846–849. doi: 10.1080/00016340902971441. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Hwang JY, Ha EH, Park H, Ha M, Lee SJ, Hong YC, Chang N. Association of maternal folate nutrition and serum c-reactive protein concentrations with gestational age at delivery. Eur J Clin Nutr. 2011;65:350–356. doi: 10.1038/ejcn.2010.267. [DOI] [PubMed] [Google Scholar]
- 14.Cebesoy FB, Balat O, Dikensoy E, Kalayci H, Ibar Y. Ca-125 and crp are elevated in preeclampsia. Hypertens Pregnancy. 2009;28:201–211. doi: 10.1080/10641950802601187. [DOI] [PubMed] [Google Scholar]
- 15.Ertas IE, Kahyaoglu S, Yilmaz B, Ozel M, Sut N, Guven MA, Danisman N. Association of maternal serum high sensitive c-reactive protein level with body mass index and severity of pre-eclampsia at third trimester. J Obstet Gynaecol Res. 2010;36:970–977. doi: 10.1111/j.1447-0756.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 16.Lowry P. 1-o-alkenyl-sn-glyceryl-3-phosphorylcholine may be a novel posttranslational modification used by the placenta. Biopolymers. 2011;96:189–192. doi: 10.1002/bip.21407. [DOI] [PubMed] [Google Scholar]
- 17.Page NM. Neurokinin b and pre-eclampsia: A decade of discovery. Reprod Biol Endocrinol. 2010;8:4. doi: 10.1186/1477-7827-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lykidis A, Baburina I, Jackowski S. Distribution of ctp:Phosphocholine cytidylyltransferase (cct) isoforms: Identification of a new cct splice variant. Journal of Biological Chemistry. 1999;274:26992–27001. doi: 10.1074/jbc.274.38.26992. [DOI] [PubMed] [Google Scholar]
- 19.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sflt-1) production response to placental ischemia/hypoxia: Role of tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol. 2013;304:R130–135. doi: 10.1152/ajpregu.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakada E, Walley KR, Nakada T, Hu Y, von Dadelszen P, Boyd JH. Toll-like receptor-3 stimulation upregulates sflt-1 production by trophoblast cells. Placenta. 2009;30:774–779. doi: 10.1016/j.placenta.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Nillawar AN, Joshi KB, Patil SB, Bardapurkar JS, Bardapurkar SJ. Evaluation of hs-crp and lipid profile in copd. J Clin Diagn Res. 2013;7:801–803. doi: 10.7860/JCDR/2013/5187.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlipak MG. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2002;107:87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 23.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: Effects of maternal body mass index. Cytokine. 2014;S1043-4666(14):00205–1. doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catarino C, Santos-Silva A, Belo L, Rocha-Pereira P, Rocha S, Patricio B, Quintanilha A, Rebelo I. Inflammatory disturbances in preeclampsia: Relationship between maternal and umbilical cord blood. J Pregnancy. 2012;2012:684384. doi: 10.1155/2012/684384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rampersad R, Barton A, Sadovsky Y, Nelson DM. The c5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta. 2008;29:855–861. doi: 10.1016/j.placenta.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 27.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of interleukin 6. Hypertension. 2006;48:711–716. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 28.Dhillion P, Wallace K, Herse F, Scott J, Wallukat G, Heath J, Mosely J, Martin JN, Jr., Dechend R, Lamarca B. Il-17-mediated oxidative stress is an important stimulator of at1-aa and hypertension during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2012;303:R353–358. doi: 10.1152/ajpregu.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page NM, Woods RJ, Gardiner SM, Lomthaisong K, Gladwell RT, Butlin DJ, Manyonda IT, Lowry PJ. Excessive placental secretion of neurokinin b during the third trimester causes pre-eclampsia. Nature. 2005;405:797–800. doi: 10.1038/35015579. [DOI] [PubMed] [Google Scholar]
- 31.Geissbuehler V, Moser R, Zimmermann K, Hillermann R, Czarniecki J, Gebhardt SG, Eberhard J. Altered plasma neurokinin b levels in patients with pre-eclampsia. Arch Gynecol Obstet. 2007;276:151–157. doi: 10.1007/s00404-006-0316-y. [DOI] [PubMed] [Google Scholar]
- 32.Zulfikaroglu E, Ugur M, Taflan S, Ugurlu N, Atalay A, Kalyoncu S. Neurokinin b levels in maternal and umbilical cord blood in preeclamptic and normal pregnancies. J Perinat Med. 2007;35:200–202. doi: 10.1515/JPM.2007.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.