Abstract
Nogo-B was recently identified as a novel vascular marker; the normally high vascular expression of Nogo-B is rapidly lost following vascular injury. Here we assess the potential therapeutic effects of Ad-Nogo-B delivery to injured vessels in vivo. Nogo-B overexpression following Ad-Ng-B infection of vascular smooth muscle cells (VSMCs) was shown to block proliferation and migration in a dose-dependent manner in vitro. We next assessed the effects of Ad-Ng-B treatment on neointima formation in two in vivo models of acute vascular injury. Adventitial delivery of Ad-Ng-B to wire-injured murine femoral arteries led to a significant decrease in the intimal area [0.014 mm2 versus 0.030 mm2 (P = 0.049)] and the intima:media ratio [0.78 versus 1.67 (P = 0.038)] as compared to the effects of Ad-β-Gal control virus at 21 days after injury. Similarly, lumenal delivery of Ad-Ng-B to porcine saphenous veins prior to carotid artery grafting significantly reduced the intimal area [2.87 mm2 versus 7.44 mm2 (P = 0.0007)] and the intima:media ratio [0.32 versus 0.55 (P = 0.0044)] as compared to the effects following the delivery of Ad-β-Gal, at 28 days after grafting. Intimal VSMC proliferation was significantly reduced in both the murine and porcine disease models. Gene delivery of Nogo-B exerts a positive effect on vascular injury-induced remodeling and reduces neointimal development in two arterial and venous models of vascular injury.
Introduction
Neointimal expansion is a major contributory factor in post-angioplasty restenosis1 and in-stent restenosis2 and limits the patency of coronary artery3 and peripheral bypass vein grafts.4 Although the use of drug-eluting stents has offered relief from postangioplasty restenosis, the use of stents is not always appropriate and poses a risk of late thrombosis.5 Additionally, there is currently no established pharmacological treatment aimed at preventing vein graft neointima (NI) formation. Consequently, there remains a need for identifying safe and effective approaches to prevent NI formation.
The Nogo isoforms A, B, and C are members of the superfamily of proteins called reticulons. Whilst Nogo-A and Nogo-C are highly expressed in the central nervous system, with Nogo-C also found in skeletal muscle, Nogo-B is expressed in a ubiquitous pattern, including the central and peripheral nervous systems, spleen, skeletal muscle, testis, heart, and vessel walls.6,7 Nogo-A is a known myelin-associated inhibitor of axonal sprouting8 and, while less is known about Nogo-C, recent studies involving Nogo-B have suggested that it has roles both as a tumor suppressor9,10 and as a regulator of vascular remodeling.11–13 Nogo-B is highly expressed in murine endothelial and vascular smooth muscle cells (VSMC) in vitro and in blood vessels in vivo, and NI formation resulting from wire-injury to the mouse femoral artery was paralleled by a rapid downregulation of Nogo-B.11 Similarly, endogenous Nogo-B expression was rapidly lost in balloon-injured rabbit carotid arteries,12 and was found to be downregulated in human arteries with atherosclerotic lesions, when compared with healthy mammary arteries.13 Nogo-B is not essential for normal vessel development, as evidenced by Nogo-A/B knockout mice [Ng A/B (−/−)]. However, the injury response following wire injury has been shown to be markedly augmented in these mice, and the phenotype can be rescued by adventitial delivery of the Nogo-B gene immediately after injury.11 In vitro experiments using fusion proteins containing the N terminus of Nogo-B have shown that the extracellular domain of Nogo-B not only serves as a chemoattractant for endothelial cells but, while promoting migration of endothelial cells, also dose-dependently blocks platelet-derived growth factor (PDGF)–induced chemotaxis of VSMCs.11
On the basis of these findings we hypothesized that local overexpression of Nogo-B could have a beneficial effect on vascular injury-induced neointimal expansion. This has not been addressed to date in any models of vascular injury. We first investigated the effect of adenoviral gene delivery of Nogo-B (Ad-Ng-B) on proliferation and migration of VSMCs, and then used two in vivo models to assess both arterial and venous injury—wire-injury of the femoral artery in mice, and saphenous vein interposition grafting into the carotid artery in pigs—to elucidate the effect of Nogo-B overexpression on NI formation in vivo. In both models we report a significant decrease in NI formation, associated with decreased cell proliferation. The study data show, therefore, that augmentation of Nogo-B levels in vivo by localized vascular gene delivery is a novel approach toward the prevention of NI formation.
Results
Ad-Ng-B inhibits proliferation of VSMCs in vitro
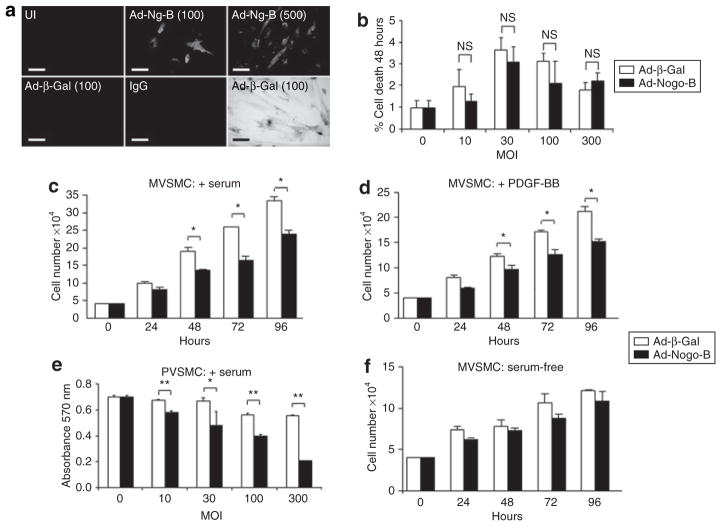
Immunofluorescent staining was used for quantifying the levels of endogenous Nogo-B expression in uninfected porcine VSMCs (PVSMCs). The expression levels increased in a dose-dependent manner following infection with Ad-Ng-B at multiplicities of infection (MOI) of 100 and 500 but not after infection with the Ad-β-Gal control virus (Figure 1a). X-gal staining cells infected with Ad-β-Gal at MOI 100 show the level of VSMC transduction at this MOI (Figure 1a). In view of the fact that previous reports have implicated Nogo-B as being a proapoptotic protein in certain cancer cells9,10,14–16 we next assessed the effect of Ad-Ng-B infection on VSMC apoptosis, using a trypan blue exclusion assay (Figure 1b) and caspase-3/7 assay (data not shown), but did not see any effect on cell death or cell survival as compared to the use of Ad-β-Gal. We next assessed whether Nogo-B affects VSMC proliferation, and observed a significant reduction in the number of viable cells as early as 48 hours after infection with Ad-Ng-B as compared to infection with Ad-β-Gal in proliferating murine VSMCs (MVSMCs) (serum- or PDGF-supplemented media) but not in quiescent cells [serum-free (SF) media], as shown in Figure 1c,d,f. This effect was also observed in PVSMCs at a dose as low as MOI 10, in a dose-dependent manner (Figure 1e). Taken together, these observations indicate that Nogo-B is a potent inhibitor of VSMC proliferation but does not regulate cell death in these cells.
Figure 1. Nogo-B inhibits vascular smooth muscle cell (VSMC) proliferation in vitro.
(a) Nogo-B and β-Gal expression in uninfected (UI), Ad-Ng-B, and Ad-β-Gal-infected [multiplicity of infection (MOI) 100 or 500] porcine VSMCs (PVSMCs). Bar =10 μm. (b) Nogo-B does not affect VSMC death, as demonstrated using a trypan blue exclusion assay. (c–e) Nogo-B blocks VSMC viability in proliferating cells, but not (f) in quiescent cells. (e) This was shown to occur in a dose-dependent manner. Mean value ± SEM of triplicates. *P < 0.05, **P < 0.01 versus Ad-β-Gal. MVSMC, murine VSMC; PDGF-BB, platelet-derived growth factor-BB.
Ad-Ng-B inhibits PDGF-induced migration of VSMCs in vitro
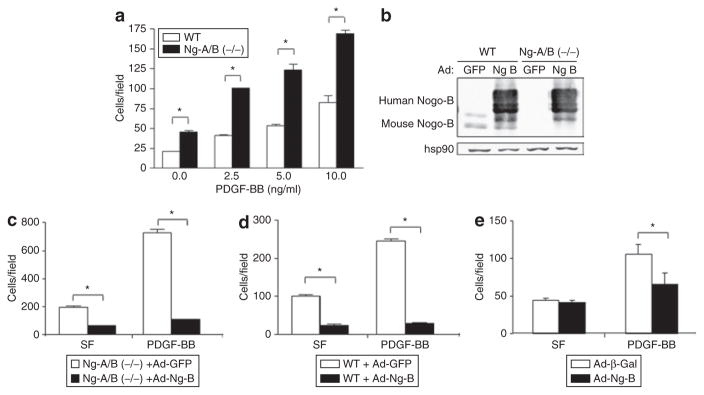
We have previously shown that the N terminus of Nogo-B dose-dependently blocks migration of MVSMC toward PDGF-BB.11 Given that migration is a key component of NI formation in vivo, we further investigated the effect of Nogo-B on VSMC migration by comparing the migration of MVSMCs derived from knockout [Ng-A/B (−/−)] mice with those from wild-type (WT) mice. As shown in Figure 2a, VSMCs from Ng-A/B (−/−) mice displayed significantly enhanced basal and PDGF-BB-induced migration when compared with VSMCs from WT mice at all the doses tested. In order to assess whether this effect can be rescued by adenoviral gene delivery of Nogo-B, we infected both WT and Ng-A/B (−/−) cells with Ad-Ng-B or Ad-GFP control virus. As confirmed by western blot analysis, infection with Ad-Ng-B at as low a dose as MOI 50 led to high levels of robust human Nogo-B expression in both WT and Ng-A/B (−/−) MVSMCs (Figure 2b). In contrast, endogenous levels of murine Nogo-B were much lower, and present only in WT MVSMCs (Figure 2b). Reconstitution of Nogo-B into Ng-A/B (−/−) MVSMCs rescued the migration phenotype induced by PDGF-BB, in addition to antagonizing migration of MVSMCs without stimulus (SF; Figure 2c). Overexpression of Nogo-B in WT MVSMCs also repressed migration, under conditions of both SF and PDGF-BB stimulation (Figure 2d). A similar effect was observed in PVSMCs; PDGF-BB-induced migration was significantly reduced as compared to that associated with the Ad-β-Gal control virus at MOI 300 (Figure 2e). At this dose there was no effect on unstimulated PVSMCs (SF; Figure 2e). Taken together, these results confirm the role of Nogo-B as a regulator of VSMC migration: overexpression of Nogo-B reduces VSMC migration both with and without stimulus in cells derived from WT and Ng-A/B (−/−) mice, as well as PDGF-BB-induced migration in PVSMCs. As VSMC migration and proliferation are key steps in the vascular remodeling that follows vascular injury, the above in vitro studies suggested that augmentation of Nogo-B levels in vivo may be a useful strategy to prevent the deleterious effects of acute vascular injury. Since this aspect has not been assessed before, we chose to address this in both arterial (murine) and venous (porcine) models.
Figure 2. Nogo-B regulates smooth muscle cell (SMC) migration in vitro.
(a) Platelet-derived growth factor-BB (PDGF-BB)-stimulated migration of vascular SMCs (VSMCs) derived from wild-type (WT) and Ng-A/B (−/−) mice. (b) Overexpression and reconstitution of Nogo-B protein in WT and Ng-A/B (−/−) murine VSMCs as verified by western blotting causes reduced migration toward serum-free (SF) media and PDGF-BB in both (c) Ng-A/B (−/−) and (d) WT mice, as compared to delivery of Ad-GFP. (e) Overexpression of Nogo-B but not β-Gal inhibits PDGF-BB-stimulated migration in porcine VSMC. Mean value ± SEM of triplicates. *P < 0.05 versus Ad-GFP or Ad-β-Gal.
Ad-Ng-B prevents wire-injury-evoked NI formation in mice
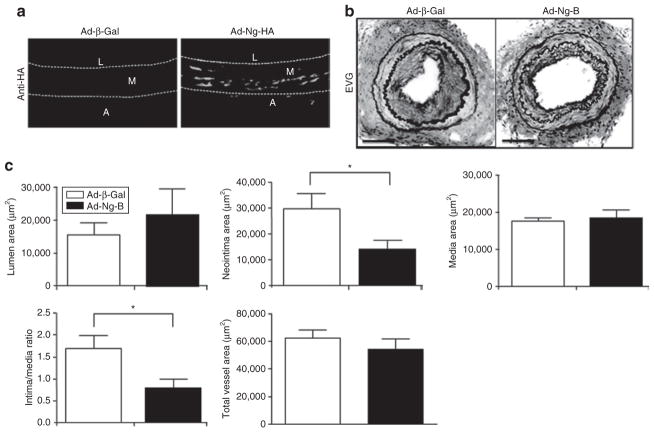
We have previously shown that the exaggerated injury response in Nogo-AB (−/−) mice can be rescued by local administration of Ad-Nogo-B at the time of injury.11 In order to assess whether Nogo-B overexpression beyond endogenous levels in WT mice can further reduce vessel damage, we next looked at the effects of Ad-Ng-B delivery to injured arteries of WT mice. Immediately after wire-injury, 3 × 108 plaque-forming units (pfu) of Ad-Ng-B or Ad-β-Gal were delivered to the adventitial side of femoral arteries using a pluronic gel. The animals were allowed to recover and, at 21 days after the injury, the animals were killed and the arteries were removed for analysis. This combination of dose and delivery route results in high levels of Nogo-B staining in medial and adventitial layers, as demonstrated by hemagglutinin (HA)-staining of vessels transduced with Ad-β-Gal versus Ad-Ng-B (HA tagged) at 5 days after transduction (Figure 3a). As shown in Figure 3b, treatment with Ad-Ng-B led to a marked reduction in neointimal thickening as compared to treatment with Ad-β-Gal. Quantitative morphometric analysis of vessel remodeling indicated a significant reduction in the mean neointimal area [0.014 mm2 versus 0.030 mm2 (P = 0.049)], as well as in the intima:media ratio [0.78 versus 1.67 (P = 0.038); Figure 3c]. There was no difference in the mean lumenal, medial, and total vessel areas (Figure 3c). We have previously shown in Nogo-A/B knockout mice, that Ad-β-Gal transduction in itself does not affect vessel morphology.11
Figure 3. Ad-Ng-B prevents wire-injury evoked neointima formation in mice.
(a) Anti-HA immunofluorescent staining of Ad-β-Gal- and Ad-Ng-B (HA tagged)-transduced injured arteries 5 days after transduction. (b) Elastic van Gieson (EVG) staining and (c) quantitative morphometry measurements of Ad-β-Gal and Ad-Ng-B-transduced arteries recovered three weeks after injury, indicating a reduction in neointima formation following treatment with Nogo-B. Bar = 100 μm. Mean value ± SEM with 10 sections analyzed from each of five mice per group. *P < 0.05 versus Ad-β-Gal.
Ad-Ng-B prevents NI formation caused by vein grafting in pigs
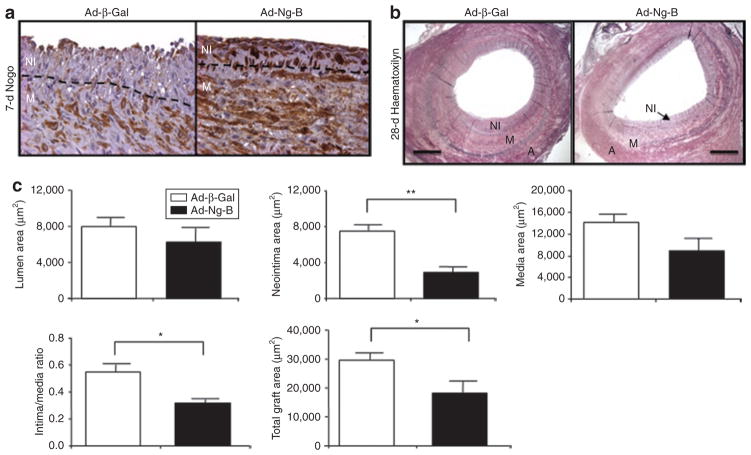
We next looked at the effect of Ad-Ng-B gene delivery to saphenous vein interposition grafts in a porcine model,17 to determine whether the protective effect of Nogo-B overexpression is limited to the murine wire-injury model, or whether it is broadly applicable to diverse strategies to prevent neointimal thickening. As described previously,18 pig saphenous veins were explanted, infused intraluminally with 2.5 × 1010 pfu of either Ad-Ng-B or Ad-β-Gal for 30 minutes prior to grafting, and the pigs were allowed to recover. The grafts were removed at 7 days after surgery to assess the levels of Nogo-B expression in treated versus control grafts. Whilst Nogo-B protein was almost completely absent from the NI and very low in the media (M) of Ad-β-Gal-transduced grafts, it was highly abundant in both layers of Ad-Nogo-B-transduced grafts (Figure 4a). In order to compare the effects of Nogo-B treatment on vascular remodeling, other grafts were removed and analyzed at 28 days after surgery. Quantitative morphometry of hematoxylin-stained sections (Figure 4b) revealed similar results to the mouse model: the neointimal area of Ad-Ng-B-treated grafts was significantly reduced as compared to Ad-β-Gal-treated grafts: Ad-Ng-B: 2.87 mm2 versus Ad-β-Gal 7.44 mm2 (P = 0.0007; Figure 4c). The total vessel area (18.09 mm2 versus 29.54 mm2, respectively, P = 0.034) and the intima:media ratio (0.32 versus 0.55, respectively, P = 0.0044) were also significantly reduced (Figure 4c), whereas there were no significant differences in lumenal or medial areas (Figure 4c). We have previously shown that Ad-β-Gal transduction in itself does not affect vessel morphometry, as compared to a vehicle control. 17,19 Collectively these data show that overexpression of Nogo-B prevents neointimal hyperplasia in both murine artery injury and pig transplantation models in vivo.
Figure 4. Ad-Ng-B prevents neointima formation caused by vein grafting in pigs.
(a) Rescue of injury-induced loss of endogenous Nogo-B with Ad-Ng-B in vein grafts 7 days after grafting. (b) Hematoxylin-staining and (c) quantitative morphometry measurements of Ad-β-Gal and Ad-Ng-B-transduced vein grafts 4 weeks after grafting. Neointima (NI), media (M), and adventitia (A). Bar = 500 μm. Mean value ± SEM with two sections analyzed from six grafts per group. *P < 0.05, **P < 0.01 versus Ad-β-Gal.
Ad-Ng-B blocks VSMC proliferation but does not affect VSMC apoptosis or endothelial regrowth in vivo
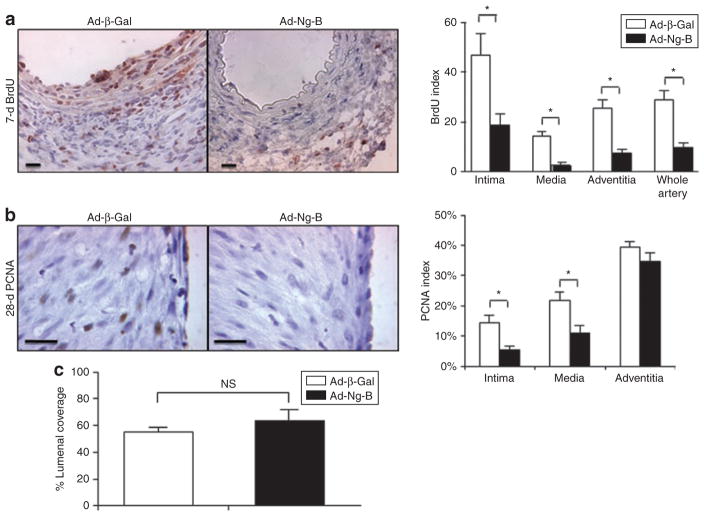
In order to gain further insight into the mechanisms through which Nogo-B affects vascular remodeling, we compared the levels of proliferation in the arteries and grafted veins of Ad-Ng-B versus Ad-β-Gal-treated animals. For the murine femoral artery injury model, 5-bromo-2′deoxyuridine (BrdU) incorporation was assessed 1 week after injury and found to be markedly reduced in Ad-Ng-B-treated animals (Figure 5a). Quantification of BrdU-positive cells revealed that this reduction was significant in all the layers of the arteries, i.e., the intimal (18.9% versus 46.8%, P = 0.028), the media (2.7% versus 14.4%, P = 0.0012), and the adventitial (7.5% versus 25.5%, P = 0.0031) layers, and in whole arteries (9.7% versus 28.9%, P = 0.0043) of Ad-Ng-B versus Ad-β-Gal-treated animals (Figure 5a). Similar results were found in porcine grafts when analyzed by proliferating cell nuclear antigen staining at 7 days with proliferative indices reduced by 10.0% (P = 0.010), 14.5% (P = 0.018), and 17.5% (P = 0.003) in intimal, medial, and adventitial layers, respectively, of Ad-Ng-B-treated grafts as compared to the Ad-β-Gal control group (data not shown). Interestingly, this reduction in proliferation was still evident in the intima and media of grafts removed at 28 days (intimal: 5.4% versus 14.5%, P = 0.040; medial: 11.1% versus 21.7%, P = 0.044 for Ad-Ng-B versus Ad-β-Gal). Together, these findings confirm the strong anti-proliferative properties of Nogo-B observed in vitro. We also assessed the levels of apoptosis in 7- and 28-day vein grafts using TUNEL staining, but did not observe significant differences in apoptotic indices between the two groups at either time point (data not shown), thereby confirming in vitro observations that regulation of apoptosis is not a mechanism for Nogo-B in VSMCs. Finally, we assessed whether accelerated endothelial regrowth is another mechanism through which Nogo-B exerts its therapeutic effects for limiting vascular injury in vivo. Twenty-eight-day vein graft sections were stained for Dolichos biflorus agglutinin lectin to highlight vascular endothelial cells, but showed no significant difference in lumenal coverage of endothelial cells between the groups (Figure 5c).
Figure 5. Ad-Ng-B blocks vascular smooth muscle cell proliferation in vivo.
The proliferative indices of cells in neointimal, medial, and adventitial layers of (a) injured mouse arteries at 1 week after injury and (b) of pig vein grafts at 4 weeks after grafting were determined using 5-bromo-2′deoxyuridine (BrdU) and proliferating cell nuclear antigen (PCNA) immunostaining respectively. (c) Lumenal endothelial cell coverage in 28-day vein grafts indicating that Ad-Nogo-B does not affect endothelial regrowth. Bar = 10 μm. *P < 0.05 versus Ad-β-Gal.
Discussion
In this study, the effects of local overexpression of Nogo-B on NI formation were investigated in two distinct and appropriate in vivo models of vascular injury. Nogo-B overexpression using an adenoviral vector resulted in reduced expansion of the NI in both models, caused by a reduction in VSMC proliferation and migration. This likely occurs because Nogo-B promotes endothelial cell migration while reducing VSMC proliferation and migration.
Wire-mediated injury of the femoral artery is an established mouse model of balloon angioplasty, whereby the artery is denuded and dilated using a straight spring wire.20 Vessels undergo an early surge of medial apoptosis just 2 hours after injury, followed by proliferation of medial VSMCs within days, migration of VSMCs to the intima by 1 week, and expansion of neointimal hyperplasia to form stable intimal lesions by 3–4 weeks.21 Saphenous vein interposition into the carotid artery of pigs is a preclinical model for coronary artery bypass graft surgery, using the saphenous vein as a conduit. In those grafts that survive the small risk of acute thrombosis, neointimal expansion is an essential adaptive reaction for arterialization of the graft. During the complex cascade of events that follow arterialization and upregulation of inflammatory markers, medial VSMCs undergo an early surge of apoptosis,22 followed by medial VSMC proliferation and myofibroblast formation by 1–2 weeks.23 Migration of VSMCs from the media into the intima is also evident by 1–2 weeks23 followed by the deposition of large amounts of extracellular matrix in the NI,24 associated with significant increase in collagenous tissue, by 1–3 months.23 Therefore, despite the unique biological responses inherent to each type of injury, both models share key elements leading to NI formation. Given that the vascular remodeling that follows injury is essential for repairing damaged endothelium and providing hemodynamic stability to vein grafts, therapeutic intervention involving increased expression of an endogenous vascular regulator is more desirable than aggressive inhibition of any isolated step of the remodeling process. Both models represent clinical procedures that have an inherent window of opportunity for local delivery. The delivery routes used in these models—i.e., intraluminal incubation of the saphenous vein ex vivo, prior to grafting, and injection of a small volume of adenoviral vector to the adventitial side of the femoral artery using a pluronic polymer—are clinically feasible, and allowed us to achieve high transgene expression levels at the site of injury without risking the hepatotoxic effects that are characteristic of systemic delivery of adenoviral vectors. We have previously shown for both models that Ad-β-Gal transduction does not affect vessel morphology compared to a vehicle control.11,19
The findings of loss of Nogo-B expression in injured mouse arteries,11 rabbit arteries,12 and human atherosclerotic plaques,13 together with the demonstration of reversal in augmentation of NI formation in Nogo-A/B (−/−) mice,11 make Nogo-B a strong candidate for therapy for prevention of NI formation. Despite sharing a 66-amino acid loop domain (Nogo-66) with the Nogo A and C isoforms, for interaction with the neural-specific Nogo-66 receptor, the vascular functions of Nogo-B are mediated via the recently identified Nogo-B receptor.25 However, structural analyses have so far proven inconclusive, with a bioinformatics and CD/NMR spectroscopy analysis of the Nogo-B receptor indicating that, of its two domains, the ectodomain is intrinsically unstructured and the cytoplasmic domain only partially folded.26 While we have shown here that Nogo-B inhibits VSMC proliferation and migration, reports of its proapoptotic role in cancer cells9,10 and its concentration in macrophage/foam cell-rich areas of human carotid plaques13 that contribute to the formation of lipid-rich necrotic cores, suggested that Nogo-B may also induce VSMC apoptosis. However we did not observe elevated apoptosis rates either in VSMCs in vitro or in Ad-Ng-B-treated grafts in vivo. This suggests the existence of cell type–dependent effects for the proapoptotic role of Nogo-B, an observation shared by a previous study that reported no Nogo-B-mediated effects on apoptosis in certain cancer cells.16 We investigated whether Nogo-B affects endothelial regrowth, as this is a mechanism used by other therapeutic genes such as eNOS,27 but found this not to be the case for Nogo-B.
With all these findings taken together, we present, for the first time, a strategy to augment Nogo-B levels in acute vascular injury models in vivo. Nogo-B overexpression thus represents a novel gene–based therapy to prevent injury-induced damage in vivo in both arterial and vein graft systems.
MATERIALS AND METHODS
Reagents were purchased from Sigma-Aldrich (Poole, UK) and tissue-culture reagents from Gibco (Paisley, UK), unless stated otherwise.
Adenoviruses
First-generation, replication-defective adenoviruses expressing HA-tagged full length human Nogo-B (Ad-Ng-B) or lacZ (Ad-β-Gal) were generated as previously described11 and purified on cesium chloride gradients. The adenoviruses were quantified for plaque-forming units per milliliter by end-point dilution as described,28 as well as for vector particles per milliliter using the Micro BCA Protein Assay Kit (Perbio Science UK Ltd., Tattenhall, UK) for protein measurement and the established formula, 1 μg protein = 4 × 109 virus particles. The MOI was calculated on the basis of the infective titer (pfu/ml).
Cell culture
PVSMCs were isolated from pig saphenous veins and maintained as previously described.29,30 MVSMCs were isolated from either C57BL/6 or Ng-A/B (−/−) mice11 and grown in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum.
Immunhistochemistry
Nogo immunofluorescent staining was performed on fixed and permeabilized PVSMCs and paraffin sections using goat anti-human Nogo (N18; Santa Cruz Biotechnology, Santa Cruz, CA) as previously described.11,13 β-Gal expression was assessed by fixing and 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside (X-gal)-staining of PVSMCs. Where stated, cells were infected with adenovirus 72 hours prior to staining. Transduction efficiency in femoral arteries was determined by immunofluorescent staining of paraffin-fixed frozen sections with anti-HA antibody (Roche Applied Science, Mannheim, Germany). Alexa Fluor 488 anti-rat secondary antibody (Invitrogen, Carlsbad, CA) was used for detection. Pictures were captured using a Carl Zeiss scanning microscope Axiovert 200M imaging system (×400), and images were digitized under constant exposure time, gain, and offset. Cell proliferation in murine arteries was determined by staining with α-BrdU (PharMingen, Franklin Lakes, NJ) staining and bound primary antibodies were detected using avidin–biotin–peroxidase (NovaRed Peroxidase Substrate Kit; Vector Laboratories, Burlington, CA) and 3-amino-9-ethyl carbazole as chromophore (Vector Laboratories, Burlington, CA). The percentages of BrdU-positive nuclei in intimal, medial, and adventitial layers were determined from the analysis of 10 sections from each of four mice. Cell proliferation in porcine vein grafts was assessed by staining for proliferating cell nuclear antigen (PC-10; Dako, Ely, UK) at 1/100 for 1 hour at 37 °C. Bound primary antibodies were detected using biotin, avidin, and peroxidase (Vectastain Elite ABC and DAB Vectastain kits; Vector Laboratories, Burlington, CA) and counterstained with hematoxylin. The percentage of proliferating cell nuclear antigen–positive nuclei in each layer was determined from four fields of view from each of seven pigs. Endothelial coverage of the lumen in 28-day vein grafts was determined by measuring the percentage of lumenal coverage, using image analysis following Dolichos biflorous agglutinin lectin staining was carried out as previously described.31
VSMC apoptosis, proliferation, and migration assays
PVSMC apoptosis was assessed by trypan blue exclusion on 70% confluent cells 72 hours after adenoviral infection. Cell proliferation in MVSMCs was assessed by cell counting at 24–96 hours after adenoviral infection (MOI 50) in growth medium supplemented with either 10% serum, 10 ng/ml PDGF-BB, or no serum. Cell proliferation in PVSMCs was assessed using the CellTiter 96 non-radioactive cell proliferation assay (Promega, Southampton, UK) at 120 hours after adenoviral infection in 15% serum-supplemented growth medium. Cell migration was assessed using modified Boyden chambers (Greiner Bio-One, Longwood, FL) as previously described.11 The migration of quiesced VSMCs toward SF media or increasing doses of PDGF-BB (Calbiochem, San Diego, CA) for 6 hours at 37 °C was assessed by counting the average number of cells in five random fields of view. Where stated, cells were infected with adenovirus at MOIs of 50 (MVSMC) or 300 (PVSMC) 18 hours prior to seeding. Nogo expression was detected by western blotting using a polyclonal antibody α-Nogo (aa14–30; Imgenex, San Diego, CA), and heat-shock protein-90 as a loading control (Santa Cruz Biotechnology, Santa Cruz, CA).
Mouse femoral artery injury and adenoviral transduction
All experiments were approved by the institutional animal care and use committees of Yale University. Eight to ten-week-old male C57BL/6 (WT) mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine and femoral artery injury was performed as previously described.11,32 Immediately after injury, Ad-β-Gal or Ad-Ng-B (3 × 108 pfu) was delivered to the adventitial side of the vessel, with 50 μl of a mixture containing 15 μl adenovirus and 35 μl pluronic-127 gel. The animals were killed and the injured femoral arteries were collected either 7 or 21 days after surgery. BrdU was injected subcutaneously (25 mg/kg) daily for 3 days before the animals were killed, and intraperitoneally (30 mg/kg) 12 hours before they were killed. Immediately after killing, perfusion-fixed or fresh femoral arteries were taken and embedded in optimum cutting temperature (Tissue-Tek, Elkhart, IN). Cryosections (5 μm) were obtained for elastic van Gieson staining, and morphometric analysis was performed on 10 sections per artery.
Adenoviral transduction and grafting of pig saphenous veins
All experiments were approved by the institutional animal care and use committees of The Chinese University of Hong Kong. Female landrace pigs (35–40 kg) were anesthetized by intramuscular administration of 30 mg ketamine and 0.6 mg atropine. Then, after endotracheal intubation, animals received 4% isoflurane in 1:1 oxygen and nitrous oxide gas. Saphenous vein recovery, infusion with adenovirus (2.5 × 1010 pfu/ml) and grafting into each common carotid artery were performed as previously described.17,19 The grafts were removed at 7 days (n = 2/group) or 28 days (n = 8/group) after surgery and embedded in paraffin wax. Serial cross-sections (6 μm) were obtained for hematoxylin staining, and quantitative morphometric analysis of vessel remodeling was performed on four sections per graft.
Statistical analysis
Results are presented as mean value ± SEM. Data were considered significant when P < 0.05, calculated using the Student’s unpaired t-test.
Acknowledgments
We thank Nicola Britton and Gregor Aitchison [British Heart Foundation Glasgow Cardiovascular Research Centre (BHF GCRC), University of Glasgow] for their technical assistance. This study was supported by the Medical Research Council for the UK, the BHF, Research Grant Council earmarked grant—CUHK 4542/06M, Hong Kong SAR, the National Institutes of Health Grants HL64793, R01 HL 61371, R01 HL 57665, and P01 HL 70295, and the National Heart, Lung, and Blood Institute–Yale Proteomics Contract N01-HV-28186 (to W.C.S.). The work was done in Glasgow and Bristol (UK), New Haven (Connecticut, USA), and Shatin (Hong Kong, China).
References
- 1.Ruygrok PN, Webster MW, de Valk V, van Es GA, Ormiston JA, Morel MA, et al. Clinical and angiographic factors associated with asymptomatic restenosis after percutaneous coronary intervention. Circulation. 2001;104:2289–2294. doi: 10.1161/hc4401.098294. [DOI] [PubMed] [Google Scholar]
- 2.Gordon PC, Gibson CM, Cohen DJ, Carrozza JP, Kuntz RE, Baim DS. Mechanisms of restenosis and redilation within coronary stents—quantitative angiographic assessment. J Am Coll Cardiol. 1993;21:1166–1174. doi: 10.1016/0735-1097(93)90241-r. [DOI] [PubMed] [Google Scholar]
- 3.Shah PJ, Gordon I, Fuller J, Seevanayagam S, Rosalion A, Tatoulis J, et al. Factors affecting saphenous vein graft patency: clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg. 2003;126:1972–1977. doi: 10.1016/s0022-5223(03)01276-5. [DOI] [PubMed] [Google Scholar]
- 4.Whittemore A. Current techniques for infrainguinal arterial reconstruction. Jpn J Surg. 1990;20:627–634. doi: 10.1007/BF02471025. [DOI] [PubMed] [Google Scholar]
- 5.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, et al. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 6.Josephson A, Trifunovski A, Widmer HR, Widenfalk J, Olson L, Spenger C. Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans. J Comp Neurol. 2002;453:292–304. doi: 10.1002/cne.10408. [DOI] [PubMed] [Google Scholar]
- 7.Oertle T, Huber C, van der Putten H, Schwab ME. Genomic structure and functional characterisation of the promoters of human and mouse nogo/rtn4. J Mol Biol. 2003;325:299–323. doi: 10.1016/s0022-2836(02)01179-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 9.Qi B, Qi Y, Watari A, Yoshioka N, Inoue H, Minemoto Y, et al. Pro-apoptotic ASY/Nogo-B protein associates with ASYIP. J Cell Physiol. 2003;196:312–318. doi: 10.1002/jcp.10297. [DOI] [PubMed] [Google Scholar]
- 10.Kuang E, Wan Q, Li X, Xu H, Zou T, Qi Y. ER stress triggers apoptosis induced by Nogo-B/ASY overexpression. Exp Cell Res. 2006;312:1983–1988. doi: 10.1016/j.yexcr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 12.Paszkowiak JJ, Maloney SP, Kudo FA, Muto A, Teso D, Rutland RC, et al. Evidence supporting changes in Nogo-B levels as a marker of neointimal expansion but not adaptive arterial remodeling. Vascul Pharmacol. 2007;46:293–301. doi: 10.1016/j.vph.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Feo JA, Hellings WE, Verhoeven BA, Moll FL, de Kleijn DP, Prendergast J, et al. Low levels of Nogo-B in human carotid atherosclerotic plaques are associated with an atheromatous phenotype, restenosis, and stenosis severity. Arterioscler Thromb Vasc Biol. 2007;27:1354–1360. doi: 10.1161/ATVBAHA.107.140913. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Qi B, Oka K, Shimakage M, Yoshioka N, Inoue H, et al. Link of a new type of apoptosis-inducing gene ASY/Nogo-B to human cancer. Oncogene. 2001;20:3929–3936. doi: 10.1038/sj.onc.1204536. [DOI] [PubMed] [Google Scholar]
- 15.Shimakage M, Inoue N, Ohshima K, Kawahara K, Oka T, Yasui K, et al. Down-regulation of ASY/Nogo transcription associated with progression of adult T-cell leukemia/lymphoma. Int J Cancer. 2006;119:1648–1653. doi: 10.1002/ijc.22011. [DOI] [PubMed] [Google Scholar]
- 16.Oertle T, Merkler D, Schwab ME. Do cancer cells die because of Nogo-B? Oncogene. 2003;22:1390–1399. doi: 10.1038/sj.onc.1206278. [DOI] [PubMed] [Google Scholar]
- 17.Angelini GD, Bryan AJ, Williams HM, Morgan R, Newby AC. Distention promotes platelet and leukocyte adhesion and reduces short-term patency in pig arteriovenous bypass grafts. J Thorac Cardiovasc Surg. 1990;99:433–439. [PubMed] [Google Scholar]
- 18.Wan S, George SJ, Nicklin SA, Yim AP, Baker AH. Overexpression of p53 increases lumen size and blocks neointima formation in porcine interposition vein grafts. Mol Ther. 2004;9:689–698. doi: 10.1016/j.ymthe.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH. Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation. 2000;101:296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- 20.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, et al. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol. 2000;32:2097–2104. doi: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- 21.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–796. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien JE, Jr, Ormont ML, Shi Y, Wang D, Zalewski A, Mannion JD. Early injury to the media after saphenous vein grafting. Ann Thorac Surg. 1998;65:1273–1278. doi: 10.1016/s0003-4975(98)00175-1. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien JE, Jr, Shi Y, Fard A, Bauer T, Zalewski A, Mannion JD. Wound healing around and within saphenous vein bypass grafts. J Thorac Cardiovasc Surg. 1997;114:38–45. doi: 10.1016/S0022-5223(97)70115-6. [DOI] [PubMed] [Google Scholar]
- 24.Francis SE, Hunter S, Holt CM, Gadsdon PA, Rogers S, Duff GW, et al. Release of platelet-derived growth factor activity from pig venous arterial grafts. J Thorac Cardiovasc Surg. 1994;108:540–548. [PubMed] [Google Scholar]
- 25.Miao RQ, Gao Y, Harrison KD, Prendergast J, Acevedo LM, Yu J, et al. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad Sci USA. 2006;103:10997–11002. doi: 10.1073/pnas.0602427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Song J. Nogo-B receptor possesses an intrinsically unstructured ectodomain and a partially folded cytoplasmic domain. Biochem Biophys Res Commun. 2007;360:128–134. doi: 10.1016/j.bbrc.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Cooney R, Hynes SO, Sharif F, Howard L, O’Brien T. Effect of gene delivery of NOS isoforms on intimal hyperplasia and endothelial regeneration after balloon injury. Gene Ther. 2007;14:396–404. doi: 10.1038/sj.gt.3302882. [DOI] [PubMed] [Google Scholar]
- 28.Nicklin SA. Simple methods for preparing recombinant adenoviruses for high-efficiency transduction of vascular cells. In: Baker AH, editor. Vascular Disease: Molecular Biology and Gene Therapy Protocols. Humana; Totowa, NJ: 1999. pp. 271–283. [DOI] [PubMed] [Google Scholar]
- 29.Southgate K, Newby AC. Serum-induced proliferation of rabbit aortic smooth muscle cells from the contractile state is inhibited by 8-Br-cAMP but not 8-Br-cGMP. Atherosclerosis. 1990;82:113–123. doi: 10.1016/0021-9150(90)90150-h. [DOI] [PubMed] [Google Scholar]
- 30.George SJ, Angelini GD, Capogrossi MC, Baker AH. Wild-type p53 gene transfer inhibits neointima formation in human saphenous vein by modulation of smooth muscle cell migration and induction of apoptosis. Gene Ther. 2001;8:668–676. doi: 10.1038/sj.gt.3301431. [DOI] [PubMed] [Google Scholar]
- 31.George SJ, Izzat MB, Gadsdon P, Johnson JL, Yim AP, Wan S, et al. Macro-porosity is necessary for the reduction of neointimal and medial thickening by external stenting of porcine saphenous vein bypass grafts. Atherosclerosis. 2001;155:329–336. doi: 10.1016/s0021-9150(00)00588-8. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Rudic RD, Sessa WC. Nitric oxide-releasing aspirin decreases vascular injury by reducing inflammation and promoting apoptosis. Lab Invest. 2002;82:825–832. doi: 10.1097/01.lab.0000018828.61722.bd. [DOI] [PubMed] [Google Scholar]