SUMMARY
Androgen deficiency in men increases body fat, but the mechanisms by which testosterone suppresses fat deposition have not been elucidated fully. Adipose tissue macrophages express the androgen receptor (AR) and regulate adipose tissue remodeling. Thus, testosterone signaling in macrophages could alter the paracrine function of these cells and thereby contribute to the metabolic effects of androgens in men. A metabolic phenotyping study was performed to determine whether the loss of AR signaling in hematopoietic cells results in greater fat accumulation in male mice. C57BL/6J male mice (ages 12–14 weeks) underwent bone marrow transplant from either wild-type (WT) or AR knockout (ARKO) donors (n = 11–13 per group). Mice were fed a high-fat diet (60% fat) for 16 weeks. At baseline, 8 and 16 weeks, glucose and insulin tolerance tests were performed, and body composition was analyzed with fat-water imaging by MRI. No differences in body weight were observed between mice transplanted with WT bone marrow [WT (WTbm)] or ARKO bone marrow [WT(ARKObm)] prior to initiation of the high-fat diet. After 8 weeks of high-fat feeding, WT(ARKObm) mice exhibited significantly more visceral and total fat mass than WT(WTbm) animals. Despite this, no differences between groups were observed in glucose tolerance, insulin sensitivity, or plasma concentrations of insulin, glucose, leptin, or cholesterol, although WT(ARKObm) mice had higher plasma levels of adiponectin. Resultant data indicate that AR signaling in hematopoietic cells influences body fat distribution in male mice, and the absence of hematopoietic AR plays a permissive role in visceral fat accumulation. These findings demonstrate a metabolic role for AR signaling in marrow-derived cells and suggest a novel mechanism by which androgen deficiency in men might promote increased adiposity. The relative contributions of AR signaling in macrophages and other marrow-derived cells require further investigation.
Keywords: androgen receptor, androgens, immunology, metabolic syndrome, obesity, testosterone
INTRODUCTION
Androgens exert metabolic effects and appear protective for the maintenance of metabolic health in men. Men with low circulating androgen levels exhibit changes in body composition characterized by decreased lean body mass and increased fat mass (Mauras et al., 1998; Pasquali, 2006; Allan et al., 2007). Low androgen levels further place men at increased risk for insulin resistance (IR) and type 2 diabetes mellitus (T2DM) (Ding et al., 2006; Keating et al., 2012). This greater metabolic risk is evident in men undergoing androgen deprivation therapy for the management of prostate cancer as well as in men with physiologic hypogonadism (Braga-Basaria et al., 2006; Keating et al., 2012). In parallel with these clinical findings, genetically male mice with global androgen receptor (AR) deficiency (Ar−/Y) exhibit greater adiposity with variable IR (Fan et al., 2005; Lin et al., 2008). Although these metabolic effects of androgen deficiency have been observed, the cellular and molecular mechanisms underlying these changes remain incompletely understood.
The anabolic effects of androgens on skeletal muscle are well recognized, and diminished muscle mass in low androgen states likely contributes to the greater susceptibility to IR and T2DM (Dubois et al., 2012; Rana et al., 2014). The mechanisms whereby androgens regulate fat mass have been only partially elucidated. AR is expressed in both pre-adipocytes and adipocytes, and AR signaling has been implicated in pre-adipocyte differentiation as well as in the regulation of lipid metabolism in mature adipocytes (Dieudonne et al., 2000; Gupta et al., 2008; Blouin et al., 2010; Chazenbalk et al., 2013). Accordingly, low androgen states could promote adiposity through direct effects on adipocytes and their precursor cells. Notably, adipose tissue comprises myriad cell types, including immune cell populations. Of the immune cells present in adipose tissue, the importance of resident macrophages in particular has been recognized in the regulation of adipocyte function and energy metabolism (Bastard et al., 2006; Lacasa et al., 2007; Suganami & Ogawa, 2010; Chazenbalk et al., 2011; Keuper et al., 2011).
Adipose tissue macrophages (ATMs) generate paracrine effectors that influence the differentiation, maturation, and survival of adipocytes (Chazenbalk et al., 2011; Keuper et al., 2011). ATMs can influence adipocyte lipid and glucose metabolism as well as adipokine secretion (Xu et al., 2003; Bastard et al., 2006; Kanda et al., 2006; Kotnik et al., 2013; Cao, 2014), providing additional means by which ATMs could influence both adipose tissue-specific and systemic insulin sensitivity. Macrophages can assume a broad spectrum of phenotypes, each characterized by differential secretion of cytokines, eicosanoids, growth factors, and other paracrine effectors. Therefore, signals that influence ATM phenotype could play a pivotal role in the regulation of adiposity and systemic insulin sensitivity.
Macrophages express AR, and androgen signaling in macrophages has been shown to influence cellular functions including chemotaxis and cytokine production (Gilliver et al., 2006; Lai et al., 2009; Figueroa et al., 2012). Thus, AR signaling in ATMs could influence the paracrine interactions between ATMs and adipocytes and thereby confer protective metabolic effects in men. Conversely, diminished androgen signaling specifically in ATMs could contribute to the metabolic dysregulation evident in both hypogonadal men and AR-deficient male mice. To date, the metabolic effects of AR signaling in macrophages remains an unexplored area of investigation. We demonstrate that the loss of AR signaling in bone marrow-derived cells promotes the accumulation of visceral fat in male mice, indicating a novel metabolic role for AR signaling specifically in hematopoietic cells.
MATERIALS AND METHODS
Animals, bone marrow transplant, and diet
The AR gene resides on the X chromosome in mice. Genetically male Ar−/Y mice were generated using a two-generation mating scheme. The B6.129S1-Artm2.1Reb/J conditional AR gene (stock number: 018450; The Jackson Laboratory, Bar Harbor, ME, USA) was converted to a null allele using the Cre-deleter strain B6.C-Tg(CMV-cre)1Cgn/J (stock number: 006054;The Jackson Laboratory). Genetically female (XX) mice, heterozygous for the deleted Ar allele, were mated with C57BL/6J males (stock number: 000664; The Jackson Laboratory) to generate genetically male (XY) wild-type (WT) controls and global AR knockout (ARKO) mutants (Ar−/Y). PCR genotyping for the Ar alleles has been described previously (Chakraborty et al., 2014), and the presence of the Y chromosome was determined using primers designed against Sry. C57BL/6J WT male mice 12–14 weeks of age underwent lethal irradiation. Bone marrow cells harvested from either ARKO male donors or WT littermate controls were transplanted to irradiated recipient mice through tail vein injection (Serreze & Leiter, 1991). Mice were jointly housed with 2–5 animals per cage. Mice were maintained on a regular chow diet for 16 weeks post transplant and then switched to a high-fat diet with 60% of calories derived from fat (D12492 formula; Research Diets Inc; New Brunswick, NJ, USA). High-fat feeding was continued over 16 weeks, with assessment of body composition and glucose homeostasis at baseline, 8 weeks, and study termination. Body weights and food intake were measured weekly. Animals were killed by cervical dislocation and exsanguination, and tissues were perfused with 10% phosphate-buffered saline prior to harvest. Tissues then were either fixed in 10% formalin or snap-frozen in liquid nitrogen. All aspects of the study were approved by the University of Washington (UW) Institutional Animal Care and Use Committee (IACUC).
Body composition assessment
Body composition was assessed by fat-water imaging using a three Tesla MR system (Philips Achieva) and was performed through the UW Nutrition Obesity Research Center Metabolic Imaging Core. Mice were anesthetized with vaporized isoflurane (1%) according to local IACUC guidelines and imaged using a single-channel solenoid mouse coil (Philips Research Laboratories, Hamburg, Germany) with built-in heating system for maintaining physiological body temperature. A modified two-point Dixon (Eggers et al., 2011) gradient echo acquisition was acquired with the following imaging parameters: FOV: 120 × 40 × 20 mm, Resolution: 0.25 × 0.25 × 1 mm, TR 17 ms, TE 6.5 and 7.65 ms, NEX 8. Total scan time per mouse was <20 min. All images were acquired in the coronal plane, and fat-only and water-only images were reconstructed. Region corresponding to the visceral fat was segmented using the anatomical information from water-only images and fat-only images. Visceral fat region was segmented semi-automatically using a snake contour and custom-designed software (Kerwin et al., 2007). The omental, renal, and gonadal fat pad regions were included in the visceral fat region. Areas of the fat-only image above the noise threshold were considered fat containing voxels. Using the visceral fat contour and fat pixel classification, fat pixels corresponding to visceral fat were automatically calculated. The remaining fat pixels were considered subcutaneous fat. The total volume of fat, subcutaneous fat volume, and visceral fat volume were calculated. The visceral to total fat ratio was also calculated. A subset (n = 4–5) of mice from each group was randomly chosen prior to study initiation to undergo this procedure. The same group of animals underwent body composition testing at all three time points. All images were analyzed by a single radiologist blinded to animal group assignment.
Glucose homeostasis
Animals were fasted for 5 h prior to glucose and insulin tolerance tests. For both tests, blood glucose was measured at time = 0, 15, 30, 60, and 90 min following intra-peritoneal injection of either dextrose (1.5 g/kg) or insulin (1 U/kg). Blood glucose was measured with a hand-held glucose meter (Accu-check, Roche Diagnostics, Indianapolis, IN, USA).
Quantitative real-time PCR
Total RNA was extracted from ~100 mg of liver and adipose tissue through previously described methods (Subramanian et al., 2011). Primers were purchased from Applied Biosystems with the exception of adiponectin, leptin, and non-POU-domain containing octamer-binding protein (NoNo), for which validated primer sets and the SYBR Green I detection system (Applied Biosystems Inc., Carlsbad, CA, USA) were used, as described elsewhere (Rubinow et al., 2013). For inguinal and epididymal fat, relative gene expression was determined through normalization to NoNo, and for liver, gene expression was normalized to β2-microglobulin. For all tissues, 2–3 different genes were evaluated as normalization genes, and those selected demonstrated the least variation among individual animals and between groups. Relative abundance of each gene was calculated using the ΔΔCt formula.
Immunohistochemistry and adipocyte size determination
Formalin-fixed livers embedded in paraffin wax were sectioned and stained with hematoxylin and eosin (H and E) or Masson’s trichrome stains for histological analyses. Liver fibrosis was quantified as previously described. (Subramanian et al., 2011) Macrophages in liver and adipose tissue were identified with a rat monoclonal antibody against MAC2 (1 : 2500; Cedarlane Laboratories, Burlington, NC, USA). Adipocyte number and cross-sectional area were determined using computer image analysis with Image J software with size criteria of 750–20 100 μm2 and circularity of 0.4–1.0.
Western blotting
Ten minutes prior to sacrifice, a subset of animals (n = 4–5 per group) underwent intra-peritoneal injection of insulin (10 U/kg) or saline vehicle. Liver and gastrocnemius muscle were harvested, and tissues were homogenized in buffer containing 0.25 m sucrose, 10 mm Tris–HCl, and 0.1 mm EGTA and protease inhibitors. For each sample, 30 μg of protein was transferred to nitrocellulose membranes, which were incubated overnight with primary antibody for phospho-AKT (1 : 000; Cell Signaling Technology, Danvers, MA, USA) and total AKT (1 : 1000; Cell Signaling Technology). Membranes were washed, incubated with horseradish protein-conjugated secondary antibody, and developed with ECL reagent (Pierce, Rockford, IL, USA).
Other analytical procedures
Hepatic lipids were extracted using a modified Folch method, and both plasma and liver triglyceride and cholesterol levels were measured with commercially available colorimetric assays (Folch et al., 1957; Subramanian et al., 2011). Plasma concentrations of insulin, leptin, adiponectin, TNF-α, and IL-6 were measured by ELISA (Millipore, Billerica, MA, USA). Plasma testosterone concentrations were measured by radioimmunoassay (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA).
Statistical analyses
Statistical analyses were performed with GraphPad Prism 5.0 Software (La Jolla, CA, USA), and values are expressed as mean ± standard error. Unpaired Student’s t-test was used for between-group comparisons at a single time point, and two-way anova was used for between-group comparisons at different time points. A p value of <0.05 was considered statistically significant.
RESULTS
Male mice with bone marrow AR deficiency exhibit accelerated visceral fat accumulation during high-fat feeding
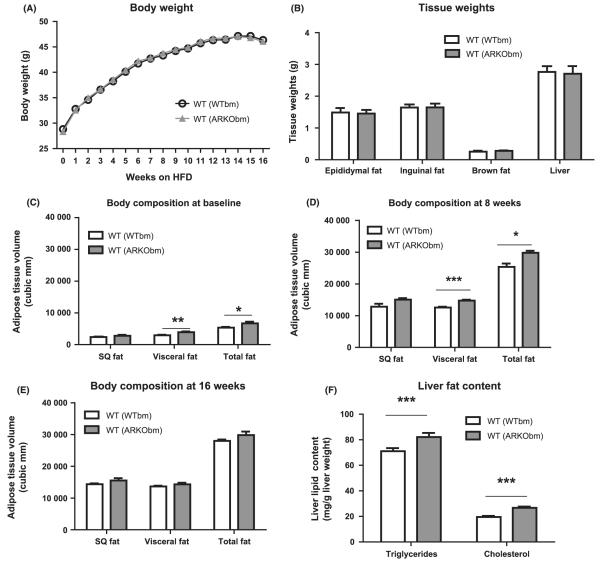
Wild-type mice transplanted with bone marrow cells from WT and Ar−/Y donors [hereafter referred to as WT(WTbm) and WT (ARKObm), respectively] did not exhibit differences in baseline body weight prior to the initiation of the high-fat diet. Body weight increased as expected over the course of high-fat feeding with comparable weight gain in both groups (Fig. 1A), and no differences in food intake were observed between the two groups (data not shown). Consistent with these findings, at study termination no differences were evident between groups for inguinal fat, epididymal fat, brown fat, or liver tissue weights (Fig. 1B).
Figure 1.
WT(WTbm) and WT(ARKObm) mice did not exhibit differences in body weight over the course of the feeding period (A) nor differences in tissue weight at study termination (B). Greater accumulation of visceral and total fat was observed among WT(ARKObm) mice on a chow diet (C) and after 8 weeks of high-fat feeding (D) but not after 16 weeks of high-fat feeding (E). Livers from WT(ARKObm) mice had higher triglyceride and cholesterol content (F). *p < 0.05, **p < 0.01, ***p < 0.001. WT, wild-type; WTbm, WT bone marrow; ARKObm, androgen receptor knockout bone marrow.
Despite the absence of differences in body weight throughout the study, baseline body composition (16 weeks after bone marrow transplant) differed between groups, with WT(ARKObm) mice exhibiting greater visceral fat than WT(WTbm) mice while still on a regular chow diet (Fig. 1C). WT(ARKObm) mice also had higher total body fat relative to WT(WTbm) mice. These differences in visceral and total fat became more pronounced after 8 weeks of high-fat feeding (Fig. 1D). Notably, however, after 16 weeks of high-fat feeding differences in body fat no longer were observed between groups (Fig. 1E). Although liver weights and visceral fat volume were similar between WT(WTbm) and WT(ARKObm) mice by the end of the 16 week high-fat feeding period, analysis of liver fat demonstrated modestly but significantly greater triglyceride and cholesterol content in livers from WT(ARKObm) mice compared with control animals [WT (WTbm)] (Fig. 1F).
WT(ARKObm) male mice do not exhibit impairments in glucose tolerance or insulin sensitivity
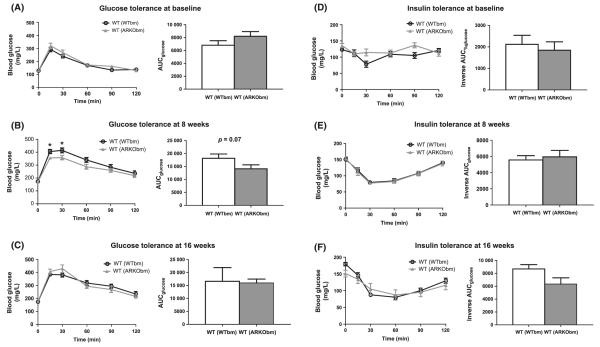
Despite the greater visceral fat observed in WT(ARKObm) mice at baseline and after 8 weeks of high-fat feeding, WT(ARKObm) mice did not exhibit impaired glucose tolerance at any time point. Furthermore, after 8 weeks on the high-fat diet, a trend was observed toward better glucose tolerance among WT(ARKObm) mice relative to WT(WTbm) mice (Fig. 2A–C). No differences in insulin sensitivity were evident at any time point (Fig. 2D–F).
Figure 2.
No differences in glucose tolerance (A) or insulin sensitivity (D) were apparent between WT(WTbm) and WT(ARKObm) mice at baseline (prior to high-fat feeding). After 8 weeks of a high-fat diet, a trend was observed toward improved glucose tolerance in WT(ARKObm) mice relative to WT(WTbm) mice (B) in the absence of differences in insulin sensitivity (E). At study termination, glucose tolerance (C) and insulin sensitivity (F) did not differ between groups. *p < 0.05. WT, wild-type; WTbm, WT bone marrow; ARKObm, androgen receptor knockout bone marrow.
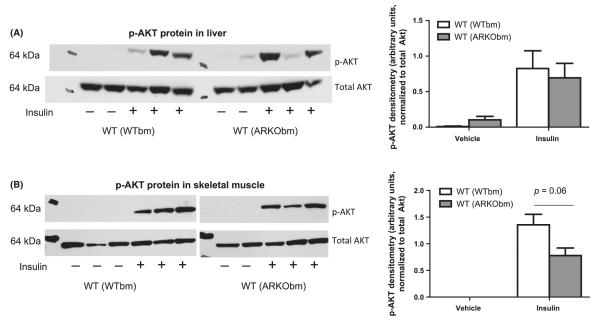
As an additional assessment of insulin sensitivity, a subset of animals were administered a supra-physiologic dose of insulin through intra-peritoneal injection immediately prior to sacrifice, and AKT phosphorylation was quantified in liver and skeletal muscle. Marked variation in AKT phosphorylation among animals was evident in liver, but no differences were found between groups (Fig. 3A). Insulin-stimulated AKT phosphorylation was similar in skeletal muscle from WT(ARKObm) and WT(WTbm) mice, as well, although a trend was observed toward decreased phosphorylation in WT(ARKObm) mice (Fig. 3B).
Figure 3.
Insulin-stimulated phosphorylation of AKT was comparable in livers harvested from WT(WTbm) and WT(ARKObm) mice (A), whereas a trend toward reduced AKT phosphorylation in skeletal muscle was evident among WT(ARKObm) mice (B). WT, wild-type; WTbm, WT bone marrow; ARKObm, androgen receptor knockout bone marrow.
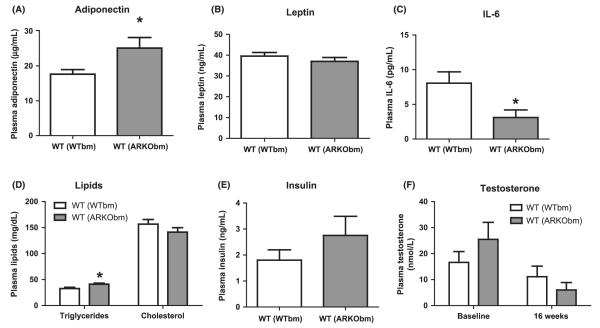
WT(ARKObm) mice have higher circulating levels of adiponectin
WT(ARKObm) mice had significantly higher plasma levels of adiponectin at study termination (Fig. 4A), whereas no differences in circulating leptin levels were observed (Fig. 4B). WT(ARKObm) mice also exhibited lower circulating levels of IL-6 and higher plasma triglycerides (Fig. 4C and D). Plasma TNF-α concentrations were measured and were undetectable in all samples (<2 pg/mL, data not shown). Consistent with glucose and insulin tolerance test results, fasting plasma insulin concentrations were similar between the groups at study termination (Fig. 4E).
Figure 4.
Circulating levels of adiponectin were higher among WT(ARKObm) than WT(WTbm) mice (A), but no differences in circulating leptin levels were observed (B). Plasma IL-6 concentrations were lower among WT(ARKObm) mice (C). Plasma triglycerides were modestly higher in WT(ARKObm) mice but no differences in plasma cholesterol levels were found (D). Fasting plasma insulin concentrations (E) were similar between groups at study termination. No differences in plasma testosterone levels were apparent at either baseline or study termination (F). *p < 0.05. WT, wild-type; WTbm, WT bone marrow; ARKObm, androgen receptor knockout bone marrow.
Male mice with global AR deficiency exhibit low circulating levels of testosterone due to atrophic gonads resulting in a feminized phenotype (Kawano et al., 2003). To determine whether isolated hematopoietic AR deficiency affects testicular testosterone production, plasma testosterone concentrations were measured in WT(WTbm) and WT(ARKObm) mice at baseline and at study termination. Considerable variation in plasma testosterone was observed among mice in both groups. Consistent with previous reports, high-fat feeding led to reductions in circulating testosterone levels (p = 0.01). However, no significant differences in plasma testosterone were evident between WT (WTbm) and WT(ARKObm) mice, either at baseline (16.6 ± 15.0 nm vs. 25.5 ± 21.6 nm, respectively) or study termination (11.1 ± 14.7 nm vS. 6.0 ± 9.1 nm, respectively, Fig. 4F).
Increased expression of lipogenic genes is evident in liver and inguinal fat from WT(ARKObm) mice
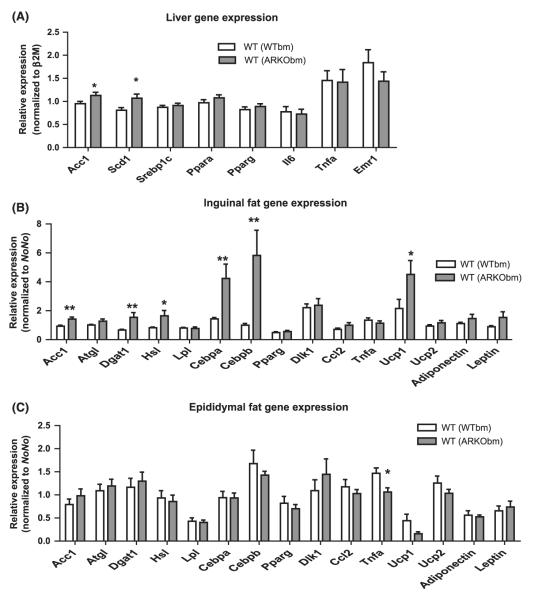
In liver, WT(ARKObm) mice exhibited higher expression of acetyl-CoA carboxylase 1 (Acc1) and stearoyl-CoA desaturase-1 (Scd1) mRNA (Fig. 5A). Gene expression differences were most striking in inguinal fat, as WT(ARKObm) mice had markedly higher expression of adipocyte differentiation markers CCAAT/enhancer-binding protein alpha and beta (Cebpa and Cebpb). Among genes implicated in lipid metabolism, significant differences were evident in Acc1 and diacylglycerol acyltransferase-1 (Dgat1), as well as hormone-sensitive lipase (Hsl), all of which were more highly expressed among WT(ARKObm) animals (Fig. 5B). Greater expression of adipose triglyceride lipase (Atgl) and leptin (Lep) among WT(ARKObm) mice were evident as trends (p = 0.09 and 0.08, respectively). Uncoupling protein-1 (Ucp1) also exhibited roughly 2-fold higher expression among WT(ARKObm) mice relative to WT(WTbm) mice. In contrast, in epididymal fat-only TNF-α (Tnfa) expression differed between groups and was significantly lower among WT(ARKObm) mice (Fig. 5C).
Figure 5.
Hepatic gene expression was notable for higher expression of lipogenic genes in WT (ARKObm) mice (A). Inguinal fat exhibited differential expression of several genes between groups (B), whereas Tnfa was the only gene differentially expressed in epididymal fat (C). *p < 0.05, **p < 0.01. WT, wild-type; WTbm, WT bone marrow; ARKObm, androgen receptor knockout bone marrow.
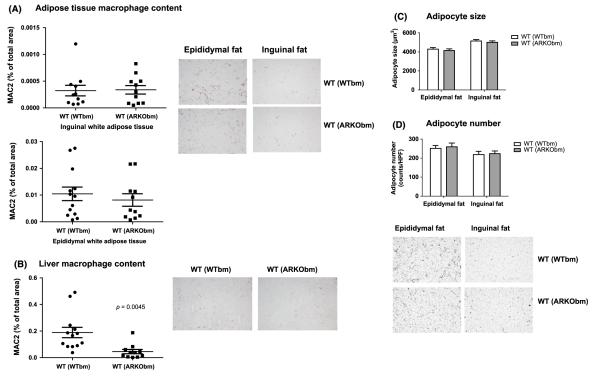
WT(ARKObm) mice exhibit reduced infiltration of tissue macrophages in liver
In both epididymal and inguinal fat, macrophage infiltration was comparable in WT(WTbm) and WT(ARKObm) mice (Fig. 6A). In contrast, MAC2 staining revealed lower tissue macrophage content in liver among WT(ARKObm) animals relative to WT(WTbm) (Fig. 6B). Only minimal liver fibrosis was seen in either group (data not shown). Adipocyte size and number were nearly identical in WT(WTbm) and WT(ARKObm) mice in both epididymal and inguinal fat depots (Fig. 6C and D).
Figure 6.
Comparable infiltration of tissue macrophages in WT(WTbm) and WT(ARKObm) mice was found in inguinal and epididymal white adipose tissue (A), whereas WT(ARKObm) mice exhibited reduced macrophage content in liver relative to WT(WTbm) mice (B). Adipocyte size (C) and number (D) were virtually identical in both groups, for both inguinal and epididymal fat. Representative images for macrophage tissue infiltration and adipocyte size and number are shown. WT, wild-type; WTbm, WT bone marrow; ARKObm, androgen receptor knockout bone marrow.
DISCUSSION
Our findings demonstrate a novel role for AR specifically in hematopoietic cells in the regulation of body composition in male mice. The absence of hematopoietic AR plays a permissive role in the accumulation of visceral and total fat. This metabolic role for AR in marrow-derived cells was evident on both chow and high-fat diets, but the effect was lost after prolonged high-fat feeding, suggesting that the protective effects of intact AR signaling in hematopoietic cells ultimately may be overwhelmed by excessive weight gain or exposure to a high-fat diet. Despite greater adiposity in WT(ARKObm) mice, no differences in glucose tolerance or insulin sensitivity were observed between WT (WTbm) and WT(ARKObm) mice.
This disassociation between adiposity and insulin sensitivity has been observed in other Ar−/Y mouse models and was attributed in part to increased adiponectin production (Fan et al., 2005; Rana et al., 2011). Higher plasma levels of adiponectin also have been found in men after testosterone deprivation (Rubinow et al., 2012) whereas androgen administration suppresses adiponectin secretion (Nishizawa et al., 2002; Page et al., 2005). Notably, our study also demonstrated higher circulating levels of adiponectin in WT(ARKObm) mice, suggesting that AR signaling in marrow-derived cells plays a key role in the paracrine regulation of adipokine secretion. As with other Ar−/Y mouse models, the insulin-sensitizing effects of adiponectin may partially underlie the preservation of glucose tolerance in WT(ARKObm) mice despite increased fat mass. Thus, our findings indicate that AR deficiency in bone marrow-derived cells alone is sufficient to recreate a metabolic phenotype similar to that observed in global Ar−/Y mice.
By study termination, differences in body composition no longer were evident between WT(WTbm) and WT(ARKObm) mice. Therefore, the nominal differences resulting from tissue-specific analyses performed at study termination are not surprising. WT(ARKObm) mice exhibited slightly albeit significantly higher triglyceride and cholesterol content in liver, but this was not sufficient to produce differences in liver weight. Notably, macrophage infiltration into liver was lower among WT(ARKObm) mice despite this higher tissue lipid content, a finding potentially consistent with previous data demonstrating that AR signaling promoted monocyte chemotaxis and macrophage tissue infiltration in a wound healing model (Lai et al., 2009). In both inguinal and epididymal adipose tissue, macrophage infiltration was similar between WT(WTbm) and WT(ARKObm) mice, as were adipocyte size and number in both depots.
Gene expression data demonstrated several differences between groups that could suggest mechanisms underlying the greater adiposity observed in WT(ARKObm) mice at earlier time points. In liver, higher expression of Acc1 and Scd1 among WT (ARKObm) mice suggests accelerated rates of fatty acid synthesis. Similarly, in inguinal white adipose tissue, WT(ARKObm) mice exhibited greater expression of lipogenic genes, as well as those implicated in adipocyte differentiation. Of note, Ucp1 expression was higher in inguinal fat from WT(ARKObm) mice, whereas expression levels were lower in both white and brown adipose tissue in mice with global AR deficiency (Yanase et al., 2008). Thus, these data support the hypothesis that AR signaling in hematopoietic cells influences energy metabolism and/or cellular differentiation in surrounding cells, suggesting that AR may play a key role in mediating the paracrine function of macrophages or other immune cell populations resident in metabolic tissues.
To our knowledge, this is the first study to evaluate the metabolic effects of AR in bone marrow-derived cells. Previous work has demonstrated a metabolic role for estrogen receptor alpha (ERa) in macrophages, as female mice with myeloid-specific ERa deficiency exhibited greater adiposity and IR on a regular chow diet (Ribas et al., 2011). Our findings indicate a parallel metabolic role for AR signaling in hematopoietic cells in male mice. However, we observed a subtler metabolic phenotype with no differences in glucose homeostasis and loss of differential adiposity by the end of the high-fat feeding period. These more modest findings could have resulted from the use of a highly obesigenic diet, which likely overwhelmed the effects of AR deficiency. As a result, findings from tissue histology and gene expression analyses undoubtedly are compromised by the timing of study termination, which occurred after differences in body composition were lost. Thus, additional work is necessary to determine whether the metabolic effects of AR deficiency vary as a function of diet. Furthermore, future studies will benefit from an earlier point of termination so that tissue analyses are performed when significant metabolic differences persist. Similarly, comprehensive metabolic analyses at earlier time points are needed, as differences in body composition were evident even prior to initiation of the high-fat diet. In addition to macrophages, AR is expressed in neutrophils and B and T lymphocytes (Lai et al., 2012) all of which infiltrate adipose tissue and have been implicated in obesity and metabolic dysregulation (Gerriets & MacIver, 2014; Shaikh et al., 2014; Tagzirt et al., 2014). Therefore, a critical objective will be to establish whether the observed phenotype derives exclusively from loss of AR in macrophages as opposed to other marrow-derived cell types. Moreover, androgens regulate cellular proliferation and differentiation in diverse stem cell populations; (Ray et al., 2008) thus, the observed phenotype also could derive in part from the loss of AR signaling in progenitor cells that reside in adipose tissue and are able to differentiate into mature adipocytes (Tang et al., 2008). Finally, as our findings demonstrate a novel metabolic role for AR signaling in hematopoietic cells, additional studies are needed to establish the specific changes in immune cell phenotype and function conferred by AR deficiency, as well as the mechanisms by which these changes lead to altered energy metabolism in key metabolic tissues.
Low circulating androgen levels consistently have been observed among men with obesity and the metabolic syndrome (Corona et al., 2011; Zarotsky et al., 2014). To date, however, controversy persists as to whether low androgen states represent a cause or effect of metabolic dysregulation (Corona et al., 2011). The present findings suggest that deficient androgen signaling could play a direct, causal role in the promotion of adiposity. Thus, this study and similar lines of investigation could lend important insight into the mechanisms by which androgen deficiency may contribute to the development of metabolic syndrome in men.
ACKNOWLEDGMENTS
KBR is the recipient of a University of Washington Nutrition Obesity Research Center Pilot and Feasibility Award P30 DK035816, American Heart Association Clinical Research Program, The Eunice Kennedy Shriver National Institute of Child Health and Development (6K12 HD053984). LJdH is supported by the National Center for Complementary and Alternative Medicine (K01 ATT007177). GJM has been funded through DK089056 and the University of Washington Nutrition Obesity Research Center (DK035816). REB and STP are recipients of a grant (HD042454) from The Eunice Kennedy Shriver National Institute of Child Health and Development, with STP additionally holding a Robert B. McMillen Professorship in Lipid Research.
Footnotes
DISCLOSURES
None.
AUTHOR CONTRIBUTIONS
KBR contributed to the study design, data acquisition, and data analysis and drafted the manuscript. SW, LJdH, and SS assisted with animal sacrifice, tissue harvest, and tissue-based analyses. GJM oversaw glucose homeostasis testing and body composition analyses. FWB, DL, NG, and REB contributed to the study design and generated all animals employed in the study. STP supervised all aspects of the study design and performance. All authors contributed manuscript revisions.
REFERENCES
- Allan CA, Strauss BJ, McLachlan RI. Body composition, metabolic syndrome and testosterone in ageing men. Int J Impot Res. 2007;19:448–457. doi: 10.1038/sj.ijir.3901552. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, Mailloux J, Luu-The V, Tchernof A. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72:176–188. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, Basaria S. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–T59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P, William Buaas F, Sharma M, Smith BE, Greenlee AR, Eacker SM, Braun RE. Androgen-dependent sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol Endocrinol. 2014;28:1055–1072. doi: 10.1210/me.2013-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk G, Bertolotto C, Heneidi S, Jumabay M, Trivax B, Aronowitz J, Yoshimura K, Simmons CF, Dumesic DA, Azziz R. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS ONE. 2011;6:e17834. doi: 10.1371/journal.pone.0017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–926. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, Maggi M. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34:557–567. doi: 10.3275/7806. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y. Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor gamma2. Endocrinology. 2000;141:649–656. doi: 10.1210/endo.141.2.7293. [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- Dubois V, Laurent M, Boonen S, Vanderschueren D, Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69:1651–1667. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011;65:96–107. doi: 10.1002/mrm.22578. [DOI] [PubMed] [Google Scholar]
- Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- Figueroa F, Davicino R, Micalizzi B, Oliveros L, Forneris M. Macrophage secretions modulate the steroidogenesis of polycystic ovary in rats: effect of testosterone on macrophage pro-inflammatory cytokines. Life Sci. 2012;90:733–739. doi: 10.1016/j.lfs.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol. 2014;5:379. doi: 10.3389/fimmu.2014.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722–732. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- Gupta V, Bhasin S, Guo W, Singh R, Miki R, Chauhan P, Choong K, Tchkonia T, Lebrasseur NK, Flanagan JN, Hamilton JA, Viereck JC, Narula NS, Kirkland JL, Jasuja R. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296:32–40. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A. 2003;100:9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating NL, O’Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2012;104:1518–1523. doi: 10.1093/jnci/djs376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin W, Xu D, Liu F, Saam T, Underhill H, Takaya N, Chu B, Hatsukami T, Yuan C. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18:371–378. doi: 10.1097/rmr.0b013e3181598d9d. [DOI] [PubMed] [Google Scholar]
- Keuper M, Blüher M, Schön MR, Möller P, Dzyakanchuk A, Amrein K, Debatin KM, Wabitsch M, Fischer-Posovszky P. An inflammatory micro-environment promotes human adipocyte apoptosis. Mol Cell Endocrinol. 2011;339:105–113. doi: 10.1016/j.mce.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kotnik P, Keuper M, Wabitsch M, Fischer-Posovszky P. Interleukin-1β downregulates RBP4 secretion in human adipocytes. PLoS ONE. 2013;8:e57796. doi: 10.1371/journal.pone.0057796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K. Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology. 2007;148:868–877. doi: 10.1210/en.2006-0687. [DOI] [PubMed] [Google Scholar]
- Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JJ, Lai KP, Zeng W, Chuang KH, Altuwaijri S, Chang C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice. Am J Pathol. 2012;181:1504–1512. doi: 10.1016/j.ajpath.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, Yeh S, Chang C. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47:1924–1935. doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85:1319–1340. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Rana K, Fam BC, Clarke MV, Pang TP, Zajac JD, MacLean HE. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab. 2011;301:E767–E778. doi: 10.1152/ajpendo.00584.2010. [DOI] [PubMed] [Google Scholar]
- Rana K, Lee NK, Zajac JD, Maclean HE. Expression of androgen receptor target genes in skeletal muscle. Asian J Androl. 2014;16:675–683. doi: 10.4103/1008-682X.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex steroids and stem cell function. Mol Med. 2008;14:493–501. doi: 10.2119/2008-00004.Ray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, Hevener AL. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci U S A. 2011;108:16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow KB, Snyder CN, Amory JK, Hoofnagle AN, Page ST. Acute testosterone deprivation reduces insulin sensitivity in men. Clin Endocrinol (Oxf) 2012;76:281–288. doi: 10.1111/j.1365-2265.2011.04189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow KB, Wall VZ, Nelson J, Mar D, Bomsztyk K, Askari B, Lai M, Smith KD, Han MS, Vivekanandan-Giri A, Pennathur S, Albert CJ, Ford DA, Davis RJ, Bornfeldt KE. Acyl-CoA Synthetase 1 is Induced by Gram-Negative Bacteria and Lipopolysaccharide and is Required for Phospholipid Turnover in Stimulated Macrophages. J Biol Chem. 2013;288:9957–9970. doi: 10.1074/jbc.M113.458372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serreze DV, Leiter EH. Development of diabetogenic T cells from NOD/Lt marrow is blocked when an allo-H-2 haplotype is expressed on cells of hemopoietic origin, but not on thymic epithelium. J Immunol. 1991;147:1222–1229. [PubMed] [Google Scholar]
- Shaikh SR, Haas KM, Beck MA, Teague H. The effects of diet-induced obesity on B cell function. Clin Exp Immunol. 2014;179:90–99. doi: 10.1111/cei.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O’Brien KD, Pennathur S, Chait A. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res. 2011;52:1626–1635. doi: 10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- Tagzirt M, Corseaux D, Pasquesoone L, Mouquet F, Roma-Lavisse C, Ung A, Lorenzi R, Jude B, Elkalioubie A, Van Belle E, Susen S, Dupont A. Alterations in neutrophil production and function at an early stage in the high-fructose rat model of metabolic syndrome. Am J Hypertens. 2014;27:1096–1104. doi: 10.1093/ajh/hpu021. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase T, Fan W, Kyoya K, Min L, Takayanagi R, Kato S, Nawata H. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol. 2008;109:254–257. doi: 10.1016/j.jsbmb.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Zarotsky V, Huang MY, Carman W, Morgentaler A, Singhal PK, Coffin D, Jones TH. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2:819–834. doi: 10.1111/andr.274. [DOI] [PubMed] [Google Scholar]