Abstract
The involvement of serotonin in mediating hunger-related changes in behavioral state has been described in many invertebrates. However, the mechanisms by which hunger signals to serotonergic cells remain unknown. We tested the hypothesis that serotonergic neurons can directly sense the concentration of glucose, a metabolic indicator of nutritional state. In the snail Lymnaea stagnalis, we demonstrate that completely isolated pedal serotonergic neurons that control locomotion changed their biophysical characteristics in response to glucose application by lowering membrane potential and decreasing the firing rate. Additionally, the excitatory response of the isolated serotonergic neurons to the neuroactive microenvironment of the pedal ganglia was significantly lowered by glucose application. Because hunger has been reported to increase the activity of select neurons and their responses to the pedal ganglia microenvironment, these responses to glucose are in accordance with the hypothesis that direct glucose signaling is involved in the mediation of the hunger-related behavioral state.
Keywords: behavioral state, serotonergic neuron, extrasynaptic release, volume transmission, Lymnaea stagnalis
Biophysical properties of individual neurons are known to be regulated by intra- and extracellular mechanisms, and depend upon the behavioral state of the organism [1–3]. Recently, we demonstrated that the hunger behavioral state could produce long-term changes in individual serotonergic neurons of the pond snail Lymnaea stagnalis that persist even after neurons are isolated from the nervous system [4–6]. Pedal serotonergic neurons of the pedal A (PeA) cluster that control locomotion [7] showed higher activity and depolarized membrane potentials after being isolated from the nervous systems of food-deprived animals. We also reported that the chemical neuroactive microenvironment of the pedal ganglia changes in accordance with the nutritional state of the animal, and produces predictable changes in single isolated neurons placed in its vicinity [3,8]. PeA cells isolated from fed preparations displayed higher excitation when placed near the PeA cluster of food-deprived snails. These hunger–induced effects can be explained by the elevated extrasynaptic serotonin release produced by an increased synthesis of serotonin in pedal serotonergic neurons [3,8–13]. The role of serotonin in mediating a hunger/feeding behavioral state has been suggested and described in many invertebrates [14–18], including mollusks [3,5,19,20]. However, the mechanisms by which hunger signals to serotonergic cells remain unknown.
Here, we tested the hypothesis that serotonergic neurons can directly sense the concentration of glucose, a metabolic indicator. Glucose is the preferred carbon and energy source for most eukaryotic cells [21–23]. In Lymnaea, it has been demonstrated that the hemolymph glucose concentration may change dramatically in response to hunger and satiety, from 57 μg/ml in starved specimens to 760 μg/ml in satiated ones [24], reflecting the quality and quantity of the food consumed. The concentrations showed variations in accord with seasonal temperature [25]. Glucose depolarized neurosecretory cells produce an insulin-like hormone, suggesting that the hormone-producing cells are endowed with glucose receptors [26].
Materials and Methods
Animals
Mature specimens of Lymnaea stagnalis (2–3 months old, 2.5 cm length) were taken from a breeding colony kept in dechlorinated tap water at room temperature and fed on lettuce. Central ganglia were dissected from snails anesthetized with an injection of 0.1 mM MgCl2. The central ganglia were placed into a 2.5 mg/ml of pronase E (Sigma) in snail physiological solution (50 mM NaCl, 1.6 mM KCl, 4 mM CaCl2, 8 mM MgCl2, 10 mM Tris, pH 7.6) for 15 min, washed in the physiological solution and pinned to Sylgard in a 4 ml chamber. The connective tissue sheath was then removed from the pedal ganglia.
Intracellular recording
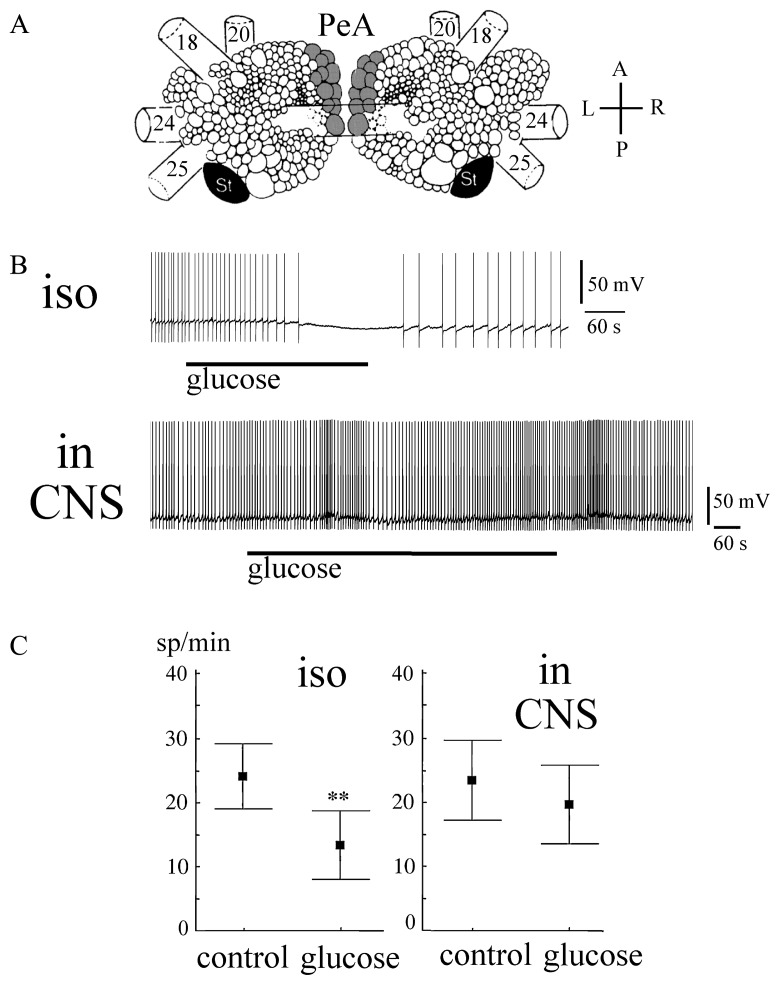
Visual identification of the PeA neurons (30–90 μm, PeA2, PeA4, PeA8, PeA11 according to classification [7]) was performed based on their location, size and coloration (Fig. 1A). The neuron was impaled with a standard glass microelectrode (10–20 MΩ filled with 3 M KCl). A standard setup for microelectrode recording was used. The electrophysiological recordings were stored in computer files using a homemade program [5,6,8,13].
Figure 1.
Effects of glucose on the activity of isolated and non-isolated serotonergic neurons of the PeA cluster. A. The positions of the PeA cluster neurons (shaded) at the dorsal surface of the paired pedal ganglia of Lymnaea stagnalis. 18, superior pedal nerve; 20, medial pedal nerve; 24, cerebro-pedal connective; 25, pleuro-pedal connective; St, statocyst St, statocyst; A, anterior; P, posterior; L, left; R, right; Modified from Slade et al., 1981. B. The records of activity of isolated (iso) and non-isolated (in CNS) PeA neurons prior to and after bath application of glucose. C. The activity of isolated (iso) and non-isolated (in CNS) serotonergic PeA neurons [mean with standard error prior to (control) and after glucose treatment (glucose), ** p<0.01, paired Wilcoxon test].
For neuron isolation, we utilized previously developed methods [13,27]. Using the intracellular microelectrode as a pull, the neuron was gently pulled out of the tissue until the separation of the proximal neurite from the neuropile was achieved. The electrical activity of the cell was monitored during isolation. Cells that demonstrated membrane injury were not used in the experiments. The isolated neuron (n=12) was placed into a continuous stream of physiological solution (0.75 ml/min). Glucose at a concentration of 250 μg/ml was added into the stream. The firing rate of a neuron was recorded prior to and after 5 min of glucose application.
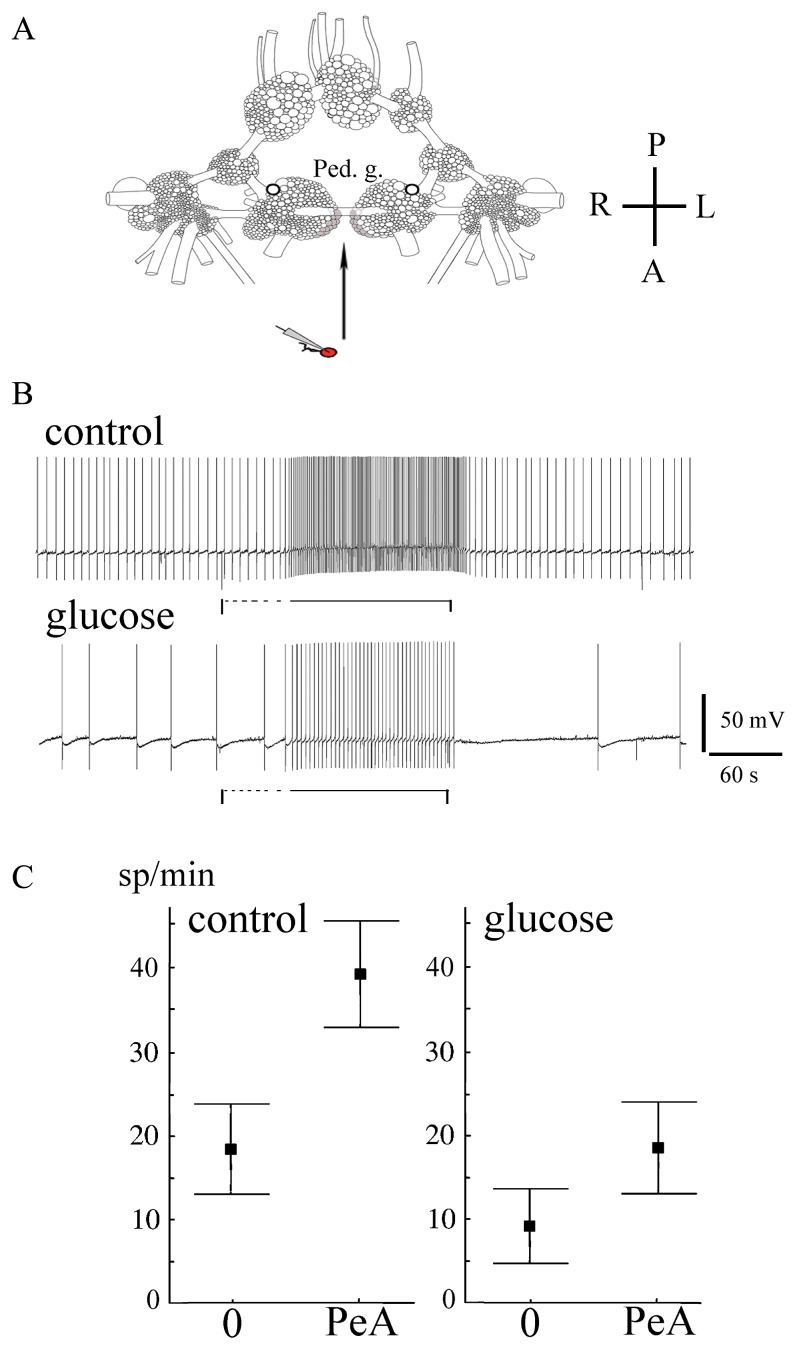
In eight experiments, the effect of glucose pretreatment for 7 minutes on the isolated neuron’s response to the nearby PeA cluster was tested. Our approach was developed based on previously described methods for the detection of extrasynaptic release of neuroactive compounds from the ganglia of Lymnaea [8,28]. Two CNS preparations were pinned down in one chamber. The first preparation was used as a source of biosensors (isolated PeA cells), the second for the investigation of the neuroactive microenvironment of the pedal ganglia. The PeA neuron impaled with the microelectrode was isolated from the first CNS preparation and placed at a distance of at least one ganglion-diameter from the CNS for 2 minutes. Then, it was moved to the intact pedal A cluster of the second CNS preparation at a distance less than half-cell size for 2 minutes and then replaced. This procedure was repeated several times in one experiment. In each position, the electrical activity of the biosensor was measured. In each experiment, 2–3 biosensors were used to check the concordance of biosensor responses. Experiments were performed in a continuous stream of either snail physiological solution (control) or 250 μg/ml glucose in physiological solution.
Statistics
The significance of differences in spike frequency was tested by the paired Wilcoxon signed-rank test for dependent samples using the STATISTICA program (StatSoft Inc. 1993, release 4.3). All values are given as the mean with the standard error and level of significance.
Results and Discussion
To test the hypothesis that metabolic signals present in a changing nutritional state may directly affect the serotonergic cellular response, we tested the effect of glucose on the activity of PeA neurons. Glucose concentrations can vary in accord with feeding state over a very broad interval (57–760 μg/ml) in Lymnaea [24]. The responses of isolated PeA neurons (n=12) exposed to an application of glucose at 250 μg/ml in a snail saline stream were tested. This concentration increases the osmolarity of the solution by approximately 2%.
Glucose caused the slow hyperpolarization of isolated PeA neurons with the maximal effect being observed at 3–5 minutes of application (Fig. 1B, C). A paired Wilcoxon test indicated a significant difference in the firing rate prior to and after 5 min of glucose application (n=12, z=3, p<0.01).
However, the same glucose concentration failed to produce a statistically significant effect on “nonisolated” PeA cells in the CNS preparation (n=10, Fig. 1B, C). This might be explained by lower glucose receptor availability in situ because the cell membrane is not entirely exposed in these conditions or by the effects of other glucose-sensitive neurons in the CNS on the PeA cells. Another explanation is that insulin is not present in isolated neurons, which aids the uptake of glucose and, therefore, allows glucose to exert more profound effects on the isolated cells than on those in situ.
In spite of the absence of a statistically significant effect of glucose on the activity of PeA neurons in the CNS, the pretreatment of the pedal ganglia for 7 minutes with glucose resulted in a significantly weaker response measured by a biosensor near the PeA cluster (n=8, Fig. 2A, B). The absolute mean frequency of electrical activity of isolated neurons near the PeA cluster was significantly lower in preparations pretreated with glucose (Fig. 2B and C, p<0.05, Wilcoxon signed-rank test). The amplitude of the isolated neuron response to the PeA cluster approach was also lower in glucose-treated preparations (Fig. 2B and C, 9±2 spike/min versus 19±4 spike/min in control, p<0.05, Wilcoxon signed-rank test). This finding suggests that glucose influences volume chemical signalization from the pedal ganglia by additional mechanisms beyond its effect on electrical activity. The present observation that extrasynaptic release of neuroactive compounds including serotonin increases the firing activity of isolated PeA neurons even after glucose pretreatment suggests that serotonin can partially compensate for the hyperpolarizing effects of glucose in the CNS.
Figure 2.
Effects of glucose on the activity of isolated PeA neurons and their responses to the nearby PeA cluster of pedal ganglia. A. A schematic representation of the experimental procedure: the isolated neuron impaled with the microelectrode was placed at a distance from the pedal ganglia of an isolated CNS then moved to the pedal A cluster (shaded) at a distance less than half-cell size for 2 minutes and replaced. A, anterior; P, posterior; L, left; R, right. B. The response of isolated serotonergic PeA neurons to the nearby pedal A cluster prior to (upper record) and 7 minutes after (lower record) bath application of glucose. The vertical lines mark the start of biosensor movement, the horizontal dashed lines mark the movement of the biosensor to the PeA cluster, and the horizontal black lines mark the unmovable state of the biosensor near the PeA cluster. C. The activity of isolated serotonergic PeA neurons (mean with standard error), left to right: prior to glucose treatment away from the pedal ganglia (0) and near the PeA cluster (PeA), 7 minutes after glucose bath application away from the pedal ganglia (0) and near the PeA cluster (PeA). Other statistical data are in the text.
Extrasynaptic neurotransmitter release plays an important role in interneuronal communication in the mammalian brain [29] and in invertebrates of various taxa [30–33]. Nutritional state-dependent extrasynaptic release of serotonin and other substances from the pedal ganglia of Lymnaea stagnalis has been suggested to play a role in the cooperation of PeA cells and in neurohormonal communication [6,8,13]. The effect observed here of glucose on intensity of extrasynaptic release from isolated ganglia is a unique example of a link between a concentration of a metabolic indicator of a nutritional state and the neuroactive chemical microenvironment of the ganglia.
In molluscs, satiation-induced inhibition of feeding is believed to be mediated by the mechanical stimuli that result from filling the gut with food [34]. In Lymnaea, gut dilation activates the mechanosensory cells, which inhibit modulatory and pattern-generating neurons and also activate radular retractor motoneurons [35]. In Aplysia californica, the feeding behavior was found to be unaffected by glucose [36], and therefore the mechanical stimulation of the gut remained the only known mechanism of satiety. It was unknown how neurons beyond the feeding system could sense changes in the nutritional state. Later, in some Lymnaea neurons from the buccal feeding network, glucose was reported to produce a hyperpolarization in situ, while in other feeding neurons there was no effect [4]. The firing activity of the serotonergic metacerebral giant cell (MGC) modulating the feeding system was decreased by glucose in the land snail Helix pomatia, and this effect could be compensated by extracellular serotonin (Hernádi et al., unpublished data). In a recent study in Lymnaea, ambiguous data on the direct effects of glucose on food-aversive learning have been obtained [37]. Injection of glucose into food-deprived snails did not affect their learning. However, if these snails were fed on sucrose, they showed learning and memory formation. It was concluded that hemolymph glucose concentration is an important factor in motivating acquisition of food-aversive learning and memory in Lymnaea. However, not only glucose but insulin and feeding motor activation are also required for memory formation [37].
Conclusion
The present experiments show that completely isolated PeA neurons decreased their firing rate in response to glucose application. Additionally, the excitatory response of isolated PeA neurons to extrasynaptic ganglia release was also significantly lowered by glucose application. Conversely, a low available glucose level (i.e., hunger) is known to increase the activity of PeA neurons and their excitatory responses to the pedal ganglia microenvironment [3,4,6]. These opposing effects of low and high glucose level are in accordance with the hypothesis that direct glucose signaling plays a role in mediating hunger-related behavioral state [4,37]. We do not know whether PeA neurons express glucoreceptors or whether they are sensitive to the secreted insulin-like hormone. Therefore, the impact of glucose on behavioral-state related properties of serotonergic neurons is not completely defined, yet should not be overlooked.
Acknowledgements
This work was supported by the RFBR grants 14-04-00537 and 14-04-00875 and by KAKENHI from JSPS (No. 25291074) to E. I.
Abbreviation
- 5-HTP
5-hydroxy-L-tryptophan
Footnotes
Conflict of Interest
All the authors declare that they have no conflict of interest.
Author Contributions
V. D. and D. S. directed the entire project. V. D. and T. D. performed the experiments. V. D., L. H. and E. I. analyzed the data. V. D., L. H., E. I. and I. Z. co-wrote the manuscript.
References
- 1.Maimon G. Modulation of visual physiology by behavioral state in monkeys, mice, and flies. Curr Opin Neurobiol. 2011;4:559–564. doi: 10.1016/j.conb.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- 3.D’yakonova VE. Neurotransmitter mechanisms of context-dependent behavior. Neurosci Behav Physiol. 2014;44:256–267. [Google Scholar]
- 4.Alania M, Dyakonova V, Sakharov DA. Hyperpolarization by glucose of feeding related neurons in snail. Acta Biol Hung. 2004;55:195–200. doi: 10.1556/ABiol.55.2004.1-4.24. [DOI] [PubMed] [Google Scholar]
- 5.Hernádi L, Hiripi L, Dyakonova V, Győri J, Vehovszky Á. The effect of food intake on the central monoaminergic system in the snail Lymnaea stagnalis. Acta Biol Hung. 2004;55:185–194. doi: 10.1556/ABiol.55.2004.1-4.23. [DOI] [PubMed] [Google Scholar]
- 6.Dyakonova VE, Hernady L, Ito E, Dyakonova TL, Chistopolsky IA, Zakharov IS, Sakharov DA. The activity of isolated neurons and the modulatory state of an isolated nervous system represent a recent behavioural state. J Exp Biol. 2015 doi: 10.1242/jeb.111930. [DOI] [PubMed] [Google Scholar]
- 7.Syed NI, Winlow W. Morphology and electrophysiology of neurons innervating the ciliated locomotor epithelium in Lymnaea stagnalis (L) Comp Biochem Physiol. 1989;93A:633–644. doi: 10.1016/0300-9629(89)90513-6. [DOI] [PubMed] [Google Scholar]
- 8.Chistopol’skii IA, Sakharov DA. Non-synaptic integration of the cell bodies of neurons into the central nervous system of the snail. Neurosci Behav Physiol. 2003;33:295–300. doi: 10.1023/a:1022163701311. [DOI] [PubMed] [Google Scholar]
- 9.Kabotyanski EA, Sakharov DA, Winlow W. Increase in serotonin production affects the electrical activity of serotonergic neurones. Biol Membrany. 1991;8:1158–1159. [Google Scholar]
- 10.Dyakonova VED, Sakharov DA. Firing of an isolated serotonergic neuron depends on the rate of neurotransmitter synthesis. Dokl Biol Sci. 2001;376:267–270. doi: 10.1023/a:1019258523049. [DOI] [PubMed] [Google Scholar]
- 11.Dyakonova VED, Sakharov DA. An isolated serotonergic neuron: the mechanism of excitation induced by enhanced synthesis of neurotransmitter. Dokl Biol Sci. 2001;378:230–232. doi: 10.1023/a:1019258523049. [DOI] [PubMed] [Google Scholar]
- 12.Hatcher NG, Zhang X, Stuart JN, Moroz LL, Sweedler JV, Gillette R. 5-HT and 5-HT-SO4, but not tryptophan or 5-HIAA levels in single feeding neurons track animal hunger state. J Neurochem. 2008;104:1358–1363. doi: 10.1111/j.1471-4159.2007.05084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyakonova VE, Chistopolsky IA, Dyakonova TL, Vorontsov DD, Sakharov DA. Direct and decarboxylation-dependent effects of neurotransmitter precursors on firing of isolated monoaminergic neurons. J Comp Physiol A. 2009;195:515–527. doi: 10.1007/s00359-009-0428-5. [DOI] [PubMed] [Google Scholar]
- 14.Lent CM, Dickinson MH. The neurobiology of feeding in leeches. Sci Am. 1988;258:98–103. doi: 10.1038/scientificamerican0688-98. [DOI] [PubMed] [Google Scholar]
- 15.Sakharov DA. Integrative function of serotonin common to distantly related invertebrate animals. In: Gustaffson M, Reuter M, editors. The Early Brain. Abo Turku, Finland: Akademi Press; 1990. pp. 73–88. [Google Scholar]
- 16.Palmer CR, Kristan WB., Jr Contextual modulation of behavioral choice. Curr Opin Neurobiol. 2011;21:520–526. doi: 10.1016/j.conb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. doi: 10.1126/science.6805073. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly JL, Clark CM, Leifer AM, Pirri JK, Haburcak M, Francis MM, Samuel AD, Alkema MJ. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 2013;11:e1001529. doi: 10.1371/journal.pbio.1001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’yakonova VE, Sakharov DA. Neurotransmitter basis of mollusc behavior: control of choice between the orienting and the defense response to the presentation of an unfamiliar object. Neurosci Behav Physiol. 1995;25:247–251. doi: 10.1007/BF02360213. [DOI] [PubMed] [Google Scholar]
- 20.Gillette R. Evolution and function in serotonergic systems. Integr Comp Biol. 2006;46:838–846. doi: 10.1093/icb/icl024. [DOI] [PubMed] [Google Scholar]
- 21.Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. J Auton Nerv Syst. 1984;10:359–372. doi: 10.1016/0165-1838(84)90033-x. [DOI] [PubMed] [Google Scholar]
- 22.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 2000;856:37–47. doi: 10.1016/s0006-8993(99)02327-6. [DOI] [PubMed] [Google Scholar]
- 23.Ruibal C, Soengas JL, Aldegunde M. Brain serotonin and the control of food intake in rainbow trout (Oncorhynchus mykiss): effects of changes in plasma glucose levels. J Comp Physiol. 2002;188A:479–484. doi: 10.1007/s00359-002-0320-z. [DOI] [PubMed] [Google Scholar]
- 24.Scheerboom JEM, Hemminga MA, Doderer A. The effects of a change of diet on consumption and assimilation and on the haemolymph-glucose concentration of the pond snail Lymnaea stagnalis (L) Proc Kon Ned Akad Wet, Ser C. 1978;81:335–346. [Google Scholar]
- 25.Karanova MV. Seasonal variation in the content of free reducing sugars in body fluids of freshwater mollusk Lymnaea stagnalis. Biol Bull. 2006;33:382–386. [PubMed] [Google Scholar]
- 26.Kits KS, Bobeldijk RC, Crest M, Lodder JC. Glucose-induced excitation in molluscan central neurons producing insulin-related peptides. Pfllugers Arch. 1991;417:597–604. doi: 10.1007/BF00372957. [DOI] [PubMed] [Google Scholar]
- 27.Dyakonova TL. Neurochemical mechanisms of the burst activity regulation in isolated endogenous oscillators of snail: role of monoamines and opioid peptides. Neirofisiologia (Kiev) 1991;23:472–480. [PubMed] [Google Scholar]
- 28.Dyakonova TL, Dyakonova VE. Coordination of rhythm-generating units via NO and extrasynaptic neurotransmitter release. J Comp Physiol A. 2010;196:529–541. doi: 10.1007/s00359-010-0541-5. [DOI] [PubMed] [Google Scholar]
- 29.Vizi ES, Kiss JP, Lendvai B. Nonsynaptic communication in the central nervous system. Neurochem Int. 2004;45:443–451. doi: 10.1016/j.neuint.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Bruns D, Jahn R. Real-time measurements of transmitter release from single synaptic vesicles. Nature. 1995;377:62–65. doi: 10.1038/377062a0. [DOI] [PubMed] [Google Scholar]
- 31.Chen G, Gavin PF, Luo G, Ewing AG. Observation and quantitation of exocytosis from the cell body of a fully developed neuron in Planorbis corneus. J Neurosci. 1995;15:7747–7755. doi: 10.1523/JNEUROSCI.15-11-07747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De-Miguel FF, Trueta C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol Neurobiol. 2005;25:297–312. doi: 10.1007/s10571-005-3061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer GE, Lukowiak K, Syed NI. Transmitter-receptor interactions between growth cones of identified Lymnaea neurons determine target cell selection in vitro. J Neurosci. 2000;20:8077–8086. doi: 10.1523/JNEUROSCI.20-21-08077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elliott CJ, Susswein AJ. Comparative neuroethology of feeding control in molluscs. J Exp Biol. 2002;205:877–896. doi: 10.1242/jeb.205.7.877. [DOI] [PubMed] [Google Scholar]
- 35.Elliott CJH, Benjamin PR. Esophageal mechanoreceptors in the feeding system of the pond snail Lymnaea stagnalis. J Neurophysiol. 1989;61:727–736. doi: 10.1152/jn.1989.61.4.727. [DOI] [PubMed] [Google Scholar]
- 36.Horn CC, Koester J, Kupfermann I. Evidence that hemolymph glucose in Aplysia californica is regulated but does not affect feeding behavior. Behav Neurosci. 1998;112:1258–1265. [PubMed] [Google Scholar]
- 37.Mita K, Okuta A, Okada R, Hatakeyama D, Otsuka E, Yamagishi M, Morikawa M, Naganuma Y, Fujito Y, Dyakonova V, Lukowiak K, Ito E. What are the elements of motivation for acquisition of conditioned taste aversion? Neurobiol Learn Mem. 2014;107:1–12. doi: 10.1016/j.nlm.2013.10.013. [DOI] [PubMed] [Google Scholar]