Abstract
Mesenchymal stem cells (MSCs) have shown their therapeutic potency for treatment of cardiovascular diseases owing to their low immunogenicity, ease of isolation and expansion, and multipotency. As multipotent progenitors, MSCs have revealed their ability to differentiate into various cell types and could promote endogenous angiogenesis via microenvironmental modulation. Studies on cardiovascular diseases have demonstrated that transplanted MSCs could engraft at the injured sites and differentiate into cardiomyocytes and endothelial cells as well. Accordingly, several clinical trials using MSCs have been performed and revealed that MSCs may improve relevant clinical parameters in patients with vascular diseases. To fully comprehend the characteristics of MSCs, understanding their intrinsic property and associated modulations in tuning their behaviors as well as functions is indispensable for future clinical translation of MSC therapy. This review will focus on recent progresses on endothelial differentiation and potential clinical application of MSCs, with emphasis on therapeutic angiogenesis for treatment of cardiovascular diseases.
1. Introduction
Cardiovascular diseases (CVDs) are one of the major causes of morbidity and mortality worldwide [1]. The idea of promoting neovascularization and improving perfusion of ischemic tissue via angiogenesis is promising for treatment of CVDs. Since ischemic diseases are primarily caused by endothelial dysfunction, the logic behind therapeutic angiogenesis is to promote spontaneous tissue reparation via endothelial cells (ECs) and growth factor administration [2]. However, due to the limitation of expanding efficiency and postnatal cell sources, angiogenic therapy with ECs is not available in most cases [2, 3]. With their tremendous differentiation potency, the application of mesenchymal stem cells (MSCs) has been suggested to be a valuable alternative source for treatment of ischemic diseases. Moreover, MSCs hold great promise for tissue regeneration and revascularization through stimulating the secretion of various cytokines responsible for proangiogenic and antiapoptotic effects [4]. Thus, elucidating the paracrine effects and endothelial differentiation of MSCs will not only enhance our understanding of vascular disease pathogenesis, but also improve our ability to facilitate endothelial differentiation of MSCs for regeneration purposes.
2. Isolation and Characterization of MSCs
MSCs were first described as stromal cells residing in the bone marrow of rats, which have the capacity to transform into fibroblast-like cells during the processes of tissue repair [5, 6]. Later in the 1970s, Friedenstein et al. demonstrated heterogeneous populations of adherent cells within bone marrow, which can replicate as undifferentiated cells and can differentiate into a variety of mesenchymal cells, including osteoblasts, chondrocytes, myocytes, and adipocytes [7, 8]. Moreover, these kinds of cells are widely distributed and can be isolated from adult tissues including bone marrow, adipose tissue, and peripheral blood or neonatal birth-associated tissues like Wharton's Jelly and placenta [9].
Bone-marrow-derived MSCs (BM-MSCs) can be isolated from bone marrow aspiration, which are among the most frequently used types in regenerative studies. However, the way to get BM-MSCs is accompanied by a risk of infection and is painful for patients, which means that finding alternatives is essential in clinical practice. Fortunately, other than bone marrow, several kinds of MSCs have been isolated successfully. For example, peripheral blood-derived MSCs (PB-MSCs) can be isolated from mononuclear cells of peripheral blood [10]. Adipose-derived MSCs are usually obtained from adipose samples by enzymatic digestion [11]. Moreover, Wharton's Jelly of umbilical cord and placenta are considered to be convenient and readily alternatives to bone marrow [12, 13]. MSCs derived from Wharton's Jelly have the weakest expression of histocompatibility complex class I genes [14] and immune-related genes [15] while they do not express major histocompatibility complex class II genes [14], which means that Wharton's Jelly-derived MSCs are promising source for tissue regeneration in clinical application.
MSCs are partly differentiated progenitor cells containing multilineage stem cells, and there are no specific and reliable markers for defining native MSCs. Isolation and purification of MSCs usually involve density gradient centrifugation or immunophenotyping [16]. The phenotype of MSCs is determined by certain surface markers including CD49a (alpha-1 integrin), CD73 (ecto-5′-nucleotidase), CD105 (endoglin), MSC antigen-1, CD271 (adapalene), CD29, CD44, CD90, and CD106 (vascular cell adhesion molecule-1), but lack of CD34, CD45, CD14, HLA-DR, CD19, and CD79 [17, 18]. However, no specific single marker can be used to identify MSCs from other kinds of cell types. The lack of specific MSCs markers has thwarted the attempts to categorize these kind of stem cells in vivo [19]. The specific genotype and proteonomic profiles' analysis of multipotential MSCs clones has been carried out to further elucidate the characterization of MSCs which are promising to understand the mechanisms for maintaining or regulating those cells from different sources [20]. CD106 is mainly expressed on blood vessel endothelium, which also is an important marker for MSCs [13]. Moreover, MSCs with high expression of transmembrane protein cadherin-2 (N-cadherin) have revealed a higher probability to differentiate into cardiomyocytes, which indicates that MSCs can improve heart function directly [21]. The unique subpopulation of MSCs possessing specific differentiation potency may contribute to designed therapeutic strategies.
3. Therapeutic Application of MSCs for Vascular Regeneration
Despite advances in medical treatment, cardiovascular diseases (CVDs) are still major causes of adult death. Stem cell-based therapy in the treatment of ischemic diseases is a fast-growing field that has been proven effective and safe [22]. MSCs can transdifferentiate into all cell lineages of three germ layers including blood vessel cells arising from mesodermal tissue, which means that it is an attractive cell type for stem cell-based therapy for treatment of CVDs [23]. The therapeutic contribution of MSCs to tissue repair includes direct differentiation into injured cells including cardiomyocytes, smooth muscle cell, and ECs; presenting cytokines in the microenvironment; and stimulating endogenous stem cell differentiation (Figure 1). The most necessary property of MSCs for treatment of ischemic diseases is their differentiation potential toward vascular phenotypes, which can be identified by specific markers or functional assays.
Figure 1.
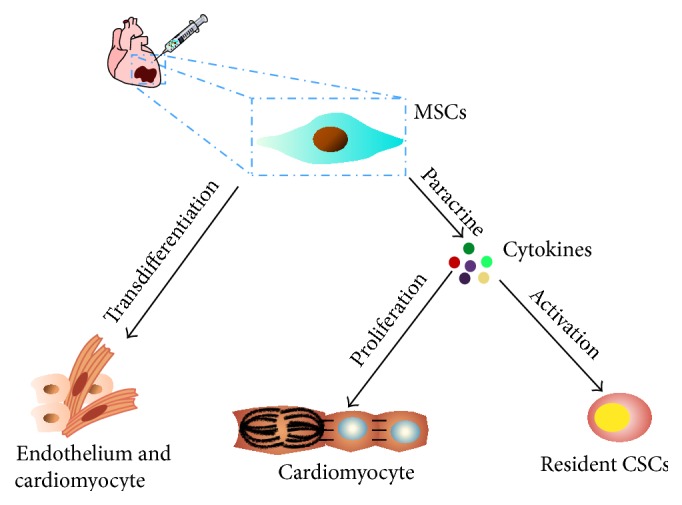
MSCs mediated therapy for myocardial infarction (MI). MSCs therapy could enhance heart function by (1) transdifferentiation into cardiomyocytes or endothelial cells (ECs) to replace the damage tissue and promote angiogenesis, respectively, (2) releasing soluble autocrine/paracrine factors, thereby activating endogenous adult cells involved in cells renewal/protection and neovascularization, and (3) stimulating endogenous resident cardiac stem cells (CSCs) proliferation and differentiation by paracrine soluble factors.
3.1. The Application of MSCs for Ischemia Heart Disease Therapy
Among the various forms of ischemic diseases, ischemic heart disease is a serious disease caused by the imbalance between myocardial oxygen supply and demand [24, 25]. MSCs transplantation could promote myocardium repair by stimulating angiogenesis, and MSCs have emerged as a promising cell type for ischemic heart disease therapy both in small and large animal models [26]. For example, the intravenously injected MSCs derived from rat fetal heart for treatment of myocardial ischemia has achieved significant improvement in cardiac function [27]. The injected cells were found to express ECs and smooth muscle cells (SMCs) markers, suggesting that MSCs can transdifferentiate into SMCs and ECs. This result was also confirmed in large animal study. In a swine model of chronic ischemic cardiomyopathy, the engrafted MSCs were found to differentiate into cardiomyocytes, SMCs, and endothelium [28]. Moreover, the efficacy of intramyocardial injection of autologous MSCs has been confirmed in a clinical trial [29]. Here, we summarized the use of MSCs in clinical trials in CVDs treatment according to https://clinicaltrials.gov/ (Table 1). A number of trials are focused on the safety and efficacy of autologous/allogeneic MSCs transplantation. The results of these trials have confirmed that MSCs injection is safe and has the capability to improve cardiac function. Despite these positive results, additional clinical trials are still encouraged to determine whether stem cell-based therapy could serve as a novel alternative tool for treatment of ischemic heart disease.
Table 1.
Completed MSC-based randomized clinical trials for ischemic heart disease therapy registered at https://clinicaltrials.gov/.
| Cell | Phase | Condition | Cell delivery route | Basis of trial design | Result | Reference |
|---|---|---|---|---|---|---|
| Autologous BM-MSCs | II/III | AMI | Intracoronary | Repairing the damaged myocardium via paracrine signaling | Autologous BM-MSCs are safe and provide modest improvement in LVEF | [102] |
|
| ||||||
| Auto-hMSCs and allo-hMSCs | I/II | CILVD | Transendocardial | Prevention remodeling of the ventricle and reduction of infarct size | Alloimmune reactions of allogeneic MSCs injection are low and improved functions are observed | [103] |
|
| ||||||
| Autologous BM-MSCs | I/II | Heart attack | Intramyocardial | Repair and restore heart function by reducing fibrosis, neoangiogenesis, and neomyogenesis | Autologous BM-MSCs could reduce scar, enhance regional function, and improve tissue perfusion | [29] |
|
| ||||||
| Allogeneic BM-MSCs | I | MI | Intravenous | Transdifferentiation of MSCs into cardiomyocytes | Intravenous allogeneic hMSCs are safe in patients after AMI | [104] |
|
| ||||||
| Allogeneic BM- MSCs | I/II | MI | Intravenous | Transdifferentiation of MSCs into cardiomyocytes and production of new blood vessels | Intravenous infusion of allogeneic BM-MSCs is safe and well-tolerated in AMI patients | [105] |
|
| ||||||
| WJ-MSCs | II | STEMI | Intracoronary | Transdifferentiation of MSCs into cardiomyocytes | Intracoronary infusion of WJ-MSCs is safe and effective in patients with AMI | [106] |
|
| ||||||
| Autologous MSCs and BMCs | I/II | LVD | Transendocardial | Stimulation of endogenous cardiac stem cells by MSCs | Transendocardial injection with MSCs or BMCs appeared to be safe for patients with ICM and LVD | [107, 108] |
|
| ||||||
| AD-MSCs | II | CMI | Not special | Angiogenesis | — | — |
|
| ||||||
| Autologous BM-MSC | I/II | CHF | Intramyocardial | Development of new myocardium and blood vessels | Intramyocardial injections of autologous culture expanded MSCs were safe and improved myocardial function | [109, 110] |
Notes. BM-MSCs: bone-marrow-derived human MSCs; WJ-MSCs: umbilical Wharton's Jelly-derived mesenchymal stem cell; AD-MSCs: adipose-derived mesenchymal stem cells; auto-hMSCs: autologous human mesenchymal stem cells; allo-hMSCs: allogeneic human mesenchymal stem cells; BMCs: bone marrow mononuclear cells; AMI: acute myocardial infarction; LVEF: left ventricular ejection fraction; STEMI: ST elevation myocardial Infarction; ICM: chronic ischemic cardiomyopathy; CMI: chronic myocardial ischemia; IHF: ischemic heart failure; CHF: congestive heart failure; and CILVD: chronic ischemic left ventricular dysfunction.
Tissue injury and/or inflammation caused by ischemia can enhance the efficacy of MSCs homing to the injury tissue, which may provide an avenue for clinical translation of MSCs in the future as a cellular tool for CVDs therapeutics [30]. MSCs expressing various surface receptors could respond to the migratory signals released by sites of injury. Meanwhile, many studies to modify and/or enhance expression of these surface markers have been explored [31]. Therefore, optimized culture conditions, biomaterials, and gene transfection can be used to enhance migration ability of MSCs homing to the sites of injury. Although MSCs are the most promising sources of regeneration medicine, their poor cell viability after transplantation imposes restrictions on their clinical applications. Therefore, another aspect of research interest is improving cell survival rate after transplantation. MSCs genetically transduced with Akt1 (protein kinase B) can improve MSCs survival while infarcted myocardium reparation was enhanced [32]. The other approaches to enhance MSCs survival in ischemic tissue include hypoxic preconditioning, genetic modification, and angiogenin expression [33, 34], and these methods highlight the utility of gene-engineered cells and preconditioning cells.
The exact pathway of acute donor cell death following transplantation is still unknown, but lack of matrix support is one of major causes [2]. Engineered microenvironments with extracellular matrix and growth factors, mimicking the establishment of cell niche in vivo, have achieved great improvement in controlling stem cell fate [35–37]. Furthermore, the use of different polymers can help delivering drugs, viruses, plasmids, and cytokines [38]. Transplantation of the construct of tissue-engineered cardiac patch with BM-MSCs for immunocompetent rats after myocardial infarction has resulted in increased fractional shortening, augmented anterior wall thickness, and reduced left ventricle interior diameter at systole [39]. A similar study of a three-dimensional scaffolds transplantation that contain more than a few layers of BM-MSCs also led to heart function improvement and formation of MSC-derived neovasculatures [40].
3.2. The Application of MSCs for Treatment of Peripheral Artery Disease (PAD)
Peripheral artery disease (PAD) is a serious disease usually caused by atherosclerotic occlusion, which can lead to critical limb ischemia, tissue injuries, and eventual limb loss [41, 42]. The patients with PAD have a very poor long-term prognosis; only 30% of patients can live for 15 years after being diagnosed with this disease [43]. Critical limb ischemia is the most serious complication of PAD, which can cause pain, on walking or even at rest, and nonhealing ulcers. Although patients with limb ischemia are treated with a combination of methods, such as statins, antiplatelet drugs, and angioplasty, these treatments are occasionally insufficient to recover sufficient arterial blood flow to maintain tissue viability [44]. Therefore, it is urgent need to develop novel treatment approaches for revascularization of ischemic limbs to repair the injured tissues.
Currently, one of the most fundamental methods for PAD treatment is therapeutic angiogenesis, which aims to promote neovascularization and restore blood flow to ischemic limbs by forming new blood vessels from preexisting ones [45]. The evidences that MSCs transplantation could achieve this purpose have been confirmed by small and large animal studies [46, 47]. And the use of MSCs has already started in the first clinical trials and the safety/efficacy of MSCs was confirmed [48, 49]. In a double-blind, controlled, randomized trial in patients with critical limb ischemia caused by diabetes [50], ulcer size, pain-free walking time, and percutaneous tissue oxygen pressure were significantly improved in MSCs treated group, and the production of VEGF and FGF-2 was significantly higher in the MSCs group compared to groups of other kinds of cells from bone marrow. Although further basic investigations should be carried out, this clinical trial proved that MSCs hold great promise for vascular regeneration.
Both animal studies and clinical trials established the evidence that therapeutic angiogenesis with MSCs can be regarded as an effective approach for treatment of PAD. The therapy potential is based on the transplantation of MSCs, which could enhance neovascularization, thereby rescuing the ischemic tissues from degeneration. However, before we apply this approach to clinical, the therapeutic potential and scientific support of MSCs transdifferentiation need to be further investigated [51]. Future studies will be undoubtedly confident that all these basic studies and clinical trials of therapeutic neovascularization will improve outcomes in PAD.
3.3. MSCs for Vascular Bioengineering
Vascular grafts are increasingly needed in the clinic for coronary disease and hemodialysis [52, 53]. Unfortunately, the acute thrombosis and subsequent occlusion often lead to the failure of the transplanted synthetic acellular vascular grafts. Common models of living vascular grafts have been developed to some different types like matrix or cells alone or hybrid vascular grafts combined with cells, matrix, and soluble factors [54]. Because of their superior proliferation capacity and lower immunogenicity as an excellent cellular candidate for off-the-shelf therapy, MSCs represent a suitable alternative stem cell source [55]. MSCs have mainly three critical functions: (1) homing to the site where tissues are damaged, (2) secreting cytokines or chemokines to facilitate the migration of host cells, and (3) transdifferentiating into functional ECs [56].
In clinical application, tissue-engineered vascular grafts (TEVGs) are supposed to have mechanically durable structure and antithrombogenesis ability. Most approaches of TEVG fabrication rely on the forms of scaffold to provide mechanical integrity upon implantation to the arterial circulation. In vivo transplantation of acellular conduits or MSC-seeded vascular grafts as interpositional grafts suggested that MSCs could block the inflammatory responses induced by nanofibrous scaffolds [57]. The combined application of polymer grafts and MSCs for developing vascular conduits confers TEVGs with mechanical strength, good tissue compatibility, and ability of remodeling.
Genetic modification of MSCs to mimic the function of ECs has been reported. Rat BM-MSCs were transduced with eNOS and can release nitric oxide (NO) subsequently. NO is normally released by ECs to offer feasible effects of angiogenesis on the native coronary arteries, as well as improve self-patency and prevent thrombogenesis [58]. Adipose-derived cells with transfection of eNOS gene could produce significant amounts of NO, which indicate the possibility of NO production of an engineered vascular [59]. This hybrid vascular prosthesis is expected to provide a therapeutic advantage by extended production of NO from the inner surfaces.
In addition to cells alone, to investigate spatial orientation of MSCs related to the engineered vascular, MSCs and human umbilical vein endothelial cells (HUVECs) were transduced, respectively, with retrovirus to express DsRed and EGFP [60]. They found that undifferentiated MSCs combined with HUVECs seeded on a graft are able to grow in vivo and function as pericytes wrapping around the endothelial tubes (Figure 2). Moreover, the angiogenic applications of MSCs are not only based on their ability to differentiate into ECs, but also on their differentiation ability toward SMCs phenotype. In response to TGF-β, MSCs have been revealed to transdifferentiate into SMCs by direct contact with vascular ECs, mechanical stress, and prostaglandin F2α (PGF2α) [61, 62]. The intramuscular injection of TGF-β1-induced human adipose tissue-derived MSCs could improve neovascularization and blood perfusion significantly [63]. These results indicate the important role of MSCs in therapeutic angiogenesis.
Figure 2.
Intravital microscope analysis revealed that MSCs behaved like pericytes wrapping around the vessel in a tissue-engineered vascular model. With multiphoton laser scanning microscopy (MPLSM), images were taken at different time points. Lumen formation and blood flow in hMSCs (EGFP+) derived cells were not able to detect (a). On the contrary, implant HUVECs (DsRed+) and hMSCs (EGFP+) in mice, hMSCs (EGFP+) could be found elongate into thin slit structures and coalesced around the HUVEC-derived vessels (b)–(f). Over time, the number of interstitial hMSCs (EGFP+) was decreased, and most of them were associated with blood vessels by day 83 (g). Reprinted with permission from [60].
4. Paracrine Mechanisms behind MSCs Angiogenic Potential
Despite the acute donor cell death after transplantation, both animal and clinical studies have demonstrated beneficial effects of the treatment, which indicate that stem cells perhaps act through paracrine pathways [3]. Cell-free conditioned medium from MSCs can induce angiogenesis through activation of VEGF-A expression and secretion in ECs [64]. Another experiments also revealed that conditioned medium from bone marrow or adipose tissue also has got positive effect on angiogenesis [65–67] profiting in which from MSCs' angiogenic paracrine secretion of HGF, bFGF, IGF-1, and VEGF [68]. Besides, exosomes released by MSCs may serve as an essential mediator of angiogenesis by transferring genetic materials and angiogenic molecules. Those results indicate that the paracrine cytokines and exosomes of MSCs have a complex composition rather than single molecule, all of which could participate in regenerative processes.
4.1. Growth Factor Production
MSCs could be recruited and mobilized to the sites of inflammation as well as injury where they can incorporate into the ischemic tissue's microenvironment. After cells transplant, the angiogenic paracrine effects of MSCs could improve tissue functions in the ischemic limb [69]. MSCs can potentiate angiogenesis via direct differentiation, cell contact interaction, or paracrine effects [70]. Angiogenic factors produced by MSCs include bFGF, VEGF, TGF-β, PDGF, angiopoietin-1, placental growth factor (PGF), IL-6, and monocyte chemotactic protein-1 (MCP-1), which facilitate tissue regeneration.
MSCs could stimulate angiogenesis in vitro and in vivo through secreted VEGF, MCP-1, and IL-6 into their condition medium, and these effects can be significantly inhibited by pretreatment with neutralizing antibodies against VEGF, MCP-1, and IL-6 [71]. IL-6 is an MSC-secreted cell factor displaying proangiogenic, progrowth, and prosurvival activities [72], which has potent proangiogenic and antiapoptotic activity. MCP-1 can be detected among MSC-secreted cytokines and is a critical chemoattractant for angiogenesis [67]. Last but not least, the most important proangiogenic factor VEGF has been shown possible to be expressed by MSCs as well as promote MSCs differentiation [73]. Meanwhile, VEGF has been revealed to regulate ECs migrations and differentiation and promote recruitment of ECs for angiogenesis and endothelialization in injured tissue [44]. The receptors of VEGF include VEGFR2 (KDR/Flk1) and tyrosine kinase receptors VEGFR1 (Flt1) [74]. VEGFR2 induces activation of various signaling pathways, including the activation of mitogen-activated protein kinase (MAPK), phosphoinositide-3-kinase and Akt (PI3K/AKT), Src, and Rac pathways. VEGFR2 is the main receptor involved in cell signaling for its strong tyrosine kinase activity [75].
MSCs are able to secrete a composite of angiogenic factors like HGF and stromal-cell-derived factor-1 (SDF-1), which can promote local angiogenesis [76]. SDF-1 is an inducible and constitutively expressed chemokine that can regulate multiple physiological processes, such as stimulating ECs proliferation and capillary tube formation [77]. HGF exerts its angiogenic activity through tyrosine phosphorylation of its specific receptor, c-Met, which can be found in ECs and SMCs [78]. The therapeutic efficacy of HGF has been investigated in a clinical trial [79] and shown to possess multiple effects in patients with critical limb ischemia.
MSCs have both autocrine and paracrine activities, which are involved in cell survival, organ function, and tissue angiogenesis [67, 80]. Growth factors mentioned above like VEGF, MCP-1, HGF, TGF-β, and IL-6 are potent angiogenic factors, which could improve angiogenesis and restoration of PAD and CVDs [81]. With these angiogenic factors, MSCs-based treatment after tissue injured could increase microvascular density and preserve organ function through increasing tissue perfusion [81]. In accordance with these observations, MSCs with various paracrine angiogenic factors are able to enhance in vivo angiogenesis and local blood flow recovery in the ischemic tissue.
4.2. Exosomes and MicroRNA Production
The angiogenic cytokines are one of the crucial factors of angiogenesis, which can support cell growth, proliferation, and communication. However, multiple studies show that the communication between MSCs and other cells is not only through cytokines but also through exerting their function via exosomes. Exosomes are small particles dynamically composed by lipids, proteins, cytoskeletal elements, molecular chaperones, and signaling molecules that can also convey biological materials to surrounding cells [82, 83]. More recently, evidences that exosomes contain genetic materials including mRNAs and microRNAs have emerged [84], and these exosomes can be functional for recipient cells. Results suggest that exosomes may serve as an essential mediator of angiogenesis by transferring genetic materials and angiogenic molecules.
Exosomes with miRNAs can be secreted by a variety of cell types such as cardiocytes, vascular cells, and MSCs in culture [85], and the altered expression of miRNAs has been correlated with CVDs. The discovery of miRNA/vesicle-mediated communication between endothelial cells and cardiovascular cells was confirmed recently [86, 87]. Moreover, miRNAs have been detected in the supernatant of human MSCs that may be associated with exosomes [88]. These exosomes were injected into a rat model of ischemia/reperfusion and revealed cardioprotective effect [89]. The result indicated that exosomes secreted by MSCs might possess therapeutic potential. Although significant progresses have been made in CVDs therapy, further investigations are needed to explore the therapeutic potency of MSCs-derived exosomes. Future clinical use of exosomes in diagnosis, monitoring disease progress, treatment, and monitoring treatment efficacy is promising.
5. Endothelial Differentiation of MSCs
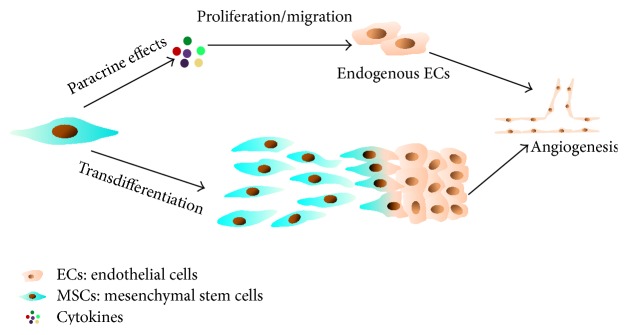
Human MSCs have been confirmed to be pluripotent progenitors that can replicate as multiple types of cells including lineages with phenotypic and functional features of ECs [90]. Further in vivo research also showed that engrafted MSCs were positive for vWF, suggesting their transdifferentiation into ECs [91]. Since MSCs can differentiate into ECs, this means that MSCs have the ability to participate in postnatal angiogenesis and form vasculature in vivo [92]. Those results confirmed that MSCs could be a viable source for the de novo generation of ECs for therapeutic angiogenesis application (Figure 3).
Figure 3.
Angiogenic potency of MSCs. MSCs could promote angiogenesis either by paracrine effects or by transdifferentiation. The secretion of cytokines could enhance the proliferation and migration of endogenous endothelial cells. Moreover, MSCs may transdifferentiate into lineage with functional and phenotypic features of ECs and participate in angiogenesis directly.
Isolated MSCs were negative for typical endothelial markers like eNOS, VE-cadherin, vWF, and pericyte markers (NG2 and platelet-derived growth factor receptor β) [93], and those markers can be used to establish the identity of endothelial differentiation of MSCs. Moreover, MSCs isolated from human Wharton's Jelly were negative for typical endothelial markers and eNOS protein expression was increased significantly after endothelial transdifferentiation [18], which has been proved in MSCs from other sources as well. Another research interest lies in the impact of NO signaling during the process of MSCs transdifferentiation into ECs [94]. It has been revealed that NO signaling is an essential regulator of vasculogenesis in MSCs. These findings offer exciting insights for MSCs-based angiogenic therapy.
VEGF is a major factor in the process of endothelial differentiation, which can induce the endothelial differentiation of MSCs [65, 95, 96]. A study implied that VEGF induces human BM-MSCs differentiation into ECs by Rho/ROCK signaling pathway. Blocking Rho/ROCK pathway could suppress the upregulation of tube formation, migration, and proliferation of ECs [97, 98]. Moreover, PDGF could induce endothelial differentiation of MSCs [99], whereas TGF-β plays an important role in cell differentiation and vascular remodeling [100]. It has been revealed that TGF-β could increase the expression of smooth muscle β-actin while decreasing the expression of gelsolin according to proteomic profiling analysis [101], which will lead to more focused and in-depth research on the influences of TGF-β on endothelial differentiation of MSCs.
6. Conclusions
Over the last decade, MSCs have been proved for engineering vascular conduits or treatment of ischemic diseases. MSCs have drawn considerable attention because of their potential to differentiate into cardiovascular lineages and their promising therapeutic prospective and beneficial properties for CVDs. MSCs can be applied to a variety of clinical scenarios not only through cell-cell interactions but also through multiple paracrine factors. Therefore, understanding the intrinsic properties and associated modulations in tuning MSCs' behaviors as well as functions is indispensable for future therapeutic applications of MSCs. Clearly elucidating the mechanisms of endothelial differentiation and therapeutic angiogenesis of MSCs will offer more simple, definitive, and effective approaches for treatment of ischemic CVDs.
Acknowledgments
This work was partially supported by grants from the National Natural Science Foundation of China (81371620, 31470951, and 81320108014), the National Basic Research Program of China (2011CB964903), and Program for Changjiang Scholars and Innovative Research Team in University (IRT13023).
Conflict of Interests
The authors declare no potential conflict of interests.
Authors' Contribution
All authors (Hongyan Tao, Zhibo Han, Zhong Chao Han, and Zongjin Li) have made substantive intellectual contributions to this study according to ICMJE guidelines. All of them have been qualified as authors. All authors read and approved the final paper.
References
- 1.Go A. S., Mozaffarian D., Roger V. L., et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daadi M. M., Li Z., Arac A., et al. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Molecular Therapy. 2009;17(7):1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z., Wilson K. D., Smith B., et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE. 2009;4(12) doi: 10.1371/journal.pone.0008443.e8443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerlin L., Park T. S., Zambidis E. T., Donnenberg V. S., Donnenberg A. D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95(12):2235–2245. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R., Everett N. B., Tyler R. Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. The Journal of Cell Biology. 1970;44(3):645–654. doi: 10.1083/jcb.44.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrakis N. L., Davis M., Lucia S. P. The in vivo differentiation of human leukocytes into histiocytes, fibroblasts and fat cells in subcutaneous diffusion chambers. Blood. 1961;17:109–118. [PubMed] [Google Scholar]
- 7.Friedenstein A. J., Gorskaja U. F., Kulagina N. N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 8.Cook D., Genever P. Regulation of mesenchymal stem cell differentiation. Advances in Experimental Medicine and Biology. 2013;786:213–229. doi: 10.1007/978-94-007-6621-1_12. [DOI] [PubMed] [Google Scholar]
- 9.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. 2011;9, article 12 doi: 10.1186/1478-811x-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassis I., Zangi L., Rivkin R., et al. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplantation. 2006;37(10):967–976. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 11.Kuhbier J. W., Weyand B., Radtke C., Vogt P. M., Kasper C., Reimers K. Bioreactor Systems for Tissue Engineering II. Vol. 123. Berlin, Germany: Springer; 2010. Isolation, characterization, differentiation, and application of adipose-derived stem cells; pp. 55–105. (Advances in Biochemical Engineering/Biotechnology). [DOI] [PubMed] [Google Scholar]
- 12.Gong W., Han Z., Zhao H., et al. Banking human umbilical cord-derived mesenchymal stromal cells for clinical use. Cell Transplantation. 2012;21(1):207–216. doi: 10.3727/096368911X586756. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z. X., Han Z.-B., Ji Y. R., et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059354.e59354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarugaser R., Hanoun L., Keating A., Stanford W. L., Davies J. E. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE. 2009;4(8) doi: 10.1371/journal.pone.0006498.e6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Bai J., Ji X., Li R., Xuan Y., Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. International Journal of Molecular Medicine. 2014;34(3):695–704. doi: 10.3892/ijmm.2014.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang N. F., Li S. Mesenchymal stem cells for vascular regeneration. Regenerative Medicine. 2008;3(6):877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams A. R., Hare J. M. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation Research. 2011;109(8):923–940. doi: 10.1161/circresaha.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguilera V., Briceño L., Contreras H., et al. Endothelium trans differentiated from Wharton's jelly mesenchymal cells promote tissue regeneration: potential role of soluble pro-angiogenic factors. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111025.e111025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv F.-J., Tuan R. S., Cheung K. M. C., Leung V. Y. L. Concise review: the surface markers and identity of human mesenchymal stem cells. STEM CELLS. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 20.Law S., Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. American Journal of Stem Cells. 2013;2(1):22–38. [PMC free article] [PubMed] [Google Scholar]
- 21.Ishimine H., Yamakawa N., Sasao M., et al. N-Cadherin is a prospective cell surface marker of human mesenchymal stem cells that have high ability for cardiomyocyte differentiation. Biochemical and Biophysical Research Communications. 2013;438(4):753–759. doi: 10.1016/j.bbrc.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 22.Das M., Sundell I. B., Koka P. S. Adult mesenchymal stem cells and their potency in the cell-based therapy. Journal of Stem Cells. 2013;8(1):1–16. [PubMed] [Google Scholar]
- 23.Chen L., Xu Y., Zhao J., et al. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0096161.e96161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q., Li Z. Angiogenesis. BioMed Research International. 2015;2015:2. doi: 10.1155/2015/135861.135861 [DOI] [Google Scholar]
- 25.Wang L., Su W., Du W., et al. Gene and MicroRNA profiling of human induced pluripotent stem cell-derived endothelial cells. Stem Cell Reviews and Reports. 2015;11:219–227. doi: 10.1007/s12015-014-9582-4. [DOI] [PubMed] [Google Scholar]
- 26.Dong F., Harvey J., Finan A., Weber K., Agarwal U., Penn M. S. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation. 2012;126(3):314–324. doi: 10.1161/CIRCULATIONAHA.111.082453. [DOI] [PubMed] [Google Scholar]
- 27.Garikipati V. N. S., Jadhav S., Pal L., Prakash P., Dikshit M., Nityanand S. Mesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: an in vivo serial pinhole gated SPECT-CT study in rats. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0100982.e100982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quevedo H. C., Hatzistergos K. E., Oskouei B. N., et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karantalis V., DiFede D. L., Gerstenblith G., et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circulation Research. 2014;114(8):1302–1310. doi: 10.1161/circresaha.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F., Huang Q., Chen J., et al. Apoptotic cells activate the ‘phoenix rising’ pathway to promote wound healing and tissue regeneration. Science Signaling. 2010;3(110, article ra13) doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maijenburg M. W., Gilissen C., Melief S. M., et al. Nuclear receptors Nur77 and Nurr1 modulate mesenchymal stromal cell migration. Stem Cells and Development. 2012;21(2):228–238. doi: 10.1089/scd.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangi A. A., Noiseux N., Kong D., et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nature Medicine. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 33.Simpson D., Liu H., Fan T.-H. M., Nerem R., Dudley S. C., Jr. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25(9):2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X.-H., Bai C.-G., Xu Z.-Y., et al. Therapeutic potential of angiogenin modified mesenchymal stem cells: angiogenin improves mesenchymal stem cells survival under hypoxia and enhances vasculogenesis in myocardial infarction. Microvascular Research. 2008;76(1):23–30. doi: 10.1016/j.mvr.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Yao X., Liu Y., Gao J., et al. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials. 2015;60:130–140. doi: 10.1016/j.biomaterials.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 36.He N., Xu Y., Du W., et al. Extracellular matrix can recover the downregulation of adhesion molecules after cell detachment and enhance endothelial cell engraftment. Scientific Reports. 2015;5 doi: 10.1038/srep10902.10902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du W., Tao H., Zhao S., He Z., Li Z. Translational applications of molecular imaging in cardiovascular disease and stem cell therapy. Biochimie. 2015;116:43–51. doi: 10.1016/j.biochi.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Nelson D. M., Hashizume R., Yoshizumi T., Blakney A. K., Ma Z., Wagner W. R. Intramyocardial injection of a synthetic hydrogel with delivery of bFGF and IGF1 in a rat model of ischemic cardiomyopathy. Biomacromolecules. 2014;15(1):1–11. doi: 10.1021/bm4010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson D. L., Dudley S. C., Jr. Modulation of human mesenchymal stem cell function in a three-dimensional matrix promotes attenuation of adverse remodelling after myocardial infarction. Journal of Tissue Engineering and Regenerative Medicine. 2013;7(3):192–202. doi: 10.1002/term.511. [DOI] [PubMed] [Google Scholar]
- 40.Wei H.-J., Chen C.-H., Lee W.-Y., et al. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29(26):3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Raval Z., Losordo D. W. Cell therapy of peripheral arterial disease: from experimental findings to clinical trials. Circulation Research. 2013;112(9):1288–1302. doi: 10.1161/circresaha.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orbay H., Zhang Y., Hong H., et al. Positron emission tomography imaging of angiogenesis in a murine hindlimb ischemia model with 64Cu-labeled TRC105. Molecular Pharmaceutics. 2013;10(7):2749–2756. doi: 10.1021/mp400191w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Zhang R., Li Y., He G., Zhang D., Zhang F. Simvastatin augments the efficacy of therapeutic angiogenesis induced by bone marrow-derived mesenchymal stem cells in a murine model of hindlimb ischemia. Molecular Biology Reports. 2012;39(1):285–293. doi: 10.1007/s11033-011-0737-y. [DOI] [PubMed] [Google Scholar]
- 44.Shimamura M., Nakagami H., Koriyama H., Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. BioMed Research International. 2013;2013:8. doi: 10.1155/2013/186215.186215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ransohoff J. D., Wu J. C. Imaging stem cell therapy for the treatment of peripheral arterial disease. Current Vascular Pharmacology. 2012;10(3):361–373. doi: 10.2174/157016112799959404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin J., Li K., Peng C., et al. MRI of iron oxide nanoparticle-labeled ADSCs in a model of hindlimb ischemia. Biomaterials. 2013;34(21):4914–4925. doi: 10.1016/j.biomaterials.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Bronckaers A., Hilkens P., Martens W., et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacology and Therapeutics. 2014;143(2):181–196. doi: 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki H., Iso Y. Clinical application of vascular regenerative therapy for peripheral artery disease. BioMed Research International. 2013;2013:6. doi: 10.1155/2013/179730.179730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell R. J., Comerota A. J., Berceli S. A., et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. Journal of Vascular Surgery. 2011;54(4):1032–1041. doi: 10.1016/j.jvs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Lu D., Chen B., Liang Z., et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Research and Clinical Practice. 2011;92(1):26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Grochot-Przeczek A., Dulak J., Jozkowicz A. Therapeutic angiogenesis for revascularization in peripheral artery disease. Gene. 2013;525(2):220–228. doi: 10.1016/j.gene.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z., Cui Y., Wang J., et al. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials. 2014;35(22):5700–5710. doi: 10.1016/j.biomaterials.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 53.Wang K., Chen X., Pan Y., et al. Enhanced vascularization in hybrid PCL/gelatin fibrous scaffolds with sustained release of VEGF. BioMed Research International. 2015;2015:10. doi: 10.1155/2015/865076.865076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.L'Heureux N., Pâquet S., Labbé R., Germain L., Auger F. A. A completely biological tissue-engineered human blood vessel. The FASEB Journal. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z.-Y., Teoh S.-H., Hui J. H. P., Fisk N. M., Choolani M., Chan J. K. Y. The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials. 2012;33(9):2656–2672. doi: 10.1016/j.biomaterials.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 56.Krawiec J. T., Weinbaum J. S., St Croix C. M., et al. A cautionary tale for autologous vascular tissue engineering: impact of human demographics on the ability of adipose-derived mesenchymal stem cells to recruit and differentiate into smooth muscle cells. Tissue Engineering Part A. 2015;21(3-4):426–437. doi: 10.1089/ten.tea.2014.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashi C. K., Zhu Y., Yang G.-Y., et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanki-Horimoto S., Horimoto H., Mieno S., et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(1):I-181–I-185. doi: 10.1161/circulationaha.105.001487. [DOI] [PubMed] [Google Scholar]
- 59.Mcilhenny S., Zhang P., Tulenko T., et al. eNOS transfection of adipose-derived stem cells yields bioactive nitric oxide production and improved results in vascular tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2015;9(11):1277–1285. doi: 10.1002/term.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Au P., Tam J., Fukumura D., Jain R. K. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunha F. F., Martins L., Martin P. K. M., Stilhano R. S., Han S. W. A comparison of the reparative and angiogenic properties of mesenchymal stem cells derived from the bone marrow of BALB/c and C57/BL6 mice in a model of limb ischemia. Stem Cell Research & Therapy. 2013;4(4, article 86) doi: 10.1186/scrt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee M. J., Kim M. Y., Heo S. C., et al. Macrophages regulate smooth muscle differentiation of mesenchymal stem cells via a prostaglandin F2α-mediated paracrine mechanism. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(11):2733–2740. doi: 10.1161/atvbaha.112.300230. [DOI] [PubMed] [Google Scholar]
- 63.Park W. S., Heo S. C., Jeon E. S., et al. Functional expression of smooth muscle-specific ion channels in TGF-β 1-treated human adipose-derived mesenchymal stem cells. American Journal of Physiology—Cell Physiology. 2013;305(4):C377–C391. doi: 10.1152/ajpcell.00404.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bussche L., Van de Walle G. R. Peripheral blood-derived mesenchymal stromal cells promote angiogenesis via paracrine stimulation of vascular endothelial growth factor secretion in the equine model. Stem Cells Translational Medicine. 2014;3(12):1514–1525. doi: 10.5966/sctm.2014-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y., Liu L., Zhang L., et al. Efficient commitment to functional CD34+ progenitor cells from human bone marrow mesenchymal stem-cell-derived induced pluripotent stem cells. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034321.e34321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhang S. H., Lee S., Shin J.-Y., Lee T.-J., Jang H.-K., Kim B.-S. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Molecular Therapy. 2014;22(4):862–872. doi: 10.1038/mt.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boomsma R. A., Geenen D. L. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PloS ONE. 2012;7(4) doi: 10.1371/journal.pone.0035685.e35685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sopko N. A., Turturice B. A., Becker M. E., et al. Bone marrow support of the heart in pressure overload is lost with aging. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0015187.e15187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlosser S., Dennler C., Schweizer R., et al. Paracrine effects of mesenchymal stem cells enhance vascular regeneration in ischemic murine skin. Microvascular Research. 2012;83(3):267–275. doi: 10.1016/j.mvr.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Watt S. M., Gullo F., van der Garde M., et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. British Medical Bulletin. 2013;108(1):25–53. doi: 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwon H. M., Hur S.-M., Park K.-Y., et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascular Pharmacology. 2014;63(1):19–28. doi: 10.1016/j.vph.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Botto S., Streblow D. N., DeFilippis V., et al. IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. Blood. 2011;117(1):352–361. doi: 10.1182/blood-2010-06-291245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beckermann B. M., Kallifatidis G., Groth A., et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. British Journal of Cancer. 2008;99(4):622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berendsen A. D., Olsen B. R. How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells. The Journal of Histochemistry and Cytochemistry. 2014;62(2):103–108. doi: 10.1369/0022155413516347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koch S., Tugues S., Li X., Gualandi L., Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. The Biochemical Journal. 2011;437(2):169–183. doi: 10.1042/bj20110301. [DOI] [PubMed] [Google Scholar]
- 76.Pasquet M., Golzio M., Mery E., et al. Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. International Journal of Cancer. 2010;126(9):2090–2101. doi: 10.1002/ijc.24886. [DOI] [PubMed] [Google Scholar]
- 77.Zhou M., Liu Z., Liu C., et al. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100(1):111–120. doi: 10.1002/jbm.b.31928. [DOI] [PubMed] [Google Scholar]
- 78.Kaga T., Kawano H., Sakaguchi M., Nakazawa T., Taniyama Y., Morishita R. Hepatocyte growth factor stimulated angiogenesis without inflammation: differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascular Pharmacology. 2012;57(1):3–9. doi: 10.1016/j.vph.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Shigematsu H., Yasuda K., Iwai T., et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Therapy. 2010;17(9):1152–1161. doi: 10.1038/gt.2010.51. [DOI] [PubMed] [Google Scholar]
- 80.Park K.-S., Kim Y.-S., Kim J.-H., et al. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 2010;89(5):509–517. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 81.Timmers L., Lim S. K., Hoefer I. E., et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Research. 2011;6(3):206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Yeh Y. Y., Ozer H. G., Lehman A. M., et al. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood. 2015;125(21):3297–3305. doi: 10.1182/blood-2014-12-618470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emanueli C., Shearn A. I., Angelini G. D., Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascular Pharmacology. 2015;71:24–30. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 85.Waldenström A., Ronquist G. Role of exosomes in myocardial remodeling. Circulation Research. 2014;114(2):315–324. doi: 10.1161/circresaha.114.300584. [DOI] [PubMed] [Google Scholar]
- 86.Hergenreider E., Heydt S., Tréguer K., et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology. 2012;14(3):249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 87.Halkein J., Tabruyn S. P., Ricke-Hoch M., et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. The Journal of Clinical Investigation. 2013;123(5):2143–2154. doi: 10.1172/jci64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen T. S., Lai R. C., Lee M. M., Choo A. B. H., Lee C. N., Lim S. K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research. 2010;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lai R. C., Arslan F., Lee M. M., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Oswald J., Boxberger S., Jørgensen B., et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. STEM CELLS. 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 91.Thakker R., Yang P. Mesenchymal stem cell therapy for cardiac repair. Current Treatment Options in Cardiovascular Medicine. 2014;16(7):p. 323. doi: 10.1007/s11936-014-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin R.-Z., Moreno-Luna R., Zhou B., Pu W. T., Melero-Martin J. M. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis. 2012;15(3):443–455. doi: 10.1007/s10456-012-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ball S. G., Shuttleworth C. A., Kielty C. M. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. Journal of Cell Biology. 2007;177(3):489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gomes S. A., Rangel E. B., Premer C., et al. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(8):2834–2839. doi: 10.1073/pnas.1220185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang M., Su Y., Sun H., et al. Induced endothelial differentiation of cells from a murine embryonic mesenchymal cell line C3H/10T1/2 by angiogenic factors in vitro. Differentiation. 2010;79(1):21–30. doi: 10.1016/j.diff.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 96.Chen M.-Y., Lie P.-C., Li Z.-L., Wei X. Endothelial differentiation of Wharton's jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Experimental Hematology. 2009;37(5):629–640. doi: 10.1016/j.exphem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 97.He Q.-W., Xia Y.-P., Chen S.-C., et al. Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Molecular Neurobiology. 2013;47(3):976–987. doi: 10.1007/s12035-013-8396-8. [DOI] [PubMed] [Google Scholar]
- 98.Wang N., Zhang R., Wang S.-J., et al. Vascular endothelial growth factor stimulates endothelial differentiation from mesenchymal stem cells via Rho/myocardin-related transcription factor—a signaling pathway. The International Journal of Biochemistry & Cell Biology. 2013;45(7):1447–1456. doi: 10.1016/j.biocel.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 99.Lin H., Shabbir A., Molnar M., et al. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. Journal of Cellular Physiology. 2008;216(2):458–468. doi: 10.1002/jcp.21414. [DOI] [PubMed] [Google Scholar]
- 100.Wang D., Park J. S., Chu J. S. F., et al. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor β1 stimulation. The Journal of Biological Chemistry. 2004;279(42):43725–43734. doi: 10.1074/jbc.m407368200. [DOI] [PubMed] [Google Scholar]
- 101.Abnaof K., Mallela N., Walenda G., et al. TGF-β stimulation in human and murine cells reveals commonly affected biological processes and pathways at transcription level. BMC Systems Biology. 2014;8, article 55 doi: 10.1186/1752-0509-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee J.-W., Lee S.-H., Youn Y.-J., et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. Journal of Korean Medical Science. 2014;29(1):23–31. doi: 10.3346/jkms.2014.29.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hare J. M., Fishman J. E., Gerstenblith G., et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. The Journal of the American Medical Association. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hare J. M., Traverse J. H., Henry T. D., et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chullikana A., Majumdar A. S., Gottipamula S., et al. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17(3):250–261. doi: 10.1016/j.jcyt.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 106.Gao L. R., Chen Y., Zhang N. K., et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Medicine. 2015;13, article 162 doi: 10.1186/s12916-015-0399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heldman A. W., DiFede D. L., Fishman J. E., et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. The Journal of the American Medical Association. 2014;311(1):62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trachtenberg B., Velazquez D. L., Williams A. R., et al. Rationale and design of the transendocardial injection of autologous human cells (bone marrow or mesenchymal) in chronic ischemic left ventricular dysfunction and heart failure secondary to myocardial infarction (TAC-HFT) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. American Heart Journal. 2011;161(3):487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 109.Mathiasen A. B., Qayyum A. A., Jorgensen E., et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial) European Heart Journal. 2015;36:1744–1753. doi: 10.1093/eurheartj/ehv136. [DOI] [PubMed] [Google Scholar]
- 110.Mathiasen A. B., Jorgensen E., Qayyum A. A., Haack-Sorensen M., Ekblond A., Kastrup J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived Mesenchymal Stromal Cells in chronic ischemic Heart Failure (MSC-HF Trial) American Heart Journal. 2012;164(3):285–291. doi: 10.1016/j.ahj.2012.05.026. [DOI] [PubMed] [Google Scholar]