Abstract
Adiponectin is a hormone secreted from adipocytes, and it demonstrates antidiabetic, anti-atherosclerotic, antiobesity and anti-inflammatory effects. However, the patterns of change in urinary adiponectin levels in various diseases remain unknown, because only trace amounts of the hormone are present in urine. In the present study, we applied an ultrasensitive ELISA coupled with thio-NAD cycling to measure urinary adiponectin levels. Spikeand-recovery tests using urine confirmed the reliability of our ultrasensitive ELISA. The limit of detection for adiponectin in urine was 2.3×10−19 moles/assay (1.4 pg/mL). The urinary adiponectin concentration ranged between 0.04 and 5.82 ng/mL in healthy subjects. The pilot study showed that the urinary adiponectin levels, which were corrected by the creatinine concentration, were 0.73±0.50 (ng/mg creatinine, N=6) for healthy subjects, versus 12.02±3.85 (ng/mg creatinine, N=3) for patients with diabetes mellitus (DM). That is, the urinary adiponectin levels were higher (P<0.05) in DM patients than in healthy subjects. Further, these urinary adiponectin levels tended to increase with the progression of DM accompanied with nephropathy. Our method is thus expected to provide a simple, rapid and reasonably priced test for noninvasive monitoring of the progression of DM without the requirement of special tools.
Keywords: adiponectin, diabetes, ELISA, nephropathy, thio-NAD cycling, urine
Adiponectin, an adipose tissue-derived hormone, is very abundant in blood relative to many hormones [1]. Epidemiological studies have revealed that adiponectin levels are lower in obese subjects than their lean counterparts [2] and that patients with diabetes mellitus (DM) and cardiovascular disease have a lower amount of adiponectin in their sera [3–5]. Thus, a low serum adiponectin level can be an excellent predictor of the development of Type 2 DM and associated cardiovascular disease in the later stage of DM [6–11].
Adiponectin has also been proposed as a biomarker of the progression of chronic kidney disease. Patients with chronic kidney disease who reached a progression endpoint, defined as doubling of serum creatinine or dialysis requirement, were reported to have displayed higher adiponectin concentrations at baseline [12]. This effect was gender-specific: the high adiponectin level was an independent predictor of the progression of chronic kidney disease in men but not in women. Among a group of patients with nephropathy as a result of Type 1 DM, those with progression of chronic kidney disease (defined as end stage renal disease (ESRD)) showed significantly higher levels of serum adiponectin at baseline [10]. In the same study, among patients with Type 1 DM nephropathy and macroalbuminuria, those who progressed to ESRD had significantly elevated adiponectin levels. However, adiponectin levels were not predictive of the progression to ESRD in patients with normoalbuminuria or microalbuminuria, which limits the use of this marker in milder cases of chronic kidney disease [13–15].
Adiponectin circulates in the form of three different oligomers that may also have distinct biological functions. The role of these oligomers in obesity and lipid metabolism has been examined after weight reduction [16]. Individuals were characterized before and after 6 months of a balanced diet. Adiponectin was determined by enzyme-linked immunosorbent assay (ELISA), and oligomers were detected by non-denaturing Western blot. The body mass index (BMI) decreased, which was associated with an improved metabolite profile, and the total adiponectin increased. The high (HMW) and medium molecular weight (MMW) adiponectin oligomers significantly increased during weight reduction, while the low molecular weight (LMW) adiponectin oligomers did not significantly change. The body weight was inversely correlated with the HMW adiponectin level and positively correlated with the LMW adiponectin level. The high-density lipoprotein (HDL) cholesterol level and the HMW adiponectin level were strongly correlated. Indeed, the HMW adiponectin and the free fatty acids levels before weight reduction predicted approximately 60% of the HDL changes during intervention. Therefore, the weight reduction resulted in a relative increase of the HMW/MMW adiponectin and a reduction of the LMW adiponectin. The total adiponectin and especially the HMW adiponectin were thus related to the circulating HDL cholesterol.
On the other hand, adiponectin is thought to be cleared rapidly from the circulation via two routes. The primary route is through the liver, at least partly via a biliary route, and the secondary route is through the kidneys [17,18]. Adiponectin monomers (28 kDa) and dimers have a molecular weight small enough to cross the normal functioning glomerular filtration barrier, which is why adiponectin is present in the urine of healthy subjects [19]. In fact, however, the urinary adiponectin concentration is unexpectedly lower than that in the serum, and thus a radioimmunoassay is required to detect urinary adiponectin [19,20]. However, the urinary adiponectin level could be a very useful biomarker if there were a noninvasive method for its measurement, and thus there is need for a new colorimetric method without special tools or chemicals such as radioisotopes or fluorescent probes.
The present study was designed with two aims in mind. The first was to examine whether an ultrasensitive ELISA coupled with thionicotinamide-adenine dinucleotide (thio-NAD) cycling [21–24] could be applied to detect adiponectin in urine. In addition, because DM or associated chronic kidney disease affects the adiponectin concentration in serum [10], the second aim was to perform a pilot study to determine whether the urinary adiponectin concentration is changed in DM patients with nephropathy.
Materials and Methods
Chemicals
The adiponectin antigen used was a recombinant human adiponectin derived from a human HMW adiponectin/Acrp30 quantikine ELISA kit (Cat #. DHWAD0; R&D Systems, Minneapolis, MN, USA). Because the antigen reference was the HMW adiponectin, we considered that the MM was 300 kDa when calculating the limit of detection and the limit of determination. The calculation by use of the MM of 300 kDa is thought to be reasonable from the following reasons: (1) the class of adiponectin with high bioactivity is HMW [25]. (2) the class of HMW adiponectin contains the multimers of >300 kDa [16]. That is, if the adiponectin in vivo is heavier than 300 kDa, the sensitivity becomes better than our present calculation. The primary and secondary antibodies for anti-adiponectin were the human adiponectin/Acrp30 monoclonal antibody (Cat #. MAB10651; R&D Systems) and the human adiponectin/Acrp30 monoclonal antibody (Cat #. MAB1065), respectively. The primary antibody was conjugated with 6-maleimidohexanoyl-2,4-dinitrophenyl-biotin, and the secondary antibody was linked to alkaline phosphatase (ALP) (EC. 3.1.3.1) [26]. Streptavidin, BSA and the creatinine assay kit (K625-100; BioVision, Milpitas, CA, USA) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Tween 20 was purchased from Nacalai Tesque (Kyoto, Japan). Thio-NAD and NADH were purchased from Roche (Mannheim, Germany). 3α-Hydroxysteroid dehydrogenase (3α-HSD; derived from Comamonas testosteroni and recombined by E.coli) was purchased from Kikkoman Biochemifa (Tokyo, Japan) and purified by BL (Numazu, Japan). From 5β-androsterone purchased from Steraloids (Newport, RI, USA), 17β-methoxy-5β-androstan-3α-ol 3-phosphate was synthesized as described previously [21]. Human urine samples were purchased from Alta Chemical (Tokyo, Japan). Six samples of urine collected from healthy subjects were used: two women in their twenties; one woman in her thirties; two men in their thirties; and one man in his fifties. Three samples of urine collected from DM patients were used: one man in his seventies and one woman in her sixties who both suffered from DM; and one man in his forties who suffered from diabetic nephropathy. In Japan, the Japan Diabetes Society has codified the diagnosis and treatment for DM in “Evidence-based Practice Guideline for the Treatment for Diabetes in Japan 2013” in their publication (see http://www.jds.or.jp/modules/en/index.php?content_id=44). All these DM patients met the relevant criteria defined in these guidelines. In particular, the last DM patient, whose serum creatinine concentration was reported to be around 10 mg/100 mL (>2 mg/100 mL = criterion of diabetic nephropathy) and who in fact underwent artificial dialysis, showed worse DM progression than the former two DM patients.
Ultrasensitive ELISA coupled with thio-NAD cycling
The experiments using human urine were performed with the permission of the Ethics Committees of Tokushima Bunri University. We purchased Immuno Module MaxiSorp F8 (Nunc, Roskilde, Denmark) as a 96-well microplate and used it without any coating. The solution including the primary antibody and secondary antibody was adjusted to contain 30 femtomoles of each in 50 μL Tris-buffered saline (TBS; its composition was 10 mM Tris-HCl (pH 7.5) and 150 mM NaCl) including 0.01% Tween 20 and 0.1% BSA (pH 7.5). The antigen or urine was also diluted with TBS including 0.01% Tween 20 and 0.1% BSA (pH 7.5). Then, 50 μL of the primary antibody and secondary antibody solution and 50 μL of the antigen or urine solution were mixed and kept overnight at 4°C. This solution is herein referred to as “immune complex” solution. A 20 μg/mL solution of streptavidin in 50 mM Na2CO3 solution (pH 9.6) was added at 100 μL per well and kept for 60 min at room temperature. The microplates were washed 3 times with TBS including 0.05% Tween 20. Then, to block nonspecific binding sites, the microplates were incubated with 200 μL of TBS including 1% BSA overnight at 4°C. The microplates were washed 3 times with TBS including 0.05% Tween 20. The immune complex solution was added at 100 μL per well and shaken for 30 min at room temperature. The microplates were washed 9 times with TBS including 0.05% Tween 20. To amplify the ELISA signals, a thio-NAD cycling solution of 100 μL was added to each well. This solution contains 1.0 mM NADH, 1.2 mM thio-NAD, 0.05 mM 17β-methoxy-5β-androstan-3α-ol 3-phosphate, and 20 U/mL 3α-HSD in 100 mM Tris-HCl (pH 9.0). The absorption of thio-NADH was measured at 405 nm with a Corona Electric MTP-500 microplate reader (Hitachinaka, Japan) thermostated at 37°C every 5 min for 60 min.
Spike-and-recovery test
All the experimental procedures for this test were the same as for the above ultrasensitive ELISA experiments without the antigen solution. Two samples of urine collected from one healthy woman in her twenties and one woman with DM in her sixties were used for the spike-and-recovery tests. We then prepared four solutions containing the following:
50 μL of TBS including 0.1% BSA and 0.01% Tween 20.
25 μL of 0.1% BSA, 0.01% Tween 20 and 25 μL of urine, which was diluted 2 – 100 times with TBS.
25 μL of recombinant human adiponectin antigen at a concentration of 2.0 ng/mL and 25 μL of TBS including 0.1% BSA and 0.01% Tween 20.
25 μL of recombinant human adiponectin antigen at a concentration of 2.0 ng/mL and 25 μL of urine, which was diluted 2–100 times with TBS.
The spike-and-recovery ratio was calculated as [{the absorbance of (4) – that of (1)} – {the absorbance of (2) – that of (1)}]/[the absorbance of (3) – that of (1)].
Limit of detection, limit of determination, and coefficient of variation
The experimental data were obtained by subtracting the mean value of the blank signals from each of the corresponding measured data points. The limit of detection was estimated from the mean of the blank, the standard deviation of the blank, and a confidence factor of 3. The limit of determination was estimated by the same method as used for the limit of detection, but with a confidence factor of 10. The coefficient of variation calculated from 3 data points was obtained for 0.1 ng/mL of adiponectin antigen.
Statistics
The data are expressed as the mean±SEM. Significant differences at P<0.05 were examined by Mann-Whitney U-test.
Results
Principle of ultrasensitive ELISA
We will first describe a substrate-cycling reaction conducted using a single dehydrogenase, 3α-HSD, as follows [27–32]. In this cycling reaction, 3α-HSD catalyzes the substrate cycling between 3α-hydroxysteroid and its corresponding 3-ketosteroid in the presence of an excess amount of NADH and thio-NAD, because 3α-HSD utilizes both NADH and thio-NAD as cofactors. In each turn of the cycle, one molecule of thio-NAD is reduced to thio-NADH, which can be measured directly by an increase in the absorbance at 400 nm (11,900 M−1 cm−1), e.g., 405 nm with a commercially available microplate reader, without any interference from other cofactors such as thio-NAD, NAD and NADH, the absorbance maximums of which are all under 340 nm. These features make it possible to determine the amount of 3α-hydroxysteroids with high sensitivity by measuring the cumulative quantity of thio-NADH. This detectable signal also changes linearly with time.
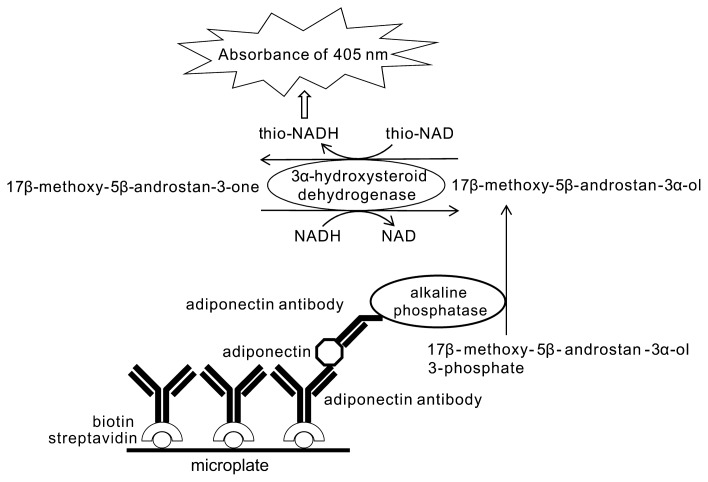
Our ultrasensitive ELISA coupled with thio-NAD cycling is performed as follows (Fig. 1) [21–24]. A sandwich method using a primary and a secondary antibody for antigens is employed in an ELISA. An androsterone derivative, 17β-methoxy-5β-androstan-3α-ol, is produced by the hydrolysis of 17β-methoxy-5β-androstan-3α-ol 3-phosphate with alkaline phosphatase linked to the secondary antibody. This 17β-methoxy-5β-androstan-3α-ol is oxidized to 17β-methoxy-5β-androstan-3-one by 3α-HSD with the cofactor thio-NAD. By the opposite reaction, 17β-methoxy-5β-androstan-3-one is reduced to 17β-methoxy-5β-androstan-3α-ol by 3α-HSD with the cofactor NADH. During this cycling reaction, thio-NADH accumulates in a quadratic function-like fashion with time. The accumulated thio-NADH can be measured directly at an absorbance of 400 nm without any interference from other cofactors.
Figure 1.
Ultrasensitive detection of adiponectin in urine by ELISA coupled with thio-NAD cycling using alkaline phosphatase, androsterone derivatives, and 3α-hydroxysteroid dehydrogenase and its coenzymes. During the cycling reaction, thio-NADH accumulates in a quadratic function-like fashion with time. Accumulated thio-NADH can be measured directly at an absorbance of 405 nm without any interference from other cofactors.
Measurement of urinary adiponectin
To pursue the first aim of the present study, we applied our ultrasensitive ELISA to measure the adiponectin concentration in urine and performed spike-and-recovery tests using urine to confirm our measurements.
Linear calibration curves and limit of detection for adiponectin
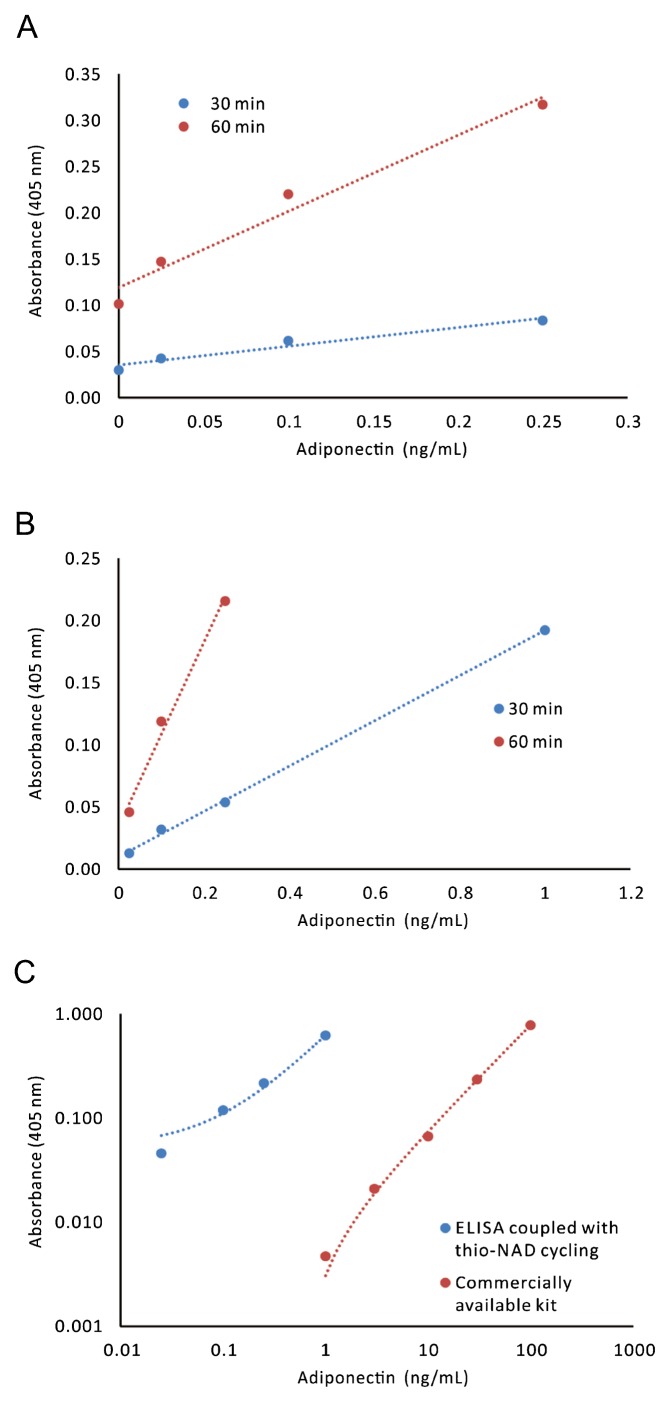
When we obtained the linear calibration curves including the blank values (i.e., the absorbance of 0 ng/mL of adiponectin) by using the data of 30-min and 60-min measurements in a Tris-buffered solution, one curve for a 30-min measurement was expressed as y=0.20x+0.04, R2=0.95, and the other one was y=0.82x+0.12, R2=0.97. From these data, we calculated that the limit of detection of adiponectin was 1.4 pg/mL (i.e., ca. 2.3×10−19 moles/assay and that the minimum limit of determination was 54.9 pg/mL (i.e., ca. 9.1×10−18 moles/assay) when the molecular mass was assumed to be 300 kDa (See Materials and methods) (Fig. 2A). These results suggest that the ultrasensitive ELISA coupled with thio-NAD cycling succeeds in detecting adiponectin at the subattomole level. Further, the coefficient variation value was 4% for 0.1 ng/mL of adiponectin antigen.
Figure 2.
Linear calibration curves for adiponectin obtained by an ultrasensitive ELISA coupled with thio-NAD cycling. (A) The linear calibration curves including the blank values (i.e., the absorbance of 0 ng/mL of adiponectin) were obtained, because these curves were needed to calculate the limit of detection and the limit of determination. The absorbance of thio-NADH was measured at 405 nm after a cycling reaction time of 30 min (blue) or 60 min (red). These curves are expressed as y=0.20x+0.04, R2=0.95 in the range of 0.025–1.0 ng/mL for 30-min measurement and y=0.82x+0.12, R2=0.97 in the range of 0.025–0.25 ng/mL for 60-min measurement. (B) Typical linear calibration curves in which the blank values (i.e., the absorbance of 0 ng/mL of adiponectin) were subtracted are shown. That is, this figure directly expresses the values corresponding to the concentrations of adiponectin. The linear calibration curve after a cycling reaction time of 30 min (blue) was y=0.18x+0.01, R2=1.00 in the range of 0.025–1.0 ng/mL; and that after a cycling reaction time of 60 min (red) was y=0.74x+0.034, R2=0.99 in the range of 0.025–0.25 ng/mL. (C) Comparison of linear calibration curves between our ultrasensitive ELISA and a commercially available kit. The kit used was the human HMW adiponectin/Acrp30 quantikine ELISA kit (Cat #. DHWAD0; R&D Systems). The linear calibration curve of our ultrasensitive ELISA (blue) was y=0.57x+0.05, R2=1.00 in the range of 0.025–1.0 ng/mL; whereas that obtained from a commercially available kit (red) was y=0.0079x–0.005, R2=1.00 in the range of 1.0–100 ng/mL. Our data (blue) were found to be at least 2 orders of magnitude more sensitive than the commercially available kit (red).
We provided two typical sets of linear calibration curves for our ultrasensitive ELISA coupled with thio-NAD cycling, one in the range of 0.025–1.0 ng/mL and the other in the range of 0.025–0.25 ng/mL of human adiponectin in a Trisbuffered solution (Fig. 2B). The curves were obtained from the absorbance of thio-NADH in cycling reactions of 30 min and 60 min, respectively. The linear calibration curve measured at 30 min in the range of 0.025–1.0 ng/mL was expressed as y=0.18x+0.01, R2=1.00, and that measured at 60 min in the range of 0.025–0.25 ng/mL was expressed as y=0.74x+0.03, R2=0.99.
We compared the data obtained by our ultrasensitive ELISA in a Tris-buffered solution with those obtained using a commercially available kit according to the manufacturer’s instruction (the human HMW adiponectin/Acrp30 quantikine ELISA kit; Cat #. DHWAD0; R&D Systems) (Fig. 2C). The comparison demonstrated that our ultrasensitive ELISA was at least 2 orders of magnitude more sensitive than the commercially available kit.
Spike-and-recovery tests for urinary adiponectin
We performed the spike-and-recovery tests for adiponectin in urine. The spike-and-recovery test is a technique for analyzing and accessing the accuracy of ELISA for particular sample types, such as serum, plasma, saliva, urine, etc. It is used to determine whether analyte detection can be affected by the difference between the diluent used for preparation and the experimental sample matrix. To perform a spike-and-recovery test, a known amount of analyte (i.e., adiponectin in our case) is added to a matrix (i.e., urine in our case). This ‘addition’ is called ‘spike’. The concentration of the added analyte in the matrix is determined from standard curves prepared. The concentrations denote the spike recovered in the matrix.
First, when using the urine collected from a healthy woman in her twenties and the adiponectin antigen at a final concentration of 1.0 ng/mL, the spike-and-recovery ratios were 6% for 1:2 dilution, 31% for 1:5 dilution, 31% for 1:10 dilution, and 89% for 1:20 dilution. Second, when using the urine collected from a woman with DM in her sixties and the adiponectin antigen at a final concentration of 1.0 ng/mL, the spike-and-recovery ratios were 88% for 1:20 dilution, 82% for 1:50 dilution, and 103% for 1:100 dilution. In general, the results of spike-and-recovery tests are accepted when they are within the range of 80% and 120% (see the following URL: http://www.woongbee.com/0NewHome/RnD/ELISA/RnD_%20SPIKEandREC2006.pdf). We therefore concluded that a 1:20 dilution of urine is needed for the detection of urinary adiponectin.
Urinary adiponectin level in healthy subjects and DM patients
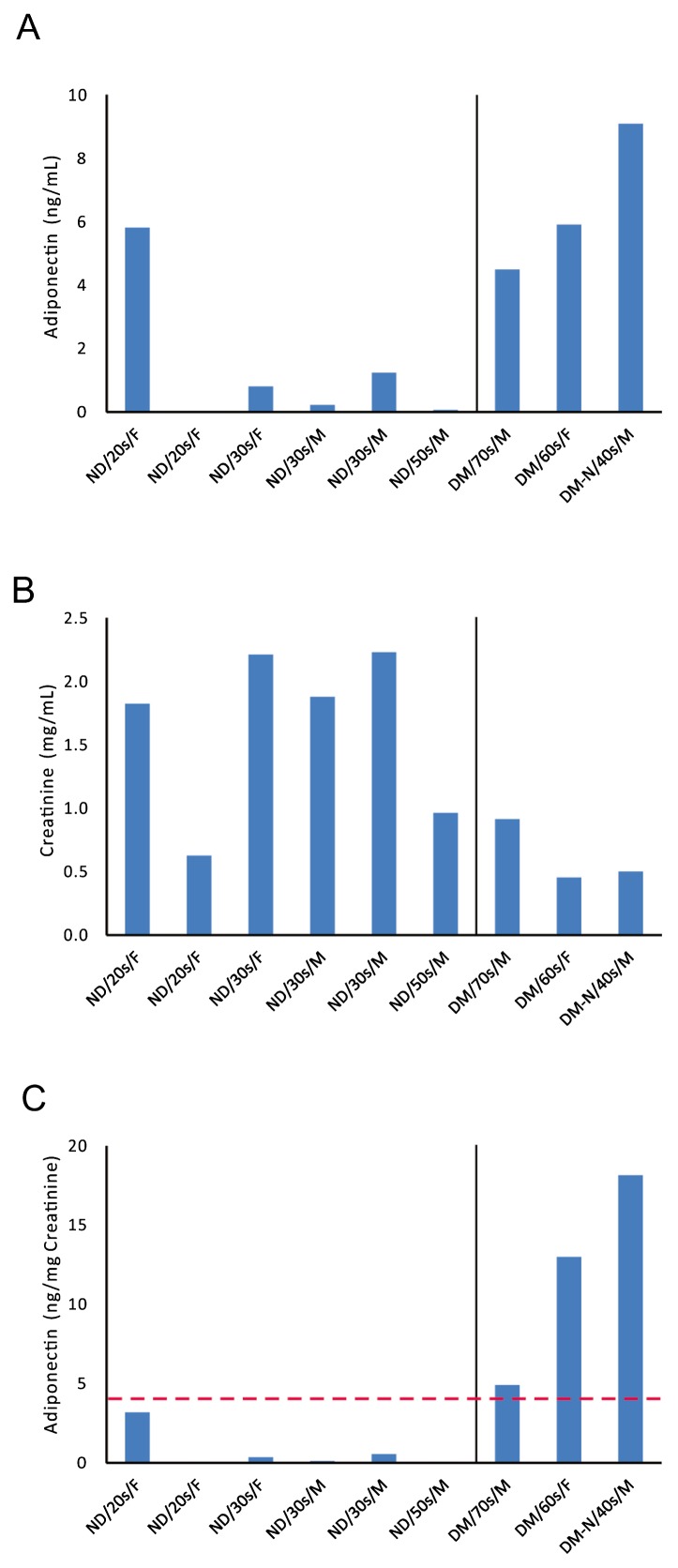
To realize the second goal of the present study, we determined the adiponectin concentrations and creatinine concentrations in urine collected from healthy subjects and DM patients (Fig. 3A,B). The adiponectin concentrations ranged from 0.04 to 5.82 ng/mL for healthy subjects, and from 4.50 to 9.10 ng/mL for DM patients (P<0.05). The creatinine concentrations ranged from 0.63 to 2.23 mg/mL for healthy subjects, and from 0.46 to 0.92 mg/mL for DM patients (P<0.05). That is, the adiponectin concentrations in the urine of DM patients were higher than those of healthy subjects, whereas the creatinine excretion amounts from DM patients were lower than those from healthy subjects. The adiponectin concentrations corrected by the creatinine concentrations were 0.73±0.50 (ng/mg creatinine, N=6) for healthy subjects, and 12.02±3.85 (ng/mg creatinine, N=3) for DM patients (Fig. 3C). That is, the urinary adiponectin levels were higher in DM patients (P<0.05) than in healthy subjects. Further, the urinary adiponectin level was 4.92 ng/mg creatinine in a man in his seventies who suffered from DM; 12.99 ng/mg creatinine in a woman in her sixties who suffered from DM; and 18.14 ng/mg creatinine in a man in his forties who suffered from diabetic nephropathy and underwent artificial dialysis (Fig. 3C). That is, the worse the progression of DM with nephropathy, the higher the urinary adiponectin levels became. Based on these results, we may set a threshold adiponectin level at 4 ng/mg creatinine to distinguish between healthy subjects and patients with DM.
Figure 3.
Urinary adiponectin levels measured by our ultrasensitive ELISA for healthy subjects and DM patients. (A) The urinary adiponectin concentrations in DM patients (DM, N=3) were higher (P<0.05) than those in healthy subjects (ND, N=6). DM-N, a DM patient with nephropathy; M, male; F, female. (B) The urinary creatinine concentrations in DM patients (N=3) were lower (P<0.05) than those in healthy subjects (N=6). (C) The urinary adiponectin levels, which were the values of adiponectin concentrations corrected by creatinine concentrations, were 0.73±0.50 (ng/mg creatinine) for heathy subjects (N=6) versus 12.02±3.85 ng/mg creatinine for DM patients (N=3). That is, the urinary adiponectin levels were higher in DM patients than in healthy subjects, and there seemed to be a threshold adiponectin level at around 4 ng/mg creatinine between healthy subjects and DM patients. The worse the progression of DM, the higher the urinary adiponectin level became.
Discussion
The present results demonstrated that our ultrasensitive ELISA coupled with thio-NAD cycling is applicable to the detection of trace amounts of urinary proteins. Thus, our ultrasensitive ELISA started stepping into a useful noninvasive test. However, the spike-and-recovery tests demonstrated that dilution of the urine sample is required to measure urinary adiponectin by our ultrasensitive ELISA. The presence of inhibitor(s) for binding between adiponectin and anti-adiponectin antibody has been discussed in salivary adiponectin measurements [33]. An increase in the measureable level of salivary adiponectin was observed on sample dilution. An inhibitor co-eluted with the dimeric form of adiponectin was capable of inhibiting ELISA measurement of salivary adiponectin. Therefore, urine may also include an inhibitor of adiponectin ELISA.
Our pilot study suggested that urinary adiponectin levels are higher in DM patients with nephropathy than in healthy subjects. These results confirm the facts reported so far in patients with DM or proteinuria by use of radioimmunoassay [19,20]. In these previous studies, radioimmunoassay was used because sensitivity was required for the urinary adiponectin detection. In their reports, the urinary adiponectin levels in Type 2 DM patients with normoalbuminuria and microalbuminuria were about 5 ng/mg creatinine, and those in Type 2 DM patients with macroalbuminuria were about 50 ng/mg creatinine [20]. The urinary adiponectin levels of control subjects, IgA-nephropathy patients and diabetic nephropathy patients were about 2 ng/mg creatinine, 60 ng/mg creatinine and 30 ng/mg creatinine, respectively [19]. We can therefore state that our ultrasensitive ELISA coupled with thio-NAD cycling can detect urinary adiponectin at the ng/mg creatinine level by a colorimetric method.
The urinary adiponectin concentrations were higher in the DM patients than in the healthy subjects (Fig. 3A). In a previous report, the opposite relation was observed for serum— i.e., the adiponectin levels were higher in the healthy sera [10]. As described in the Introduction, adiponectin may be cleared rapidly from the circulation [17,18]. On the other hand, the urinary creatinine concentrations were lower in the DM patients than in the healthy subjects (Fig. 3B). Creatinine is cleared from the body entirely by the kidneys. If kidney function is abnormal, the creatinine concentration increases in blood because less creatinine is released through urine [34]. In other words, a decrease in creatinine concentration in the urine in the DM patients may correspond to an increase in creatinine concentration in the blood, which was not measured in the present study. The urinary creatinine concentration is thought to well reflect the stages of nephropathy.
Several recent studies have reported on the measurement of salivary adiponectin concentrations [33,35–37]. These studies showed that the salivary adiponectin concentrations were on the order of ng/mL, whereas the serum adiponectin concentrations were on the order of μg/mL [35,36] and the urinary adiponectin concentration was on the order of pg - ng/mL, as observed in the present study as well. There was a positive correlation between the salivary and serum levels [36], but not between the urinary levels and either the salivary or serum levels. In any case, both the salivary adiponectin and the serum adiponectin levels can be sufficiently measured with commercially available kits. In more recent study, no significant difference was reported for salivary adiponectin between healthy individuals and patients with metabolic syndrome [37]. Further, Western blotting under non-reducing conditions revealed that the salivary adiponectin was predominantly of a super high molecular weight form [25]. The salivary adiponectin levels were significantly higher in women than in men, in agreement with the results for serum samples. On the other hand, the urinary protein levels depend on the function of the kidney. That is, we may discover new knowledge by application of our ultrasensitive ELISA to the measurement of urinary proteins.
Finally, let us compare our ultrasensitive ELISA with another highly sensitive method. For example, a matrix-assisted laser desorption ionization time-of-flight mass spectrometer (MALDI-TOF-MS) would be a potential tool for detecting trace amounts of proteins, if one could use a very large apparatus over a long period of time without pause. For insulin, a recent study using MALDI-TOF-MS reported that 106 insulin molecules could be detected in this manner, that is, 1.7×10−18 moles [38]. Although the comparison between our ultrasensitive ELISA [21,23] and the MS is not straightforward, in general our method can be considered easier, because we can detect trace amounts of proteins simply by applying thio-NAD cycling regents to the usual ELISA system.
Conclusion
Ultrasensitive ELISA coupled with thio-NAD cycling was demonstrated to be a powerful tool for the detection of trace amounts of proteins or peptides in urine or blood. The subattomole-level measurement shown in the present study is expected to play an important role in the detection of urinary target proteins, because their concentrations are very low. Our ultrasensitive ELISA, which does not employ special tools or chemicals such as radioisotopes or fluorescent probes, will pave the way toward simple, fast and noninvasive measurements for a variety of diseases.
Acknowledgments
This study was supported by a grant for the Development of Systems and Technology for Advanced Measurement and Analysis from JST, a grant for the Regional Innovation Strategy Support Program 2009 from MEXT, a grant for the New Regional Consortium Research and Development Project from METI and the Hokkaido Bureau of Economy, Trade, and Industry to E.I. and some of the coauthors.
Footnotes
Author contributions
S. W., K. N., S. H. and E. I. conceived and designed the experiments. M. M., R. N., K. M. and T. Y. performed the experiments. M. M., S. W., K. N., T. Y., T. M., S. H. and E. I. analyzed the data. S. W., K. N., T. Y., T. M., S. H. and E. I. wrote the paper.
Conflicts of interest
R. N., K. M., T. Y., T. M., S. H. and E. I. declare that they have no competing interests. M. M. and K. N. are employees of TAUNS Laboratories, Inc., and S. W. is an employee of BL Co., Ltd.
References
- 1.Matsuzawa Y, Funahashi T.&Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann NY Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 2.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 3.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 5.Ix JH.&Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406–412. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 7.Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. Erratum in: Lancet 361, 1060 (2003) [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB.&Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 10.Jorsal A, Tarnow L, Frystyk J, Lajer M, Flyvbjerg A, Parving HH, et al. Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int. 2008;74:649–654. doi: 10.1038/ki.2008.201. [DOI] [PubMed] [Google Scholar]
- 11.Ghoshal K.&Bhattacharyya M. Adiponectin: Probe of the molecular paradigm associating diabetes and obesity. World J Diabetes. 2015;6:151–166. doi: 10.4239/wjd.v6.i1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollerits B, Fliser D, Heid IM, Ritz E, Kronenberg F MMKD Study Group. Gender-specific association of adiponectin as a predictor of progression of chronic kidney disease: the mild to moderate kidney disease study. Kidney Int. 2007;71:1279–1286. doi: 10.1038/sj.ki.5002191. [DOI] [PubMed] [Google Scholar]
- 13.Looker HC, Krakoff J, Funahashi T, Matsuzawa Y, Tanaka S, Nelson RG, et al. Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:4010–4017. doi: 10.1210/jc.2003-031916. [DOI] [PubMed] [Google Scholar]
- 14.Kronenberg F. Emerging risk factors and markers of chronic kidney disease progression. Nat Rev Nephrol. 2009;5:677–689. doi: 10.1038/nrneph.2009.173. [DOI] [PubMed] [Google Scholar]
- 15.Saraheimo M, Forsblom C, Thorn L, Wadén J, Rosengård-Bärlund M, Heikkilä O, et al. Serum adiponectin and progression of diabetic nephropathy in patients with type 1 diabetes. Diabetes Care. 2008;31:1165–1169. doi: 10.2337/dc07-2306. [DOI] [PubMed] [Google Scholar]
- 16.Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 17.Tacke F, Wüstefeld T, Horn R, Luedde T, Srinivas Rao A, Manns MP, et al. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol. 2005;42:666–673. doi: 10.1016/j.jhep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, et al. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58:1961–1970. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimotomai T, Kakei M, Narita T, Koshimura J, Hosoba M, Kato M, et al. Enhanced urinary adiponectin excretion in IgA-nephropathy patients with proteinuria. Ren Fail. 2005;27:323–328. [PubMed] [Google Scholar]
- 20.Koshimura J, Fujita H, Narita T, Shimotomai T, Hosoba M, Yoshioka N, et al. Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem Biophys Res Commun. 2004;316:165–169. doi: 10.1016/j.bbrc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Watabe S, Kodama H, Kaneda M, Morikawa M, Nakaishi K, Yoshimura T, et al. Ultrasensitive enzyme-linked immunosorbent assay (ELISA) of proteins by combination with the thio-NAD cycling method. BIOPHYSICS. 2014;10:49–54. doi: 10.2142/biophysics.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakatsuma A, Kaneda M, Kodama H, Morikawa M, Watabe S, Nakaishi K, et al. Detection of HIV-1 p24 at attomole level by ultrasensitive ELISA with thio-NAD cycling. PLoS One. 2015;10:e0131319. doi: 10.1371/journal.pone.0131319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito E, Kaneda M, Kodama H, Morikawa M, Tai M, Aoki K, et al. Immunoreactive insulin in DM-patient sera detected by ultrasensitive ELISA with thio-NAD cycling. BioTechniques. 2015 doi: 10.2144/000114355. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuma A, Kaneda M, Kodama H, Morikawa M, Watabe S, Nakaishi K, et al. Ultrasensitive colorimetric detection of HIV-1 p24. Clin Lab Int. 2015;(October Issue):20–22. [Google Scholar]
- 25.Lin H, Maeda K, Fukuhara A, Shimomura I.&Ito T. Molecular expression of adiponectin in human saliva. Biochem Biophys Res Commun. 2014;445:294–298. doi: 10.1016/j.bbrc.2014.01.163. [DOI] [PubMed] [Google Scholar]
- 26.Hashida S, Kitamura K, Nagatomo Y, Shibata Y, Imamura T, Yamada K, et al. Development of an ultrasensitive enzyme immunoassay for human proadrenomedullin N-terminal 20 peptide and direct measurement of two molecular forms of PAMP in plasma from healthy subjects and patients with cardiovascular disease. Clin Biochem. 2004;37:14–21. doi: 10.1016/j.clinbiochem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Skålhegg BA. 3α-hydroxysteroid dehydrogenase from Pseudomonas testosteroni: kinetic properties with NAD and its thionicotinamide analogue. Eur J Biochem. 1975;50:603–609. doi: 10.1111/j.1432-1033.1975.tb09901.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura T, Kurosawa T, Ikegawa S.&Tohma M. Substrate specificity of 3α-hydroxysteroid dehydrogenase for the oxidation of fetal bile acids. Bunseki Kagaku. 1995;44:865–869. [Google Scholar]
- 29.Ueda S, Oda M, Imamura S.&Ohnishi M. Kinetic study of the enzymatic cycling reaction conducted with 3α-hydroxysteroid dehydrogenase in the presence of excessive thio-NAD+ and NADH. Anal Biochem. 2004;332:84–89. doi: 10.1016/j.ab.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Zhang GH, Cong AR, Xu GB, Li CB, Yang RF.&Xia TA. An enzymatic cycling method for the determination of serum total bile acids with recombinant 3α-hydroxysteroid dehydrogenase. Biochem Biophys Res Commun. 2005;326:87–92. doi: 10.1016/j.bbrc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka T, Ueda S, Matsumoto H.&Kawakami M. An ultrasensitive enzymatic method for measuring mevalonic acid in serum. J Lipid Res. 2012;53:1987–1992. doi: 10.1194/jlr.D028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwai A, Yoshimura T, Wada K, Watabe S, Sakamoto Y, Ito E, et al. Spectrophotometric method for the assay of steroid 5α-reductase activity of rat liver and prostate microsomes. Anal Sci. 2013;29:455–459. doi: 10.2116/analsci.29.455. [DOI] [PubMed] [Google Scholar]
- 33.Akuailou EN, Vijayagopal P, Imrhan V.&Prasad C. Measurement and validation of the nature of salivary adiponectin. Acta Diabetol. 2013;50:727–730. doi: 10.1007/s00592-012-0388-z. [DOI] [PubMed] [Google Scholar]
- 34.Kowalski A, Krikorian A.&Lerma EV. Diabetic nephropathy for the primary care provider: new understandings on early detection and treatment. Ochsner J. 2014;14:369–379. [PMC free article] [PubMed] [Google Scholar]
- 35.Toda M, Tsukinoki R.&Morimoto K. Measurement of salivary adiponectin levels. Acta Diabetol. 2007;44:20–22. doi: 10.1007/s00592-007-0236-8. [DOI] [PubMed] [Google Scholar]
- 36.Mamali I, Roupas ND, Armeni AK, Theodoropoulou A, Markou KB.&Georgopoulos NA. Measurement of salivary resistin, visfatin and adiponectin levels. Peptides. 2012;33:120–124. doi: 10.1016/j.peptides.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Thanakun S, Watanabe H, Thaweboon S.&Izumi Y. An effective technique for the processing of saliva for the analysis of leptin and adiponectin. Peptides. 2013;47:60–65. doi: 10.1016/j.peptides.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Chao TC, Nelson R.&Ros A. Direct detection of peptides and proteins on a microfluidic platform with MALDI mass spectrometry. Anal Bioanal Chem. 2012;404:1681–1689. doi: 10.1007/s00216-012-6257-3. [DOI] [PubMed] [Google Scholar]