Abstract
One of the major topics in biophysics and physicobiology is to understand and utilize biological functions using various advanced techniques. Taking advantage of the photoreactivity of the seven-transmembrane rhodopsin protein family has been actively investigated by a variety of methods. Rhodopsins serve as models for membrane-embedded proteins, for photoactive proteins and as a fundamental tool for optogenetics, a new technology to control biological activity with light. In this review, we summarize progress of microbial rhodopsin research from the viewpoint of distribution, diversity and potential.
Keywords: retinal, rhodopsin, π-conjugation, visible light, vitamin-A
Rhodopsins
In the early morning, we usually wake up with bright sunlight arising from the horizon, take breakfast and go to the office or school. On the way to the office or school, we are able to see colorful plants and animals. We then make a living for several hours under illumination with fluorescent and/or light emitting diode (LED) bulbs or with sunlight. On the way back home, we can be fascinated by the incredible view of a setting sun. At night, beautiful fireworks in the sky give us hope for tomorrow. Thus, light is one of the most important stimuli for organisms.
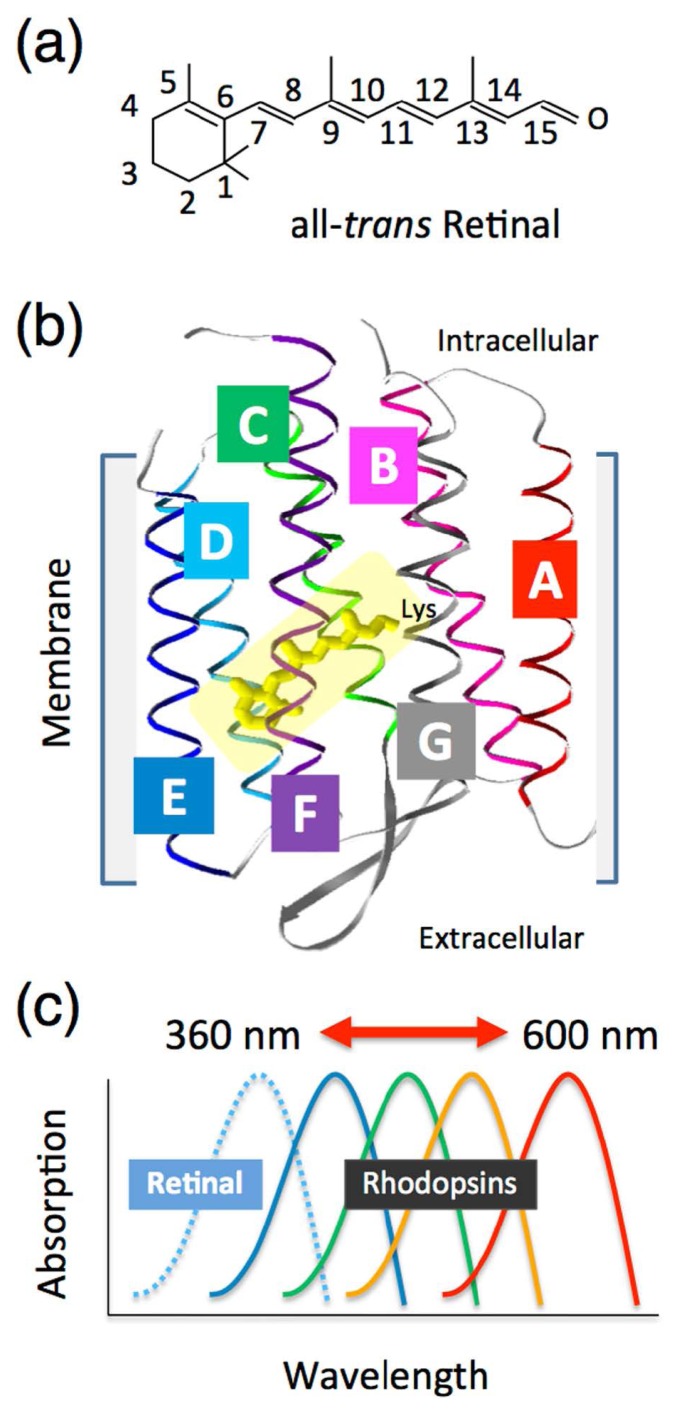
In organisms, including human beings, biological protein molecules are responsible for the light reception. However, in general, proteins are transparent and are thus not responsive in the visible light region. Therefore, photoreceptive proteins have evolved that can bind a chromophore molecule, which can absorb visible wavelengths of light. One of the largest groups of photoreceptive proteins is the rhodopsin protein family (also called retinal protein), where vitamin-A aldehyde retinal is employed as a chromophore [1]. The retinal chromophore forms the all-trans configuration in organic solvents as the most favorable conformer and it absorbs blue light (~360 nm) (Fig. 1a) [1]. The retinal is incorporated into the protein moiety (called an “opsin”) that consists of seven-transmembrane α-helices by the formation of a protonated Schiff base linkage to a specific conserved lysine residue in the opsin (Fig. 1b). The interaction between retinal and opsin induces spectral red-shifts in a wide range of the visible region (400~600 nm) [2] (Fig. 1c). The opsin-induced spectral shift is called an “opsin-shift”. The visible light absorption of rhodopsins triggers the trans-cis or cis-trans isomerization of the retinal chromophore [1]. The isomerization process appears within a few hundreds of a femtosecond time frame. The stored energy of the excited retinal chromophore induces sequential conformational changes of the protein moiety required for its biological functions, such as visual responses, ion transportation and photosensing [1,2].
Figure 1.
Introduction of the photoactive rhodopsin molecule. (a) Chemical structure of the retinal chromophore with numbering of the carbons. (b) Crystal structure of a typical type-1 microbial rhodopsin (bacteriorhodopsin, BR: PDB code, 1C3W) [71]. The retinal chromophore (yellow) that is covalently attached to the opsin via a specific lysine residue is surrounded by seven-transmembrane α-helices (A, B, C, D, E, F and G). (c) Schematic of the opsin-induced spectral shift of the retinal chromophore, named the “opsin-shift”. The absorption spectra from the retinal chromophore colored yellow in solution are greatly affected by interaction with a variety of opsins.
It is well-known that there are two types of rhodopsins in nature [3]. One is type-1 rhodopsins, which are widespread in the microbial world [4,5], while the other is type-2 rhodopsins, which are widely distributed in animals [6,7] and are categorized as members of the G-protein-coupled receptor (GPCR) family. As mentioned above, all-trans retinal is the most stable conformer, and therefore many type-1 rhodopsins have the all-trans configuration in the dark (Fig. 1b) [1–3]. On the other hand, many type-2 rhodopsins have the 11-cis configuration due to the specific interaction with the opsins [1,6,7]. In addition to the chromophore difference, sequence homology between type-1 and type-2 rhodopsins is low, though both possess similar chromophore (retinal) and protein (seven-transmembrane helices) structures [8].
In this review, we would like to focus on type-1 microbial rhodopsins. Please see extensive reviews for type-2 rhodopsins [1,6,7,9,10].
Classical type-1 rhodopsins from the archaeon Halobacterium salinarum
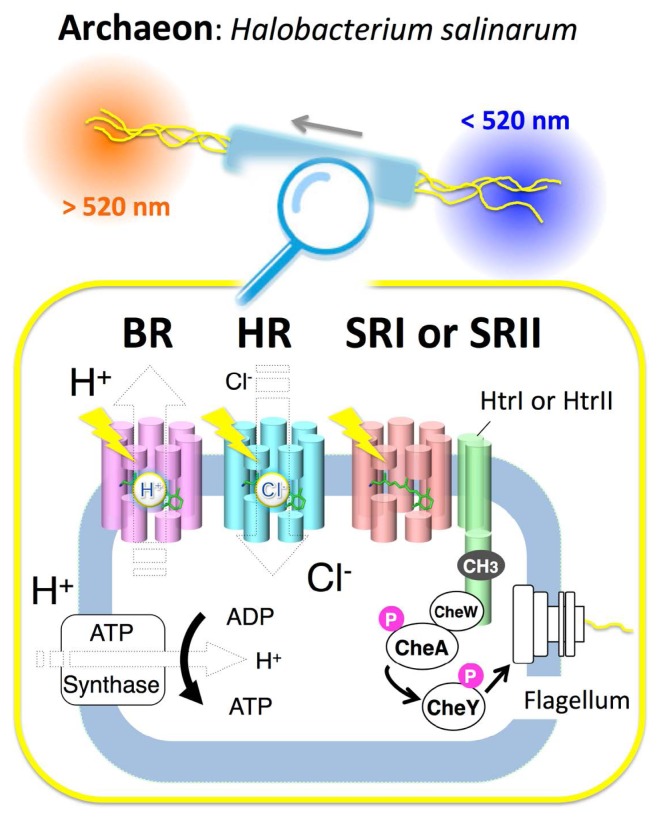
In 1971, the first type-1 rhodopsin, named bacteriorhodopsin (BR), was found in the halophilic archaeon Halobacterium salinarum (formerly halobium) [11]. BR works as a light-driven outward electrogenic proton pump in the cell membrane, and one H+ moves from the intracellular to the extracellular side during a cyclic photoreaction called a “photocycle” [12]. The proton gradient produced is utilized by adenosine triphosphate (ATP) synthase. In other words, organisms having light-driven pumps can produce ATP under light illumination (Fig. 2). It is well-known that ATP is a multifunctional nucleotide used in organisms as a coenzyme, and is often called the molecular unit of currency of intracellular energy transfer.
Figure 2.
The classical four microbial rhodopsins from the archaeon Halobacterium salinarum. The membrane of H. salinarum contains four rhodopsins, bacteriorhodopsin (BR), halorhodopsin (HR), sensory rhodopsin-I (SRI) and sensory rhodopsin-II (SRII, also called phoborhodopsin, pR). BR and HR work as a light-driven proton pump and a halide ion pump, respectively, while SRI and SRII work as photo-sensors, and form signaling complexes with their cognate transducer proteins, HtrI and HtrII, respectively, in the membrane. Light signals are transmitted from the SRI-HtrI and SRII-HtrII complexes to a cytoplasmic two-component signal transduction cascade that consists of the adaptor protein CheW, the kinase CheA and the response regulator CheY, which regulates the rotational direction of the flagellar motor, resulting in attractant or repellent phototaxis responses. Adaptation is also essential for the detection of temporal changes of stimuli and high sensitivity to stimuli over a wide dynamic range. Covalent modifications of Htrs (i.e., methylation and demethylation), which are involved in adaptation, are illustrated as “CH3”. “P” indicates the phosphate functional group. Using those signaling systems, cells move toward longer wavelengths of light (λ>520 nm) where BR and HR work to produce ATP, while they avoid shorter wavelengths of light (λ<520 nm), which contain harmful near-UV.
From the same organism, three other rhodopsins, halorhodopsin (HR), sensory rhodopsin-I (SRI) and sensory rhodopsin-II (SRII), were discovered in 1977 [13], 1982 [14] and 1985 [15], respectively (Fig. 2). HR works as a light-driven inward electrogenic chloride pump and produces ATP as does BR [16,17]. However the physiological role of HR is not fully understood. HR-induced ATP synthesis is sustained for only few minutes. Therefore, besides the ATP production, the Cl− transport has been suggested to contribute in maintaining the osmotic balance during the cell growth [16]. SRI and SRII initiate positive or negative phototaxis responses, respectively [18]. When a cell moves toward a certain wavelength of light, this behavior is designated as positive phototaxis, while behavior in which a cell avoids a certain wavelength of light is designated as negative phototaxis. SRI and SRII form a 2 : 2 signaling complex in the membrane with their cognate transducer proteins HtrI and HtrII, respectively (Fig. 2) [19–21]. Light absorption by SRI and SRII triggers the trans-cis photo-isomerization of retinal chromophores, leading to the cognate photocycle. During the photocycle, light signals are transmitted from the SRI-HtrI and SRII-HtrII complexes to the cytoplasmic two-component signal transduction cascade, which consists of the kinases CheA and CheY with an adaptor protein CheW (Fig. 2) [18]. Finally, the rotational direction of the flagellar motor is regulated by the phosphorylation state of CheY (Fig. 2) [18]. SRII absorbs a shorter wavelength of light (~500 nm), whereas BR, HR and SRI absorb a longer one (~580 nm) [18,22,23]. As a result, the archaeon Halobacterium salinarum is attracted to light with wavelengths longer than 520 nm and avoids light with wavelengths shorter than 520 nm (Fig. 2) [18]. Light of >520 nm can activate BR and HR to produce ATP, and cells avoid shorter wavelengths of light which contain harmful near-UV [18]. The close relationship between these four archaeal rhodopsins (BR, HR, SRI and SRII) has been experimentally demonstrated by functional conversion with replacement of some amino acid residues (i.e., conversion from BR into HR [24], from BR into SRII [25] and from SRI and SRII into BR [26,27]).
These classical rhodopsins have become a model both of membrane proteins and photoactive proteins, because of several characteristics (e.g., they are relatively small membrane proteins consisting of only ~250 amino acid residues with a single retinal chromophore and their photoabsorption abilities allow to control the biological activity by light at high temporal (~ tens of fs) and spatial (~ tens of nm) resolutions). In fact, high resolution tertiary structures of BR, HR and SRII, both in the ground states and in many of the photointermediates during the photocycle, have been reported by X-ray crystallography, electron microscopy and NMR spectroscopy [1,28]. Time-resolved pump-probe spectroscopy was utilized to investigate the structure and structural changes both in the excited states and in photointermediates in a wide range of time frames from femtoseconds to seconds [1,29,30]. The detailed structures and structural changes, both of the protein moiety and the retinal chromophore, have also been extensively analyzed by vibrational spectroscopies and theoretical studies [1,31–34].
Thus, the archael rhodopsins have become models for the simplest and most essential features both of photoactive proteins and of membrane-embedded proteins.
New aspects for microbial rhodopsins
Wide distribution in organisms
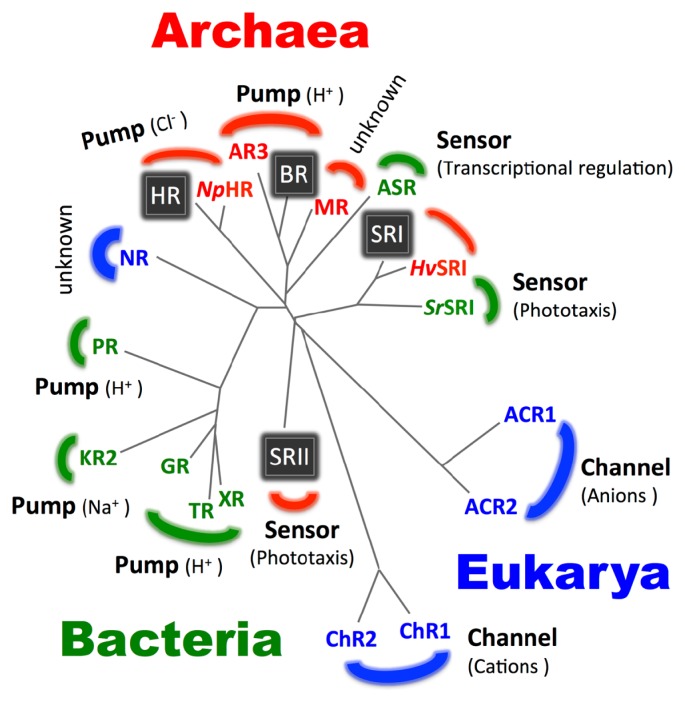
Although a large number of genes encoding microbial type-1 opsins have been identified in archaea, no opsin gene from bacteria or eukarya has been identified until 1997. In 1997, two groups have made a breakthrough regarding the distribution of microbial rhodopsins. They have identified microbial rhodopsin homologs from fungi [35,36]. In 1999, Borkovich and coworkers have found a novel gene that encodes an opsin from the eukaryotic filamentous fungus Neurospora crassa, and named it Neurospora rhodopsin (NR) (Fig. 3) [37,38]. Since then, hundreds of new rhodopsin have been found between 1997 and today. For instance, two light-driven proton pumps, Proteorhodopsin (PR) and Xanthorhodopsin (XR), have been found in the eubacterial marine bacterioplankton in 2000 [39] and in the halophile eubacterial Salinibacter ruber in 2005 [40], respectively (Fig. 3). Interestingly, XR possesses a secondary chromophore called salinixanthin (SX) as a light-harvesting antenna in the native membrane, and the efficiency of energy transfer from SX to retinal has been calculated as approximately 45% [40]. Those findings encouraged us to seek unidentified microbial rhodopsins from nature. Regarding the contributions of our group, we found (or characterized) several novel microbial rhodopsins, including highly stable SRI homologs from the eubacterium Salinibacter ruber (SrSRI) in 2008 [41] and from the archaeon Haloarcula vallismortis (HvSRI) in 2010 [42], on the basis of progress in environmental genomics. Further, we identified Middle rhodopsin (MR) from the archaeon Haloquadratum walsbyi as an evolutionary intermediate between BR and SRII in 2011 [43] and Thermophilic rhodopsin (TR) as a thermally stable light-driven proton pump derived from a thermophilic organism in 2013 [44]. Please see extensive reviews for the contributions of other groups [1,2,4,5]. These newly discovered microbial type-1 rhodopsins are shown in Figure 3 and are also summarized in Table 1 along with some of their photochemical and biological properties.
Figure 3.
The wide distribution of microbial rhodopsins in all three domains of life. By 1999, type-1 rhodopsins have only been described in halophilic archaea. The four classical rhodopsins (BR, HR, SRI and SRII) are shown as boxes with white letters. After 1999, environmental genomics revealed that microbial rhodopsins are broadly distributed among bacteria (green) and eukarya (blue) in addition to archaea (red).
Table 1.
Examples of newly discovered microbial rhodopsins
| Opsin type | Year identified | Origin | λmax [nm] | Function |
|---|---|---|---|---|
| NRa | 1999 | Neurospora crassa | 534 | unknown |
| PRb | 2000 | Marine bacterioplankton | 520 | H+ pump |
| ChRc | 2002 | Chlamydomonas reinhardtii | 505 | Cation (H+, Na+, etc) channel |
| ASRd | 2003 | Anabaena (Nostoc) sp. PCC7120 | 543 | Transcriptional regulator |
| XRe | 2005 | Salinibacter ruber | 560 | H+ pump |
| SrSRIf | 2008 | Salinibacter ruber | 558 | Phototaxis sensor |
| MRg | 2011 | Haloquadratum walsbyi | 485 | unknown |
| TRh | 2013 | Thermus thermophilus | 530 | H+ pump |
| KR2i | 2013 | Krokinobacter eikastus | 524 | Na+ pump |
| ACR2j | 2015 | Guillardia theta | 470 | Anion (Cl−, Br−, etc) channel |
Thus today, a variety of type-1 rhodopsin genes have been identified from all domains of life (i.e., archaea, eubacteria and eukarya) and the number of genes is up to tens of thousands, indicating the great physiological significance of the widely distributed microbial rhodopsins for organisms (Fig. 3) [45].
Rich functional diversity
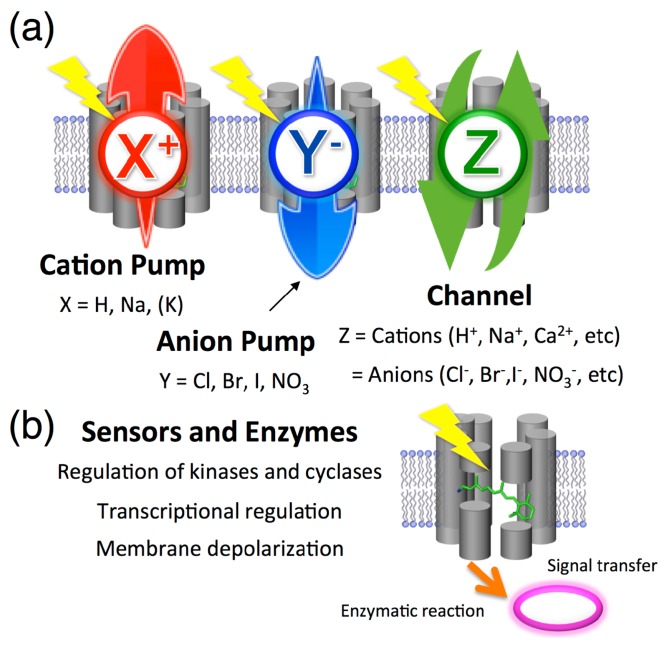
The wide distribution of microbial rhodopsins has also revealed their rich functional diversity (Fig. 4). It is well-known that there are two types of membrane-embedded ion transport machineries in nature. Ion pumps generate an electrochemical potential by energy-coupled active ion transportation, while one of the functions of ion channels is to produce an action potential by passive ion transportation. By 1999, two types of ion pumping rhodopsins, for H+ and Cl−, are known to exist only in archaea [3]. The Cl− pumping rhodopsins can also transport other anions such as Br− and I− [16], whereas, only proton (H+) can be a substrate ion but not other cations including Na+, K+ and Rb+. This is reasonable because of a characteristic property of H+. It is known that H+ can be rapidly transferred through several functional groups, including the OH group of the water molecule by the Grotthuss mechanism, but direct ion translocation is required for other cations. However, in 2013, a light-driven Na+ pumping rhodopsin (KR2) was first identified from the marine bacterium Krokinobacter eikastus (Fig. 3 and 4a) [46]. That unique ion transport mechanism was characterized by X-ray crystallography in 2015 [47,48]. In short, no binding of Na+ to the vicinity of the Schiff base under the unphotolyzed state is observed for the Na+ pumping rhodopsin. For ion channels, in 2002 and 2003, two light-gated cation channels, Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2), were found in the eukaryotic green alga Chlamydomonas reinhardtii [49]. In 2015, two other light-gated anion channels, anion channelrhodopsin-1 (ACR1) and anion channelrhodopsin-2 (ACR2), were found in the eukaryotic cryptophyte Guillardia theta (Fig. 3 and 4a) [50]. Thus, the control of ion concentrations by the ion transporters is one of the most essential functions of microbial rhodopsins. For instance, the discovery of PR indicated that a previously unsuspected mode of bacterially mediated light-driven ATP production commonly occurs in oceanic surface waters worldwide [51]. How can pumps and channels function differently? In 2015, we succeeded in the functional conversion of an H+ pump Archaerhodopsin-3 (AR3) from the archaeon Halorubrum sodomense to an H+ channel by replacing only three amino acid residues around the retinal chromophore [52]. That result indicates that essential differences between pumps and channels in the rhodopsin family are much smaller than previously imagined.
Figure 4.
Multitalented microbial rhodopsins. The type-1 microbial rhodopsins are roughly categorized as ion transporters (i.e., cation pump, anion pump and ion channel) and as sensors. (a) For ion transporters, the number of substrates (X, Y and Z) greatly increased in the early 21st century. (b) For sensors and coenzymes, the photosensory function was also extended from only the regulation of kinases to the control of cyclases, transcriptional regulation and depolarization of the plasma membrane that accounts for the phototactic behaviors of the prokaryote Chlamydomonas reinhardtii.
In addition to ion transportation, rhodopsins are also involved in photoreception for signal transduction (Fig. 4b) [53]. As shown in Figure 2, SRI and SRII form signaling complex with their cognate transducer proteins (HtrI for SRI and HtrII for SRII). The complexes are responsible for positive and negative phototaxis via the control of kinase activity inside cells [53]. As the other type of sensory signal transduction, depolarization by the light-gated cation transport activity of ChRs is utilized to control the motility of cells through regulation of the flagellar motor apparatus [54]. As a novel type of photoreceptor, Spudich and coworkers identified Anabaena sensory rhodopsin (ASR) from the cyanobacterium Anabaena (Nostoc) sp. PCC7120 in 2003 [55] and we demonstrated its function as a photo-dependent transcriptional regulator in 2012 [56]. As other functions, a photochromic histidine kinase rhodopsin (HKR) [57] and the rhodopsin-guanylyl cyclase (RhGC) [58] have been identified in 2012 and 2015, respectively. It is noteworthy that the biological function(s) of many microbial rhodopsins, including NR and MR, is still unclear (Fig. 3). In other words, the expanded biological roles of microbial rhodopsins should become obvious in the future.
Great potential for optogenetics
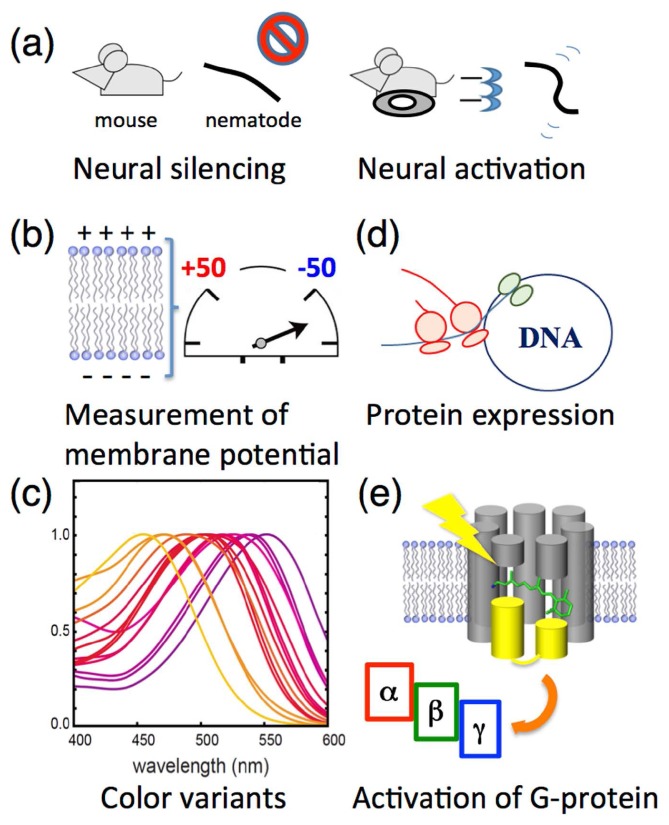
In 2005, Boyden and coworkers made a breakthrough regarding a new technology named “optogenetics” (Fig. 5) [59]. In that study, they expressed ChR2 in mammalian cells to excite neurons by cation influx upon photoillumination. In 2007 and 2010, the same group succeeded in light-induced neural silencing using a Cl− pumping rhodopsin HR from the archaeon Natronomonas pharaonis (NpHR) [60] and an H+ pumping rhodopsin AR3 [61], respectively. Upon photoexcitation, the ion pumps induce a hyperpolarization across the membrane in a nerve cell, results in its neural silencing. Thus, these ion transport machineries are biotechnologically utilized as a molecular switch to control neural activity by light (Fig. 5a). It is noteworthy that in 2012, Cohen and coworkers utilized the endogenous fluorescence of AR3 to monitor the single action potential in cultured rat hippocampal neurons [62]. Since then, they succeeded in directly measuring the membrane voltage at the millivolt-level (Fig. 5b) [63].
Figure 5.
Optogenetic applications. (a) The photo-activation of ion transporting rhodopsins induces a hyperpolarization used for neural silencing and a depolarization used for neural activation both in vivo and in vitro. (b) The genetically modified H+ pump AR3 is utilized to measure the membrane potential in vivo. (c) The color variants of ion transporting rhodopsins allow optogenetics control in a wide range of wavelengths of light. (d) One of the sensory rhodopsins, Anabaena sensory rhodopsin (ASR), can be utilized as a tool for arbitrary protein expression in vivo regulated by visible light. (e) Chimeric proteins of Gloeobacter rhodopsin (GR) and bovine visual rhodopsin (Rh) are utilized to activate the trimeric G-protein.
As for luminescent and fluorescent proteins, the color variants of rhodopsins make it possible to use various wavelengths of visible light. For rhodopsins, the absorption maximum corresponds to the most probable transition from its ground to its excited state. All microbial rhodopsins reported are quite similar in their primary and tertiary structures, especially in the chromophore-binding site [8], which suggests that the absorption maximum is mainly controlled by the slight structural difference(s) of their side chain(s). Regarding the retinal chromophore, if the polyene chain possesses a planar conformation, a π-conjugation of the retinal becomes long (see Fig. 1a). A rotation of the β-ionone ring can, despite breaking the π-conjugation, easily occur in an isolated condition because of the steric repulsions between the methyl groups of the β-ionone ring at positions 1 and 5 and the hydrogens of the polyene chain at positions 7 and 8 (see Fig. 1a) [1]. To characterize this in the rhodopsins, we prepared a variety of rhodopsin mutants. As a result, we succeeded in developing blue-shifted color variants both of AR3 and ChR with a few mutations (~3) on the basis of rational design at atomic resolution, achieved through accurate molecular simulations, electrophysiology and X-ray crystallography [64,65]. The molecular simulation models and the crystal structure revealed the precisely designed conformational changes of the chromophore induced by combinatory mutations. The mutants shrink its π-conjugated system which produce large blue shifts (maximally 100 nm) of the absorption spectra (Fig. 5c). Of note, in the mutants, photosensitive ion transport activities are maintained. Thus, we successfully produced blue-shifted color variants both for neural silencing and excitation. It should be noted that some groups in USA and Germany have also succeeded in production of blue-shifted variants [66–68].
In addition to the control of neural activity, the photo-activation of an intracellular signaling cascade is also useful, and is recognized as one aspect of optogenetics. In 2012, we demonstrated that ASR represses the transcription of the protein expression in the cytosol and that this is fully inhibited by the light activation of ASR [56]. The system provides an advantage in controlling the amount of protein at temporal/spatial resolution and in providing control of the timing, compared with other techniques (Fig. 5d). In the future, ASR might be a novel tool for optogenetics, next to the ion transporting rhodopsins. Another interesting and demanding target is the hetero-trimeric G-protein, which is activated by GPCR, because various signaling processes in mammalian cells are regulated by GPCR [10]. Although the activity is still low, Kandori and coworkers have reported that chimeric proteins between a type-1 proton pump rhodopsin from the eubacterium Gloeobacter violaceus (GR) and bovine visual type-2 rhodopsin (Rh) can activate a type of G-protein by light like native Rh (Fig. 5e) [69,70]. However, its relation to the optogenetics is still unclear. Thus, compared with the ion transporting rhodopsins, further progress is needed to apply sensory rhodopsins as optogenetic tools.
Future perspectives
In the 20th century, microbial rhodopsins were research models both for membrane-embedded proteins and for photoactive proteins. Subsequently, in the early 21st century, they have become a fundamental template for optogenetics technology. Progress has been continuously assisted by a variety of scientific approaches, including biophysics, biochemistry and molecular biology. Of note, the United Nations determined the year 2015 as the International Year of Light and Light-based Technologies (IYL 2015: http://www.light2015.org/Home.html). Because light is a powerful tool, both for observing and controlling physical, chemical and biological phenomena in a variety of samples with various spatial dimensions from the atom to the organism, we strongly believe that making maximum use of light will open the door for the next generation of rhodopsin research.
Acknowledgements
YS wishes to thank collaborators, especially Profs. Naoki Kamo, John Spudich and Hideki Kandori, for giving me the opportunity to study microbial rhodopsins extensively. Our original publications were supported by a Grant-in-Aid from the Japanese Ministry of Education, Science, Technology, Sports and Cultures. Support for this work was provided by the Foundation of the Promotion of Ion Engineering and Research Foundation for Opto-science and Technology. We also thank DASS Manuscript for the expert English language review.
Footnotes
Conflicts of Interest
All authors declare that they have no conflict of interest.
Author Contributions
M. K., and Y. S. prepared the figures and wrote the manuscript.
References
- 1.Ernst OP, Lodowski DT, Elstner M, Hegemenn P, Brown LS.&Kandori H. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem Rev. 2014;114:126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudo Y. CRC Handbook of Org Photochem Photobiol. 3rd eds. CRC Press; Boca Raton: 2012. Transport and sensory rhodopsins in microorganisms; pp. 1173–1193. [Google Scholar]
- 3.Spudich JL, Yang CS, Jung KH.&Spudich EN. Retinylidene proteins: structures and functions from archaea to humans. Annu Rev Cell Dev Biol. 2000;16:365–392. doi: 10.1146/annurev.cellbio.16.1.365. [DOI] [PubMed] [Google Scholar]
- 4.Brown LS. Eubacterial rhodopsins—unique photosensors and diverse ion pumps. Biochim Biophys Acta. 2014;1837:553–561. doi: 10.1016/j.bbabio.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Brown LS.&Jung KH. Bacteriorhodopsin-like proteins of eubacteria and fungi: the extent of conservation of the haloarchaeal proton-pumping mechanism. Photochem Photobiol Sci. 2006;5:538–546. doi: 10.1039/b514537f. [DOI] [PubMed] [Google Scholar]
- 6.Terakita A. The opsins. Genome Biol. 2005;6:213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shichida Y.&Matsuyama T. Evolution of opsins and photo-transduction. Philos Trans R Soc Lond, B, Biol Sci. 2009;364:2881–2895. doi: 10.1098/rstb.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouyama T.&Murakami M. Structural divergence and functional versatility of the rhodopsin superfamily. Photochem Photobiol Sci. 2010;9:1458–1465. doi: 10.1039/c0pp00236d. [DOI] [PubMed] [Google Scholar]
- 9.Terakita A.&Nagata T. Functional properties of opsins and their contribution to light-sensing physiology. Zool Sci. 2014;31:653–659. doi: 10.2108/zs140094. [DOI] [PubMed] [Google Scholar]
- 10.Manglik A.&Kobilka B. The role of protein dynamics in GPCR function: insights from the β2AR and rhodopsin. Curr Opin Cell Biol. 2014;27:136–143. doi: 10.1016/j.ceb.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oesterhelt D.&Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 12.Lanyi JK. Bacteriorhodopsin. Annu Rev Physiol. 2004;66:665–688. doi: 10.1146/annurev.physiol.66.032102.150049. [DOI] [PubMed] [Google Scholar]
- 13.Matsuno-Yagi A.&Mukohata Y. Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977;78:237–243. doi: 10.1016/0006-291x(77)91245-1. [DOI] [PubMed] [Google Scholar]
- 14.Bogomolni RA.&Spudich JL. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci USA. 1982;79:6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Mochizuki Y, Kamo N.&Kobatake Y. Evidence that the long-lifetime photointermediate of s-rhodopsin is a receptor for negative phototaxis in Halobacterium halobium. Biochem Biophys Res Commun. 1985;127:99–105. doi: 10.1016/s0006-291x(85)80131-5. [DOI] [PubMed] [Google Scholar]
- 16.Schobert B.&Lanyi JK. Halorhodopsin is a light-driven chloride pump. J Biol Chem. 1982;257:10306–10313. [PubMed] [Google Scholar]
- 17.Mukohata Y, Ihara K, Tamura T.&Sugiyama Y. Halobacterial rhodopsins. J Biochem. 1999;125:649–657. doi: 10.1093/oxfordjournals.jbchem.a022332. [DOI] [PubMed] [Google Scholar]
- 18.Hoff WD, Jung KH.&Spudich JL. Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu Rev Biophys Biomol Struct. 1997;26:223–258. doi: 10.1146/annurev.biophys.26.1.223. [DOI] [PubMed] [Google Scholar]
- 19.Chen X.&Spudich JL. Demonstration of 2:2 stoichiometry in the functional SRI-HtrI signaling complex in Halobacterium membranes by gene fusion analysis. Biochemistry. 2002;41:3891–3896. doi: 10.1021/bi015966h. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XN, Zhu J.&Spudich JL. The specificity of interaction of archaeal transducers with their cognate sensory rhodopsins is determined by their transmembrane helices. Proc Natl Acad Sci USA. 1999;96:857–862. doi: 10.1073/pnas.96.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudo Y, Yamabi M, Kato S, Hasegawa C, Iwamoto M, Shimono K, et al. Importance of specific hydrogen bonds of archaeal rhodopsins for the binding to the transducer protein. J Mol Biol. 2006;357:1274–1282. doi: 10.1016/j.jmb.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Yan B, Mazur P, Derguini F, Nakanishi K.&Spudich JL. Color regulation in the archaebacterial phototaxis receptor phoborhodopsin (sensory rhodopsin II) Biochemistry. 1990;29:8467–8474. doi: 10.1021/bi00488a038. [DOI] [PubMed] [Google Scholar]
- 23.Sudo Y, Yuasa Y, Shibata J, Suzuki D.&Homma M. Spectral tuning in sensory rhodopsin I from Salinibacter ruber. J Biol Chem. 2011;286:11328–11336. doi: 10.1074/jbc.M110.187948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki J, Brown LS, Chon YS, Kandori H, Maeda A, Needleman R, et al. Conversion of bacteriorhodopsin into a chloride ion pump. Science. 1995;269:73–75. doi: 10.1126/science.7604281. [DOI] [PubMed] [Google Scholar]
- 25.Sudo Y.&Spudich JL. Three strategically placed hydrogen-bonding residues convert a proton pump into a sensory receptor. Proc Natl Acad Sci USA. 2006;103:16129–16134. doi: 10.1073/pnas.0607467103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogomolni RA, Stoeckenius W, Szundi I, Perozo E, Olson KD.&Spudish JL. Removal of transducer HtrI allows electrogenic proton translocation by sensory rhodopsin I. Proc Natl Acad Sci USA. 1994;91:10188–10192. doi: 10.1073/pnas.91.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo Y, Iwamoto M, Shimono K, Sumi M.&Kamo N. Photo-Induced Proton Transport of Pharaonis Phoborhodopsin (Sensory Rhodopsin II) Is Ceased by Association with the Transducer. Biophys J. 2001;80:916–922. doi: 10.1016/S0006-3495(01)76070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanyi JK. X-ray diffraction of bacteriorhodopsin photocycle intermediates. Mol Membr Biol. 2004;21:143–150. doi: 10.1080/09687680410001666345. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi T, Yabushita A, Saito T, Ohtani H.&Tsuda M. Sub-5-fs real-time spectroscopy of transition states in bacteriorhodopsin during retinal isomerization. Photochem Photobiol. 2007;83:363–368. doi: 10.1562/2006-08-19-IR-1006. [DOI] [PubMed] [Google Scholar]
- 30.Shim S, Dasgupta J.&Mathies RA. Femtosecond time-resolved stimulated Raman reveals the birth of bacteriorhodopsin’s J and K intermediates. J Am Chem Soc. 2009;131:7592–7597. doi: 10.1021/ja809137x. [DOI] [PubMed] [Google Scholar]
- 31.Brown LS, Sasaki J, Kandori H, Maeda A, Naadleman R.&Lanyi JK. Glutamic acid 204 is the terminal proton release group at the extracellular surface of bacteriorhodopsin. J Biol Chem. 1995;270:27122–27126. doi: 10.1074/jbc.270.45.27122. [DOI] [PubMed] [Google Scholar]
- 32.Kötting C.&Gerwert K. Proteins in action monitored by time-resolved FTIR spectroscopy. Chemphyschem. 2005;6:881–888. doi: 10.1002/cphc.200400504. [DOI] [PubMed] [Google Scholar]
- 33.Altun A, Yokoyama S.&Morokuma K. Quantum Mechanical/Molecular Mechanical Studies on Spectral Tuning Mechanisms of Visual Pigments and Other Photoactive Proteins. Photochem Photobiol. 2008;84:845–854. doi: 10.1111/j.1751-1097.2008.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katayama K, Sekharan S.&Sudo Y. Color Tuning in Retinylidene Proteins. Optogenetics: Light-Sensing Proteins and Their Applications. 2015;Chapter 7:89–107. [Google Scholar]
- 35.Saranak J.&Foster KW. Rhodopsin guides fungal phototaxis. Nature. 1997;387:465–466. doi: 10.1038/387465a0. [DOI] [PubMed] [Google Scholar]
- 36.Graul RC.&Sadée W. Evolutionary relationships among proteins probed by an iterative neighborhood cluster analysis (INCA). Alignment of bacteriorhodopsins with the yeast sequence YRO2. Pharm Res. 1997;14:1533–1541. doi: 10.1023/a:1012166015402. [DOI] [PubMed] [Google Scholar]
- 37.Bieszke JA, Braun EL, Bean LE, Kang S, Natvig DO.&Borkovich KA. The nop-1 gene of neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc Natl Acad Sci USA. 1999;96:8034–8039. doi: 10.1073/pnas.96.14.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bieszke JA, Spudich EN, Scott KL, Borkovich KA.&Spudich JL. A eukaryotic protein, NOP-1, binds retinal to form an archaeal rhodopsin-like photochemically reactive pigment. Biochemistry. 1999;38:14138–14145. doi: 10.1021/bi9916170. [DOI] [PubMed] [Google Scholar]
- 39.Béja O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, et al. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science. 2000;289:1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 40.Balashov SP, Imasheva ES, Bochenko VA, Anton J, Wang JM.&Lanyi JK. Xanthorhodopsin: a proton pump with a light-harvesting carotenoid antenna. Science. 2005;309:2061–2064. doi: 10.1126/science.1118046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitajima-Ihara T, Furutani Y, Suzuki D, Ihara K, Kandori H, Hamma M, et al. Salinibacter sensory rhodopsin: sensory rhodopsin I-like protein from a eubacterium. J Biol Chem. 2008;283:23533–23541. doi: 10.1074/jbc.M802990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagasaki J, Suzuki D, Ihara K, Inoue K, Kikukawa T, Sasaki M, et al. Spectroscopic studies of a sensory rhodopsin I homologue from the archaeon Haloarcula vallismortis. Biochemistry. 2010;49:1183–1190. doi: 10.1021/bi901824a. [DOI] [PubMed] [Google Scholar]
- 43.Sudo Y, Ihara K, Kobayashi S, Suzuki D, Irieda H, Kikukawa T, et al. A microbial rhodopsin with a unique retinal composition shows both sensory rhodopsin II and bacteriorhodopsin-like properties. J Biol Chem. 2011;286:5967–5976. doi: 10.1074/jbc.M110.190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukamoto T, Inoue K, Kandori H.&Sudo Y. Thermal and spectroscopic characterization of a proton pumping rhodopsin from an extreme thermophile. J Biol Chem. 2013;288:21581–21592. doi: 10.1074/jbc.M113.479394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spudich JL, Sineshchekov OA.&Govorunova EG. Mechanism divergence in microbial rhodopsins. Biochim Biophys Acta. 2014;1837:546–552. doi: 10.1016/j.bbabio.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue K, Ono H, Abe-Yoshizumi R, Yoshizawa S, Ito H, Kogure K, et al. A light-driven sodium ion pump in marine bacteria. Nat Commun. 2013;4:1678. doi: 10.1038/ncomms2689. [DOI] [PubMed] [Google Scholar]
- 47.Gushchin I, Shevchenko V, Polovinkin V, Kovalev K, Alekseev A, Round E, et al. Crystal structure of a light-driven sodium pump. Nat Struct Mol Biol. 2015;22:390–395. doi: 10.1038/nsmb.3002. [DOI] [PubMed] [Google Scholar]
- 48.Kato HE, Inoue K, Abe-Yoshizumi R, Kato Y, Ono H, Konno M, et al. Structural basis for Na(+) transport mechanism by a light-driven Na(+) pump. Nature. 2015;521:48–53. doi: 10.1038/nature14322. [DOI] [PubMed] [Google Scholar]
- 49.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 50.Govorunova EG, Sineshchekov OA, Janz R, Liu X.&Spudich JL. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 2015;349:647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Béjà O, Spudich EN, Spudich JL, Leclerc M.&DeLong EF. Proteorhodopsin phototrophy in the ocean. Nature. 2001;411:786–789. doi: 10.1038/35081051. [DOI] [PubMed] [Google Scholar]
- 52.Inoue K, Tsukamoto T, Shimono K, Suzuki Y, Miyauchi S, Hayashi S, et al. Converting a light-driven proton pump into a light-gated proton channel. J Am Chem Soc. 2015;137:3291–3299. doi: 10.1021/ja511788f. [DOI] [PubMed] [Google Scholar]
- 53.Inoue K, Tsukamoto T.&Sudo Y. Molecular and evolutionary aspects of microbial sensory rhodopsins. Biochim Biophys Acta. 2014;1837:562–577. doi: 10.1016/j.bbabio.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Sineshchekov OA, Jung KH.&Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonasreinhardtii. ProcNatl Acad Sci USA. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung KH, Trivedi VD.&Spudich JL. Demonstration of a sensory rhodopsin in eubacteria. Mol Microbiol. 2003;47:1513–1522. doi: 10.1046/j.1365-2958.2003.03395.x. [DOI] [PubMed] [Google Scholar]
- 56.Irieda H, Morita T, Maki K, Homma M, Aiba H.&Sudo Y. Photo-induced regulation of the chromatic adaptive gene expression by Anabaena sensory rhodopsin. J Biol Chem. 2012;287:32485–32493. doi: 10.1074/jbc.M112.390864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luck M, Mathes T, Bruun S, Fudim R, Hagedorn R, Tran Nguyen TM, et al. A photochromic histidine kinase rhodopsin (HKR1) that is bimodally switched by ultraviolet and blue light. J Biol Chem. 2012;287:40083–40090. doi: 10.1074/jbc.M112.401604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheib U, Stehfest K, Gee CE, Körshen HG, Fudim R, Oertner TG, et al. The rhodopsin-guanylyl cyclase of the aquatic fungus Blastocladiella emersonii enables fast optical control of cGMP signaling. Sci Signal. 2015;8:rs8. doi: 10.1126/scisignal.aab0611. [DOI] [PubMed] [Google Scholar]
- 59.Boyden ES, Zhang F, Bamberg E, Nagel G.&Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 60.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 61.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D.&Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods. 2012;9:90–95. doi: 10.1038/nmeth.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen AE.&Hochbaum DR. Measuring membrane voltage with microbial rhodopsins. Methods Mol Biol. 2014;1071:97–108. doi: 10.1007/978-1-62703-622-1_8. [DOI] [PubMed] [Google Scholar]
- 64.Sudo Y, Okazaki A, Ono H, Yagasaki J, Sugo S, Kamiya M, et al. A blue-shifted light-driven proton pump for neural silencing. J Biol Chem. 2013;288:20624–20632. doi: 10.1074/jbc.M113.475533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kato HE, Kamiya M, Sugo S, Ito J, Taniguchi R, Orito A, et al. Atomistic design of microbial opsin-based blue-shifted optogenetics tools. Nat Commun. 2015;6:7177. doi: 10.1038/ncomms8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prigge M, Schneider F, Tsunoda SP, Shilynsky C, Wietek J, Deisseroth K, et al. Color-tuned channelrhodopsins for multiwavelength optogenetics. J Biol Chem. 2012;287:31804–31812. doi: 10.1074/jbc.M112.391185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods. 2014;11:825–833. doi: 10.1038/nmeth.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, et al. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 2014;344:409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- 69.Sasaki K, Yamashita T, Yoshida K, Inoue K, Shichida Y.&Kandori H. Chimeric proton-pumping rhodopsins containing the cytoplasmic loop of bovine rhodopsin. PLoS One. 2014;9:e91323. doi: 10.1371/journal.pone.0091323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakatsuma A, Yamashita T, Sasaki K, Kawanabe A, Inoue K, Furutani Y, et al. Chimeric microbial rhodopsins containing the third cytoplasmic loop of bovine rhodopsin. Biophys J. 2011;100:1874–1882. doi: 10.1016/j.bpj.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luecke H, Schobert B, Richter HT, Cartailler JP.&Lanyi JK. Structure of bacteriorhodopsin at 1.55 Å resolution. J Mol Biol. 1999;291:899–911. doi: 10.1006/jmbi.1999.3027. [DOI] [PubMed] [Google Scholar]