Abstract
Background:
Salvianolic acid B (Sal B) is a bioactive water-soluble compound of Salviae miltiorrhizae, a traditional herbal medicine that has been used clinically for the treatment of cardiovascular diseases. This study sought to evaluate the effect of Sal B on matrix metalloproteinase-9 (MMP-9) and on the underlying mechanisms in tumor necrosis factor-α (TNF-α)-activated human coronary artery endothelial cells (HCAECs), a cell model of Kawasaki disease.
Methods:
HCAECs were pretreated with 1–10 μmol/L of Sal B, and then stimulated by TNF-α at different time points. The protein expression and activity of MMP-9 were determined by Western blot assay and gelatin zymogram assay, respectively. Nuclear factor-κB (NF-κB) activation was detected with immunofluorescence, electrophoretic mobility shift assay, and Western blot assay. Protein expression levels of mitogen-activated protein kinase (c-Jun N-terminal kinase [JNK], extra-cellular signal-regulated kinase [ERK], and p38) were determined by Western blot assay.
Results:
After HCAECs were exposed to TNF-α, 1–10 μmol/L Sal B significantly inhibited TNF-α-induced MMP-9 expression and activity. Furthermore, Sal B significantly decreased IκBα phosphorylation and p65 nuclear translocation in HCAECs stimulated with TNF-α for 30 min. In addition, Sal B decreased the phosphorylation of JNK and ERK1/2 proteins in cells treated with TNF-α for 10 min.
Conclusions:
The data suggested that Sal B suppressed TNF-α-induced MMP-9 expression and activity by blocking the activation of NF-κB, JNK, and ERK1/2 signaling pathways.
Keywords: Endothelial Cell Injury, Kawasaki Disease, Mitogen-activated Protein Kinase, Matrix Metalloproteinase-9, Nuclear factor-κB, Salvianolic Acid B
INTRODUCTION
Kawasaki disease (KD) is a febrile vascular inflammatory disease that occurs in children.[1] Its incidence has increased in many countries over the last 30 years.[2] Its major complication is coronary arterial damage, including coronary artery inflammation, coronary artery aneurysms, and coronary artery occlusion.[3] The incidence of coronary artery complications (CAC) was 20–25% in children without intravenous immunoglobulin (IVIG) therapy.[4] Therefore, CAC has become the leading cause of acquired cardiovascular disease in children in developed countries.[4]
Although the pathogenesis of KD is still unclear, endothelial cell injury has been proved to be the major pathological basis of CAC, and cytokine cascade amplification is a key step in the endothelial cell injury.[5] Among the cytokines, tumor necrosis factor-α (TNF-α) is a key inflammatory mediator responsible for immune responses and systemic inflammation in KD,[6] and has been used successfully to generate a cell model of KD in vitro.[7] Matrix metalloproteinase-9 (MMP-9), one of effector molecules of TNF-α signaling, plays an important role in the pathogenesis of elastin breakdown.[8,9,10] In a previous study, we confirmed that MMP-9 plays an important role in the formation of CAC in a mouse model of KD.[11] However, the underlying mechanism of CAC in cells level requires further elucidation. Nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs), including extra-cellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38, are the intracellular signaling pathways that participate in various immune regulation.[12] These pathways may be involved in modulating the expression of MMP-9.
Currently, IVIG and aspirin are the standard therapies for KD.[13] Although these therapies have greatly reduced the incidence of coronary artery lesions, approximately 15−25% of children with KD do not respond to these therapies.[14] Therefore, there is a great need to find alternative agents for the treatment of KD.
Salvianolic acid B (Sal B) is a bioactive water-soluble compound of Salviae miltiorrhizae,[15] a traditional herbal medicine that has been used clinically for the treatment of cardiovascular and cerebrovascular diseases in Asian countries for centuries.[16,17,18,19] Studies have reported that Sal B displays a potent anti-inflammatory activity.[20,21,22,23] In our previous studies, we found that herbal medicines containing Sal B had therapeutic effects on both children with KD and a mouse model of KD. However, whether Sal B affects MMP-9 expression and its mechanism needs to be further evaluated.
Therefore, in this study, we aimed to detect the effects of Sal B on the expression and activity of MMP-9 in TNF-α-induced human coronary artery endothelial cells (HCAECs) and to identify its possible intracellular molecular targets. This study may provide evidence for a new integrated treatment for KD resistant to IVIG therapy.
METHODS
Reagents
Sal B (purity N 98% determined by high-performance liquid chromatography) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Recombinant human TNF-α was purchased from Cell Signaling Technology (#8902, Danvers, MA, USA). U0126 (#9903), SP600125 (#8177), SB203580 (#5633) were purchased from Cell Signaling Technology (Beverly, MA, USA). BAY 11-7082 was purchased from Merck (Darmstadt, Germany). Polyvinylidenedifluoride (PVDF) membranes were purchased from Millipore (Billerica, MA, USA). All other chemicals and reagents used in the study were of analytical grade and commercially available.
Cell culture
HCAECs were purchased from the American Type Culture Collection (ATCC) (Lot #61492256, Manassas, VA, USA). Cells were cultured in Endothelial Cell Growth Kit-vascular endothelial growth factor (ATCC) supplemented with 10% fetal bovine serum, at 37°C in a 5% CO2 incubator. Experiments were performed using cells between passages 3 and 5.
Cell viability assay
The cell survival was detected by Cell Counting Kit-8 (CCK-8) kit (Dojindo, Kyushu Island, Kumamoto, Japan) according to the manufacturer's instructions. HCAECs were incubated in 96-well plates with Sal B in different concentrations. After 48 h treatment, CCK-8 was added, and cells continued to incubation for 2 h. The plates were read at 450 nm using an iMARK type 680 microplate reader (Bio-Rad technology, Hercules, CA, USA). Culture medium without cells was used as background controls.
Matrix metalloproteinase-9 activity assay
After 24 h of Sal B treatment, culture medium was collected and centrifuged at 16,000 × g for 5 min at 4°C to remove cell debris. The supernatants were detected in duplicate with a Gelatin Zymogram Assay Kit (Applygen Technologies, Beijing, China) for MMP-9 activities according to the manufacturer's instructions. Gelatinolytic activity was manifested as horizontal white bands on a blue background by using Gel pro 4.0 Software (Media Cybernetics Technology, Warrendale, PA, USA).
Preparation of total and nuclear fraction proteins
Cells were collected and centrifuged at 1000 × g for 5 min at 4°C to remove the supernatant, and stored at −70°C. The total cellular protein was extracted by using a Total Protein Extraction Kit (Promab Technology, No. SJ-200501, Shanghai, China). The cytoplasmic and nuclear proteins were extracted, respectively, by using NucBuster™ Protein Exaction Kit (MERCK, No. 71183-3, Darmstadt, Germany) according to the manufacturer's instructions. Protein concentrations in whole cell lysates and fractions were measured using a bicinchoninic acid method. All protein samples were stored at −70°C until use.
Western blot analysis
HCAECs were treated with 1–10 μmol/L Sal B for 24 h, and then stimulated with 100 ng/ml TNF-α for 10 min (for JNK and ERK1/2), 30 min (for p-IκBα, p65), or 8 h (for MMP-9). The concentration and induction time of TNF-α were chosen based on previous reports[24] and our preliminary data. Equal amounts of total cell lysates (for MMP-9, IκBα, and GAPDH) and cytoplasmic (for p65 and GAPDH) or nuclear (for p65, p38, JNK, ERK1/2, and lamin B) fractions were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto PVDF membranes.[25] After blocking antigens with 5% nonfat milk for 1 h at room temperature, the membranes were incubated with a primary antibody at 4°C overnight and subsequently with a horseradish peroxidase-conjugated second antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 1 h. The immune complexes were detected using enhanced chemiluminescence reagents, analyzed by Gel-pro 4.0 version Gel Analysis Software (Media Cybernetics, MD, USA), and quantified by integrated optical density. Primary antibodies used included mouse anti-MMP-9 monoclonal antibody (1:800), Goat anti-p-JNK monoclonal antibody (1:400), Mouse anti-p-p38 monoclonal antibody (1:500), rabbit anti-p-ERK1/2 monoclonal antibody (1:500), mouse anti-JNK monoclonal antibody (1:400), rabbit anti-p38 monoclonal antibody (1:500), rabbit anti-ERK monoclonal antibody (1:500), mouse anti-GAPDH antibody (1:800), mouse anti-lamin B monoclonal antibody (1:800) (Santa Cruz Biotechnology), rabbit anti-NF-κB p65 antibody (1:1000), and mouse anti-p-IκBα antibody (1:400) (Cell Signaling Technology, Beverly, MA, USA).
Confocal laser scanning of nuclear factor-κB p65
NF-κB expression in HCAECs was also detected by immunofluorescence as described previously.[26] Cells were seeded onto sterilized coverslips in a 96-well culture plate. After being treated with TNF-α for 1 h, the cells were fixed for 15 min in 4% (w/v) paraformaldehyde and permeabilized by 0.2% Triton X-100 for 15 min. After blocking overnight at 4°C, cells were incubated with rabbit anti-NF-κB p65 monoclonal antibody (1:100) for 2 h at 37°C, and then fluorescein-conjugated anti-rabbit IgG antibody (1:500) for 0.5 h at 37°C. Finally, cells were incubated with propidium iodide for 20 min to stain the nucleus. NF-κB p65 was imaged by a confocal laser scanning microscope (Lecia TCS SP8, Frankfurt, Germany). NF-κB p65 was observed as green fluorescence and the nucleus as red fluorescence.
Statistical analysis
GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. Results were reported as the mean ± standard deviation (SD). Data were analyzed by one-way analysis of variance (ANOVA), followed by least significance difference or Tamhane's T2 multiple comparison test using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). A P < 0.05 was considered statistically significant.
RESULTS
Effect of salvianolic acid B on cell survival
Before analysis of MMP-9, we first determined the cytotoxicity of Sal B. When compared to the untreated baseline control, 1–10 μmol/L Sal B showed no cytotoxic effect on cell viability. However, 50 μmol/L and 100 μmol/L Sal B significantly reduced cell viability (both P < 0.05; Figure 1a). Therefore, in the following experiments, we used the doses of Sal B during 1–10 μmol/L.
Figure 1.
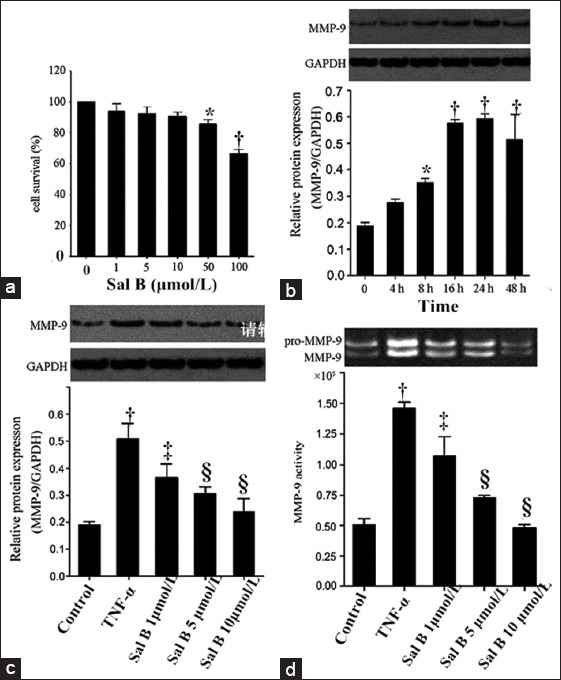
Effects of Sal B on MMP-9 activity and expression in TNF-α-induced human coronary artery endothelial cells. (a) Effect of Sal B on human coronary artery endothelial cells survival. Cells were incubated with Sal B (1–100 μmol/L) for 48 h and Cell Counting Kit-8 assay was performed to evaluate the cytotoxicity; (b) Cells were stimulated with 100 ng/ml TNF-α for 2–48 h. MMP-9 protein was measured by Western blot assay; (c) Cells were pretreated with Sal B (1–10 μmol/L) for 24 h and stimulated with 100 ng/ml TNF-α for 8 h. MMP-9 protein was measured by Western blot assay; (d) Secreted MMP-9 in the cell-free culture media was measured by gelatin zymogram assay. *P < 0.05, †P < 0.01 versus the untreated baseline control. ‡P < 0.05, §P < 0.01 versus the TNF-α group. Sal B: Salvianolic acid B; MMP-9: Matrix metalloproteinas-9; TNF-α: Tumor necrosis factor-α.
Effects of salvianolic acid B on tumor necrosis factor-α-stimulated matrix metalloproteinase-9 expression and activity in human coronary artery endothelial cells
Western blot analysis was performed to determine the protein level of MMP-9. Gelatin zymography was performed in order to determine the MMP-9 activity. HCAECs were treated with 100 ng/ml TNF-α in the indicated time periods in order to detect if TNF-α increases the MMP-9 protein expression. As shown in Figure 1b, MMP-9 expression was induced by TNF-α 8 h after and persisted for at least 24 h. As expected, in comparison to the baseline control, TNF-α stimulation for 24 h significantly upregulated both MMP-9 protein expression [Figure 1c] and activity [Figure 1d]. For TNF-α-activated cells, three doses of Sal B (1–10 μmol/L) significantly reduced TNF-α-enhanced MMP-9 activity and protein expression (MMP-9 activity: TNF-α group vs. control group, P = 0.001; Sal B 1 μmol/L vs. TNF-α group, P = 0.049; Sal B 5 μmol/L vs. TNF-α group, P = 0.000; Sal B 10 μmol/L vs. TNF-α group, P = 0.000. MMP-9 expression: TNF-α group vs. control group, P = 0.000; Sal B 1 μmol/L vs. TNF-α group, P = 0.038; Sal B 5 μmol/L vs. TNF-α group, P = 0.002; Sal B 10 μmol/L vs. TNF-α group, P = 0.005) in a dose-dependent manner.
Involvement of mitogen-activated protein kinases and nuclear factor-κB in the induction of matrix metalloproteinase-9 by tumor necrosis factor-α
To understand the role of MAPKs and NF-κB in the regulation of MMP-9 by Sal B, we first assessed the role of MAPKs and NF-κB in the induction of MMP-9 by TNF-α using several MAPK and NF-κB inhibitors. As shown in Figure 2a, TNF-α-induced MMP-9 protein expression was significantly inhibited by SP600125 (50 μmol/L), a JNK inhibitor (P = 0.001), and U0126 (10 μmol/L), a specific ERK inhibitor and Bay 11-1072 (2 μmol/L), an inhibitor of NF-κB signaling (P = 0.000), but not by SB203580 (10 μmol/L), a specific p38 inhibitor (P = 0.19), suggesting NF-κB, JNK, and ERK signaling are mainly involved in the regulation of MMP-9 expression, except p38.
Figure 2.
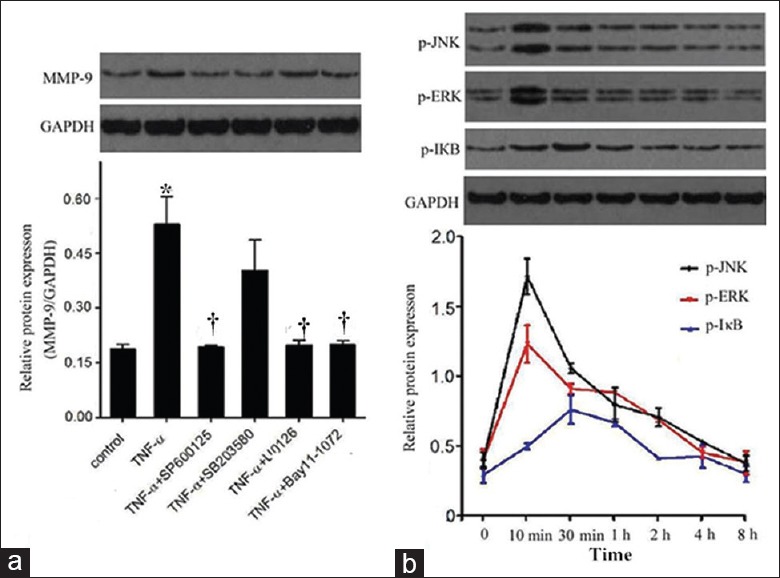
Involvement of MAPKs and nuclear factor-κB in the induction of MMP-9 by TNF-α. (a) Cells were treated with SP600125 at 50 μmol/L for 30 min, SB203580 at 10 μmol/L, Bay 11-1072 at 2 μmol/L and U0126 at 10 μmol/L for 1 h, and then co-treated with TNF-α for 8 h. Protein level of MMP-9 was detected by Western blot assay; (b) Cells were treated with TNF-α for various times (10 min to 8 h) extracted and the protein levels of JNK, ERK, and IκB were determined by Western blot. The band intensities were assessed by scanning densitometry. Significance compared with untreated group, *P < 0.01; significance compared with TNF-α group, †P < 0.01. MAPK: Mitogen-activated protein kinase; JNK: c-Jun N-terminal kinase; ERK: Extra-cellular signal-regulated kinase; MMP-9: Matrix metalloproteinase-9; TNF-α: Tumor necrosis factor-α.
Effect of salvianolic acid B on activation of nuclear factor-κB and mitogen-activated protein kinases-induced by tumor necrosis factor-α in human coronary artery endothelial cells
Western blot analysis was performed to determine the protein level of phosphorylation of IκB, JNK, and ERK1/2. NF-κB p65 translocation was detected by Western blot and immunofluorescence. As shown in Figure 2b, TNF-α exposure enhanced the phosphorylation of JNK and ERK1/2, which peaked at around 10 min, and the phosphorylation of IκB, which peaked at about 30 min, followed by a gradual decrease.
To understand the mechanisms behind the regulation of MMP-9 by Sal B, we first determined the effect of NF-κB activation and translocation. TNF-α treatment for 0.5 h induced p65 translocation as indicated by increased p65 in the nucleus and decreased p65 in cytoplasm [Figure 3a]. Sal B (1–10 μmol/L) significantly suppressed p65 translocation when cells were exposed to TNF-α (NF-κB p65 [nuleus]: TNF-α group vs. control group, P = 0.001; Sal B 1 μmol/L vs. TNF-α group, P = 0.019; Sal B 5 μmol/L vs. TNF-α group, P = 0.002; Sal B 10 μmol/L vs. TNF-α group, P = 0.0001. NF-κB p65 [cytoplasma]: TNF-α vs. control group, P = 0.001; Sal B 1 μmol/L vs. TNF-α group, P = 0.008; Sal B 5 μmol/L vs. TNF-α group, P = 0.001; Sal B 10 μmol/L vs. TNF-α group, P = 0.0001). These results were further confirmed by immune-fluorescence of NF-κB (p65) [Figure 3b]. Furthermore, after TNF-α treatment for 0.5 h, Sal B significantly suppressed IκBα activation (TNF-α group vs. control group, P = 0.001; Sal B 1 μmol/L vs. TNF-α group, P = 0.048; Sal B 5 μmol/L vs. TNF-α group, P = 0.006; Sal B 10 μmol/L vs. TNF-α group, P = 0.001). Next, we determined the effect of Sal B on TNF-α-induced MAPK activation. Compared to TNF-α alone, the induction of p-ERK1/2 and p-JNK were inhibited by Sal B in a concentration-dependent manner [Figure 4].
Figure 3.
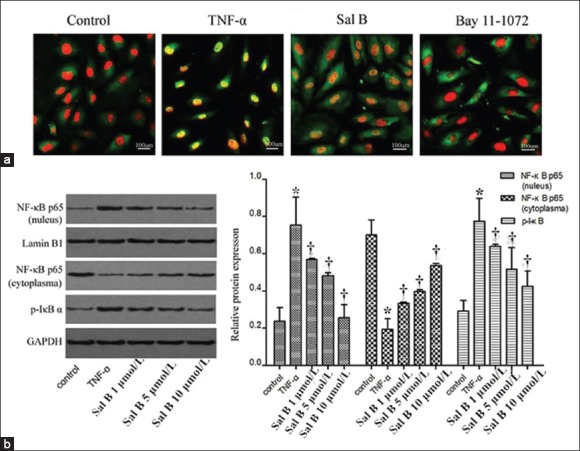
Effect of Sal B on activation of nuclear factor-κB pathway induced by TNF-α in human coronary artery endothelial cells. (a) Cells were pretreated with Sal B at various concentrations and then co-treated with TNF-α for 30 min. Phosphorylated IκBα protein and p65 protein in nucleus and cytoplasm were detected by Western blot assay; (b) Human umbilical vein endothelial cells were analyzed by immunofluorescence using nuclear factor-κB p65 subunit antibody and DAPI (×20). Red: Nucleus; green: Nuclear factor-κB p65. Images were representatives of three independent experiments. Significance compared with untreated group, *P < 0.01; significance compared with TNF-α, †P < 0.01. Sal B: Salvianolic acid B; TNF-α: Tumor necrosis factor-α.
Figure 4.
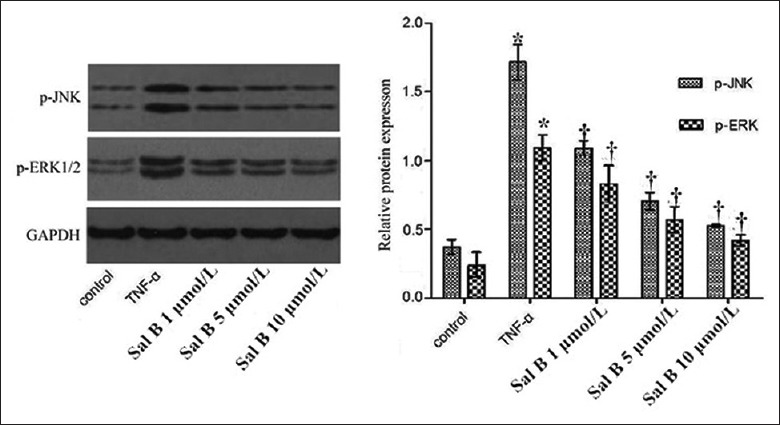
Effect of Sal B on activation of MAPKs pathway induced by TNF-α in human coronary artery endothelial cells. Cells were pretreated with Sal B at various concentrations and then co-treated with TNF-α for 10 min. Cells were extracted and protein levels were determined by Western blot analysis. Significance compared with untreated group, *P < 0.01; significance compared with TNF-α group, †P < 0.01. Sal B: Salvianolic acid B; TNF-α: Tumor necrosis factor-α; MAPK: Mitogen-activated protein kinase.
DISCUSSION
We found that Sal B significantly reduced MMP-9 protein expression and activity in TNF-α-induced HCAECs. Furthermore, we found that Sal B markedly inhibited the activation of NF-κB and the phosphorylation of JNK and ERK1/2 in TNF-α-induced HCAECs. These data suggested that Sal B reduces TNF-α-induced MMP-9 expression via the inhibition of multiple upstream signaling pathways, including NF-κB, JNK, and ERK1/2.
TNF-α largely contributes to the inflammation progress of KD at the coronary arteries.[6] This was demonstrated in a TNF-α knockout KD rat model which is resistant to coronary arteries vasculitis.[27] In this study, TNF-α was used to induce HCAECs as a coronary arteries inflammation model of KD.
Although the mechanisms of KD are not totally understood, it has been found the involvement of MMP-9 in the vasculitis process of KD. MMP-9, as a major component of the enzyme cascade for degradation of the extracellular matrix, participates in the processes of elastin breakdown, which is related to the development of coronary artery aneurysms in KD.[8] MMP-9 is inducible by TNF-α in vascular endothelial cells.[7] We demonstrated that the activity and expression of MMP-9 induced by TNF-α were decreased by 1–10 μmol/L Sal B in dose-dependent degrees. Since cell viability was not influenced by the same doses of Sal B, suggesting that the inhibitory effect of Sal B on MMP-9 is not due to the reduction of cell number, but through other mechanisms.
After demonstrating the inhibitory effect of Sal B on MMP-9 expression, we subsequently detected the involvement of NF-κB and MAPKs in MMP-9 expression. To do this, several specific inhibitors of NF-κB and MAPKs were used to determine which signaling pathways might be involved in the expression of MMP-9. Our data showed that except the inhibitor of p38, another inhibitor can decrease the MMP-9 expression induced by TNF-α. This means NF-κB, ERK1/2 and JNK pathways were involved in MMP-9 expression.
In NF-κB activation, there are several key steps, including IκB kinase activation, IκBα degradation, p65 nuclear translocation, and DNA binding activity of NF-κB.[28] In this study, Sal B was found to decrease IκBα phosphorylation and p65 nuclear translocation in TNF-α-induced HCAECs. Furthermore, Sal B inhibited TNF-α-induced ERK1/2 and JNK activation in a concentration-dependent manner. Our study indicated that NF-κB, JNK, and ERK signaling pathways may be related to the pathogenesis of KD. This provides a new idea on the study of the pathogenesis of KD. This can be further confirmed in animal and clinical studies. Sal B, a bioactive compound of S. miltiorrhizae, had a regulatory effect on TNF-α-induced MMP-9 expression via NF-κB, JNK, and ERK signaling pathways. Our findings provide evidence that Sal B may be an alternative agent for the treatment of KD that does not respond to IVIG therapy, which have potential clinical significance. Of course, this requires further confirmation.
In conclusion, our data showed that Sal B reduced MMP-9 expression and activity through inhibiting NF-κB, JNK, and ERK activation. Our findings may provide insight into the pathogenesis of KD and suggests a novel adjunctive therapy for KD.
Financial support and sponsorship
This study was supported by the grants from National Natural Science Foundation of China (No. 81274109, 30973238), Key Research Project of Beijing Natural Science Foundation (B)/Beijing Education Committee (No. KZ201010025024), and Project for Science and Technology Innovation, Beijing Education Committee (No. PXM2011_014226_07_000085).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Medjaden and Editage for its linguistic assistance during the preparation of this manuscript.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 2.Kawasaki T. Kawasaki disease. Int J Rheum Dis. 2014;17:597–600. doi: 10.1111/1756-185X.12408. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 4.Du ZD, Zhao D, Du J, Zhang YL, Lin Y, Liu C, et al. Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr Infect Dis J. 2007;26:449–51. doi: 10.1097/01.inf.0000261196.79223.18. [DOI] [PubMed] [Google Scholar]
- 5.Yeung RS. Lessons learned from an animal model of Kawasaki disease. Clin Exp Rheumatol. 2007;25(1 Suppl 44):S69–71. [PubMed] [Google Scholar]
- 6.Hui-Yuen JS, Duong TT, Yeung RS. TNF-alpha is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of Kawasaki disease. J Immunol. 2006;176:6294–301. doi: 10.4049/jimmunol.176.10.6294. [DOI] [PubMed] [Google Scholar]
- 7.Ichiyama T, Ueno Y, Isumi H, Niimi A, Matsubara T, Furukawa S. An immunoglobulin agent (IVIG) inhibits NF-kappaB activation in cultured endothelial cells of coronary arteries in vitro . Inflamm Res. 2004;53:253–6. doi: 10.1007/s00011-004-1255-3. [DOI] [PubMed] [Google Scholar]
- 8.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–27. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 9.Lau AC, Duong TT, Ito S, Yeung RS. Matrix metalloproteinase 9 activity leads to elastin breakdown in an animal model of Kawasaki disease. Arthritis Rheum. 2008;58:854–63. doi: 10.1002/art.23225. [DOI] [PubMed] [Google Scholar]
- 10.Lau AC, Rosenberg H, Duong TT, McCrindle BW, Yeung RS. Elastolytic matrix metalloproteinases and coronary outcome in children with Kawasaki disease. Pediatr Res. 2007;61:710–5. doi: 10.1203/pdr.0b013e318053418b. [DOI] [PubMed] [Google Scholar]
- 11.Shangguan W, Du Z, Yang H, Zhang Y, Song M, Dong W. Effects of intravenous immunoglobulin upon the overexpression and over-activation of nuclear factor-?B and matrix metalloproteinase-9 in murine model of Kawasaki disease (in Chinese) Nat Med J China. 2014;94:938–43. [PubMed] [Google Scholar]
- 12.Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci U S A. 2001;98:6476–81. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 2001;324:1633–9. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 14.Okada K, Hara J, Maki I, Miki K, Matsuzaki K, Matsuoka T, et al. Pulse methylprednisolone with gammaglobulin as an initial treatment for acute Kawasaki disease. Eur J Pediatr. 2009;168:181–5. doi: 10.1007/s00431-008-0727-9. [DOI] [PubMed] [Google Scholar]
- 15.Hu P, Liang QL, Luo GA, Zhao ZZ, Jiang ZH. Multi-component HPLC fingerprinting of radix Salviae miltiorrhizae and its LC-MS-MS identification. Chem Pharm Bull (Tokyo) 2005;53:677–83. doi: 10.1248/cpb.53.677. [DOI] [PubMed] [Google Scholar]
- 16.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008;117:280–95. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000;21:1089–94. [PubMed] [Google Scholar]
- 19.Zhou L, Zuo Z, Chow MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–59. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, et al. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem. 2001;82:512–21. doi: 10.1002/jcb.1176. [DOI] [PubMed] [Google Scholar]
- 21.Stumpf C, Fan Q, Hintermann C, Raaz D, Kurfürst I, Losert S, et al. Anti-inflammatory effects of danshen on human vascular endothelial cells in culture. Am J Chin Med. 2013;41:1065–77. doi: 10.1142/S0192415X13500729. [DOI] [PubMed] [Google Scholar]
- 22.Chen SC, Lin YL, Huang B, Wang DL, Cheng JJ. Salvianolic acid B suppresses IFN-γ-induced JAK/STAT1 activation in endothelial cells. Thromb Res. 2011;128:560–4. doi: 10.1016/j.thromres.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Chen YL, Hu CS, Lin FY, Chen YH, Sheu LM, Ku HH, et al. Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J Cell Biochem. 2006;98:618–31. doi: 10.1002/jcb.20793. [DOI] [PubMed] [Google Scholar]
- 24.Lin CC, Tseng HW, Hsieh HL, Lee CW, Wu CY, Cheng CY, et al. Tumor necrosis factor-alpha induces MMP-9 expression via p42/p44 MAPK, JNK, and nuclear factor-kappaB in A549 cells. Toxicol Appl Pharmacol. 2008;229:386–98. doi: 10.1016/j.taap.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Seok Yang W, Lee J, Woong Kim T, Hye Kim J, Lee S, Hee Rhee M, et al. Src/NF-κB-targeted inhibition of LPS-induced macrophage activation and dextran sodium sulphate-induced colitis by Archidendron clypearia methanol extract. J Ethnopharmacol. 2012;142:287–93. doi: 10.1016/j.jep.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Shen T, Aneas I, Sakabe N, Dirschinger RJ, Wang G, Smemo S, et al. Tbx20 regulates a genetic program essential to adult mouse cardiomyocyte function. J Clin Invest. 2011;121:4640–54. doi: 10.1172/JCI59472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son MB, Gauvreau K, Burns JC, Corinaldesi E, Tremoulet AH, Watson VE, et al. Infliximab for intravenous immunoglobulin resistance in Kawasaki disease: A retrospective study. J Pediatr. 2011;158:644–649.e1. doi: 10.1016/j.jpeds.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Ivanenkov YA, Balakin KV, Lavrovsky Y. Small molecule inhibitors of NF-kB and JAK/STAT signal transduction pathways as promising anti-inflammatory therapeutics. Mini Rev Med Chem. 2011;11:55–78. doi: 10.2174/138955711793564079. [DOI] [PubMed] [Google Scholar]


