Abstract
Background:
Intravoxel incoherent motion (IVIM) has the potential to provide both diffusion and perfusion information without an exogenous contrast agent, its application for the brain is promising, however, feasibility studies on this are relatively scarce. The aim of this study is to assess the feasibility of IVIM perfusion in patients with acute ischemic stroke (AIS).
Methods:
Patients with suspected AIS were examined by magnetic resonance imaging within 24 h of symptom onset. Fifteen patients (mean age was 68.7 ± 8.0 years) who underwent arterial spin labeling (ASL) and diffusion-weighted imaging (DWI) were identified as having AIS with ischemic penumbra were enrolled, where ischemic penumbra referred to the mismatch areas of ASL and DWI. Eleven different b-values were applied in the biexponential model. Regions of interest were selected in ischemic penumbras and contralateral normal brain regions. Fast apparent diffusion coefficients (ADCs) and ASL cerebral blood flow (CBF) were measured. The paired t-test was applied to compare ASL CBF, fast ADC, and slow ADC measurements between ischemic penumbras and contralateral normal brain regions. Linear regression and Pearson's correlation were used to evaluate the correlations among quantitative results.
Results:
The fast ADCs and ASL CBFs of ischemic penumbras were significantly lower than those of the contralateral normal brain regions (1.93 ± 0.78 μm2/ms vs. 3.97 ± 2.49 μm2/ms, P = 0.007; 13.5 ± 4.5 ml·100 g-1·min-1 vs. 29.1 ± 12.7 ml·100 g-1·min-1, P < 0.001, respectively). No significant difference was observed in slow ADCs between ischemic penumbras and contralateral normal brain regions (0.203 ± 0.090 μm2/ms vs. 0.198 ± 0.100 αμm2/ms, P = 0.451). Compared with contralateral normal brain regions, both CBFs and fast ADCs decreased in ischemic penumbras while slow ADCs remained the same. A significant correlation was detected between fast ADCs and ASL CBFs (r = 0.416, P < 0.05). No statistically significant correlation was observed between ASL CBFs and slow ADCs, or between fast ADCs and slow ADCs (r = 0.111, P = 0.558; r = 0.200, P = 0.289, respectively).
Conclusions:
The decrease in cerebral blood perfusion primarily results in the decrease in fast ADC in ischemic penumbras; therefore, fast ADC can reflect the perfusion situation in cerebral tissues.
Keywords: Arterial Spin Labeling, Cerebral Blood Flow, Intravoxel Incoherent Motion, Ischemic Penumbra
INTRODUCTION
Magnetic resonance imaging (MRI) is a sensitive tool by which to detect the perfusion abnormalities in patients with acute ischemic stroke (AIS). Diffusion-weighted imaging (DWI) is the most sensitive MRI technique by which to identify the acute ischemic core.[1] When used together, perfusion-weighted imaging (PWI) and DWI can identify brain tissue with hypoperfusion, which is defined as “ischemic penumbra,” from normal diffusion.[2] The role of perfusion neuroimaging in the management of AIS is to confirm the presence of reduced regional blood flow and contribute to the identification of the ischemic penumbra that might be salvaged by thrombolytic and/or endovascular recanalization therapy.[3]
Dynamic susceptibility contrast imaging, an MRI perfusion technique, is the most commonly used protocol in clinical practice. Moreover, arterial spin labeling (ASL) is a noninvasive alternative for imaging whole-brain cerebral perfusion that uses radiofrequency pulses instead of gadolinium to label inflowing arterial blood.[4] Recent studies demonstrate that ASL can be used to quantify cerebral blood flow (CBF) values in the ischemic core and areas with perfusion/diffusion mismatch in patients with AIS.[5,6,7,8] In this study, ischemic penumbra was defined by decreased ASL CBF area, except in the restricted diffusion area.
Intravoxel incoherent motion (IVIM) imaging was introduced by Le Bihan et al.[9] as a joint method by which to measure perfusion and diffusion. IVIM is an extension of the DWI that allows the simultaneous acquisition of both microcirculatory and diffusivity information. The capillary network lacks the spatial orientation, and the molecular motion within the microcirculation can be viewed as “pseudo-diffusion.” Perfusion indices are derived by modeling the signal attenuation as a composite outcome of interstitial water diffusion and intravascular capillary blood flow. IVIM imaging was assessed for measuring sensitivity in the rat brain[10] and has been applied in measuring sensitivity in several body organs.[11,12,13,14] Federau et al.[15] showed that IVIM can quantitatively measure perfusion in the human brain, however, feasibility studies on this are relatively scarce.
Because IVIM has the potential to provide both diffusion and perfusion information without an exogenous contrast agent, its application for the brain is promising. It might be used to detect ischemic penumbra in patients with AIS, identify the type of a cerebral tumor, classify the stage of a malignant tumor, or evaluate the effects of chemotherapy or radiotherapy in patients with a cerebral tumor.
To our knowledge, few studies have specifically validated the relationship between the perfusion index derived from IVIM (fast apparent diffusion coefficients [ADC]) and ASL CBF in the human brain. In this study, ASL is used to assess cerebral blood perfusion. Based on the above literature, we hypothesized that fast ADC would decrease and slow ADC would remain the same in the ischemic penumbra as compared with contralateral normal cerebral tissue. Moreover, a significant correlation would be found between fast ADC and ASL CBF.
METHODS
Patient selection
All tests were approved by our Institutional Review Board, and informed consent was obtained from all participants. The analysis was performed on data collected from August 2012 to July 2014 in an ongoing prospective registry of patients who were evaluated using diffusion-perfusion MRI at our medical center. Image data were included in this study if: (1) The patient presented with symptoms of AIS; (2) Cerebral hemorrhage was excluded by CT examination; (3) Baseline MRI was performed within 6 h of symptom onset in patients with contraindication for intravenous thrombolysis, or > 6 h but within 24 h; (4) Both ASLs and multi b-value DWIs were performed; (5) Obvious ischemic penumbra was observed; the area of decreased ASL CBF was 1.5 times larger than the area of the restricted diffusion.
During the 24-month study, 102 consecutive suspected AIS patients had a baseline MRI that included ASL and multi b-value DWI sequences, 15 of whom met the inclusion and exclusion criteria. The mean age was 68.7 ± 8.0 years; 11 were males. The median time from symptom onset to the MRI examination was 11.8 ± 7.0 h.
Magnetic resonance imaging protocols
All examinations were performed on a discovery MR750 3.0 T whole-body MR scanner (Milwaukee, Wisconsin, USA) with an 8-channel head coil. The imaging protocols included an axial T2 propeller, axial T2 flair, axial T1 flair, axial multi b-value DWI, sagittal T1 flair, and three-dimensional (3D) ASL.
Pseudocontinuous 3D ASL was performed using an interleaved 3D stack of spiral fast-spin echo sequence with background suppression. The labeling period was 1500 ms with a postlabeling delay time of 1525 ms. Multiarm spiral imaging was used, with 8 arms and 512 points acquired on each arm. A high level of background suppression was achieved by using 4 separate inversion pulses spaced around the pseudocontinuous labeling pulse. The entire process took 269 s to complete, which included proton attenuation. Other 3D ASL parameters were as follows: 36 axial slices; repetition time (TR), 4632 ms; echo time (TE), 10.5 ms; slice thickness 4.0 mm; field-of-view (FOV), 24 cm × 24 cm; number of excitation (NEX), 3, and bandwidth, 62.5 kHz. Axial multi b-value DWIs were performed using single-shot spin-echo echo planar sequences with the following parameters: TR, 4000 ms; TE, Min; b-value and NEX, 0/2, 50/2, 100/2, 200/2, 400/2, 600/2, 800/2, 1000/3, 2000/3, 3000/4, 4000/4 s/mm2; slice thickness, 5.0 mm; slice spacing, 1.5 mm; and FOV, 24 cm × 24 cm. The diffusion gradient strengths were applied along the X, Y, and Z axes. The duration of the sequence was 320 s.
Axial T2 propeller parameters were as follows: TR, 4242 ms; TE, 93 ms; slice thickness, 5.0 mm; slice spacing, 1.5 mm; FOV, 24 cm × 24 cm; and NEX, 1.5. Axial T2 flair parameters were as follows: TR, 8500 ms; TE, 162 ms; TI, 2100 ms; slice thickness, 5.0 mm; slice spacing, 1.5 mm; FOV, 24 cm × 24 cm; and NEX, 1. Axial T1 flair parameters were as follows: TR, 1750 ms; TE, 24 ms; TI, 780 ms; slice thickness, 5.0 mm; slice spacing, 1.5 mm; FOV, 24 cm × 24 cm; and NEX, 1. Sagittal T1 flair parameters were as follows: TR, 1941 ms; TE, 24 ms; TI, 681 ms; slice thickness, 5.0 mm; slice spacing, 1.5 mm; FOV, 24 cm × 24 cm; and NEX, 1.
Postprocessing and evaluation
Data postprocessing was performed on the GE 4.5 Workstation. Regions of interest (ROIs) were delineated as large as possible in the area of ischemic penumbra and the contralateral normal brain tissue of the same size. ROIs were located in the plane of ischemic penumbra within a maximum area, which avoided the sulcus. Sagittal T1 flair was used as a cross reference to ensure that the ROIs of DWIs and ASL CBF were located at nearly the same position. Fast ADCs and slow ADCs were quantitatively measured from multi b-value DWIs applied to the biexponential model; CBFs were quantitatively measured from the 3D ASL [Figure 1]. The biexponential model fits the following equation:
Figure 1.
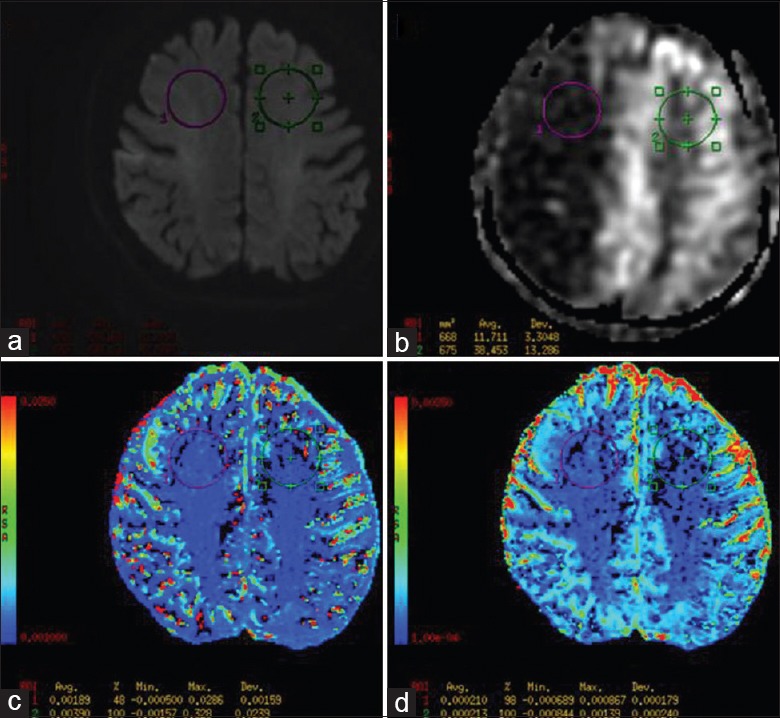
A 72-year-old man presented with left-sided weakness and slurred speech for 6.0 h. Magnetic resonance imaging scan showed acute ischemia stroke with ischemic penumbra. (a-d) The diffusion-weighted imaging, arterial spin labeling cerebral blood flow map, fast apparent diffusion coefficient map and slow apparent diffusion coefficient map in the area of ischemic penumbra respectively.
M/M0 = Pfast × exp (−b × fast ADC) + Pslow × exp (−b × slow ADC).
where M is the signal in the presence of diffusion sensitization, M0 is that in the absence of diffusion sensitization, fast ADC and slow ADC are ADC values, and Pfast and Pslow are the percentages of contributions to the signal from the fast- and slow-diffusing water compartments (Pfast = 1 − Pslow).[16]
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA). A paired t-test was applied to compare the differences of the ASL CBF, fast ADC, and slow ADC measurements between ischemic penumbras and contralateral normal brain regions. Linear regression and Pearson's correlation were used to evaluate the correlations among ASL CBF, fast ADC, and slow ADC measurements in all selected ROIs (both ischemic penumbras and contralateral normal brain regions). The significance level was defined as P < 0.05 (two-tailed). Values are expressed as the mean ± standard deviation (SD).
RESULTS
Fast ADC, slow ADC and ASL CBF measurements of ischemic penumbras and contralateral normal brain regions are presented in Table 1. The ROI area is 197 mm ± 160 mm. Fast ADCs and ASL CBFs of ischemic penumbras were statistically significantly lower (P < 0.05) than those of the contralateral normal brain regions. No statistically significant difference (P > 0.05) was observed in slow ADCs between ischemic penumbras and contralateral normal brain regions. Compared with contralateral normal brain regions, both fast ADCs and ASL CBFs decreased in ischemic penumbras while slow ADCs remained the same.
Table 1.
Biexponential parameters and ASL CBF in patients with acute stroke and statistical analysis (n = 15)
| Parameters | Ischemia penumbras | Contralateral normal brain | t | P |
|---|---|---|---|---|
| Fast ADC (μm2/ms) | 1.93 ± 0.78 | 3.97 ± 2.49 | −3.147 | 0.007* |
| Slow ADC (μm2/ms) | 0.203 ± 0.090 | 0.198 ± 0.100 | 0.776 | 0.451 |
| ASL CBF | 13.5 ± 4.5 | 29.1 ± 12.7 | −5.777 | <0.001* |
| (ml·100 g−1·min−1) |
Values are presented as mean ± SD. *The significance level was defined as P < 0.05. ADC: Apparent diffusion coefficient; SD: Standard deviation; ASL CBF: Arterial spin labeling cerebral blood flow.
The results of Pearson's correlation are presented in Table 2. A significant correlation (r = 0.416, P < 0.05) was detected between fast ADC and ASL CBF [Figure 2]. No statistically significant correlation was observed between ASL CBF and slow ADC, or between fast ADC and slow ADC.
Table 2.
The results of Pearson's correlation between measurements
| Parameters | r | P | n |
|---|---|---|---|
| Fast ADC and ASL CBF | 0.416 | 0.022* | 30 |
| Slow ADC and ASL CBF | 0.111 | 0.558 | 30 |
| Fast ADC and slow ADC | 0.200 | 0.289 | 30 |
*The significance level was defined as P < 0.05. ADC: Apparent diffusion coefficient; ASL CBF: Arterial spin labeling cerebral blood flow.
Figure 2.

The scatter plot of fast apparent diffusion coefficient (fast ADC) and arterial spin labeling cerebral blood flow (CBF) (r = 0.416, P < 0.05) (n = 30).
DISCUSSION
In the present study, fast ADCs derived from multi b-value DWIs applied to the biexponential model can reflect cerebral perfusion. To our knowledge, few studies had combined multi b-value DWIs and ASL techniques in the evaluation of the human brain. Although diffusion imaging has proved to be largely useful in a wide variety of clinical applications, as well as in more advanced applications, such as diffusion tensor imaging and tractography, perfusion measurement with IVIM is not common because of its low signal-to-noise ratio,[17] with the blood volume in the brain estimated to be in the low single-digit percentage range.[15,18] Recently, with the continuous upgrading of MRI hardware and software, the biexponential model is promising for application in multi b-value DWIs of the central nervous system.
ASL is a noncontrast perfusion imaging method that relies on the magnetic labeling of arterial water.[19,20] Radiofrequency pulses are used to magnetically alter the status of water protons in arterial blood with respect to those in stationary tissue, thereby generating an endogenous intravascular tracer.[20,21] Presuming that the magnetically labeled arterial blood exchanges with tissue water at the capillary level, CBF can, in principle, be quantified from a labeling experiment and a control experiment without magnetically labeled water protons, based on the theory of diffusible tracer kinetics.[20,22,23] Recent studies have demonstrated that ASL can be used to quantify CBF values in the ischemic core and ischemic penumbra in patients with AIS.[5,6,7,8]
The ischemic penumbra is functionally impaired, yet still viable, tissue surrounding the ischemic core.[24,25] Several studies have indicated that for the purpose of defining the tissue at risk of infarction, the ischemic penumbra can be operationally defined as the mismatch between the lesion volumes detected with PWI and that detected with DWI.[3,26] With PWI, perfusion decreases in the ischemic penumbra but is normal in DWI. In this study, compared with contralateral normal brain regions, both ASL CBFs and fast ADCs decreased in ischemic penumbras, but slow ADC remained the same, which was in accord with the perfusion and diffusion situation of the ischemic penumbras and contralateral normal brain regions. The cerebral perfusion and fast ADC decreased in the ischemic penumbra, which inferred that the fast ADC might reflect the perfusion in cerebral tissue. In addition, a significant correlation was detected between fast ADCs and ASL CBFs, which indicates that fast ADCs could reflect the perfusion in cerebral tissue. This is an important step in confirming the feasibility of fast ADC as a quantitative index to measure brain perfusion.
IVIM[27] is a term that designates the microscopic translational motions that occur in each image voxel in MRI. In biological tissues, these motions include molecular diffusion of water and microcirculation of blood in the capillary network. Molecular diffusion results from random micromolecular motion called “Brownian motion.” Perfusion results from blood microcirculation in the capillary network. Perfusion can be considered an incoherent motion resulting from the pseudorandom orientation of capillaries at the voxel level. In other words, it assumes an isotropic and sufficiently randomly laid microvasculature network so that water molecules in the blood experience “many random” direction changes during the motion-sensitizing period of the diffusion sequence. Under these assumptions, a so-called pseudo-diffusion (fast ADC), which describes macroscopically the incoherent movement of blood in the microvasculature compartment, can be measured with a standard diffusion sequence if an adequate set of parameters is used. A biexponential model is used to extract perfusion parameters from multi b-value DWIs. A linear relationship between IVIM perfusion parameters and classic perfusion parameters was derived under given assumptions by Le Bihan et al.[28] In brief, a fast ADC is inversely proportional to mean transit time (MTT), which is consistent with the result that fast ADCs decreased in ischemic penumbra in this study. Because CBF is inversely proportional to MTT, a fast ADC is proportional to CBF, which explains the reason for the significant correlation detected between fast ADCs and ASL CBFs in this study.
Although the results of the data from this study are exciting and encouraging, several limitations should be considered. First, the cohort (15 patients) was small, and the study was performed at a single center. Second, no CBF threshold other than visual assessment was used to evaluate the penumbra in ASL perfusion. Third, ASL perfusion might overestimate the perfusion defects[6,29] and areas of benign oligemia might be included in the penumbra. Fourth, lacking suitable postprocess software to accurately fuse multi b-value DWIs and ASLs, ROIs, rather than the entire ischemic penumbra, were part of the ischemic penumbras.
In conclusion, we present our initial clinical experience with multi b-value DWIs of the brain, which demonstrate that fast ADCs have a significant correlation with ASL CBFs. Although the advantage and disadvantage of fast ADCs as they compare to ASL CBFs must be studied further, fast ADCs derived from multi b-value DWIs applied to a biexponential model supply another noninvasive method by which to create an image of brain cerebral perfusion. Moreover, multi b-value DWIs can provide both diffusion and perfusion information. The present study represents an initial step in exploring the clinical value of applying multi b-value DWIs to the cerebral tissue.
Financial support and sponsorship
This work was supported by Key Projects in the National Science and Technology Pillar Program during the 12th 5. year Plan Period of China (No. 2011BAI08B10).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang and Xiu-Yuan Hao
REFERENCES
- 1.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: A preliminary analysis. J Cereb Blood Flow Metab. 1996;16:53–9. doi: 10.1097/00004647-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Kidwell CS, Alger JR, Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2004;35(11 Suppl 1):2662–5. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 3.Schlaug G, Benfield A, Baird AE, Siewert B, Lövblad KO, Parker RA, et al. The ischemic penumbra: Operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–37. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 4.Niibo T, Ohta H, Yonenaga K, Ikushima I, Miyata S, Takeshima H. Arterial spin-labeled perfusion imaging to predict mismatch in acute ischemic stroke. Stroke. 2013;44:2601–3. doi: 10.1161/STROKEAHA.113.002097. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez DA, Bokkers RP, Mirasol RV, Luby M, Henning EC, Merino JG, et al. Pseudocontinuous arterial spin labeling quantifies relative cerebral blood flow in acute stroke. Stroke. 2012;43:753–8. doi: 10.1161/STROKEAHA.111.635979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YC, Liu HL, Lee JD, Yang JT, Weng HH, Lee M, et al. Comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MRI in patients with acute stroke. PLoS One. 2013;8:e69085. doi: 10.1371/journal.pone.0069085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang DJ, Alger JR, Qiao JX, Hao Q, Hou S, Fiaz R, et al. The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: Comparison with dynamic susceptibility contrast-enhanced MRI. Stroke. 2012;43:1018–24. doi: 10.1161/STROKEAHA.111.631929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokkers RP, Hernandez DA, Merino JG, Mirasol RV, van Osch MJ, Hendrikse J, et al. Whole-brain arterial spin labeling perfusion MRI in patients with acute stroke. Stroke. 2012;43:1290–4. doi: 10.1161/STROKEAHA.110.589234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 10.Neil JJ, Bosch CS, Ackerman JJ. An evaluation of the sensitivity of the intravoxel incoherent motion (IVIM) method of blood flow measurement to changes in cerebral blood flow. Magn Reson Med. 1994;32:60–5. doi: 10.1002/mrm.1910320109. [DOI] [PubMed] [Google Scholar]
- 11.Sigmund EE, Cho GY, Kim S, Finn M, Moccaldi M, Jensen JH, et al. Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magn Reson Med. 2011;65:1437–47. doi: 10.1002/mrm.22740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klauss M, Gaida MM, Lemke A, Grünberg K, Simon D, Wente MN, et al. Fibrosis and pancreatic lesions: Counterintuitive behavior of the diffusion imaging-derived structural diffusion coefficient d. Invest Radiol. 2013;48:129–33. doi: 10.1097/RLI.0b013e31827ac0f1. [DOI] [PubMed] [Google Scholar]
- 13.Luciani A, Vignaud A, Cavet M, Nhieu JT, Mallat A, Ruel L, et al. Liver cirrhosis: Intravoxel incoherent motion MR imaging – Pilot study. Radiology. 2008;249:891–9. doi: 10.1148/radiol.2493080080. [DOI] [PubMed] [Google Scholar]
- 14.Sumi M, Van Cauteren M, Sumi T, Obara M, Ichikawa Y, Nakamura T. Salivary gland tumors: Use of intravoxel incoherent motion MR imaging for assessment of diffusion and perfusion for the differentiation of benign from malignant tumors. Radiology. 2012;263:770–7. doi: 10.1148/radiol.12111248. [DOI] [PubMed] [Google Scholar]
- 15.Federau C, Maeder P, O’Brien K, Browaeys P, Meuli R, Hagmann P. Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology. 2012;265:874–81. doi: 10.1148/radiol.12120584. [DOI] [PubMed] [Google Scholar]
- 16.Steier R, Aradi M, Pál J, Perlaki G, Orsi G, Bogner P, et al. A biexponential DWI study in rat brain intracellular oedema. Eur J Radiol. 2012;81:1758–65. doi: 10.1016/j.ejrad.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 17.Pekar J, Moonen CT, van Zijl PC. On the precision of diffusion/perfusion imaging by gradient sensitization. Magn Reson Med. 1992;23:122–9. doi: 10.1002/mrm.1910230113. [DOI] [PubMed] [Google Scholar]
- 18.Kim T, Kim SG. Quantification of cerebral arterial blood volume and cerebral blood flow using MRI with modulation of tissue and vessel (MOTIVE) signals. Magn Reson Med. 2005;54:333–42. doi: 10.1002/mrm.20550. [DOI] [PubMed] [Google Scholar]
- 19.Dixon WT, Du LN, Faul DD, Gado M, Rossnick S. Projection angiograms of blood labeled by adiabatic fast passage. Magn Reson Med. 1986;3:454–62. doi: 10.1002/mrm.1910030311. [DOI] [PubMed] [Google Scholar]
- 20.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 21.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89:212–6. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calamante F, Williams SR, van Bruggen N, Kwong KK, Turner R. A model for quantification of perfusion in pulsed labelling techniques. NMR Biomed. 1996;9:79–83. doi: 10.1002/(SICI)1099-1492(199604)9:2<79::AID-NBM399>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383–96. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 24.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12:723–5. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 25.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–65. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 26.Sunshine JL, Tarr RW, Lanzieri CF, Landis DM, Selman WR, Lewin JS. Hyperacute stroke: Ultrafast MR imaging to triage patients prior to therapy. Radiology. 1999;212:325–32. doi: 10.1148/radiology.212.2.r99au52325. [DOI] [PubMed] [Google Scholar]
- 27.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 28.Le Bihan D, Turner R. The capillary network: A link between IVIM and classical perfusion. Magn Reson Med. 1992;27:171–8. doi: 10.1002/mrm.1910270116. [DOI] [PubMed] [Google Scholar]
- 29.Nael K, Meshksar A, Liebeskind DS, Coull BM, Krupinski EA, Villablanca JP. Quantitative analysis of hypoperfusion in acute stroke: Arterial spin labeling versus dynamic susceptibility contrast. Stroke. 2013;44:3090–6. doi: 10.1161/STROKEAHA.113.002377. [DOI] [PMC free article] [PubMed] [Google Scholar]


