Abstract
Background:
Pulmonary alveolar proteinosis (PAP) is a rare lung disease, the most common type of which is autoimmune PAP. The gold standard therapy for PAP is whole lung lavage (WLL). Few studies have reported the optimal technique with which to evaluate the response to WLL. In this study, we aimed to identify parameters with which to assess the need for repeat WLL during a long-term 8-year follow-up.
Methods:
We conducted a retrospective analysis of 120 patients with autoimmune PAP with 80 of whom underwent WLL. Physiologic, serologic, and radiologic features of the patients were analyzed during an 8-year follow-up after the first WLL treatment.
Results:
Of the 40 patients without any intervention, 39 patients either achieved remission or remained stable and only one died of pulmonary infection. Of the 56 patients who underwent WLL for 1 time, 55 remained free from a second WLL and 1 patient died of cancer. Twenty-four required additional treatments after their first WLL. The baseline PaO2 (P = 0.000), PA-aO2 (P = 0.000), shunt fraction rate (P = 0.001), percent of predicted normal diffusing capacity of the lung for carbon monoxide (DLCO%Pred) (P = 0.016), 6-min walk test (P = 0.013), carcinoembryonic antigen (CEA) (P = 0.007), and neuron-specific enolase (NSE) (P = 0.003) showed significant differences among the three groups. The need for a second WLL was significantly associated with PaO2 (P = 0.000), CEA (P = 0.050), the 6-minute walk test (P = 0.026), and DLCO%Pred (P = 0.041). The DLCO%Pred on admission with a cut-off value of 42.1% (P = 0.001) may help to distinguish whether patients with PAP require a second WLL.
Conclusions:
WLL is the optimal treatment method for PAP and provides remarkable improvements for affected patients. The DLCO%Pred on admission with a cut-off value of 42.1% may distinguish whether patients with PAP require a second WLL.
Keywords: Diffusing Capacity of the Lung for Carbon Monoxide, Prognosis, Pulmonary Alveolar Proteinosis, Treatment, Whole Lung Lavage
INTRODUCTION
Pulmonary alveolar proteinosis (PAP) is a rare lung disease characterized by the accumulation of massive quantities of phospholipid, protein, and other amorphous periodic acid-Schiff (PAS)-positive materials within the alveolar spaces.[1] PAP causes restrictive ventilatory defects and decreased diffusion capacity.[2] There are three clinical categories of this disorder: Primary (autoimmune or idiopathic), secondary, and hereditary.[3] Although various treatments have been investigated, including granulocyte-macrophage colony-stimulating factor (GM-CSF), rituximab, plasmapheresis, stem cell transplantation, pulmonary transplantation of macrophage progenitors, marrow transplantation, and lung transplantation surgery, the current gold standard therapy for PAP is whole lung lavage (WLL).[4] Studies of the clinical characteristics and treatment options for PAP have already reported in other countries.[5,6,7,8,9] However, few studies have provided information on PAP therapy in Chinese patients. In this study, we evaluated a cohort of 120 Chinese patients with PAP to identify parameters with which to assess the responsiveness to WLL therapy at a long-term follow-up of 8 years.
METHODS
Study population
The diagnosis of PAP was based on the typical “crazy paving pattern” on chest high-resolution computed tomography (HRCT) images, milky bronchoalveolar lavage fluid (BALF), and the presence of amorphous PAS-positive material in the BALF or lung biopsy tissue specimens.[2,10] Patients with characteristic chest HRCT findings and confirmed histologic findings in the BALF or biopsy specimens were included. Patients aged <18 years or with confirmed secondary conditions such as silicosis and other inhalational syndromes, autoimmune diseases, malignancies, and hematopoietic disorders were excluded.
In total, 120 patients with autoimmune PAP hospitalized from January 1, 1990 to January 1, 2014 at our hospital in China were enrolled in this retrospective study. The patients information was recorded from their medical records. We contacted the patients by telephone to ascertain the follow-up information and outcomes. This study was approved by the review board of our hospital.
Assessment
Arterial blood gas analysis (including the partial pressure of arterial oxygen [PaO2], alveolar-arterial oxygen gradient [PA-aO2], and shunt fraction rate) and measurements of various serum biochemical markers (lactate dehydrogenase [LDH], carcinoembryonic antigen [CEA], neuron specific enolase [NSE], total cholesterol [TC], and triglycerides [TGs]) were routinely performed in the clinical chemistry laboratories according to internal standard operative procedures. Pulmonary function testing was performed in accordance with the American Thoracic Society and European Respiratory Society standards and included the following parameters: Percent of predicted normal forced vital capacity (FVC%Pred), percent of predicted normal forced expiratory volume in 1 s (FEV1%Pred), FEV1/FVC%Pred ratio, percent of predicted normal total lung capacity (TLC%Pred), and percent of predicted normal diffusing capacity of the lung for carbon monoxide (DLCO%Pred). We objectively assessed patients’ exercise capacity by performing the 6-min walk test according to the criteria in the American Thoracic Society statement on the 6-min walk test.[11] Chest HRCT was conducted in all patients, and the findings were downloaded from our hospital's image bank. Two radiological specialists reached a consensus on the HRCT imaging findings.
Management
Close observation without intervention was recommended for patients with mild and moderate symptoms, as reported in most studies.[2] We conducted WLL following specific criteria, including a resting PaO2 <65 mmHg (1 mmHg = 0.133 KPa), PA-aO2 >40 mmHg, or shunt fraction >10%.[12] An additional WLL was conducted when a patient exhibited worsening symptoms or progressive respiratory failure (>10 mmHg decrease in PaO2 or need for oxygen treatment or exercise desaturation) or chest images showed a worsening of previous findings or the appearance of new infiltrates characteristics of PAP. A stable condition was defined as the absence of new PAP symptoms, or no worsening of the previous symptoms and no new radiological infiltrates after treatment or spontaneously. Remission was defined as clinical improvement during the follow-up period, while for the patients who accepted WLL, which was particularly defined as without additional therapy after the first WLL. The duration of remission for the patients who accepted WLL was defined as the period of time from the initial WLL to the second WLL.
Statistical analysis
Quantitative variables are expressed as the mean value and standard deviation (SD) or the median value and interquartile range (IQR). The Kolmogorov–Smirnov test was used to test the normal distribution of quantitative variables. Qualitative variables are summarized as counts and percentages. The paired t-test was used to compare the data before and after the WLL. Multiple comparison analysis of variance and the Kruskal–Wallis test were performed to investigate differences in parameters among the three groups according to WLL times. The correlations between DLCO%Pred and PaO2, CEA, and NSE were assessed by Pearson correlation analysis. Cox regression analysis was used to distinguish patients with the need for repeated WLL by univariate and multivariate analyses. Receiver operating characteristic curve analysis was used to determine the most suitable cut-off value of DLCO%Pred. The Kaplan–Meier method with log-rank test was employed to compare the difference in the cumulative rate of patients who did not require repeated WLL between the high and low DLCO%Pred groups. A two-sided P < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS version 19 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Clinical features and examination results
In total, 120 patients with autoimmune PAP were identified (88 male, 32 female). Their age (mean ± SD) was 43 ± 11 years. Fifty-seven patients (47.5%) had a history of smoking. The most common symptoms at presentation were dyspnea (75.8%) and cough (70.8%). Other symptoms included expectoration (54.2%), fever (21.7%), chest distress (15.8%), chest pain (9.2%), and fatigue (1.7%). Ground glass opacification (91.7%) was the most common finding in chest HRCT among the 120 patients with PAP, followed by the crazy paving pattern (75.0%). Less commonly, reticular interstitial opacity (25.0%), patching (17.5%), and consolidation (7.5%) were seen. The mean FVC%Pred was 77.3% ± 18.4%, and the mean TLC%Pred was 79.0% ± 12.0%. The mean DLCO%Pred was 57.2% ± 19.6%. The average value of the 6-min walk test was 490.9 ± 131.5 m. The patients were mildly hypoxemic with a mean PaO2 of 64.5 ± 13.8 mmHg and a median PA-aO2 of 41.4 mmHg (IQR, 32.6–54.2 mmHg). The serum LDH level was mildly elevated, with a median value of 269 IU/L (IQR, 213–390 IU/L). The cancer biomarkers CEA and NSE were also elevated, as shown in Table 1.
Table 1.
Clinical features of 120 Chinese PAP patients
| Characteristics | n | Baseline |
|---|---|---|
| Age (year, mean ± SD) | 120 | 43 ± 11 |
| Pulmonary function test (%, mean ± SD) | ||
| FVC%Pred | 92 | 77.3 ± 18.4 |
| FEV1%Pred | 92 | 76.8 ± 17.5 |
| FEV1/FVC%Pred | 92 | 85.8 ± 9.4 |
| DLCO%Pred | 92 | 57.2 ± 19.6 |
| TLC%Pred | 92 | 79.0 ± 12.0 |
| 6-min walk test (m, mean ± SD) | 81 | 490.9 ± 131.5 |
| Arterial blood gas | ||
| PaO2 (mmHg, mean ± SD) | 106 | 64.5 ± 13.8 |
| PA-aO2 (mmHg, median) | 106 | 41.4 (32.6–54.2)* |
| Shunt fraction rate (mean ± SD) | 106 | 21.2 ± 13.0 |
| Serum biochemical markers | ||
| LDH (IU/L, median) | 99 | 269 (213–390)* |
| CEA (ng/ml, median) | 82 | 6.8 (4.4–15.0)* |
| NSE (ng/ml, mean ± SD) | 89 | 12.6 ± 7.1 |
| TC (mmol/L, mean ± SD) | 93 | 5.3 ± 1.3 |
| TG (mmol/L, median) | 93 | 1.8 (1.3–2.4) |
*Range. SD: Standard deviation; PAP: Pulmonary alveolar proteinosis; %Pred: Percentage of the predicted value; FVC: Forced vital capacity; FEV1: Forced expiratory volume in one second; DLCO: Diffusing capacity of the lung for carbon monoxide; TLC: Total lung capacity; PaO2: Partial pressure of oxygen in arterial blood; PA-aO2: Alveolar-arterial oxygen gradient; LDH: Lactate dehydrogenase; CEA: Carcinoembryonic antigen; NSE: Neuron-specific enolase; TC: Cholesterol; TG: Triglyceride. 1 mmHg = 0.133 KPa.
Diagnostic methods
In this study, the median time from the onset of symptoms to diagnosis was 9.5 months. BALF analysis was the most frequently applied diagnostic method in 102 (85.0%) patients; it was used alone in 37 (36.3%) patients and combined with transbronchial lung biopsy in 65 (63.7%) patients. Surgical lung biopsy was conducted in 9 patients. Computed tomography guided percutaneous lung biopsy and video assisted thoracic surgery were used for diagnosis in seven and 2 patients, respectively.
Patients’ outcomes
According to therapeutic criteria, 56 of 120 patients with PAP (46.7%) underwent a single WLL, 24 (20%) underwent multiple WLL, and 40 (33.3%) underwent surveillance without any intervention. None of the 80 patients developed severe complications after WLL. During the 8.6-year follow-up period, 1 patient among those who did not undergo WLL died of severe pulmonary infection and respiratory failure 21 days after diagnosis. Eleven patients underwent spontaneous remission, and the remaining 28 patients (no intervention) were in stable condition. Only one patient among those who underwent a single WLL died of lung cancer about 3 years after the diagnosis of PAP, the other 55 patients who underwent a single WLL remained in remission. Of the 24 patients who underwent multiple WLL, 13 (54%) were in stable condition, 6 (25%) underwent remission, and the remaining 5 (21%) had developed progression at the end of the follow-up period.
Comparison of baseline parameters among the three groups
We compared the baseline parameters among the three groups according to the WLL times. The arterial blood gas analysis results, DLCO%Pred (P = 0.016), 6-min walk test (P = 0.013), CEA (P = 0.007), and NSE (P = 0.003) showed statistically significant differences among the three groups. Although LDH, TGs, and TC were elevated, the differences in these parameters among the three groups failed to reach statistical significance [Table 2]. There was no correlation between DLCO%Pred and age (P = 0.316), sex (P = 0.240), smoking history (P = 0.551) or 6-min walk test (P = 0.363), but correlations were found between DLCO%Pred and PaO2 (P = 0.023), CEA (P = 0.019), and NSE (P = 0.005).
Table 2.
Analysis of baseline parameters among PAP patients according to the WLL times
| Characteristics | WLL 0 | WLL 1 | WLL > 1 | F | P |
|---|---|---|---|---|---|
| (n = 40) | (n = 56) | (n = 24) | |||
| Pulmonary function test | |||||
| FVC%Pred (%, mean ± SD) | 79.3 ± 18.6 | 80.2 ± 18.0 | 68.9 ± 17.2 | 2.119 | 0.129 |
| FEV1%Pred (%, mean ± SD) | 78.0 ± 17.9 | 80.2 ± 16.7 | 69.1 ± 16.9 | 2.238 | 0.115 |
| FEV1/FVC%Pred (%, mean ± SD) | 83.5 ± 11.7 | 86.5 ± 9.0 | 87.9 ± 6.0 | 1.076 | 0.347 |
| DLCO%Pred (%, mean ± SD) | 64.0 ± 19.8 | 57.7 ± 16.8 | 45.7 ± 20.3 | 4.400 | 0.016 |
| TLC%Pred (%, mean ± SD) | 80.6 ± 13.1 | 80.5 ± 10.8 | 73.2 ± 12.2 | 1.984 | 0.647 |
| 6-min walk test (m, mean ± SD) | 547.0 ± 139.4 | 495.1 ± 60.6 | 389.4 ± 166.1 | 4.834 | 0.013 |
| Arterial blood gas | |||||
| PaO2 (mmHg, mean ± SD) | 72.2 ± 13.2 | 62.4 ± 13.2 | 58.1 ± 11.2 | 9.414 | 0.000 |
| PA-aO2 (mmHg, median) | 33.0 (24.9–39.2)* | 47.8 (37.2–60.5)* | 49.4 (38.8–57.6)* | NA | 0.000 |
| Shunt fraction rate (mean ± SD) | 14.1 ± 9.3 | 24.3 ± 13.5 | 26.9 ± 12.9 | 7.482 | 0.001 |
| Serum biochemical markers | |||||
| LDH (IU/L, median) | 241 (214–337)* | 279 (210–383)* | 338 (214–461)* | NA | 0.383 |
| CEA (ng/ml, median) | 4.3 (3.0–6.6)* | 7.6 (5.1–15.2)* | 11.5 (8.3–30.6)* | NA | 0.007 |
| NSE (ng/ml, mean ± SD) | 9.4 ± 4.2 | 12.9 ± 5.9 | 19.2 ± 9.7 | 6.811 | 0.003 |
| TC (mmol/L, mean ± SD) | 5.1 ± 1.6 | 5.4 ± 1.1 | 5.7 ± 1.1 | 1.006 | 0.371 |
| TG (mmol/L, mean ± SD) | 1.6 (1.0–2.5) | 1.8 (1.3–2.3) | 1.8 (1.4–3.1) | 0.005 | 0.659 |
P value was calculated by comparing the baseline parameters among the three groups according to the WLL times; *Range. PAP: Pulmonary alveolar proteinosis; WLL: Whole lung lavage; PaO2: Partial pressure of oxygen in arterial blood; PA-aO2: Alveolar-arterial O2 gradient; FVC: Forced vital capacity; %Pred: Percentage of the predicted value; FEV1: Forced expiratory volume in one second; DLCO: Diffusing capacity of the lung for carbon monoxide; TLC: Total lung capacity; LDH: Lactate dehydrogenase; CEA: Carcinoembryonic antigen; NSE: Neuron-specific enolase; TC: Cholesterol; TG: Triglyceride; SD: Standard deviation. 1 mmHg = 0.133 KPa.
Comparison of test results before and after whole lung lavage
We analyzed the serum biochemical markers, pulmonary function test results, and 6-min walk test results before and after WLL in the 80 patients who underwent WLL therapy. PaO2 (P = 0.000), FVC%Pred (P = 0.032), TLC%Pred (P = 0.020), DLCO%Pred (P = 0.001), and the 6-min walk test (P = 0.016) improved after WLL. In contrast, PA-aO2 (P = 0.002) and LDH (P = 0.000) decreased after WLL [Table 3].
Table 3.
Prelavage and postlavage parameters for 80 PAP patients accepted the WLL
| Characteristics | Before lavage | After lavage | t | P |
|---|---|---|---|---|
| Pulmonary function test | ||||
| FVC%Pred (%) | 77.8 ± 18.7 | 81.3 ± 15.3 | −2.263 | 0.032 |
| FEV1%Pred (%) | 78.8 ± 18.0 | 81.0 ± 14.1 | −1.276 | 0.213 |
| FEV1/FVC%Pred (%) | 86.4 ± 9.5 | 84.1 ± 6.2 | 1.913 | 0.067 |
| DLCO%Pred (%) | 56.2 ± 17.6 | 66.8 ± 22.7 | −3.612 | 0.001 |
| TLC%Pred (%) | 78.3 ± 11.2 | 83.6 ± 13.4 | −2.475 | 0.020 |
| 6-min walk test (m) | 445.0 ± 132.4 | 546.2 ± 88.1 | −2.681 | 0.016 |
| Arterial blood gas | ||||
| PaO2 (mmHg) | 59.0 ± 12.0 | 68.2 ± 12.9 | −4.713 | 0.000 |
| PA-aO2 (mmHg) | 73.3 ± 88.6 | 52.6 ± 43.5 | 2.526 | 0.002 |
| Shunt fraction rate | 27.4 ± 13.2 | 19.4 ± 10.9 | 3.280 | 0.002 |
| Serum biochemical markers | ||||
| LDH (IU/L) | 339.7 ± 119.0 | 250.3 ± 75.5 | 5.371 | 0.000 |
| CEA (ng/ml) | 18.4 ± 13.3 | 16.1 ± 14.0 | 0.632 | 0.551 |
| NSE (ng/ml) | 16.2 ± 6.0 | 11.7 ± 9.3 | 1.989 | 0.118 |
| TC (mmol/L) | 5.5 ± 1.3 | 4.8 ± 1.0 | 1.906 | 0.081 |
| TG (mmol/L) | 2.8 ± 3.5 | 1.6 ± 0.7 | 7.723 | 0.388 |
P value was calculated by comparing the parameters before and after WLL in the 80 patients who underwent WLL therapy. Data are presented as a mean ± standard deviation. PAP: Pulmonary alveolar proteinosis; WLL: Whole lung lavage; PaO2: Partial pressure of oxygen in arterial blood; PA-aO2: Alveolar-arterial oxygen gradient; FVC: Forced vital capacity; FEV1: Forced expiratory volume in one second; DLCO: Diffusing capacity of the lung for carbon monoxide; TLC: Total lung capacity; %Pred: Percentage of the predicted value; LDH: Lactate dehydrogenase; CEA: Carcinoembryonic antigen; NSE: Neuron-specific enolase; TC: Cholesterol; TG: Triglyceride. 1 mmHg = 0.133 KPa.
Predictive value of baseline percent of predicted normal diffusing capacity of the lung for carbon monoxide for the necessity of a second whole lung lavage
Comparison of the patients who underwent a single WLL and those who underwent multiple WLL therapies revealed significant differences in PaO2 (P = 0.000), DLCO%Pred (P = 0.041), the 6-min walk test (P = 0.026), and CEA (P = 0.050). When the DLCO%Pred cut-off level was set at 42.1%, the baseline DLCO%Pred predicted a second WLL with a sensitivity of 66.7% and specificity of 80.0% [Figure 1]. For Kaplan–Meier analysis of the time to a second WLL, we divided the patients into two groups: The low DLCO%Pred group (<42.1%) and the high DLCO%Pred group (>42.1%). A significant difference in the need for a second WLL was observed between the two groups (P = 0.001) when the entire follow-up period was compared [Figure 2].
Figure 1.
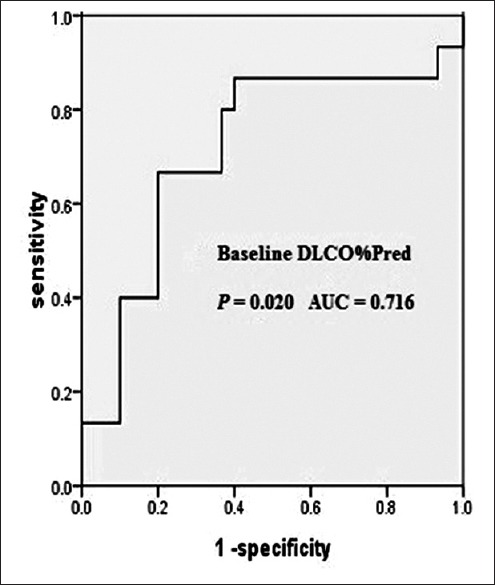
Receiver operating curve of DLCO%Pred. The baseline DLCO%Pred with the cut-off level of 42.1% could predict a second WLL with a sensitivity of 66.7% and specificity of 80.0%. WLL: Whole lung lavage; AUC: Area under the receiver operating curve; DLCO%Pred: Percent of predicted normal diffusing capacity of the lung for carbon monoxide.
Figure 2.
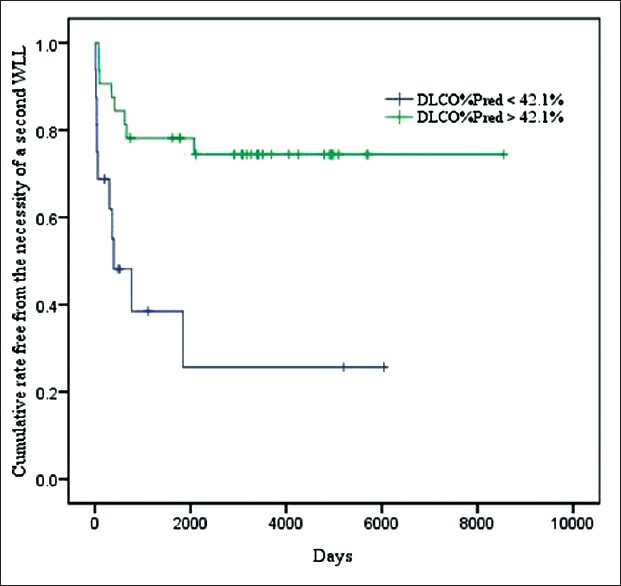
Kaplan–Meier plot showing patients between two groups. The green line shows the cumulative rate free from the necessity of a second WLL for the high DLCO%Pred (>42.1%) group, the blue line shows the cumulative rate free from the necessity of a second WLL for the low DLCO%Pred (<42.1%) group. The P value of the need for a second WLL between the two groups was 0.001. WLL: Whole lung lavage; AUC: Area under the receiver operating curve; DLCO%Pred: Percent of predicted normal diffusing capacity of the lung for carbon monoxide.
The univariate analysis results demonstrated that the DLCO%Pred (P = 0.015, odds ratio [OR] = 0.961, 95% confidence interval [CI] = 0.931–0.992), 6-min walk test (P = 0.025, OR = 0.995, 95% CI = 0.991-0.999), CEA (P = 0.009, OR = 1.053, 95% CI = 1.013-1.059), and NSE (P = 0.041, OR = 1.090, 95% CI = 1.003-1.184) could identify patients who needed a second WLL, whereas no correlations were found between a second WLL and age, sex, smoking history, PaO2, or LDH. In the multivariate model taking into account baseline demographics (age, sex, and smoking history), DLCO%Pred (P = 0.015, OR = 0.961, 95% CI = 0.931–0.992) could also identify patients who needed a second WLL. However, when the data were adjusted for covariates (PaO2, LDH, 6-min walk test, CEA, and NSE), DLCO%Pred failed to reach statistical significance.
DISCUSSION
PAP was first recognized in 1958 by Rosen et al.[1] Since then, increasing numbers of reports on PAP have been published; however, only a few studies have evaluated Asian populations[6,8] and the response to WLL treatment with a long-term follow-up. In this study, we retrospectively reviewed 120 Chinese adults with autoimmune PAP, 80 of whom underwent WLL.
Our results seem to be different from those of previous large studies in many aspects. The mean age at diagnosis in our study was 43 years. However, in the studies of Asian populations from Japan[6] and Korea,[8] the mean age was older at 51 and 52 years, respectively. A low proportion of our cohort constituted smokers (48%), while the proportion of smokers in a Germany cohort (79%) was nearly 2 times higher than this. Spontaneous remission occurred in 9% of patients (n = 11) in this study, which is lower than that (13%) in a Korean study.[8] Only 2 patients died in our cohort, representing the lowest percentage of 2%. Because the morbidity rate was low, we did not analyze the overall survival rate.
Comparison of the results before and after WLL shows that patients experienced objective improvements after WLL. The results of the arterial blood gas analysis are corroborated by the data in a Korean[8] and Italian study.[13] The improvement of all parameters in the pulmonary function tests except TLC%Pred is also in agreement with the results of the above mentioned Korean[8] and Italian studies.[13] The results of the 6-min walk test support the increase in the treadmill distance in the Italian study.[13] Considering that we reexamined the results about 4–6 weeks after the first WLL, not only were the materials in the alveolar spaces removed, but the engorgement of the lymphatic vessels within the interlobular septae was almost completely relieved, which is the basis for the improvement in the test results described above.[13] Multiple WLL treatments were conducted in 24 (30%) of the 80 patients, which is similar to the frequency reported in the Italian study.[9]
Although WLL has been performed in many centers for almost 50 years,[14] no consensus on how to best evaluate the effectiveness of WLL has been established. In practice, many physicians evaluate the indications for treatment and establish the management strategy intuitively. In the present study, a 42.1% cut-off value for DLCO%Pred might help to distinguish patients who require a second WLL and possibly allow for early prognostic assessment of the responsiveness of PAP to WLL. Large quantities of surfactant lipids and proteins accumulate in the alveolar spaces, leading to a decrease in the alveolar capillary membrane surface area, an increase in the diffusion path length, and ventilation perfusion mismatch, which are the main reasons for the disproportionate reduction of DLCO%Pred in patients with PAP.[15] Consequently, DLCO%Pred is well correlated with the severity of PAP. When combined with other parameters, DLCO%Pred failed to reach statistical difference in our study, which might have been caused by the potential correlation between DLCO%Pred and PaO2, CEA, and NSE. However, DLCO%Pred is still associated with the need for lavage, as reported in the above mentioned study from Italy[9] and another from Germany.[16] Although the serum KL-6 level seems to predict the outcome of PAP,[16] measurement of the serum KL-6 level is not widely used as a routine test in most hospitals in China. In contrast, nearly all hospitals can routinely conduct pulmonary function tests at reasonable cost; thus, DLCO%Pred may provide higher practicability.
During the 8.6-year follow-up period of this study, no patients in the multiple WLL group died; however, all of these patients required additional WLLs or other methods to relieve symptoms. Two patients (one female[17] and one male) exhibited a good initial response to WLL. However, multiple sessions of WLL were subsequently required. These patients then underwent several sessions of plasmapheresis with very little improvement. Finally, supplementation with inhaled and subcutaneously injected GM-CSF was performed. The patients were in stable condition at the end of the follow-up period.
As reported, up to 10% of patients are resistant to WLL, and this treatment is associated with various complications, including hypoxemia, hydropneumothorax, acute respiratory distress syndrome, and infections. However, various modern techniques are being applied to increase the effectiveness and safety, and the percentage of complications is decreasing.[18] Other approaches or combined therapy is recommended for patients who develop recurrence after WLL or cannot accept the risks and potential complications of an invasive procedure.[10] GM-CSF followed by WLL could be attempted; the response rate to this combination in one study varied from 43% to 92%, and the relapse rate in GM-CSF responders was 29.7%.[19] Inhaled GM-CSF was effective in 62–92% of patients with few complications such as fever or upper respiratory infection.[20,21] In contrast, subcutaneously administered GM-CSF was effective in 43–75% of patients in other studies.[22,23,24] However, local reactions at the injection sites and other minor toxicities occurred in 85% of patients,[5] and 45% of patients required additional WLL.[24] Consequently, with the better responsiveness and tolerance, inhaled GM-CSF is more highly recommended.[19] If there is an inadequate response to GM-CSF or unacceptable side effects, rituximab or plasmapheresis can be attempted. Rituximab has a high response rate ranging from 78% to 100% and is associated with minor adverse reactions such as fatigue, nausea, and nasal congestion.[25,26,27] Plasmapheresis removes circulating antibodies with Gram-negative sepsis as a complication.[23,28,29] However, the approaches mentioned above are not yet validated for routine use. Systematic evaluation and standard principles should be conducted in future research.
Our study was limited by its retrospective nature. First, not all data were collected from the patients’ clinical notes, affecting the quality in some cases. Second, we could not test and monitor the GM-CSF autoantibody levels. Finally, the number of patients enrolled was limited because of the single-center nature of the study. Given the rarity of autoimmune PAP, we collected a remarkable number of samples to clarify the epidemiology, clinical features, and prognosis in Chinese patients and shared our experience with WLL to promote the development of a better management strategy.
In conclusion, the prognosis of patients with PAP is relatively good. Some patients achieve remission and remain stable without any intervention. WLL is the optimal treatment method and provides remarkable improvements. The need for a second WLL is significantly associated with CEA, NSE, the 6-min walk test, and DLCO%Pred. The DLCO%Pred on admission with a cut-off value of 42.1% may distinguish patients with PAP, who require multiple WLL, which provides a promising approach for clinicians in evaluating the responsiveness to WLL in patients with PAP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang and Jian Gao
REFERENCES
- 1.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–42. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 2.Leth S, Bendstrup E, Vestergaard H, Hilberg O. Autoimmune pulmonary alveolar proteinosis: Treatment options in year 2013. Respirology. 2013;18:82–91. doi: 10.1111/j.1440-1843.2012.02274.x. [DOI] [PubMed] [Google Scholar]
- 3.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–39. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 4.Luisetti M, Kadija Z, Mariani F, Rodi G, Campo I, Trapnell BC. Therapy options in pulmonary alveolar proteinosis. Ther Adv Respir Dis. 2010;4:239–48. doi: 10.1177/1753465810378023. [DOI] [PubMed] [Google Scholar]
- 5.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: Progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–35. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177:752–62. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonella F, Bauer PC, Griese M, Ohshimo S, Guzman J, Costabel U. Pulmonary alveolar proteinosis: New insights from a single-center cohort of 70 patients. Respir Med. 2011;105:1908–16. doi: 10.1016/j.rmed.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Byun MK, Kim DS, Kim YW, Chung MP, Shim JJ, Cha SI, et al. Clinical features and outcomes of idiopathic pulmonary alveolar proteinosis in Korean population. J Korean Med Sci. 2010;25:393–8. doi: 10.3346/jkms.2010.25.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campo I, Mariani F, Rodi G, Paracchini E, Tsana E, Piloni D, et al. Assessment and management of pulmonary alveolar proteinosis in a reference center. Orphanet J Rare Dis. 2013;8:40. doi: 10.1186/1750-1172-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Dov I, Segel MJ. Autoimmune pulmonary alveolar proteinosis: Clinical course and diagnostic criteria. Autoimmun Rev. 2014;13:513–7. doi: 10.1016/j.autrev.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 11.Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med. 2003;167:1287. doi: 10.1164/ajrccm.167.9.950. [DOI] [PubMed] [Google Scholar]
- 12.Michaud G, Reddy C, Ernst A. Whole-lung lavage for pulmonary alveolar proteinosis. Chest. 2009;136:1678–81. doi: 10.1378/chest.09-2295. [DOI] [PubMed] [Google Scholar]
- 13.Beccaria M, Luisetti M, Rodi G, Corsico A, Zoia MC, Colato S, et al. Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J. 2004;23:526–31. doi: 10.1183/09031936.04.00102704. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez J, Kieffer RF, Jr, Ball WC., Jr Bronchopulmonary lavage in man. Ann Intern Med. 1965;63:819–28. doi: 10.7326/0003-4819-63-5-819. [DOI] [PubMed] [Google Scholar]
- 15.Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med. 2010;61:105–19. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonella F, Ohshimo S, Miaotian C, Griese M, Guzman J, Costabel U. Serum KL-6 is a predictor of outcome in pulmonary alveolar proteinosis. Orphanet J Rare Dis. 2013;8:53. doi: 10.1186/1750-1172-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Hy, Sun Xf, Wang Yx, Xu Zj, Huang H. Whole lung lavage combined with Granulocyte-macrophage colony stimulating factor inhalation for an adult case of refractory pulmonary alveolar proteinosis. BMC Pulm Med. 2014;14:87. doi: 10.1186/1471-2466-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Abraham R, Greenfeld A, Rozenman J, Ben-Dov I. Pulmonary alveolar proteinosis: Step-by-step perioperative care of whole lung lavage procedure. Heart Lung. 2002;31:43–9. doi: 10.1067/mhl.2002.119831. [DOI] [PubMed] [Google Scholar]
- 19.Khan A, Agarwal R, Aggarwal AN. Effectiveness of granulocyte-macrophage colony-stimulating factor therapy in autoimmune pulmonary alveolar proteinosis: A meta-analysis of observational studies. Chest. 2012;141:1273–83. doi: 10.1378/chest.11-0951. [DOI] [PubMed] [Google Scholar]
- 20.Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, Nasuhara Y, et al. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2010;181:1345–54. doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tazawa R, Inoue Y, Arai T, Takada T, Kasahara Y, Hojo M, et al. Duration of benefit in patients with autoimmune pulmonary alveolar proteinosis after inhaled granulocyte-macrophage colony-stimulating factor therapy. Chest. 2014;145:729–37. doi: 10.1378/chest.13-0603. [DOI] [PubMed] [Google Scholar]
- 22.Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, Doyle IR, et al. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J Respir Crit Care Med. 2001;163:524–31. doi: 10.1164/ajrccm.163.2.2003146. [DOI] [PubMed] [Google Scholar]
- 23.Bonfield TL, Kavuru MS, Thomassen MJ. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin Immunol. 2002;105:342–50. doi: 10.1006/clim.2002.5301. [DOI] [PubMed] [Google Scholar]
- 24.Venkateshiah SB, Yan TD, Bonfield TL, Thomassen MJ, Meziane M, Czich C, et al. An open-label trial of granulocyte macrophage colony stimulating factor therapy for moderate symptomatic pulmonary alveolar proteinosis. Chest. 2006;130:227–37. doi: 10.1378/chest.130.1.227. [DOI] [PubMed] [Google Scholar]
- 25.Malur A, Kavuru MS, Marshall I, Barna BP, Huizar I, Karnekar R, et al. Rituximab therapy in pulmonary alveolar proteinosis improves alveolar macrophage lipid homeostasis. Respir Res. 2012;13:46. doi: 10.1186/1465-9921-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavuru MS, Malur A, Marshall I, Barna BP, Meziane M, Huizar I, et al. An open-label trial of rituximab therapy in pulmonary alveolar proteinosis. Eur Respir J. 2011;38:1361–7. doi: 10.1183/09031936.00197710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amital A, Dux S, Shitrit D, Shpilberg O, Kramer MR. Therapeutic effectiveness of rituximab in a patient with unresponsive autoimmune pulmonary alveolar proteinosis. Thorax. 2010;65:1025–6. doi: 10.1136/thx.2010.140673. [DOI] [PubMed] [Google Scholar]
- 28.Garber B, Albores J, Wang T, Neville TH. A plasmapheresis protocol for refractory pulmonary alveolar proteinosis. Lung. 2015;193:209–11. doi: 10.1007/s00408-014-9678-2. [DOI] [PubMed] [Google Scholar]
- 29.Luisetti M, Rodi G, Perotti C, Campo I, Mariani F, Pozzi E, et al. Plasmapheresis for treatment of pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1220–2. doi: 10.1183/09031936.00097508. [DOI] [PubMed] [Google Scholar]


