Abstract
Background:
Pathophysiological processes, such as malignancy, can lead to the formation of stiffer tissue in lung cancers. Endobronchial ultrasound (EBUS) elastography is a novel technique for measuring tissue stiffness during EBUS-guided transbronchial needle aspiration (EBUS-TBNA). The current study was conducted to investigate the diagnostic value of EBUS elastography for mediastinal and hilar lymph node metastasis in lung cancers.
Methods:
From January 2014 to January 2015, 40 patients suspected of lung cancer were enrolled, and a total of 68 lymph nodes were evaluated by EBUS-TBNA. EBUS-guided elastography of lymph nodes was performed prior to EBUS-TBNA. Standard EBUS characteristics were also described. Pathological determination of malignant or benign lymph nodes was used as the gold standard for this study. If EBUS-TBNA did not result in a formal pathological diagnosis of malignancy, patients were referred for a surgical procedure. Comparisons of elastography and standard EBUS characteristics were made between benign and malignant lymph nodes.
Results:
Elastography grading scores and strain ratios showed significant differences between benign and malignant lymph nodes (P = 0.000). The elastography strain ratio was more sensitive and specific for determining malignant lymph nodes than elastography grading score or standard EBUS criteria. The receiver operating characteristic curve for the elastography strain ratio showed an area under the curve of 0.933. The best cut-off point of the strain ratio for differentiating malignant from benign lymph nodes was 32.07. The elastography strain ratio had a sensitivity of 88.1%, the specificity of 80.8%, positive predictive value of 88.1%, and negative predictive value of 80.8% for distinguishing malignant from benign nodes. The overall accuracy of elastography strain ratio was 85.3%. The strain ratio of malignant and benign lymph nodes positively correlated with the elastography grading score (r = 0.561, P = 0.000).
Conclusions:
EBUS elastography can be effectively used to predict mediastinal and hilar lymph node metastases in lung cancer. This noninvasive technique may thus complement standard EBUS and help guide EBUS-TBNA procedures.
Keywords: Elastography, Endobronchial Ultrasound, Lung Cancer, Mediastinal and Hilar Lymph Node, Strain Ratio
INTRODUCTION
Lung cancer is one of the most common malignant tumors and the leading cause of cancer death, with a 5-year survival rate of only 16%.[1] The appropriate treatment and outcomes of lung cancer depend on proper staging and diagnosis. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was recently introduced as a novel tool for lung cancer staging. Under the guidance of ultrasound images, the safety, and accuracy of TBNA have been greatly improved.[2] Consequently, EBUS-TBNA has been recommended as an important tool for lung cancer staging by the National Comprehensive Cancer Network (NCCN).[3]
Ultrasound elastography technology has gradually been applied to the clinical setting.[4] Elastography can be used to determine the elasticity of lesions and pathophysiological processes, such as malignancy, that make tissues stiffer or less deformable; thus, the technique exhibits potential application to the differential diagnosis of benign and malignant breast lesions. However, whether or not, EBUS elastography can be used for the noninvasive discrimination of benign and malignant thoracic lymph nodes as a guide for EBUS-TBNA remains unknown. The purpose of this study is to assess the value of EBUS elastography in diagnosing mediastinal and hilar lymph node metastases in lung cancer.
METHODS
Patients
A total of 40 patients suspected of lung cancer by chest X-ray and computed tomography (CT) were assessed by EBUS-TBNA at our hospital between 1 January 2014 and 31 January 2015. Among these patients, 26 were male, and 14 were female; the average age was 65 ± 11 years. All patients were evaluated by chest CT with single contrast injection before EBUS-TBNA. A total of 68 lymph nodes were assessed by EBUS-TBNA. Elastography was performed on all lymph nodes that were candidates for EBUS-TBNA. Written and informed consent was obtained from all subjects. This study was approved by the Ethics Review Committee of the First People's Hospital of Nantong.
Elastography procedure and EBUS-TBNA
Ultrasonic bronchoscopy examination was performed under general anesthesia with etomidate and fentanyl via a laryngeal mask. The convex probe EBUS (CP-EBUS; EB-1970UK, Pentax, Japan) was inserted through an oral connecting tube. Scanning was performed at an ultrasound frequency of 7.5 MHz, and images were generated on a new dedicated ultrasound processor (HI VISION Avius, Hitachi company, Japan). After the appearance of target lymph nodes, the diameter, shape, edge definition, internal echo distribution, and location of the lymph nodes were recorded in conventional B-mode by ultrasound. Ultrasound image characteristics were independently recorded and analyzed by two skilled endoscopists with formal training.
Elastography was performed on all lymph nodes that were candidates for EBUS-TBNA. After recording of the ultrasound image characteristics in B-mode, the procedure was switched to elastography mode. The scan range included the entire lymph node and surrounding normal tissue. Elastographic images were generated based on the compressive action generated by the pulsation of vessels in the thoracic cavity and respiratory movement. The elasticity of tissue within the scanned area was reconstructed by comparing it with the surrounding tissue; observations were translated into a color signal that was overlaid on the B-mode image. The colors associated with hard, intermediate, and soft tissues were blue, green, and yellow/red, respectively. Elastographic and B-mode images were simultaneously displayed side-by-side on the monitor. Elastographic patterns were described according to the dominant colors and their distribution within the target lymph node. The following grading standard was used: 1 point when over 80% of the section was green and yellow/red; 2 points when over 50% but <80% of the section was green and yellow/red; 3 points when over 50% but <80% of the section was blue; and 4 points when over 80% of the section was blue [Figure 1].[5]
Figure 1.
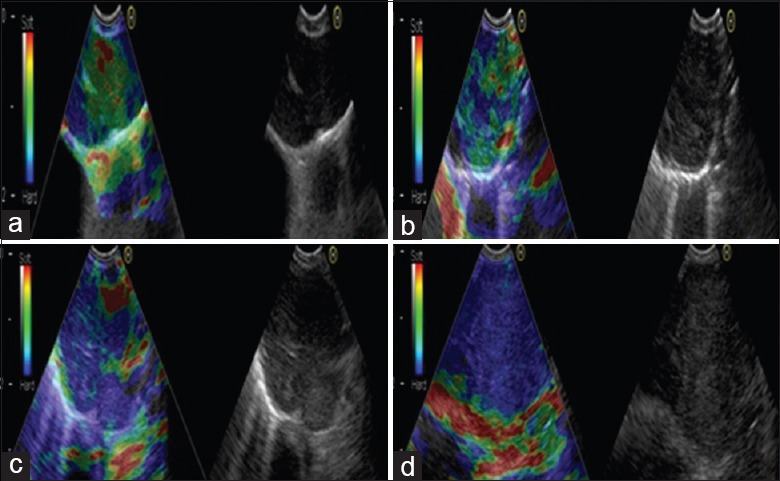
Representative lymph nodes on endobronchial ultrasound elastography. (a) Representative images showing that the lymph node had a distinct boundary, low echo, and homogeneous echo. The elastography grading score in this figure was 1 point. Histopathological specimen from endobronchial ultrasound-guided transbronchial needle aspiration demonstrated the existence of inflammation. (b) Representative images showing that the lymph node was round, the boundary was clear and the internal echo was low. The herein elastography grading score was 2 points. The pathological result confirmed the existence of granuloma lesion. (c) Representative images showing that the lymph node had a distinct boundary, medium echo and uneven echo. The elastography grading score in this figure was 3 points. The pathological result showed the diagnosis of small cell lung cancer. (d) Representative images showing that the lymph node had a distinct boundary, medium echo and uneven echo. The herein elastography grading score was 4 points. The pathological result showed the diagnosis of poorly differentiated adenocarcinoma.
The strain ratio was only measured when good contact and appropriate compression of the transducer were achieved, as indicated by the elastography image on the ultrasound processor. The largest possible area of the node was outlined from the superimposed elastography image; the same procedure was performed on a similar-sized area that was surrounded by apparently normal tissue. The ultrasound processor measured the strain of each area as a quantitative figure, and the strain ratio between the two areas was calculated. The strain ratio was recorded a minimum of 3 times prior to EBUS-TBNA. The means of these recordings were also calculated.
After elastography, EBUS-TBNA was performed with a Cook 22 gauge needle (c976006, Cook Ireland Limited Liability Company, Ireland). Three passes were obtained, and histological and cytological specimens were collected and sent to the laboratory for subsequent analysis by pathologists who were blinded to the elastography values. A definitive diagnosis of malignancy from the EBUS-TBNA specimens was considered a positive result. No clear evidence of malignancy or inadequate specimen by EBUS-TBNA was deemed a negative result. Thoracoscopy or thoracotomy was performed for confirmation of each negative case.
Statistical analysis
All the data were showed as a mean ± standard deviation (SD). Comparison of the demographic variables between histologically or cytologically proven benign and malignant nodes was conducted via contingency table (Chi-square) analysis or the Mann–Whitney test, as appropriate receiver operating characteristic (ROC) was analyzed to compare the relative sensitivity and specificity of EBUS elastography and conventional EBUS criteria with EBUS-TBNA in terms of malignant lymph node detection. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy rate were calculated from the standard definitions used for malignancy diagnosis. Statistical analyses were performed by using SPSS software (Version 17.0, SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant when P < 0.05.
RESULTS
Characteristics of study patients
A total of 40 patients (26 males, 14 females; mean age 65 ± 11 years) underwent elastography, and EBUS-TBNA was performed on 68 hilar and mediastinal lymph nodes. Histological and cytological results confirmed 42 malignant lymph nodes; among these specimens, 12 lymph nodes were squamous cell carcinoma, 11 lymph nodes were adenocarcinoma, 9 lymph nodes were small-cell lung cancer, and 10 lymph nodes were low-differentiation cancer. Only 26 lymph nodes were benign. Among the observed lymph nodes, 12 were located in group 2R, 15 in groups 4R and 4L, 21 in group 7, 11 in groups 10R and 10L, and 9 in groups 11s, 11i, and 11L. The clinical characteristics of the 40 study patients were summarized in Table 1.
Table 1.
Clinical characteristics of the 40 study patients
| Characteristics | No. |
|---|---|
| Gender, n | |
| Male | 26 |
| Female | 14 |
| Age (years), median | 65 |
| Short axis diameter of the LNS (mm), median | 17 |
| Location | |
| Upper paratracheal (2R) | 12 |
| Lower paratracheal (4R,4L) | 15 |
| Subcarinal (7) | 21 |
| Hilar (10R,10L) | 11 |
| Interlobar (11s, 11i, 11L) | 9 |
| Pathology | |
| Malignant | 42 |
| Squamous cell carcinoma | 12 |
| Adenocarcinoma | 11 |
| Small-cell lung cancer | 9 |
| Low differentiation cancer | 10 |
| Benign | 26 |
LNS: Lymph nodes; 2R,4R,10R: Right side of station 2,4,10 lymph node; 4L,10L,11L: Left side of station 4,10,11 lymph node; 11s: Lymph node between the right upper bronchus and bronchus intermedius; 11i: Lymph node between the right middle and lower bronchi
Comparison of elastography strain ratio and elastography grading score between benign and malignant lymph nodes
Among the 68 lymph nodes included in this work, 42 malignant and 26 benign lymph nodes were confirmed by pathological diagnosis. The strain ratio of malignant lymph nodes was significantly higher than that of their benign counterparts (87.69 ± 49.15 vs. 20.60 ± 17.14, P = 0. 000). The elastography grading score was higher in the malignant lymph node group than in the benign lymph node group (3.35 ± 0.91 vs. 1.84 ± 0.97, P = 0.000), and the strain ratio for malignant and benign lymph nodes was confirmed to be positively correlated with the elastography grading score (r = 0.561, P = 0.000) [Figure 2].
Figure 2.
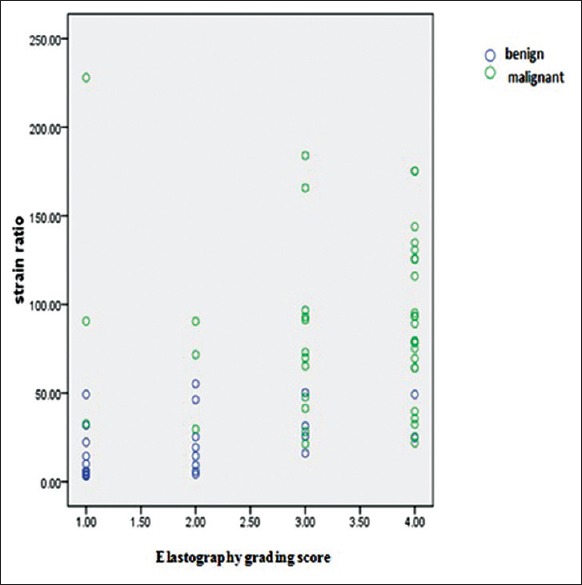
Correlations of elastography strain ratio and elastography grading score in benign or malignant lymph nodes.
Diagnostic value of elastography strain ratio
The area under the curve (AUC) based on the elastography strain ratio was 0.933 [Figure 3]. The strain ratio showed an optimal cut-off point of 32.07 to distinguish malignant nodes from benign ones. The sensitivity, specificity, PPV, NPV, and accuracy were 88.1% (37/42), 80.8% (21/26), 88.1% (37/42), and 85.3% (58/68), respectively.
Figure 3.
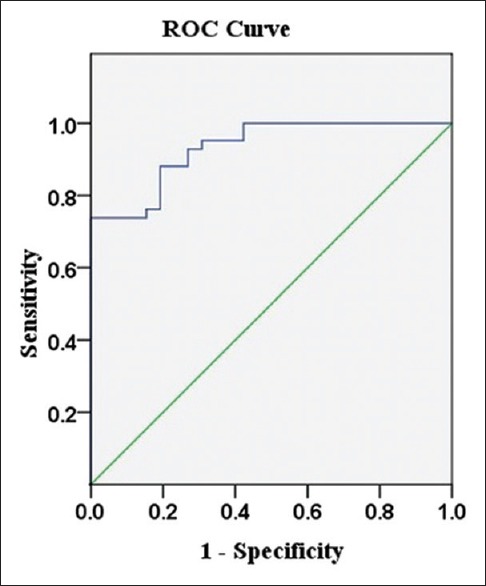
Receiver operating characteristic curve for elastography strain ratio.
Comparison of elastography strain ratio versus other ultrasound methods for distinguishing benign from malignant lymph nodes
Comparisons revealed that the AUCs of hypoechoic, distinct boundary, uneven echo, short diameter ≥1 cm, elastography grading score, and strain ratio were 0.662, 0.705, 0.561, 0.732, 0.852, and 0.933, respectively. There was statistic difference between stain ratio and elastography grading score (P = 0.011), hypoechoic (P = 0.000), distinct boundary (P = 0.001), uneven echo (P = 0.000), and short diameter ≥1 cm (P = 0.000). Compared with all other ultrasound parameters surveyed, the elastography strain ratio was the best diagnostic parameter for distinguishing benign from malignant nodes. Compared with other nodal assessments, the elastography strain ratio showed more favorable results on account of its sensitivity, specificity, PPV, NPV, and accuracy [Figure 4 and Table 2].
Figure 4.
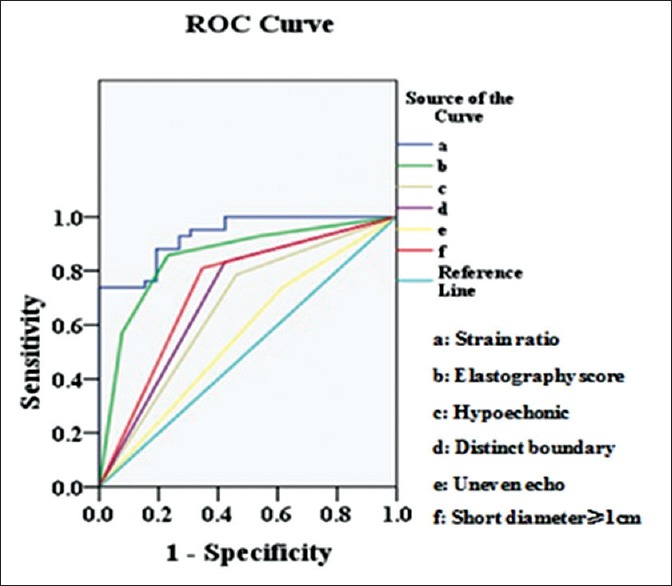
Receiver operating characteristic curve comparison of elastography strain ratio against elastography grading score and conventional endoscopic ultrasound criteria.
Table 2.
Diagnostic values of different ultrasound parameters
| Ultrasound parameters | Specificity (%), n/n | Sensitivity (%), n/n | PPV (%), n/n | NPV (%), n/n | Accuracy (%), n/n | AUC | P* |
|---|---|---|---|---|---|---|---|
| Hypoechonic | 53.8 (14/26) | 78.5 (33/42) | 73.3 (33/45) | 60.8 (14/23) | 69.1 (47/68) | 0.662 | 0.000 |
| Distinct boundary | 57.6 (15/26) | 83.3 (35/42) | 76.1 (35/46) | 68.1 (15/22) | 73.5 (50/68) | 0.705 | 0.001 |
| Uneven echo | 38.4 (10/26) | 73.8 (31/42) | 65.9 (31/47) | 47.6 (10/21) | 60.3 (41/68) | 0.561 | 0.000 |
| Short diameter ≥1cm | 76.9 (20/26) | 80.9 (34/42) | 85.0 (34/40) | 71.4 (20/28) | 79.4 (54/68) | 0.732 | 0.000 |
| Elastography score ≥2.5 | 76.9 (20/26) | 85.7 (36/42) | 85.7 (36/42) | 76.9 (20/26) | 82.3 (56/68) | 0.852 | 0.011 |
| Strain ratio ≥32.07 | 80.8 (21/26) | 88.1 (37/42) | 88.1 (37/42) | 80.8 (21/26) | 85.3 (58/68) | 0.933 | - |
*P versus strain ratio (AUC). PPV: Positive predictive value; NPV: Negative predictive value; AUC: Area under the curve
DISCUSSION
Accurate TNM staging of lung cancer determines the treatment and prognosis of cancer patients.[6] Noninvasive and invasive examinations are used to evaluate the mediastinal lymph nodes of patients with lung cancer. Noninvasive examinations mainly include chest computer tomography (CT) and positron emission tomography/CT (PET/CT), whereas invasive methods can be classified into mediastinoscopy and EBUS-TBNA examinations. All of these examinations demonstrate different accuracies in mediastinal staging. Previous studies have shown that the sensitivity and specificity of CT for diagnosing mediastinal lymph node metastasis are 51–61% and 85%, respectively.[7] However, the high false positive rate of PET/CT during lymph node metastasis diagnosis renders this method controversial.
EBUS-TBNA is a relatively new technique that has rapidly developed in recent years. Needle aspiration guided by ultrasound has greatly increased the positive rate of mediastinal lymph node biopsy. EBUS-TBNA plays a crucial role in mediastinal staging.[8] NCCN guidelines recommend the use of mediastinoscopy and EBUS-TBNA for mediastinal staging and pathological examination as both methods reveal no significant statistical differences in terms of diagnostic sensitivity and accuracy of judging mediastinal lymph node metastasis.[9] Given the obvious advantages of EBUS-TBNA in terms of operation safety, the gold standard status of mediastinoscopy has been questioned.
EBUS-TBNA is widely used in the diagnosis and preoperative staging of lung cancers. Lymph nodes are punctured according to the N3, N2, and N1 sequence, as well as the selection of obvious enlargements in the chest CT and lymph nodes that are easy to puncture (e.g., groups 4R and 7). Changing the needle after puncturing each lymph node is impossible; thus, EBUS-TBNA may cause tumor planting and the emergence of false positives. Consequently, ultrasonic images are used to predict lymph node metastasis. Fujiwara et al.[10] suggested that round, heterogeneous echoes, edge clarity, and coagulation necrosis are independent predictors for assessing malignant lymph nodes in lung cancer patients. However, the accuracy of conventional ultrasound image characteristics in the diagnosis of malignant lymph nodes is not particularly high, and the subjective judgment of the technician is often required.[11] Our present study showed that conventional ultrasound images produce low or uneven echoes, clear boundaries, and short diameters >1 cm; these characteristics present statistical significance in the identification of benign and malignant lymph nodes. Our results are consistent with published data from Fujiwara's group.[10] In fact, the accuracy of a single ultrasound image in the diagnosis of malignant lymph nodes is not especially high (60.3–79.4%).
Elastography is a technology that can reflect the hardness of the tissue. Using this technique, structural deformations caused by compression or vibration are mapped to produce color images representing the relative elasticity or stiffness of tissue.[12] Tissues with a low elastic coefficient are displayed in red, whereas higher values are displayed in blue. In principle, pathophysiological processes, such as malignancy, make tissues stiffer or less deformable. Malignant tumors are usually hard, and this characteristic allows identification of malignant lesions by elastosonography. Ultrasound elastosonography has been widely used in breast and prostate cancer, especially for diagnosing malignant breast lesions.[13,14] Endoscopic ultrasound elastosonography was originally used for the differential diagnosis of pancreatic lesions. Some researchers have used endoscopic elastosonography to differentiate benign tissues from malignancies in pancreatic masses and lymph nodes. When diagnosing benign and malignant pancreatic masses, a sensitivity and specificity of 92.3% and 80% were respectively obtained, and, when diagnosing benign and malignant lymph nodes, a sensitivity and specificity of 91.8% and 92.3% were respectively obtained. In conventional ultrasound, the diagnostic sensitivity and specificity for determining pancreatic masses are 92.3% and 68.9%, respectively, while the corresponding values for lymph nodes are 78.6% and 50%, respectively.[15]
The data demonstrate that endoscopic ultrasound elastography presents more advantages than conventional ultrasound in the diagnosis of malignant pancreatic masses and lymph nodes. However, few studies have focused on the role of endoscopic ultrasound elastography in the diagnosis of benign and malignant hilar and mediastinal lymph nodes. In this study, the technology was applied to measure the hilar and mediastinal lymph nodes of lung cancer patients. EBUS elastography with a strain ratio cut-off of ≥32.07 for malignancy was used to obtain a sensitivity and specificity of 88.1% and 80.8%, respectively. Compared with all of the conventional EBUS criteria surveyed, the strain ratio showed the highest ROC AUC. Compared with the strain ratio, the diagnostic value of elastography grading score decreased. An elastic grade of ≥2.5 was selected as the diagnostic standard. Our results showed a diagnostic accuracy of 82.3%, specificity of 76.9%, sensitivity of 85.7%, PPV of 85.7%, and NPV of 76.9%, and the strain ratio of malignant and benign lymph nodes was positively correlated with elastography grading score. Compared with the characteristics of conventional lymph node B imaging, the elastosonography technology presented obvious advantages in the diagnosis of benign and malignant lymph nodes.
Elastography has been shown to effectively distinguish benign from malignant lymph nodes, thereby providing a novel noninvasive method with which to identify both types of lymph nodes. Elastography also directs the order of lymph node puncture based on the strain ratio and image features. For the preoperative staging of lung cancer lymph nodes by EBUS-TBNA, we suggest that lymph nodes with a strain ratio <32.07 be punctured first, followed by lymph nodes with a strain ratio of ≥32.07, to avoid tumor planting and false positives. For cases that present no indications of operation but require a definitive diagnosis, we suggest that lymph nodes with a strain ratio of ≥32.07 be punctured first. This selection procedure can improve the diagnostic positive rate to some extent and reduce unnecessary damage and pain.
Elastography is mainly used to detect the hardness of tissues. However, central necrosis and vascular invasion often occur in a lymph node; thus, the strain ratio within the node may decrease as the “softer” components of the node are assessed, thereby ultimately influencing the application of elastography techniques. For example, out of the 42 pathologically determined malignant cases reviewed in this work, 5 cases had an elastography strain ratio that presented a false negative result. In addition, calcification of the lymph nodes may increase their hardness. This phenomenon may explain why the strain ratio of the lymph nodes significantly increased in two patients with tuberculosis.
The elastography grading score can be affected by subjective factors because it is usually recorded according to the elastogram color distribution characteristics.[16] The use of a strain ratio is an objective measure because it is not limited by interobserver variability. However, technical issues that can challenge ultrasound elastography examinations include breathing exercises and adjacent vascular pulsations that produce certain compressive displacements.[17] Therefore, completely identical elastic recordings or diagnoses for two inspections are impossible to obtain. Possible resolutions include: (1) Selection of image frames with a relatively stable pressure curve and amplitude and (2) repeated and dynamic recording to minimize the errors.
In summary, this study showed that EBUS elastography, especially the stain ratio, is more accurate than traditional EBUS imaging for distinguishing malignancies in the hilar and mediastinal lymph nodes of lung cancers. Under the guidance of elastosonography, the positive diagnosis rate of TBNA can be improved, unnecessary punctures can be reduced, the time of operation can be shortened, and the occurrence of false positives can be avoided.
Financial support and sponsorship
This work was supported by the grant from Health Bureau of Nantong (No. WQ2015002).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Horeweg N, de Koning H. The importance of screening for lung cancer. Expert Rev Respir Med. 2014;8:597–614. doi: 10.1586/17476348.2014.937428. [DOI] [PubMed] [Google Scholar]
- 2.Vaidya PJ, Kate AH, Yasufuku K, Chhajed PN. Endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer diagnosis and staging. Expert Rev Respir Med. 2015;9:45–53. doi: 10.1586/17476348.2015.992784. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw. 2014;12:1738–61. doi: 10.6004/jnccn.2014.0176. [DOI] [PubMed] [Google Scholar]
- 4.Barr RG. Elastography in clinical practice. Radiol Clin North Am. 2014;52:1145–62. doi: 10.1016/j.rcl.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa MK, Kubota A, Hanamura H, Furukawa M. Clinical application of real-time tissue elastography to head and neck cancer - Evaluation of cervical lymph node metastasis with real-time tissue elastography. Nihon Jibiinkoka Gakkai Kaiho. 2007;110:503–5. doi: 10.3950/jibiinkoka.110.503. [DOI] [PubMed] [Google Scholar]
- 6.McPhail S, Johnson S, Greenberg D, Peake M, Rous B. Stage at diagnosis and early mortality from cancer in England. Br J Cancer. 2015;112(Suppl 1):S108–15. doi: 10.1038/bjc.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2 nd edition) Chest. 2007;132(3 Suppl):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 8.Navani N, Nankivell M, Lawrence DR, Lock S, Makker H, Baldwin DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: An open-label, pragmatic, randomised controlled trial. Lancet Respir Med. 2015;3:282–9. doi: 10.1016/S2213-2600(15)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142:1393–400.e1. doi: 10.1016/j.jtcvs.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara T, Yasufuku K, Nakajima T, Chiyo M, Yoshida S, Suzuki M, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: A standard endobronchial ultrasound image classification system. Chest. 2010;138:641–7. doi: 10.1378/chest.09-2006. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Olivé I, Radua J, Serra P, Andreo F, Sanz-Santos J, Monsó E, et al. Intra- and interobserver agreement among bronchial endosonographers for the description of intrathoracic lymph nodes. Ultrasound Med Biol. 2012;38:1163–8. doi: 10.1016/j.ultrasmedbio.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Saftoiu A, Vilman P. Endoscopic ultrasound elastography - A new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161–5. [PubMed] [Google Scholar]
- 13.Jiang Q, Zhang Y, Chen J, Zhang YX, He Z. Technical evaluation of Virtual Touch™ tissue quantification and elastography in benign and malignant breast tumors. Exp Ther Med. 2014;8:1059–64. doi: 10.3892/etm.2014.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Ma X, Zhan W, Zhu F, Li M, Huang J, et al. Real-time elastography in the diagnosis of patients suspected of having prostate cancer: A meta-analysis. Ultrasound Med Biol. 2014;40:1400–7. doi: 10.1016/j.ultrasmedbio.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World J Gastroenterol. 2009;15:1587–93. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fritscher-Ravens A. Blue clouds and green clouds: Virtual biopsy via EUS elastography? Endoscopy. 2006;38:416–7. doi: 10.1055/s-2006-925277. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Defrias DV, Alasadi R, Nayar R. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA): Experience of an academic centre in the USA. Cytopathology. 2010;21:35–43. doi: 10.1111/j.1365-2303.2009.00664.x. [DOI] [PubMed] [Google Scholar]


