Abstract
Background:
This study was to investigate the relationship among aortic artery calcification (AAC), cardiac valve calcification (CVC), and mortality in maintenance hemodialysis (MHD) patients.
Methods:
All MHD patients in Shanghai Ruijin Hospital in July 2011 were included. To follow up for 42 months, clinical data, predialysis blood tests, echocardiography, and lateral lumbar X-ray plain radiography results were collected. Plasma FGF23 level was measured using a C-terminal assay.
Results:
Totally, 110 MHD patients were involved in this study. Of which, 64 (58.2%) patients were male, the mean age was 55.2 ± 1.4 years old, and the median dialysis duration was 29.85 (3.0–225.5) months. About 25.5% of the 110 MHD patients had CVC from echocardiography while 61.8% of the patients had visible calcification of aorta from lateral lumbar X-ray plain radiography. After 42 months follow-up, 25 (22.7%) patients died. Kaplan–Meier analysis showed that patients with AAC or CVC had a significant greater number of all-cause and cardiovascular deaths than those without. In multivariate analyses, the presence of AAC was a significant factor associated with all-cause mortality (hazard ratio [HR]: 3.149, P = 0.025) in addition to lower albumin level and lower 25-hydroxy Vitamin D (25(OH)D) level. The presence of CVC was a significant factor associated with cardiovascular mortality (HR: 3.800, P = 0.029) in addition to lower albumin level and lower 25(OH)D level.
Conclusion:
Lateral lumbar X-ray plain radiography and echocardiography are simple methods to detect AAC and CVC in dialysis patients. The presence of AAC and CVC was independently associated with mortality in MHD patients. Regular follow-up by X-ray and echocardiography could be a useful method to stratify mortality risk in MHD patients.
Keywords: FGF23, Hemodialysis, Mortality, Vascular Calcification
INTRODUCTION
Cardiovascular disease (CVD) is the main cause of death in maintenance hemodialysis (MHD) patients. Both aortic artery calcification (AAC) and cardiac valve calcification (CVC) have a high incidence in dialysis patients. Now, different vascular calcification scores have been evaluated in dialysis patient. The diagnosis of vascular calcification is usually based on very expensive and highly technical devices such as electron beam computed tomography (EBCT) or multislice spiral computed tomography (CT). However, lateral lumbar X-ray is a useful approach to detect AAC with cheap price and low radiation. In Addition, the use of plain radiographic films of bone has already been suggested in kidney disease improve global outcomes (KDIGO) chronic kidney disease mineral and bone disorder (CKD-MBD) clinic practice guideline.[1] KDIGO CKD-MBD guideline also suggests detecting CVC through echocardiography.
Our previous studies have already showed the high incidence of AAC and CVC in dialysis patients and increased FGF23 (fibroblast growth factor 23) was associated with AAC and CVC. Now, in this study, we aimed to investigate the relationship among AAC, CVC, and mortality, and to figure out that, which factor could predict the outcome of MHD patients.
METHODS
Patients
This study was a cohort study. Two hundred forty-seven MHD patients were treated in Ruijin Hospital affiliated to Shanghai Jiao Tong University, School of Medicine in July 2011. Two hundred seventeen patients met the following inclusion criteria: (1) Age over 18 years, (2) patients received hemodialysis 3 times a week, on a 4 h schedule, using a dialysate calcium concentration of 1.5 mmol/L, (3) no rapidly progressive kidney disease. Among these patients, 74 patients refused to take part in this study, 18 patients with cancer, 15 patients dialysis vintage less than 3 months. Totally, 107 patients were excluded from this study. At last, 110 dialysis patients were included in our study. These patients were followed up for 42 months. This study was approved by the Institutional Review Board of the Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine and was in accordance with the principle of the Helsinki Declaration.
All clinic data of MHD patients were collected, including blood pressure, which were recorded using the mean of the previous 1-month, height and weight, and medical history.
Laboratory measurements
Predialysis blood tests were collected, which include prealbumin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total protein, albumin, blood urea nitrogen, serum creatinine, uric acid, parathyroid hormone (PTH), 25-hydroxy vitamin D (25(OH)D), triglyceride, cholesterol, high density lipoprotein, low density lipoprotein, serum phosphate, and calcium. PTH was measured using an intact assay by a chemiluminescent method (Abbott i2000); serum 25(OH)D was measured by electrochemiluminescence immunoassay (Roche Cobas e601). The samples for measuring FGF23 were ethylenediaminetetraacetic acid plasma. We collected all the samples with other blood test samples on the same day in July 2011 before dialysis. After centrifugation for 10 min at 2000 rpm, all plasmas were stored at −80°C as soon as possible. Plasma FGF23 level was measured using a C-terminal assay (FGF23 [C-Term] ELISA, Immutopics Inc., San Clamente, CA, USA). Body mass index was calculated as weight in kilograms divided by height in meters squared.
Echocardiography
All echocardiographic measurements were performed according to the recommendations of the American Society of Echocardiography by 2 sonographers unaware of biochemical results. Two-dimensional assessment of the aortic valve and mitral valve, together with continuous-wave Doppler ultrasonography, was performed according to parasternal long-axis and short-axis views. CVC is defined as bright echoes of more than 1 mm on 1 or more cusps of the aortic or mitral valve or mitral annulus. Echocardiography is a sensitive and specific method for the detection of valve calcifications.
Lateral lumbar X-ray plain radiography
AAC was detected by a lateral lumbar X-ray plain radiography at a voltage of 70 kV in 120 MHD patients and read by two radiologists using a semi-quantitative score [Figure 1]. This semi-quantitative score also used by others[1,2,3,4] and summarized as follows: Calcified deposits along the anterior and posterior longitudinal walls of the abdominal aorta adjacent to each lumbar vertebra from L1 to L4 were assessed using the midpoint of the intervertebral space above and below the vertebrae as the boundaries. Calcifications were graded as follows: 0, no aortic calcific deposits; 1, less than one-third of the corresponding vertebral length; 2, one-third or more, but less than two-thirds of the corresponding length; 3, more than two-thirds of corresponding length. Each patient's radiological semi-quantitative score ranged from 0 to 4 for segment affected, 0–6 for each vertebral level, and 0–24 for the total score. The blood tests and X-ray plain radiography were done within 1 month.
Figure 1.
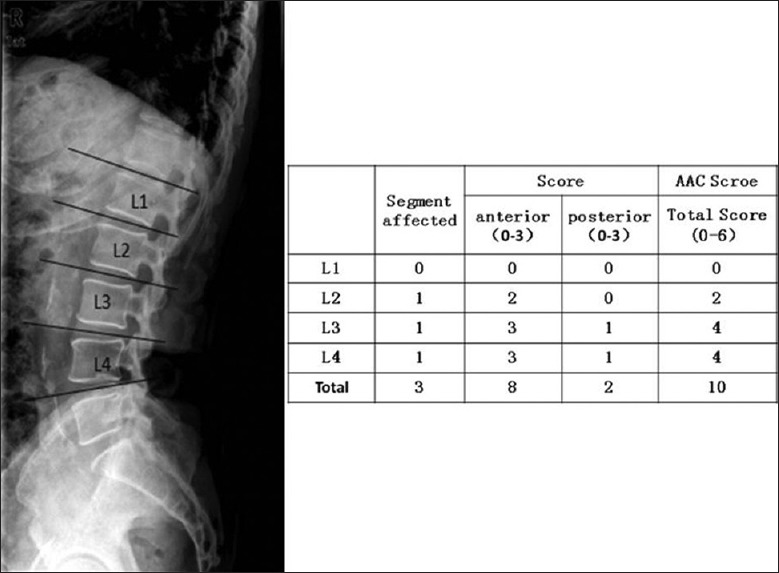
Aortic artery calcification score.
Statistical analysis
Statistical Package for Social Analysis (SPSS for Windows, IBM Corp, USA) version 19.0 was used for data analysis. Results are expressed as mean ± standard error of mean, median (and range), or frequency (as percentage). Comparison between groups was performed by an unpaired t-test or the nonparametric Wilcoxon rank sum test in case of nonnormally distributed variables. Categorical data were compared between the groups by the χ2 test. The Kaplan–Meier method was used to estimate survival probabilities using the log-rank test. Variables relevant to survival were identified by the univariate Cox proportional hazards method. Significant variables were then selected for further analysis using multivariate Cox proportional hazard models. All statistical tests were performed at the two-sided 0.05 level of significance.
RESULTS
Totally, 110 MHD patients were involved in this study. Of whom, 64 (58.2%) patients were male, the mean age was 55.2 ± 1.4 years, and the median dialysis duration was 29.85 (3.0–225.5) months. The demographic and clinical characteristics of MHD patients are shown in Table 1. Among 110 MHD patients, only one patient received parathyroidectomy and 15 (13.6%) patients had diabetes mellitus.
Table 1.
Clinical characteristics of MHD patients (n = 110)
| Parameters | Results | Reference range | |
|---|---|---|---|
| Male, n (%) | 64 (58.2) | – | |
| Age (years), mean ± SD | 55.2 ± 1.4 | – | |
| BMI (kg/m2), mean ± SD | 21.3 ± 0.5 | – | |
| Dialysis duration (month) | 29.85 (3.00–225.5) | – | |
| (range) | |||
| Cause of kidney disease | |||
| Glomerular nephritis, n (%) | 63 (57.3) | – | |
| Diabetes mellitus, n (%) | 15 (13.6) | – | |
| Polycystic kidney | 9 (8.2) | – | |
| disease, n (%) | |||
| Gout, n (%) | 5 (4.5) | – | |
| Hypertension | 4 (3.6) | – | |
| nephropathy, n (%) | |||
| Obstructive nephropathy, | 4 (3.6) | – | |
| n (%) | |||
| Unknown, n (%) | 10 (9.1) | – | |
| Pre-ALB (mg/L), mean ± SD | 305.9 ± 6.9 | 180–380 | |
| ALT (IU/L), range | 11 (5–52) | 16–64 | |
| AST (IU/L), range | 13 (5–33) | 8–40 | |
| ALP (IU/L), range | 67.5 (33–1001) | 38.0–126.0 | |
| TP (g/L), range | 63 (35–78) | 60–83 | |
| ALB (g/L), range | 34 (21–58) | 35.0–55.0 | |
| BUN (mmol/L), | 24.9 ± 0.6 | 2.5–7.1 | |
| mean ± SD | |||
| SCR (μmol/L), mean ± SD | 1052.6 ± 24.9 | Male: 62–115; female: 53–97 | |
| UA (μmol/L), mean ± SD | 451.4 ± 7.5 | 160–430 | |
| iPTH (pg/ml) (range) | 243.35 (8.5–2219.0) | 130–600* | |
| 25(OH)D (nmol/L), mean ± SD | 51.6 ± 2.53 | 50.0–80.0 | |
| TG (mmol/L), mean ± SD | 2.32 ± 0.22 | 0.56–1.70 | |
| TC (mmol/L), mean ± SD | 4.07 ± 0.10 | 2.33–5.7 | |
| HDL (mmol/L), mean ± SD | 1.08 ± 0.03 | 0.80–1.80 | |
| LDL (mmol/L), mean ± SD | 2.22 ± 0.07 | 1.30–4.30 | |
| cFGF23 (RU/ml) (range) | 28,683.1 (69.9–351,947.3) | <100* | |
| LgFGF23 (mean ± SD) | 3.86 ± 0.07 | – | |
| P (mg/dl), mean ± SD | 5.77 ± 0.18 | 2.5–4.5† | |
| Ca (mg/dl), mean ± SD | 9.00 ± 0.09 | 8.5–10.0† |
*Our previous study has shown that FGF23 level in general population is <100 Ru/ml, which is consistent with other study; †Reference range according to KDIGO CKD-MBD guideline. BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; TP: Total protein; BUN: Blood urea nitrogen; SCR: Serum creatinine; UA: Uric acid; iPTH: Intact parathyroid hormone; 25(OH)D: 25-hydroxy Vitamin D; TG: Triglyceride; TC: Total cholesterol; HDL: High density lipoprotein; LDL: Low density lipoprotein; FGF23: Fibroblast growth factor 23; P: Phosphate; Ca: Calcium; MHD: Maintenance hemodialysis; ALB: Albumin; CKD-MBD: Chronic kidney disease mineral and bone disorder; KDIGO: Kidney Disease Improve Global Outcomes: Improving Global Outcomes. –: Not available.
Cardiac valve calcification
Twenty-eight (25.5%) of 110 MHD patients had CVC from echocardiography, 25 (22.7%) with aortic valve calcification, 10 (9.1%) with mitral valve calcification, and only 1 (0.9%) with tricuspid valve calcification.
Sixteen (25.0%) of male and 12 (26.1%) of female patients have CVC in our study. There is no gender difference in the incidence of CVC (P = 0.897).
Aortic artery calcification
Sixty-eight (61.8%) of 110 MHD patients had visible calcification of aorta from lateral lumbar X-ray plain radiography, and the mean involved segments were 1.59 with mean AACs 4.21 ± 0.51 scores. In analysis of the incidence of each segment of AAC, L1 segment was 25.5%, L2 41.8%, L3 42.7% and L4 49.1%. The mean AACs of anterior and posterior were 2.29 and 1.92, respectively.
Thirty-eight (59.4%) of male and 30 (65.2%) of female have AAC. There is no gender difference in the incidence of AAC (P = 0.534).
Mortality
After 42 months follow-up, 25 (22.7%) patients died, including 16 cases from cardiovascular events, 6 respiratory failure, 2 abandon treatment, and 1 deep venous thrombosis of lower extremity. In our study, 19 (29.7%) male patients and 6 (13.0%) female patients died during follow-up. There is a significant difference between two genders (P = 0.040).
Kaplan–Meier analyses
Kaplan–Meier analyses were performed to examine the univariate association between the presence of abdominal aortic calcification, CVC, and outcome. Figure 2 shows the relationship among AAC, CVC, and death from all-causes mortality and cardiovascular mortality. Patients with AAC had a significantly greater number of deaths from all-cause than those without AAC (Log-rank test, P = 0.002). Similarly, patients with valve calcification also had a significantly greater number of deaths (Log-rank test, P = 0.001). Figure 3 shows the Kaplan–Meier analysis of cardiovascular mortality (Log-rank test, P = 0.049 in AAC and P < 0.001 in CVC).
Figure 2.
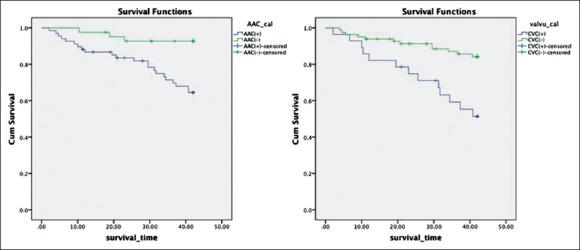
Kaplan–Meier analysis of all-cause mortality (P = 0.002 and P = 0.001).
Figure 3.
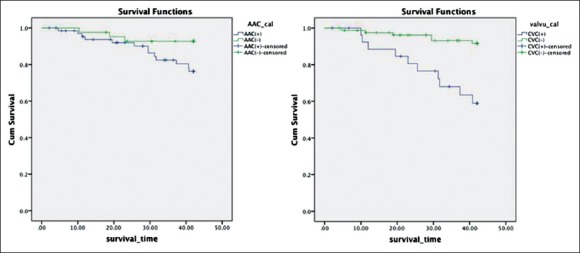
Kaplan–Meier analysis of cardiovascular mortality (P = 0.049 and P < 0.001).
Multivariate analysis with Cox proportional hazards models
Multivariate Cox proportional hazards analyses were performed to identify factors associated with mortality. In multivariate analyses, factors that showed P < 0.05 on univariate analyses were entered as possible factors associated with mortality. Univariate Cox proportional hazards analysis for AAC, CVC, and mortality are shown in Figures 4 and 5. The presence of AAC was a significant factor associated with all-cause mortality (hazard ratio [HR]: 3.149, P = 0.025) in addition to lower albumin level and lower 25(OH)D level. The presence of CVC was a significant factor associated with cardiovascular mortality (HR: 3.800, P = 0.029) in addition to lower albumin level and lower 25(OH)D level. Univariate analyses and Cox proportional hazards models are shown in Tables 2 and 3.
Figure 4.
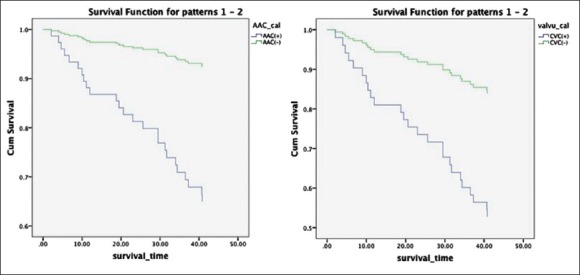
Univariate Cox proportional hazards analysis for aortic artery calcification, cardiac valve calcification, and all-cause mortality.
Figure 5.
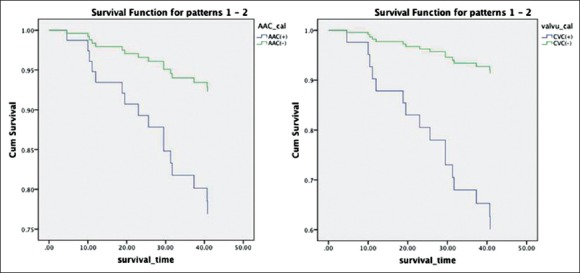
Univariate Cox proportional hazards analysis for aortic artery calcification, cardiac valve calcification, and cardiovascular mortality.
Table 2.
Univariate and multivariate Cox proportional hazards analysis for all-cause mortality
| Items | All-cause mortality | Cox proportional hazards model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex (male vs. female) | 2.553 | 1.019–6.395 | 0.045 | 1.673 | 0.634–4.417 | 0.299 |
| Age | 1.059 | 1.024–1.095 | 0.001 | 1.034 | 0.992–1.078 | 0.112 |
| Dialysis duration | 1.002 | 0.991–1.013 | 0.778 | – | – | – |
| Pre-ALB | 0.996 | 0.991–1.002 | 0.206 | – | – | – |
| TP | 0.980 | 0.924–1.039 | 0.495 | – | – | – |
| ALB | 0.751 | 0.671–0.841 | <0.001 | 0.820 | 0.725–0.927 | 0.002 |
| BUN | 0.979 | 0.916–1.046 | 0.526 | – | – | – |
| SCR | 0.999 | 0.997–1.000 | 0.128 | – | – | – |
| UA | 0.998 | 0.993–1.003 | 0.384 | – | – | – |
| Ca | 0.481 | 0.094–2.461 | 0.380 | – | – | – |
| P | 0.855 | 0.439–1.664 | 0.664 | – | – | – |
| iPTH | 0.999 | 0.997–1.001 | 0.198 | – | – | – |
| 25(OH)D | 0.979 | 0.962–0.996 | 0.014 | 0.981 | 0.965–0.998 | 0.024 |
| TG | 0.988 | 0.820–1.189 | 0.897 | – | – | – |
| TC | 0.749 | 0.495–1.132 | 0.170 | – | – | – |
| HDL | 0.284 | 0.074–1.085 | 0.006 | – | – | – |
| LDL | 0.798 | 0.475–1.342 | 0.395 | – | – | – |
| LgFGF23 | 1.098 | 0.661–1.822 | 0.719 | – | – | – |
| BMI | 1.036 | 0.951–1.129 | 0.414 | – | – | – |
| CVC (presence vs. absence) | 3.638 | 1.658–7.982 | 0.001 | 1.563 | 0.637–3.836 | 0.330 |
| AAC (presence vs. absence) | 5.422 | 1.622–18.129 | 0.006 | 3.149 | 0.889–11.159 | 0.025 |
BMI: Body mass index; TP: Total protein; ALB: Albumin; BUN: Blood urea nitrogen; SCR: Serum creatinine; UA: Uric acid; iPTH: Intact parathyroid hormone; 25(OH)D: 25-hydroxy Vitamin D; TG: Triglyceride; TC: Total cholesterol; HDL: High density lipoprotein; LDL: Low density lipoprotein; FGF23: Fibroblast growth factor 23; P: Phosphate; Ca: Calcium; CVC: Cardiac valve calcification; AAC: Aortic artery calcification; HR: Hazard ratio; CI: Confidence interval.
Table 3.
Univariate and multivariate Cox proportional hazards analysis for cardiovascular mortality
| Items | Cardiovascular mortality | Cox proportional hazards model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex (male vs. female) | 3.508 | 0.999–12.315 | 0.050 | – | – | – |
| Age | 1.051 | 1.009–1.094 | 0.017 | 1.008 | 0.975–1.062 | 0.757 |
| Dialysis duration | 0.996 | 0.981–1.012 | 0.629 | – | – | – |
| Pre-ALB | 0.999 | 0.992–1.006 | 0.718 | – | – | – |
| TP | 0.995 | 0.920–1.077 | 0.908 | – | – | – |
| ALB | 0.774 | 0.658–0.911 | 0.002 | 0.847 | 0.728–0.984 | 0.030 |
| BUN | 0.977 | 0.899–1.062 | 0.581 | – | – | – |
| SCR | 0.999 | 0.997–1.001 | 0.325 | – | – | – |
| UA | 1.000 | 0.994–1.006 | 0.952 | – | – | – |
| Ca | 0.907 | 0.121–6.804 | 0.924 | – | – | – |
| P | 1.044 | 0.464–2.348 | 0.918 | – | – | – |
| iPTH | 0.999 | 0.997–1.001 | 0.270 | – | – | – |
| 25(OH)D | 0.966 | 0.943–0.989 | 0.004 | 0.962 | 0.941–0.984 | 0.001 |
| TG | 1.041 | 0.856–1.265 | 0.689 | – | – | – |
| TC | 0.997 | 0.634–1.569 | 0.990 | – | – | – |
| HDL | 0.313 | 0.060–1.628 | 0.168 | – | – | – |
| LDL | 0.919 | 0.488–1.729 | 0.794 | – | – | – |
| LgFGF23 | 1.246 | 0.661–2.350 | 0.496 | – | – | – |
| BMI | 1.014 | 0.913–1.126 | 0.796 | – | – | – |
| CVC (presence vs. absence) | 5.665 | 2.056–15.606 | 0.001 | 3.800 | 1.150–12.558 | 0.029 |
| AAC (presence vs. absence) | 3.264 | 0.929–11.465 | 0.045 | 2.391 | 0.631–9.060 | 0.200 |
BMI: Body mass index; TP: Total protein; ALB: Albumin; BUN: Blood urea nitrogen; SCR: Serum creatinine; UA: Uric acid; PTH: Parathyroid hormone; 25(OH)D: 25-hydroxy Vitamin D; TG: Triglyceride; TC: Total cholesterol; HDL: High density lipoprotein; LDL: Low density lipoprotein; FGF23: Fibroblast growth factor 23; P: Phosphate; Ca: Calcium; CVC: Cardiac valve calcification; AAC: Aortic artery calcification; HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
Cardiovascular disease is very common in CKD patients, especially in MHD patients. High prevalence of AAC and CVC has been reported in MHD patients. The goal of our study is to investigate the relationship among AAC, CVC and the mortality in Chinese MHD patients, and which would be the better method to predict the outcome.
Now different vascular calcification scores have been evaluated in dialysis patient by various methods, such as X-ray plain radiography, B-mode ultrasound, and CT. The use of CT for diagnosis of AAC is highly reliable and sensitive, but expensive and delivering a substantial dose of radiation. Lateral lumbar X-ray is a simple method for detecting AAC with cheap price, available device, and low radiation. AAC score is a convenient score for clinic doctors to evaluate the severity of calcification and associated with mortality.[4,5,6] About 94.4%[1] of the MHD patients in Australia, 56.5%[6] in Japan, and 81.0%[3] in Europe had visible AAC. Our previous study showed that the prevalence of AAC was 60.83% in Chinese MHD patients. In general population, AAC was associated with congestive heart failure. Some studies also showed that AAC is associated with coronary artery calcification.[2] Aortic arch calcification could also predict cardiovascular and all-cause mortality in MHD patients. Komatsu et al.[7] found that aortic arch calcification is linked to an increased risk of CAD and is associated with cardiovascular risk factors such as age, hypertension, dyslipidemia, and DM in general population.[8]
The incidence of CVC in dialysis patients is also high, and the incidence of AVC is higher than the MVC, which is similar to the result of our study. CVC is associated with the presence and severity of CAD in predialysis CKD.[9] In dialysis patients, CVC is related to mortality[10] and age; calcium-phosphorus product and hypoprealbuminemia are independent risk factors for CVC.[11] The post-hoc analysis of ADVANCE study showed that CVC is a predictor of CAC progression, and of greater cardiovascular vulnerability.[12]
In our study, we analyzed the association among AAC, CVC, and mortality. In Kaplan–Meier analyses and univariate analyses, both AAC and CVC could predict all-cause and cardiovascular mortality in MHD patients. However, in Cox proportional hazards models, only AAC was a significant factor associated with all-cause mortality and CVC was a significant factor associated with cardiovascular mortality. This may be because the semi-quantitative method for evaluating AAC and small size of our study. In spite of this, the results of the present study still can support the additional value of lateral lumbar X-ray films and echocardiography in MHD patients to predict their short-term outcome. We also found that male had a higher mortality than female in our study. It is well-known that the incidence of cardiovascular disease is relative to gender. Allison et al.[13] performed whole-body EBCT scans on 650 asymptomatic subjects and found that male had higher percentage of calcification than female. Although there is no gender difference in the incidence of AAV and CVC in our study, male dialysis patients had a higher mortality than female.
FGF23 is a novel bone-derived phosphaturic factors involved in mineral metabolism disorder and increased with the decreased kidney function and phosphate accumulates.[14] It promotes renal phosphorus wasting and inhibits conversion of 25(OH)D to the active 1,25-dihydroxy vitamin D form.[15,16] Many studies showed that FGF23 has been linked to cardiovascular disease such as left ventricle hypertrophy[17,18] and vascular calcification[19,20] in CKD. Our previous study also found that FGF23 is associated with the presence of AAC in MHD patients. Not just in CKD patients, a French cohort study, including 1130 healthy males, also shows that circulating FGF23 is associated with severe AAC independent of other traditional risk factors.[21] Many studies show that FGF23 is related to increased mortality[22,23] in dialysis patients. However, we did not find this relationship between FGF23 and all-cause mortality. This may be caused by the method of qualitative measurement of aortic calcification in the present study, and this study is only short-term follow-up (42 months). We need to continue to follow-up MHD patients to investigate FGF23 and long-term outcome.
In CKD patients, hyperphosphatemia and calcium load are key risk factors and that impaired bone remodeling leads to vascular calcification. The treatment of hyperphosphatemia with phosphate binder would give beneficial effect on vascular calcification progression.[24] In our study, we did not find that phosphate and calcium are related to all-cause mortality.
In Cox analyses, albumin level is a protective factor for AAC. Albumin level is not only a nutrition factor but also an inflammation factor. The decreased albumin level is associated with an inflammation situation. Huang et al.[25] found that C-reactive protein is a predictor of AAC. Other inflammation factor, such as MCP-1, was also related to all-cause and cardiovascular mortality in peritoneal dialysis patients.[26] S100A12, the pro-inflammatory RAGE-ligand, elevated in CKD 5 stage patients and is an independent predictor of mortality risk.[27] Hence, in our study, patients with low albumin level may have severe inflammation reaction and may have a link to AAC.
One of the important findings of this study is the association between 25(OH)D level and short-term outcome. We found that 25(OH)D is an independent predictor of cardiovascular and all-cause mortality. Vitamin D deficiency has been linked to cardiovascular disease and early mortality in patients on hemodialysis, and the same association exists at earlier stages of CKD. Ravani et al.[28] found that plasma 25(OH)D predicted both time to death and ESRD. In some studies, low doses and more physiology doses of active vitamin D have been found to have a cardioprotective effect.[29,30] Previous studies demonstrated that alfa-calcidol therapy was associated with a significantly lower risk of cardiovascular and all-cause mortality in chronic HD patients.[31,32] Shoji et al.[33] reported that MHD patients treated with alfa-calcidol were at reduced risk of cardiovascular death. Naves-Díaz et al.[34] reported that a mean daily oral vitamin D dose below 0.25 μg can reduce the mortality rate by 53% in MHD patients whose serum PTH levels were below 150 pg/ml. All these findings suggest that low 25(OH)D level and vitamin D3 therapy may improve cardiovascular and all-cause mortality.
There were a few limitations in our study. First, evaluation of AAC is the semi-quantitative method. Therefore, the true calcium deposition in the aortic wall may be underestimated. Second, the population size in this study was small. In the future, a larger multiple-center, prospective study should be done to analyze the value of AAC in dialysis patients.
In conclusion, lateral lumbar X-ray plain radiography and echocardiography are simple methods to detect AAC and CVC in dialysis patients. The presence of AAC and CVC was independently associated with mortality in MHD patients. Regular follow-up by X-ray and echocardiography could be useful method to stratify mortality risk in MHD patients.
Financial support and sponsorship
This study was supported by Shanghai Science Foundation, China (No. 14ZR1425400).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Toussaint ND, Pedagogos E, Lau KK, Heinze S, Becker GJ, Beavis J, et al. Lateral lumbar X-ray assessment of abdominal aortic calcification in Australian haemodialysis patients. Nephrology (Carlton) 2011;16:389–95. doi: 10.1111/j.1440-1797.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 2.Bellasi A, Ferramosca E, Muntner P, Ratti C, Wildman RP, Block GA, et al. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int. 2006;70:1623–8. doi: 10.1038/sj.ki.5001820. [DOI] [PubMed] [Google Scholar]
- 3.Honkanen E, Kauppila L, Wikström B, Rensma PL, Krzesinski JM, Aasarod K, et al. Abdominal aortic calcification in dialysis patients: Results of the CORD study. Nephrol Dial Transplant. 2008;23:4009–15. doi: 10.1093/ndt/gfn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis. 1997;132:245–50. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 5.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 6.Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2007;49:417–25. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu M, Okazaki M, Tsuchiya K, Kawaguchi H, Nitta K. Aortic arch calcification predicts cardiovascular and all-cause mortality in maintenance hemodialysis patients. Kidney Blood Press Res. 2014;39:658–67. doi: 10.1159/000368476. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Galvin HK, Johnson SC, Langston CS, Sclamberg J, Preston CA. Aortic calcification on plain chest radiography increases risk for coronary artery disease. Chest. 2002;121:1468–71. doi: 10.1378/chest.121.5.1468. [DOI] [PubMed] [Google Scholar]
- 9.Kim IY, Kim MJ, Lee DW, Lee SB, Shin MJ, Rhee H, et al. Cardiac valve calcification is associated with presence and severity of coronary artery disease in patients with pre-dialysis chronic kidney disease. Clin Exp Nephrol. 2015 doi: 10.1007/s10157-015-1104-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed BA, Yang W, Litt H, Rosas SE. Valvular calcification, inflammation, and mortality in dialysis patients. J Heart Valve Dis. 2013;22:584–90. [PubMed] [Google Scholar]
- 11.Wang C, Jiang L, Feng S, Shi Y, Shen H, Shi X, et al. Risk factor analysis of calcification in aortic and mitral valves in maintenance peritoneal dialysis patients. Kidney Blood Press Res. 2013;37:488–95. doi: 10.1159/000355729. [DOI] [PubMed] [Google Scholar]
- 12.Bellasi A, Reiner M, Pétavy F, Goodman W, Floege J, Raggi P. Presence of valvular calcification predicts the response to cinacalcet: Data from the ADVANCE study. J Heart Valve Dis. 2013;22:391–9. [PubMed] [Google Scholar]
- 13.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–6. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 14.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–8. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–9. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 16.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 17.Canziani ME, Tomiyama C, Higa A, Draibe SA, Carvalho AB. Fibroblast growth factor 23 in chronic kidney disease: Bridging the gap between bone mineral metabolism and left ventricular hypertrophy. Blood Purif. 2011;31:26–32. doi: 10.1159/000321368. [DOI] [PubMed] [Google Scholar]
- 18.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UA. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25:2679–85. doi: 10.1093/ndt/gfq089. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Chen X, Xie J, Ma X, Zhong F, Hou L, et al. Fibroblast growth factor 23 is a predictor of aortic artery calcification in maintenance hemodialysis patients. Ren Fail. 2013;35:660–6. doi: 10.3109/0886022X.2013.781844. [DOI] [PubMed] [Google Scholar]
- 21.Schoppet M, Hofbauer LC, Brinskelle-Schmal N, Varennes A, Goudable J, Richard M, et al. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: The STRAMBO study. J Clin Endocrinol Metab. 2012;97:E575–83. doi: 10.1210/jc.2011-2836. [DOI] [PubMed] [Google Scholar]
- 22.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant. 2009;24:2792–6. doi: 10.1093/ndt/gfp191. [DOI] [PubMed] [Google Scholar]
- 24.Frazão JM, Adragão T. Treatment of hyperphosphatemia with sevelamer hydrochloride in dialysis patients: Effects on vascular calcification, bone and a close look into the survival data. Kidney Int Suppl. 2008;111:S38–43. doi: 10.1038/ki.2008.544. [DOI] [PubMed] [Google Scholar]
- 25.Huang JW, Lien YC, Yang CY, Liu KL, Wu CF, Yen CJ, et al. Osteoprotegerin, inflammation and dyslipidemia are associated with abdominal aortic calcification in non-diabetic patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis. 2014;24:236–42. doi: 10.1016/j.numecd.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Ko KI, Park KS, Lee MJ, Doh FM, Kim CH, Koo HM, et al. Increased dialysate MCP-1 is associated with cardiovascular mortality in peritoneal dialysis patients: A prospective observational study. Am J Nephrol. 2014;40:291–9. doi: 10.1159/000368201. [DOI] [PubMed] [Google Scholar]
- 27.Isoyama N, Leurs P, Qureshi AR, Bruchfeld A, Anderstam B, Heimburger O, et al. Plasma S100A12 and soluble receptor of advanced glycation end product levels and mortality in chronic kidney disease Stage 5 patients. Nephrol Dial Transplant. 2015;30:84–91. doi: 10.1093/ndt/gfu259. [DOI] [PubMed] [Google Scholar]
- 28.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–88. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272(4 Pt 2):H1751–8. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa M, Ogawa T, Inoue T, Otsuka K, Nitta K. Effect of alfacalcidol therapy on the survival of chronic hemodialysis patients. Ther Apher Dial. 2012;16:248–53. doi: 10.1111/j.1744-9987.2012.01061.x. [DOI] [PubMed] [Google Scholar]
- 32.Andress DL. Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33–43. doi: 10.1038/sj.ki.5000045. [DOI] [PubMed] [Google Scholar]
- 33.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant. 2004;19:179–84. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 34.Naves-Díaz M, Alvarez-Hernández D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74:1070–8. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]


