Abstract
Background:
Hypertrophic scar is one of the most common complications and often causes the disfigurement or deformity in burn or trauma patients. Therapeutic methods on hypertrophic scar treatment have limitations due to the poor understanding of mechanisms of hypertrophic scar formation. To throw light on the molecular mechanism of hypertrophic scar formation will definitely improve the outcome of the treatment. This study aimed to illustrate the negative role of eukaryotic initiation factor 6 (eIF6) in the process of human hypertrophic scar formation, and provide a possible indicator of hypertrophic scar treatment and a potential target molecule for hypertrophic scar.
Methods:
In the present study, we investigated the protein expression of eIF6 in the human hypertrophic scar of different periods by immunohistochemistry and Western blot analysis.
Results:
In the hypertrophic scar tissue, eIF6 expression was significantly decreased and absent in the basal layer of epidermis in the early period, and increased slowly and began to appear in the basal layer of epidermis by the scar formation time.
Conclusions:
This study confirmed that eIF6 expression was significantly related to the development of hypertrophic scar, and the eIF6 may be a target molecule for hypertrophic scar control or could be an indicator of the outcomes for other treatment modalities.
Keywords: Eukaryotic Initiation Factor 6, Hypertrophic Scar, Therapeutic Target
INTRODUCTION
A hypertrophic scar is one of the fibrotic diseases, arising from fibroproliferation disorder which occurs after the damage of deep dermis by burns or trauma. Patients with hypertrophic scar often suffer from difficulties in daily life and psycho-social dysfunction.[1,2,3,4] The main pathological characteristics of the hypertrophic scar are an overproduction of extracellular matrix (ECM) and collagens.[5,6] Due to the severe functional disabilities caused by a hypertrophic scar,[7] burn rehabilitation is very important for patients after burns, especially occupational therapy which bridges the transfer from hospitals into the community. Pressure therapy is considered as the first line noninvasive treatment for the hypertrophic scar.[8,9,10,11,12,13,14] It facilitates the scar maturation, improves the appearance of a hypertrophic scar, reduces the erythema, as well as alleviates pain and pruritus.[12,13,15,16] However, the outcome of hypertrophic scar treatment was poor, and the mechanisms of how pressure therapy influences the scar maturation are not fully reviewed.[12,17] Lai et al. demonstrated that a static pressure of more than 20 mmHg could accelerate the hypertrophic scar remodeling.[12] Further work of this research team also illustrated the histopathological response of pressure therapy in patients with burn scar. Suppression of dermal myofibroblasts by induction of apoptosis would result in reduced collagen overproduction.[10]
To investigate the mechanisms of pressure therapy on hypertrophic scar maturation, it is important for both surgeons and occupational therapists to identify the key molecule which promotes or inhibits the hypertrophic scar formation. Our previous in vitro study demonstrated that the eukaryotic initiation factor 6 (eIF6) expression was significantly decreased in fibroblasts derived from hypertrophic scar when compared with that from normal skin (unpublished data). eIF6 (also known as integrin β4 binding protein), is a ribosome anti-association factor that regulates translational initiation and ribosome synthesis due to its capacity to modulate ribosome 60S availability and 80S subunit, and plays a very important role in ribosome formation.[18] Additionally, eIF6 was involved in connection with cytoskeleton, cell apoptosis, and cell cycle progression.[18] Moreover, eIF6 has been identified as an interacting protein of the hypertrophic scar-related protein P311,[19,20] which indicates that it may be involved in the regulation of hypertrophic scar formation as well as myofibroblast differentiation.[21] However, the spatial and temporal expression of eIF6 in the process of human hypertrophic scar formation remains unknown. The current study was designed to study the eIF6 protein expression pattern during the process of hypertrophic scar formation.
METHODS
Clinical specimens
This was a retrospective study. Because of the possible variation among the individuals, the cases enrolled in our study were only those who received hypertrophic scar excision followed by autoskin grafting, and there was remaining donor skin tissue after grafting, that is, the hypertrophic scar tissue and normal skin tissue were the homobody. Immediately after the surgical procedure, the samples of hypertrophic scar tissues and pair-matched normal skin tissues were both kept either in liquid nitrogen or in 4% paraformaldehyde. Therefore, total 18 cases (whose hypertrophic scar tissues and remaining donor skin tissues were kept in the Institute of Burn Research, Southwest Hospital, Chongqing, China) were enrolled in this study from October 2013 to August 2014. The study was conducted according to the Helsinki Declaration and approved by the Ethics Committee of Southwest Hospital. Samples used in this study were anonymous, and it is impossible for anyone to link the samples to the sources. Therefore, the Ethics Committee of Southwest Hospital waivered the informed consent.
Patient groups
Due to the individual variation, there are no clear staging criteria for hypertrophic scar for the present.[22] We used Vancouver Scar Scale (VSS) to evaluate for height and color, and visual analog scale (VAS) for pain and itch of the hypertrophic scar (data not shown). All the patients were divided into three groups according to both clinical manifestation (decided by VSS and VAS) and scar age [Table 1].
Table 1.
The demographic information of the HS patients
| Variables | PP (n = 6) | MP (n = 4) | RP (n = 8) |
|---|---|---|---|
| Age, years | 38.17 ± 18.43 | 30.25 ± 21.50 | 19.75 ± 8.75 |
| Gender (male/female), n | 2/4 | 1/3 | 2/6 |
| Scar age, months | 6.83 ± 3.25 | 17.75 ± 2.75 | 98.75 ± 119.74 |
| (minimum, maximum) | (3.00, 11.00) | (15.00, 21.00) | (29.00, 384.00) |
Data are presented as the mean ± SD. PP: Proliferative phase; MP: Mature phase; RP: Regressive phase; SD: Standard deviation; HS: Hypertrophic scar.
Group I: The scar age was within 1-year, and the clinical manifestation of scars was raised, erythematous and hard with or without itch and pain. We defined this period of the hypertrophic scar as proliferative phase.
Group II: The scar age was about 1–2 years. Scars in this phase were stable and less erythematous and hard. The itch and pain were a relief. We defined this period of the hypertrophic scar as mature phase.
Group III: The scar age was more than 2 years. Scars became flat and soft, and the color was closer to the normal skin. We defined this period of the hypertrophic scar as regressive phase.
Immunohistochemistry study
Mouse polyclonal antibody against eIF6 (Abnova, Taiwan, China) was used. Tissue samples were fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffered saline at pH 7.4 and embedded in paraffin. Serial 4 μm sections of the paraffin-embedded tissues were then collected. For immunohistochemical staining, the sections were incubated with Proteinase K (Millipore, USA) for 20 min (37°C), and endogenous peroxidase was quenched with 3% H2 O2 in methanol for 20 min at room temperature. The sections were blocked with normal goat serum (Mouse SP Kit, ZSGB-Bio, China) for 30 min and then incubated in mouse polyclonal antibody against eIF6 at a final dilution of 1:400 at 4°C overnight. Lastly, sections were incubated with a peroxidase-coupled secondary antibody plus streptavidin–peroxidase complex and followed by diaminobenzidine staining.
Western blot analysis
Western blot analysis was performed exactly according to the previously reported protocol.[23] Briefly, total proteins were extracted in lysis buffer (50 mmol/L Tris-HCl pH 7.5; 2% sodium deoxycholate stock) from the frozen tissue samples, and the extracted proteins were quantified by the bicinchoninic acid method (Pierce, USA). Twenty micrograms of proteins prepared in Laemmli buffer, were loaded on 12% acrylamide reducing denaturing gel, transferred to polyvinylidene fluoride membranes (Millipore), and blotted with the mouse polyclonal antibody eIF6 (at a 1:2000 dilution with 3% bovine serum albumin). The revelation was performed with the ECL method (Pierce). Additionally, we used glyceraldehyde-3-phosphate dehydrogenase for the normalization of data.
Morphometric analysis of eukaryotic initiation factor 6 in hypertrophic scar and normal skin tissues
Pictures of each section were taken with a Leica Confocal Microscope (Leica Microsystems, Wetzlar, Germany). For the immunohistochemical staining analysis, the expression of eIF6 was observed, and digital images were randomly obtained from five fields under ×400 magnification each section. The expression intensity of the positive-labeled cells was measured and analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, USA). Data are expressed as the average optical density (AOD), AOD = integral optical density (IOD)/area of the total field (IOD = optical intensity of positive cells × area of positive cells).
Statistical analysis
We performed statistical analyses using one-way analysis of variance followed by Turkey multiple comparison. The data are presented as the mean ± standard deviation. P < 0.05 was considered to be a significant difference.
RESULTS
Demography of the hypertrophic scar patients
Table 1 illustrates the demographic data of three patients’ groups. There were total 18 patients enrolled in this study with scar age ranging from 3 months to 32 years: Group I, proliferative phase: n = 6; Group II, mature phase: n = 4; and Group III, regressive phase: n = 8. Patient age varied from 2 years to 49 years. Hypertrophic scar sites included face, neck, preclavicle, hand, upper arm, lower limb, foot, perineum, and presternal.
Eukaryotic initiation factor 6 expression was morphologically absent in the basal layer of epidermis of hypertrophic scar
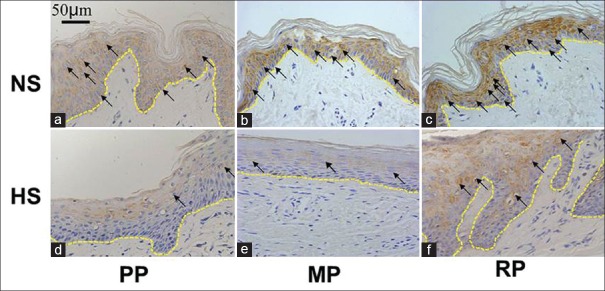
In normal skin, eIF6 was mainly distributed in the cytoplasm of the basal layer of keratinocytes [Figure 1a–c]. It is also expressed in other cell layers of the epidermis. In contrast, eIF6 was mainly expressed in granular layer and stratum spinosum, as well as stratum corneum, but no obvious positive expression in basal layer and in the dermis of the hypertrophic scar [Figure 1d–f]. However, there is a small amount of keratinocytes in basal layer express eIF6 in the hypertrophic scar of regressive phase [Figure 1f]. The expression level of eIF6 was significantly decreased in the proliferative phase when standardized to normal skin [Figure 2 and Table 2], eIF6 expression level was significantly increased in regressive phase compared with proliferative phase (P = 0.001) and mature phase (P = 0.012). There was no significant difference of eIF6 expression between proliferative phase and mature phase of hypertrophic scar tissue (P = 0.066).
Figure 1.
Immunohistochemistry staining of eukaryotic initiation factor 6 in a different phase of hypertrophic scar and normal skin. (a) Normal skin of hypertrophic scar in proliferative phase; (b) Normal skin of hypertrophic scar in mature phase; (c) Normal skin of hypertrophic scar in regressive phase; (d) Hypertrophic scar in proliferative phase; (e) Hypertrophic scar in mature phase; (f) Hypertrophic scar in regressive phase; the arrows point to the eukaryotic initiation factor 6 positive cells, and the yellow dotted lines presented the basement membrane (basal layer was a single cell layer above the basement membrane). Bar: 50 μm. NS: Normal skin; HS: Hypertrophic scar; PP: Proliferative Phase; MP: Mature Phase; RP: Regressive Phase.
Figure 2.
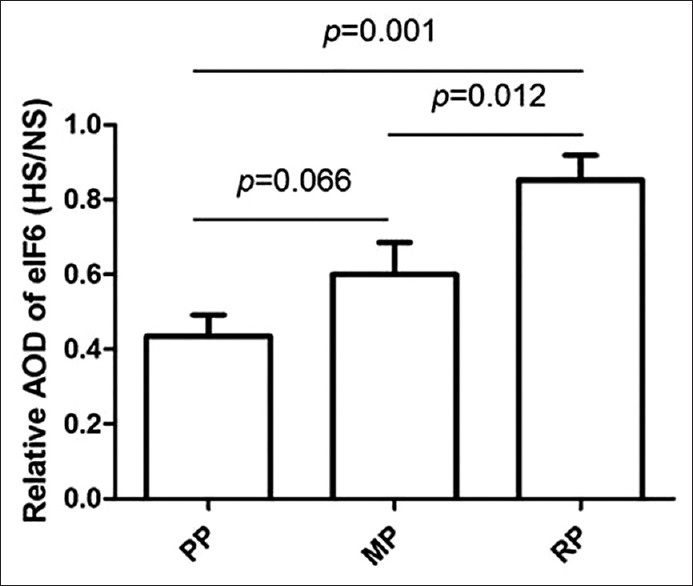
Relative average optical density ratio of eukaryotic initiation factor 6 in hypertrophic scar/normal skin. The data are presented as the mean ± standard deviation, and the statistic result was determined by one-way analysis of variance followed by Turkey multiple comparison. AOD: Average optical density; NS: Normal skin; HS: Hypertrophic scar; PP: Proliferative Phase; MP: Mature Phase; RP: Regressive Phase.
Table 2.
The P values between different stages of HS
| Phase | Morphometric analysis | Western blot analysis | ||||
|---|---|---|---|---|---|---|
| PP | MP | RP | PP | MP | RP | |
| PP | – | 0.066 | 0.001 | – | 0.01 | 0.001 |
| MP | 0.066 | – | 0.012 | 0.01 | – | 0.05 |
| RP | 0.001 | 0.012 | – | 0.001 | 0.05 | – |
HS: Hypertrophic scar; PP: Proliferative phase; MP: Mature phase; RP: Regressive phase.
Eukaryotic initiation factor 6 was down-regulated during the hypertrophic scar development
Western blot analysis showed eIF6 expression level was decreased in hypertrophic scar tissue compared with normal skin [Figure 3]. Furthermore, when standardized to normal skin, the protein level of eIF6 was significantly increased in mature phase and regressive phase of hypertrophic scar compared with proliferative phase of hypertrophic scar (P = 0.01 and P = 0.001, respectively) [Figure 3b and Table 2]. The degree of eIF6 expression was markedly increased in the mature phase of hypertrophic scar compared with that in regressive phase (P = 0.05) [Figure 3b and Table 2].
Figure 3.
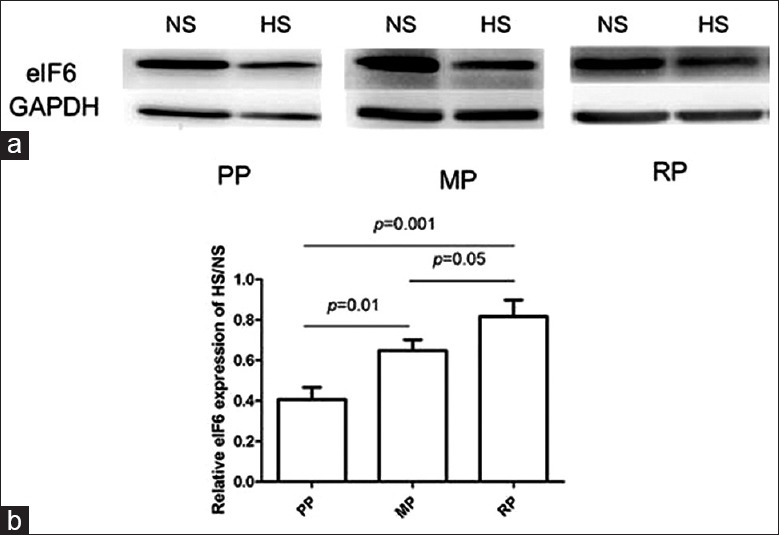
The Western blot analysis of eukaryotic initiation factor 6 expression in hypertrophic scar and normal skin. (a) Western blot analysis of eukaryotic initiation factor 6 expression in normal skin and hypertrophic scar in proliferative phase, mature phase, and regressive phase. (b) Relative eukaryotic initiation factor 6 levels on Western blot analysis in normal skin and hypertrophic scar in proliferative phase, mature phase, and regressive phase. The data were presented as the mean ± standard deviation, and the statistic result was determined by one-way analysis of variance followed by Turkey multiple comparison. NS: Normal skin; HS: Hypertrophic scar; PP: Proliferative Phase; MP: Mature Phase; RP: Regressive Phase.
DISCUSSION
Hypertrophic scar has been a problem since human being came to the earth, and it has been still a big challenge for human being, as we do not know much about the mystery of hypertrophic scar formation as well as we do not have great progress in the control of hypertrophic scar development. Our previous pilot study found that the eIF6 expression was significantly decreased in fibroblasts derived from hypertrophic scar compared with that from the normal skin in vitro. Moreover, blocking transforming growth factor-β (TGF-β) signaling inhibits scar formation in rat cutaneous wounds.[24] eIF6 deficiency also promoted α-smooth muscle actin and TGF-β1 expression in fibroblasts, and cutaneous fibrosis in an animal model (unpublished data). These unpublished data implied the involvement of eIF6 in hypertrophic scar formation in a human being. Therefore, the possible relationship between eIF6 and hypertrophic scar formation was studied by the analysis of eIF6 expression distribution and quantity in the development of hypertrophic scar in the human being through immunohistochemistry and Western blot.
In the current study, eIF6 was down-regulated in human hypertrophic scar tissues compared to normal skin tissues as demonstrated by Western blot and immunohistochemistry, which is consistent with our previous findings in scar-derived fibroblasts in vitro. Importantly, eIF6 expression was related to the moments of hypertrophic scar development, that is, eIF6 was down-regulated in the proliferative phase of hypertrophic scar formation, and gradually increased during the regressive phase. Why does eIF6 have this dynamic expression pattern along with hypertrophic scar development? It is known that eIF6 regulates the biosynthesis of ribosome 60S as well as protein translation.[18,23,25,26] eIF6 is also highly expressed in the cycling cells and has an impact on cell cycle.[27,28] It means that increased eIF6 expression may promote cell proliferation. On the other hand, it is reasonable to speculate that decreased eIF6 expression may impede the cell cycling, but promote the cell function, such as producing more ECM and cell differentiation, like epithelium-to-mesenchymal transition. Therefore, down-regulated eIF6 in the proliferative phase of hypertrophic scar formation may be related to the active function of both epidermal cells and fibroblasts. While, the gradually increased expression of eIF6 in the regressive phase of hypertrophic scar formation indicates the process of reconstruction of the newly-formed regenerative tissues is close to the end. Taken together, eIF6 might be involved in the hypertrophic scar formation, and could be a marker for identifying the moment of hypertrophic scar formation. Restoration of eIF6 level in the cells might be helpful for hypertrophic scar control in the future.
Another finding in our present study was that eIF6 was deficient in the basal layer of epidermis of proliferative phase and recurrence in regressive phase. eIF6 is originally identified in mammals as a cytoplasmic interactor of β4 integrin, which is crucial in hemidesmosome formation, cell adhesion, and responsible for the blister formation of hypertrophic scar surface.[29,30] In the epidermis of the skin, integrins are essential for tissue structure and integrity.[31] The reduction of eIF6 in the early stage of hypertrophic scar implies that connection between newly-formed epidermis and dermis is fragile, and the hypertrophic scar remodeling is yet to be finished. Based on the above analysis, we propose that eIF6 expression level and distribution might be an indicator for the progress of hypertrophic scar development and be helpful for the evaluation of therapy effects, such as pressure therapy and massage. However, further evidence is needed to confirm this hypothesis. A future longitudinal study will be designed to investigate the effectiveness of pressure therapy on scars and the relationship between the clinical expressions and the change of eIF6 in the cells of hypertrophic scar.
In conclusion, eIF6 expression was significantly related to the development of human hypertrophic scar. eIF6 could be one of the target molecules for hypertrophic scar control as well as a potential indicator for evaluation of hypertrophic scar management effects, such as pressure therapy and massage.
Financial support and sponsorship
This work was supported by grants from “863” Project (No. 2012AA020504), the National Natural Science Foundation of China (Nos. 81372082, 81401603).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Zhu Z, Ding J, Shankowsky HA, Tredget EE. The molecular mechanism of hypertrophic scar. J Cell Commun Signal. 2013;7:239–52. doi: 10.1007/s12079-013-0195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns. 2009;35:15–29. doi: 10.1016/j.burns.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Rumsey N, Clarke A, White P. Exploring the psychosocial concerns of outpatients with disfiguring conditions. J Wound Care. 2003;12:247–52. doi: 10.12968/jowc.2003.12.7.26515. [DOI] [PubMed] [Google Scholar]
- 4.Leblebici B, Adam M, Bagis S, Tarim AM, Noyan T, Akman MN, et al. Quality of life after burn injury: The impact of joint contracture. J Burn Care Res. 2006;27:864–8. doi: 10.1097/01.BCR.0000245652.26648.36. [DOI] [PubMed] [Google Scholar]
- 5.Ray S, Ju X, Sun H, Finnerty CC, Herndon DN, Brasier AR. The IL-6 trans-signaling-STAT3 pathway mediates ECM and cellular proliferation in fibroblasts from hypertrophic scar. J Invest Dermatol. 2013;133:1212–20. doi: 10.1038/jid.2012.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle) 2015;4:119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai JK, Song HF, Chen ML, Chen BJ, Jing S, Xu MH, et al. The treatment of deformity of axillary scar contracture after burns (in Chinese) Natl Med J Chin. 2004;84:830–2. [PubMed] [Google Scholar]
- 8.Monstrey S, Middelkoop E, Vranckx JJ, Bassetto F, Ziegler UE, Meaume S, et al. Updated scar management practical guidelines: Non-invasive and invasive measures. J Plast Reconstr Aesthet Surg. 2014;67:1017–25. doi: 10.1016/j.bjps.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Rabello FB, Souza CD, Farina Júnior JA. Update on hypertrophic scar treatment. Clinics (Sao Paulo) 2014;69:565–73. doi: 10.6061/clinics/2014(08)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li-Tsang CW, Feng B, Huang L, Liu X, Shu B, Chan YT, et al. A histological study on the effect of pressure therapy on the activities of myofibroblasts and keratinocytes in hypertrophic scar tissues after burn. Burns. 2015;41:1008–16. doi: 10.1016/j.burns.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Li-Tsang CW, Feng BB, Li KC. Pressure therapy of hypertrophic scar after burns and related research (in Chinese) Chin J Burns. 2010;26:411–5. [PubMed] [Google Scholar]
- 12.Lai HY, Li-Tsang CW, Zheng YP. Effect of different pressure magnitudes on hypertrophic scar in a Chinese population. Burns. 2010;36:1234–41. doi: 10.1016/j.burns.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Zhao H, Chen Y, Fu X. Modifications of traditional pressure gloves for improved performance in scar flexion contracture prevention and fingertip circulation inspection. Burns Trauma. 2014;2:146. doi: 10.4103/2321-3868.134083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng BB, Pao WY, Wu A, Li HC, Li-Tsang CW. Are “smart pressure monitored suits” “smarter” than conventional garments in clinical applications? Hong Kong J Occup Ther. 2013;23:82–8. [Google Scholar]
- 15.Wienert V. Compression treatment after burns. Wien Med Wochenschr. 1999;149:581–2. [PubMed] [Google Scholar]
- 16.Engrav LH, Heimbach DM, Rivara FP, Moore ML, Wang J, Carrougher GJ, et al. 12-Year within-wound study of the effectiveness of custom pressure garment therapy. Burns. 2010;36:975–83. doi: 10.1016/j.burns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Anzarut A, Olson J, Singh P, Rowe BH, Tredget EE. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: A meta-analysis. J Plast Reconstr Aesthet Surg. 2009;62:77–84. doi: 10.1016/j.bjps.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 18.Miluzio A, Beugnet A, Volta V, Biffo S. Eukaryotic initiation factor 6 mediates a continuum between 60S ribosome biogenesis and translation. EMBO Rep. 2009;10:459–65. doi: 10.1038/embor.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng X, Yuan S, Tan J, Ma B, Bian X, Xu C, et al. Identification of ITGB4BP as a new interaction protein of P311. Life Sci. 2012;90:585–90. doi: 10.1016/j.lfs.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Tan J, Peng X, Luo G, Ma B, Cao C, He W, et al. Investigating the role of P311 in the hypertrophic scar. PLoS One. 2010;5:e9995. doi: 10.1371/journal.pone.0009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan J, Luo G, Wu J. The biological roles of ITGB4BP and its potential effect on fibrosis. Int J Burns Trauma. 2011;1:51–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ZY, Zhang J, Lu SL. Objective evaluation of burn and post-surgical scars and the accuracy of subjective scar type judgment. Chin Med J. 2008;121:2517–20. [PubMed] [Google Scholar]
- 23.Sanvito F, Piatti S, Villa A, Bossi M, Lucchini G, Marchisio PC, et al. The beta4 integrin interactor p27(BBP/eIF6) is an essential nuclear matrix protein involved in 60S ribosomal subunit assembly. J Cell Biol. 1999;144:823–37. doi: 10.1083/jcb.144.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Chua CH, Wu XL, Wang DR, Yin DM, Cui L, et al. Inhibiting scar formation in rat cutaneous wounds by blocking TGF-beta signaling (in Chinese) Natl Med J Chin. 2003;83:31–6. [PubMed] [Google Scholar]
- 25.Ceci M, Gaviraghi C, Gorrini C, Sala LA, Offenhäuser N, Marchisio PC, et al. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature. 2003;426:579–84. doi: 10.1038/nature02160. [DOI] [PubMed] [Google Scholar]
- 26.Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science. 2011;334:941–8. doi: 10.1126/science.1211204. [DOI] [PubMed] [Google Scholar]
- 27.Brina D, Grosso S, Miluzio A, Biffo S. Translational control by 80S formation and 60S availability: The central role of eIF6, a rate limiting factor in cell cycle progression and tumorigenesis. Cell Cycle. 2011;10:3441–6. doi: 10.4161/cc.10.20.17796. [DOI] [PubMed] [Google Scholar]
- 28.Vaccaro MC, Cuccaro M, De Marco N, Campanella C. Expression of p27BBP/eIF6 is highly modulated during Xenopus laevis embryogenesis. Mol Reprod Dev. 2006;73:482–90. doi: 10.1002/mrd.20449. [DOI] [PubMed] [Google Scholar]
- 29.Fang IM, Yang CH, Yang CM, Chen MS. Overexpression of integrin alpha6 and beta4 enhances adhesion and proliferation of human retinal pigment epithelial cells on layers of porcine Bruch's membrane. Exp Eye Res. 2009;88:12–21. doi: 10.1016/j.exer.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Biffo S, Sanvito F, Costa S, Preve L, Pignatelli R, Spinardi L, et al. Isolation of a novel beta4 integrin-binding protein (p27(BBP)) highly expressed in epithelial cells. J Biol Chem. 1997;272:30314–21. doi: 10.1074/jbc.272.48.30314. [DOI] [PubMed] [Google Scholar]
- 31.Kenny FN, Connelly JT. Integrin-mediated adhesion and mechano-sensing in cutaneous wound healing. Cell Tissue Res. 2015;360:571–82. doi: 10.1007/s00441-014-2064-9. [DOI] [PubMed] [Google Scholar]