Abstract
Background:
Genetic factors are the main cause of early miscarriage. This study aimed to investigate aneuploidy in spontaneous abortion by fluorescence in situ hybridization (FISH) using probes for 13, 16, 18, 21, 22, X and Y chromosomes.
Methods:
A total of 840 chorionic samples from spontaneous abortion were collected and examined by FISH. We analyzed the incidence and type of abnormal cases and sex ratio in the samples. We also analyzed the relationship between the rate of aneuploidy and parental age, the rate of aneuploidy between recurrent abortion and sporadic abortion, the difference in incidence of aneuploidy between samples from previous artificial abortion and those from no previous induced abortion.
Results:
A total of 832 samples were finally analyzed. 368 (44.23%) were abnormal, in which 84.24% (310/368) were aneuploidies and 15.76% (58/368) were polyploidies. The first was trisomy16 (121/310), followed by trisomy 22, and X monosomy. There was no significant difference in the rate of aneuploidy in the advanced maternal age group (≥35 years old) and young maternal age group (<35 years old). However, the rate of trisomy 22 and the total rate of trisomies 21, 13, and 18 (the number of trisomy 21 plus trisomy 13 and trisomy 18 together) showed significantly different in two groups. We found no skewed sex ratio. There was no significant difference in the rate of aneuploidy between recurrent miscarriage and sporadic abortion or between the samples from previous artificial abortion and those from no previous artificial abortion.
Conclusions:
Aneuploidy is a principal factor of miscarriage and total parental age is a risk factor. There is no skewed sex ratio in spontaneous abortion. There is also no difference in the rate of aneuploidy between recurrent abortion and sporadic abortion or between previous artificial abortion and no previous induced abortion.
Keywords: Aneuploidy, Artificial Abortion, In Situ Hybridization, Miscarriage, Parental Age, Recurrent Abortion, Sex Ratio
INTRODUCTION
Approximately, 15–20% of clinical pregnancies will be a spontaneous miscarriage (SM) during the first trimester. Chorionic samples of spontaneous abortion can be examined by various methods to investigate the etiology of SM, especially genetic factors. Other issues such as a skewed sex ratio in miscarriage and a previous history of artificial abortion that affects the outcome of the subsequent pregnancy and so on were also deserved to be investigated in SM. Fetal chromosomal abnormalities are the primary etiology of SM, especially aneuploidies.[1,2] Chromosome aneuploidy and polyploidy consist of more than 96% of chromosomal abnormalities in spontaneous abortion and X, Y, 13, 16, 18, 21, and 22 are frequently involved. Although conventional karyotyping is the gold standard of diagnosis. However, culture failure, microbial infection of the sample, maternal cell contamination and poor chromosomal preparations often result in failure of conventional karyotyping, with an overall failure rate of 21%.[3] Fluorescence in situ hybridization (FISH) is a reliable diagnostic method for chromosome aneuploidy.[4,5,6]
In this study, we tested chorionic samples from miscarriage to identify aneuploidies or abnormal numbers of 13, 16, 18, 21, 22, X and Y by FISH. This prospective study aimed to apply FISH to detect chromosome aneuploidies. We also aimed to determine the sex ratio in the first trimester and compare the rate of aneuploidy in sporadic abortion with that of recurrent abortion. In addition, we attempted to determine if there was any difference in the rate of aneuploidy between samples from previous artificial abortion and those from no previous artificial abortion. Finally, we aimed to investigate the relationship between the rate of the aneuploidy and total parental age.
METHODS
Sample collection and ethical approval
From 2009 to 2013, we collected 840 chorionic samples from patients who had spontaneous abortion from our hospital. We excluded samples from patients with structural abnormality of genital organs and major diseases, such as diabetes, and thyroid hypofunction. All of the samples were collected from patients who had spontaneous abortion in a natural pregnancy. In order to analyze the relationship between parental age, we divided the samples into five age groups (<25, 25–29, 30–34, 35–39, ≥40 years) according to maternal and paternal age. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committees of Beijing Obstetrics and Gynecology Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Moreover, we got informed consent in writing from each participant before we collected and tested the samples.
Preparation of samples
Each chorionic sample was obtained by curettage and examined under microscopy to avoid contamination of the maternal decidua. Samples were then washed in normal saline. Finally, samples were cut into small pieces, digested in 37°C collagenase for 30 min, centrifuged (500 ×g) and the supernatant was removed. The precipitate was incubated with a hypotonic solution, centrifuged, and the supernatant was removed. The precipitate was fixed by methanol/acetic acid (3:1) for 15 min twice. Finally, the precipitate of each sample was smeared onto three slides for FISH.
Fluorescence in situ hybridization test
Probe Kits for FISH were provided by GP Medical Technologies, Beijing, China. They included three kit sets of FISH probes. One set included GLP 13 (green) and GLP 21 (red), which were labeled by fluorescein isothiocyanate and tetramethylrhodamine, respectively. Another kit included CSP 18 (blue)/CSP X (green)/CSP Y (red), which were labeled by diethyl aminocoumarin, fluorescein isothiocyanate, and tetramethylrhodamine, respectively. The third kit included GLP 16 (red)/GLP 22 (green).
Before FISH testing, the slides were incubated at 46°C for 60 min. The prepared slides were washed with 2 × saline sodium citrate (SSC, pH 7.0) for 5 min twice, treated with 0.1 mol/L HCl for 5 min, and incubated with pepsin in 0.01 mol/L HCl at 37°C for 8 min. The slides were washed again with 2 × SSC for 5 min, dehydrated with ethanol at 70%, 85%, and 100% in sequence, and air-dried. The probe mixtures (each probe mixture included 2 ml probe, 7 ml hybridizing buffer, and 1 ml deionized water) were denatured at 76°C for 5 min. The slides were denatured separately in 70% formamide/2 × SSC at 76°C for 10 min. After denaturation, the slides were dehydrated with −20°C precooled ethanol at 70%, 85%, and 100% in sequence, and air-dried.
The denatured probe mixtures were individually placed onto the prepared slides, and the slides were covered by a cover glass, and then sealed with glue. Hybridization was performed in a wet box at 42°C overnight. After being washed with 50% formamide/2 × SSC at 46°C for 10 min 3 times, 2 × SSC for 10 min, and 2 × SSC/0.1% NP-40 for 5 min, the air-dried slides were restained with 4′,6-diamidino-2-phenylindole for 10–20 min before being analyzed.
Analytical criteria
For each specimen, at least 100 nuclei were evaluated. Our analytic criteria were defined as disomic. If 90% of detected cells were normal, the specimen was defined as normal. If 60% were abnormal, the specimen was diagnosed as having an anomaly. The results were reported as uninformative if the above criteria were not met.
Statistical analysis
We used Logistic regression to analyze relationships of multiple factors. Data are presented as a mean ± standard deviation (SD). SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Significance was set at P < 0.05.
RESULTS
Frequency and type of chromosome aneuploidy
Among 840 samples, we obtained FISH results of 832 samples. Among these, there were 368 (44.23%) abnormal cases. Of these 368 abnormal cases, we found that 310 (84.24%) were aneuploidies involved in the 13, 16, 18, 21, 22, X, and Y chromosomes. A total of 58/368 (15.76%) were polyploidies. The most frequent aneuploidy was trisomy 16 (121/310), followed by trisomy 22. The most frequent monosomy was X monosomy [Table 1].
Table 1.
Distribution of 368 abnormal cases
| Abnormalities | Cases, n |
|---|---|
| Aneuploidy | 310 |
| X monosomy | 57 |
| 21 monosomy | 3 |
| 22 monosomy | 1 |
| 16 trisomy | 121 |
| 21 trisomy | 23 |
| 13 trisomy | 18 |
| 18 trisomy | 11 |
| 22 trisomy | 56 |
| XXY | 2 |
| X trisomy | 2 |
| Trisomy or monosomy involved | 16 |
| in two or more chromosomes | |
| Polyploidy | 58 |
| Total | 368 |
Sex ratio
Of the 832 samples, 415 patients were women and 417 were men. Therefore, the ratio of males to females was approximately 1:1.
Association of aneuploidy with parental age
The relationship between aneuploidy and maternal ages was shown in Tables 2–4. There was no significant difference between the advanced maternal age group (≥35 years old) and the young maternal age group (<35 years old). However, the rate of aneuploidy of the advanced maternal age group (50.9%, as usually advanced maternal age is ≥ 35) was slightly higher than that of the young maternal age group (42.6%). When we further classified aneuploidy, we found that the rate of trisomy 16 was similar in the advanced maternal age and young maternal age groups, and no significant difference was found in the rates of monosomy X in these two groups. However, the rates of trisomy 22 and the total rate of trisomies 21, 13, and 18 of the advanced maternal age group were significantly higher than those of the young maternal age group (P = 0.01, P = 0.027).
Table 2.
Maternal age and aneuploidy
| Maternal age | Cases, n | Percentage of abnormality, n (%) |
|---|---|---|
| <35 | 673 | 287 (42.6) |
| ≥35 | 159 | 81 (50.9) |
| P | 0.058 | |
There was no significant difference between the advanced maternal age group and the young maternal age group (P=0.058).
Table 4.
The relationship between total parental age and aneuploidy
| B | SE | Wals | df | Significant | Exp(B) | |
|---|---|---|---|---|---|---|
| Total age | 0.017 | 0.008 | 4.286 | 1 | 0.038 | 1.017 |
| B | −1.295 | 0.521 | 6.189 | 1 | 0.013 | 0.274 |
The sum of parental age (maternal age plus paternal age) was a risk factor of aneuploidy (P=0.038, OR=1.017). OR=Odds ratio; SE: Standard error.
Table 3.
Maternal age and the ratios of 16, 22, 21, 13, and 18 trisomies and X monosomy
| Maternal age | 16 trisomy, n (%) | 22 trisomy, n (%) | 21+13+18 trisomy, n (%) | X monosomy, n (%) |
|---|---|---|---|---|
| <35 | 98 (14.6) | 38 (5.6) | 35 (5.2) | 45 (6.7) |
| ≥35 | 23 (14.5) | 18 (11.3) | 16 (10.1) | 12 (7.5) |
| P | 0.975 | 0.01* | 0.027† | 0.699 |
The rate of trisomy 16, as well as the rate of monosomy X, was similar in the advanced maternal age and young maternal age groups (P=0.975, P=0.699). The rates of trisomy 22 and the total rate of trisomies 21, 13 and 18 of the advanced maternal age group were significantly higher than those of the young maternal age group (*P=0.01, †P=0.027).
We further divided the samples into five age groups (<25, 25–29, 30–34, 35–39, ≥40 years) according to maternal and paternal age, and compared their rate of aneuploidy among the age groups [Figure 1 and Figure 2]. Consequently, we found the trend that the rate of aneuploidy increased with maternal age. Specifically, the rate of aneuploidy is 29.41% when maternal age <25 years old, and 50.0% when maternal age ≥40 years old [Figure 1], although we did not have significant difference between each two groups. The same trend in paternal could be seen until 40 years old, more specifically, the rate of aneuploidy is 50% when paternal age is 35–39 years old, while 44.33% when paternal age ≥40 years old [Figure 2].
Figure 1.
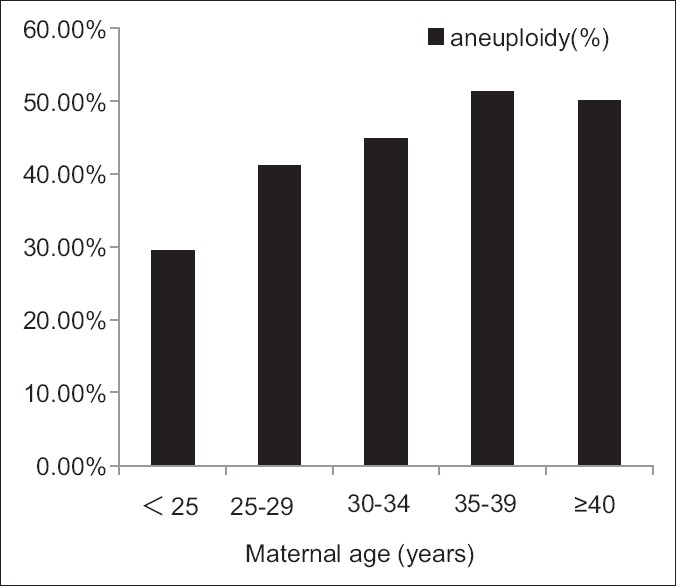
The rate of aneuploidy increased with maternal age, but there was no significant difference between the groups.
Figure 2.
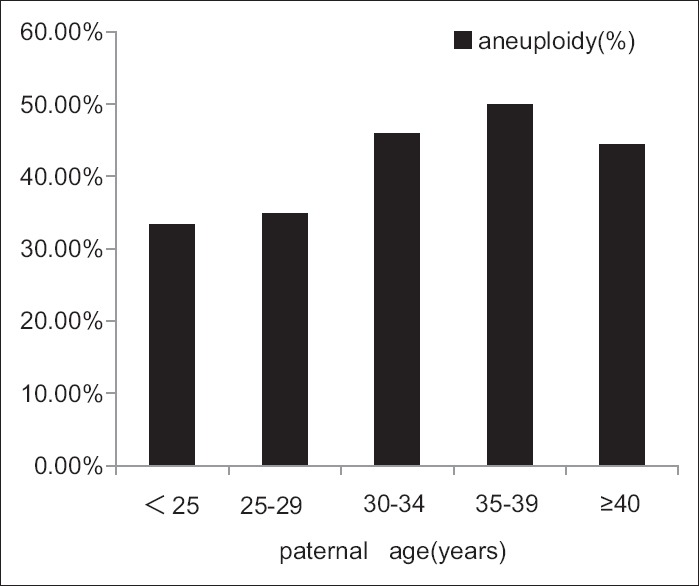
The rate of aneuploidy increased until 40 years old. According to paternal age, the rate of aneuploidy was higher at the age of 35–39 years than at 25–29 years (P = 0.005, X2 test).
We also analyzed the relationship between total parental age (maternal age plus paternal age) and aneuploidy. We found that the sum of parental age was a risk factor of aneuploidy [Table 4]. The Logistic regression equation was as follows: y = total age × 0.017–1.295.
Comparison of aneuploidy between recurrent miscarriage and sporadic abortion
To examine whether common aneuploidy is one of the main causes of recurrent miscarriage, we divided the 832 cases into two groups. One group included cases with no or one previous miscarriage and the other included those with two previous miscarriages. There was no significant difference in previous miscarriage between the two groups (P = 0.497). We also analyzed the relationships between aneuploidy and confounding factors, such as parental age, gravidity, and parity, by Logistic regression. The rate of aneuploidy was not related to the number of miscarriages, paternal age, gravidity, or parity, but it was related to maternal age (odds ratio = 1.055).
Artificial abortion and subsequent spontaneous abortion
We indirectly determined if artificial abortion was a cause of spontaneous abortion of subsequent pregnancy. We divided the cases into four groups according to the number of previous artificial abortions (0, 1, 2, ≥3). We compared the rate of aneuploidy/polyploidy of the four groups but did not find any significant difference among the groups.
DISCUSSION
Frequency and type of chromosome aneuploidy
Approximately, half of miscarriages in the first trimester are caused by fetal chromosomal abnormalities, especially numerical chromosome abnormalities.[7,8] Some researchers have reported that the rate of abnormality involving in chromosone nunber in spontaneous abortion was 23–61%.[9,10] Shearer reported that the rate of numerical chromosome abnormality was 48% in 3361 successful karyotype samples and 93% in abnormal samples.[11] In our study, 832 abortion samples were analyzed, where 44.23% of cases had an abnormal chromosomal complement. This finding is in accordance with the results of research mentioned above[9,10,11] that described using the FISH technique. In this study, in 310 abnormal cases, the commonest kind of aneuploidy is trisomy, especially trisomy 16, which is called “miscarriage chromosome.” Polyploidy was the next most common aneuploidy, followed by X monosomy. However, in the second trimester, the most common aneuploidy is 21-trisomy.[12] This finding indicates that most aneuploidies of fetal miscarriage spontaneously occur during the first trimester.
Skewed sex ratio
Conflicting results have been shown for the sex ratio of normal karyotype spontaneous abortions.[13,14] However, most studies have shown a greater number of females than males.[15] Our study indicated that 415 cases were females and 417 cases were males, with an approximate ratio of males to females of 1:1. This difference between studies may be due to the fact that we only analyzed seven chromosomes.
Association of aneuploidy with parental age
Many reports have suggested that advanced maternal age is an important factor related to chromosomal aneuploidies. Our study showed no significant difference in the rate of aneuploidy between the advanced maternal age group (≥35 years) and the young maternal age group (<35 years). However, when we compared the ratios of trisomy 16, trisomy 22, trisomies 21 + 13 + 18, and X monosomy between the two age groups, we found that the rates of trisomy 22 and trisomies 21 + 13 + 18 were significantly higher in the advanced maternal age group than in the young maternal age group. However, trisomy 16 and X monosomy showed no differences between the two age groups. These findings suggest that not all chromosome aneuploidies are related to advanced age. However, when we grouped the cases in five subgroups according to maternal age, we found a trend that the rate of aneuploidy increased with an increase in maternal age, but there was no significant difference among the groups. Moreover, paternal age has been reported to be involved in fetal aneuploidy.[16] We also analyzed the association paternal age with aneuploidy and found that the rate of aneuploidy increased with an increase in paternal age until 40 years old, when this rate then decreased. The rate of aneuploidy was significantly higher at the age of 35–39 years than at 25–29 years. All of these findings indicated that parental age was the main factor for inducing aneuploidy, and paternal age >35 years old may be the critical age, from which the aneuploidy increased significantly. Therefore, we examined the association between aneuploidy and total parental age (maternal age plus parental age). We observed that the sum of parental age was a risk factor of aneuploidy, and the Logistic regression equation was as follows: y = total age × 0.017–1.295.
Comparison of aneuploidy between recurrent miscarriage and sporadic abortion
The definition of recurrent pregnancy loss is when at least two or more miscarriages have occurred.[17,18] The rate of recurrent pregnancy loss is approximately 5% in all couples.[3] In women with recurrent miscarriage, the prevalence of chromosome aneuploidy greatly varies,[19,20] whether tested by the gold standard or molecular genetics techniques. To determine whether aneuploidy is the main cause of recurrent miscarriage, we compared the rate of aneuploidy of recurrent miscarriage with that of sporadic abortion and found no significant difference between them. Therefore, there may be an alternative mechanism responsible for the majority of recurrent miscarriages. Considering confounding factors related to miscarriage, such as parental age, gravidity, and parity, we only found that aneuploidy was related to maternal age.
Artificial abortion and subsequent spontaneous abortion
There are few data on whether artificial abortion may be related to subsequent miscarriage. BALB/c mice experiment result showed that repeated early medical abortions led to spontaneous abortion and pregnancy loss during subsequent pregnancies.[21] In humans, artificial abortion might be associated with an increased risk of first-trimester miscarriage in subsequent pregnancies. To indirectly determine whether artificial abortion is a cause of spontaneous abortion of subsequent pregnancy, we compared the rate of aneuploidy/polyploidy according to the number of previous artificial abortions. However, we did not find any significant difference in the rate of aneuploidy/polyploidy according to this number. Therefore, artificial abortion may not be related to subsequent miscarriage.
A limitation of this study was that we did not obtain all parental karyotypes. However, the rate of abnormal karyotypes in parents with a history of an adverse outcome of pregnancy was approximately 2–3%. Therefore, we consider that karyotypes of parents may not have severely affected the results of this study.
In conclusion, chromosomal abnormalities are still a major cause of miscarriage. More chromosomal abnormalities may be found by conventional karyotyping than by the use of FISH. FISH is still a reliable and quick method to test aneuploidy in miscarriage. With the advent of array comparative genomic hybridization and array-single nucleotide, an increasing amount of genetic factors may be found in spontaneous abortion.
Financial support and sponsorship
This work was supported by grants from the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (No. ZYLX201510) and the Beijing Municipal Science and Technology Commission (No. Z121107001012165).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Hassold T, Jacobs P. Trisomy in man. Ann Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- 2.Petracchi F, Colaci DS, Igarzabal L, Gadow E. Cytogenetic analysis of first trimester pregnancy loss. Int J Gynaecol Obstet. 2009;104:243–4. doi: 10.1016/j.ijgo.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 3.van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta. 2012;1822:1951–9. doi: 10.1016/j.bbadis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Bryndorf T, Christensen B, Vad M, Parner J, Carelli MP, Ward BE, et al. Prenatal detection of chromosome aneuploidies in uncultured chorionic villus samples by FISH. Am J Hum Genet. 1996;59:918–26. [PMC free article] [PubMed] [Google Scholar]
- 5.Mann K, Donaghue C, Fox SP, Docherty Z, Ogilvie CM. Strategies for the rapid prenatal diagnosis of chromosome aneuploidy. Eur J Hum Genet. 2004;12:907–15. doi: 10.1038/sj.ejhg.5201224. [DOI] [PubMed] [Google Scholar]
- 6.Jia CW, Wang SY, Ma YM, Lan YL, Si YM, Yu L, et al. Fluorescence in situ hybridization in uncultured amniocytes for detection of aneuploidy in 4210 prenatal cases. Chin Med J (Engl) 2011;124:1164–8. [PubMed] [Google Scholar]
- 7.Dória S, Carvalho F, Ramalho C, Lima V, Francisco T, Machado AP, et al. An efficient protocol for the detection of chromosomal abnormalities in spontaneous miscarriages or foetal deaths. Eur J Obstet Gynecol Reprod Biol. 2009;147:144–50. doi: 10.1016/j.ejogrb.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho B, Dória S, Ramalho C, Brandão O, Sousa M, Matias A, et al. Aneuploidies detection in miscarriages and fetal deaths using multiplex ligation-dependent probe amplification: An alternative for speeding up results? Eur J Obstet Gynecol Reprod Biol. 2010;153:151–5. doi: 10.1016/j.ejogrb.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Menten B, Swerts K, Delle Chiaie B, Janssens S, Buysse K, Philippé J, et al. Array comparative genomic hybridization and flow cytometry analysis of spontaneous abortions and mors in utero samples. BMC Med Genet. 2009;10:89. doi: 10.1186/1471-2350-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomax B, Tang S, Separovic E, Phillips D, Hillard E, Thomson T, et al. Comparative genomic hybridization in combination with flow cytometry improves results of cytogenetic analysis of spontaneous abortions. Am J Hum Genet. 2000;66:1516–21. doi: 10.1086/302878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shearer BM, Thorland EC, Carlson AW, Jalal SM, Ketterling RP. Reflex fluorescent in situ hybridization testing for unsuccessful product of conception cultures: A retrospective analysis of 5555 samples attempted by conventional cytogenetics and fluorescent in situ hybridization. Genet Med. 2011;13:545–52. doi: 10.1097/GIM.0b013e31820c685b. [DOI] [PubMed] [Google Scholar]
- 12.Jia CW, Lan Y, Si YM, Ma YM, Lang Y, Wang SY, et al. The analysis of the factors related to abnormal karyotyes of fetus. Chin J Med Genet (Chin) 2013;30:635–7. [Google Scholar]
- 13.Hassold T, Chen N, Funkhouser J, Jooss T, Manuel B, Matsuura J, et al. A cytogenetic study of 1000 spontaneous abortions. Ann Hum Genet. 1980;44(Pt 2):151–78. doi: 10.1111/j.1469-1809.1980.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 14.Songster GS, Sun L, Chang SC, Cheung SW. Chromosome analysis in spontaneous pregnancy loss: Use of placental villus mesodermal core cell cultures. Am J Med Genet. 1992;42:785–8. doi: 10.1002/ajmg.1320420607. [DOI] [PubMed] [Google Scholar]
- 15.Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril. 2010;93:1234–43. doi: 10.1016/j.fertnstert.2009.01.166. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs PA, Hassold TJ. The origin of numerical chromosome abnormalities. Adv Genet. 1995;33:101–33. doi: 10.1016/s0065-2660(08)60332-6. [DOI] [PubMed] [Google Scholar]
- 17.van den Boogaard E, Kaandorp SP, Franssen MT, Mol BW, Leschot NJ, Wouters CH, et al. Consecutive or non-consecutive recurrent miscarriage: Is there any difference in carrier status? Hum Reprod. 2010;25:1411–4. doi: 10.1093/humrep/deq089. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan AE, Silver RM, LaCoursiere DY, Porter TF, Branch DW. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol. 2004;104:784–8. doi: 10.1097/01.AOG.0000137832.86727.e2. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73:300–4. doi: 10.1016/s0015-0282(99)00495-1. [DOI] [PubMed] [Google Scholar]
- 20.Carp HJ. Recurrent miscarriage: Genetic factors and assessment of the embryo. Isr Med Assoc J. 2008;10:229–31. [PubMed] [Google Scholar]
- 21.Lv F, Xu X, Zhang S, Wang L, Wang N, He B, et al. Repeated abortion affects subsequent pregnancy outcomes in BALB/c mice. PLoS One. 2012;7:e48384. doi: 10.1371/journal.pone.0048384. [DOI] [PMC free article] [PubMed] [Google Scholar]


