Abstract
Background:
Although human parainfluenza virus (HPIV) has been determined as an important viral cause of acute respiratory infections (ARIs) in infants and young children, data on long-term investigation are still lacking to disclose the infection pattern of HPIV in China.
Methods:
Nasopharyngeal aspirates were collected from 25,773 hospitalized pediatric patients with ARIs from January 2004 through December 2012 for respiratory virus screen by direct immuno-fluorescence assay.
Results:
Out of these specimens, 1675 (6.50%, 1675/25,773) showed HPIV positive, including 261 (1.01%, 261/25,773) for HPIV1, 28 (0.11%, 28/25,773) for HPIV2, and 1388 (5.39%, 1388/25,773) for HPIV3, 2 of the samples were positive for both HPIV1 and HPIV3, and 36 were co-detected with other viruses. The positive rates of HPIVs were higher in those younger than 3 years old. HPIV3 was detected from all age groups, predominantly from patients under 3 years of age, and the highest frequency was found in those 6 months to 1-year old (352/4077, 8.63%). HPIV3 was the dominant type in each of the years detected between May and July. HPIV1 showed a peak in every odd year, mainly in August or September. HPIV was detected most frequently from patients with upper respiratory infection (12.49%, 157/1257), followed by bronchitis (11.13%, 176/2479), asthma (9.31%, 43/462), bronchiolitis (5.91%, 150/2536), pneumonia (6.06%, 1034/17,068), and those with underlying diseases (1.0%, 15/1506). HPIV3 is the dominant type in these six disease groups referred above, especially in the asthma group.
Conclusions:
HPIV is one of the important viral causes of ARIs in infants and young children in Beijing based on the data from the hospitalized children covering a 9-year term. HPIV3 is the predominant type in all these years and in most of the disease groups. HPIVs with different types show different seasonality.
Keywords: Acute Respiratory Infection, China, Human Parainfluenza Virus, Infants and Young Children
INTRODUCTION
Human parainfluenza viruses (HPIVs) are enveloped, negative-sense RNA viruses that belong to the family Paramyxoviridae for their poor growth in embryonated eggs and different antigenic sites from myxoviridae. Four genetically different types have been defined, including HPIV1 and 3 belonging to the genus Respirovirus and HPIV2 and 4 to the genus Rubulavirus.[1] HPIV4 is further divided into 4A and 4B subtypes by hemagglutination inhibition and neutralizing tests,[2] while HPIV1 was divided into two major linages[3] and HPIV3 was divided into three major lineages.[4,5]
Although HPIV4 is rarely reported, HPIVs1-3, first discovered in 1959, are important causes of various acute respiratory infections (ARIs) worldwide, including rhinitis, otitis, croups, bronchitis, bronchiolitis, and pneumonia, which can be found in both children and adults, and can cause severe symptoms in immunocompromised hosts, especially in those undergoing bone marrow transplantation.[6] Risk factors that increase the incidence and severity of lower respiratory infections (LRIs) in patients living in developing countries include large family size, crowded living condition, low birth weight, malnutrition, Vitamin A deficiency, lack of breastfeeding, pollution, and young age.[7]
The Naval Medical Research Unit No. 6 conducted surveillance of viral respiratory pathogens in several Latin American countries over the past decades,[8,9] and Villaran et al. provided epidemiologic and phylogenetic information about HPIVs that circulated from Central and South America.[10] Mizuta et al. evaluated the HPIVs infection in Japan between 2001 and 2011.[11] While only a few epidemiological studies and data on HPIVs were reported in relatively short period in China.[12,13,14] HPIV infections follow both endemic and epidemic patterns, and the seasonality of HPIVs varies depending on regions, distinct variations being observed from year to year,[11] therefore more data covering a longer period should be accumulated to disclose the regional HPIV infection pattern and the importance of these viruses to pediatric patients with ARIs in China.
METHODS
Patients and specimens
Nasopharyngeal aspirates (NPAs) from 25,773 hospitalized pediatric patients in the affiliated Children's Hospital to Capital Institute of Pediatrics for ARIs during January 2004 to December 2012 were enrolled. Age of these patients ranged from 2 h to 18 years old, and 16,277 boys and 9496 girls.
Clinical diagnoses for the enrolled patients were defined by the pediatric doctors in the hospital and the diagnostic standards were referred to the seventh edition of Zhu Futang Practical Pediatrics.[15] The patients whose specimens were enrolled in this research were categorized retrospectively into the following groups according to their clinical diagnosis: Fever only, upper respiratory infection (URI), bronchitis, bronchiolitis, pneumonia, asthma, ARIs complicated with underlying diseases, such as Kawasaki disease, rheumatoid arthritis, acute lymphatic leukemia, idiopathic thrombocytopenic purpura, infectious mononucleosis, Langerhans cell hyperplasia, and epilepsy.
Screening for respiratory viruses including human parainfluenza virus 1–3
Upon arrival, all NPAs were processed routinely and then centrifuged at 500 ×g for 10 min. The pellets from NPAs were re-suspended by several drops of sterile PBS and spotted onto an acetone-cleaned slide for viral antigen detection. Individual monoclonal antibody reagents, labeled with fluorescein-isothiocyanate (FITC) against respiratory syncytial virus (RSV), human adenovirus (HAdV), influenza virus (Flu) A and B, and HPIV1–3, were used for specific virus identification by direct immuno-fluorescence assay (DFA) with D3® Ultra™ DFA Respiratory Virus Screening and ID Kit (Diagnostic Hybrids, Inc., USA). In addition, FITC labeled monoclonal antibody against human metapneumovirus (HMPV) (Diagnostic Hybrids, Inc., USA) was used simultaneously for HMPV identification.
The study was approved by the Ethics Committee of Capital Institute of Pediatrics.
Statistical analysis
For comparisons of categorical data, Chi-square test and Fisher's exact test in SPSS version 17.0 (SPSS Inc., USA) were used where appropriate. All tests were two-tailed and a P < 0.05 was considered as statistically significant.
RESULTS
Human parainfluenza virus 1–3 screening from children with acute respiratory infection
Out of these 25,773 specimens collected from patients with ARIs and tested for respiratory viruses, 1675 (6.50%, 1675/25,773) showed HPIV positive, including 261 (1.01%, 261/25,773) positive for HPIV1, 28 (0.11%, 28/25,773) for HPIV2, and 1388 (5.39%, 1388/25,773) for HPIV3. Two of these HPIV positive samples were positive for both HPIV1 and 3, therefore the sum of these positive for HPIV types is 1677 instead of 1675. In addition, 36 of the HPIV positive NPAs were co-detected with other viral pathogens determined by the DFA, including 8 positive for HPIV1 (6 RSV, 1 FluA, and 1 HAdV), 27 positive for HPIV3 (16 RSV, 7 HAdV, 2 HMPV, 1 FluB, and 1 FluA), and 1 positive for three pathogens (HPIV3, RSV, and FluB). Viral pathogens with the highest frequency of co-infection with HPIVs were RSV (23/36, 63.89%) followed by HAdV (8/36, 22.22%).
Age distribution of patients with human parainfluenza virus infection
HPIVs were more frequently detected in specimens from children younger than 3 years old [Table 1]. Among them, HPIV3 was detected from all age groups, predominantly from patients under 3 years of age, and the highest frequency (8.63%; 352/4077) was found in those 6 months to 1-year old. In general, positive rates of HPIV1 and 2 were lower than that of HPIV3 in all age groups. More HPIV1 was detected in children younger than 4 years old. The frequency of HPIV2 infection was the lowest among that of HPIV1–3, and no HPIV2 positive specimen was detected from children older than 8 years [Table 1].
Table 1.
Age distribution of HPIV positive patients, n (%)
| Groups | n | HPIV1 | HPIV2 | HPIV3 | HPIVs |
|---|---|---|---|---|---|
| ≤6 months | 8538 | 60 (0.70) | 4 (0.05) | 469 (5.49) | 533 (6.24) |
| ~1-year | 4077 | 62 (1.52) | 7 (0.17) | 352 (8.63) | 421 (10.33) |
| ~2 years | 3675 | 60 (1.63) | 5 (0.14) | 309 (8.41) | 374 (10.18) |
| ~3 years | 2464 | 38 (1.54) | 3 (0.12) | 124 (5.03) | 165 (6.70) |
| ~4 years | 1980 | 23 (1.16) | 3 (0.15) | 62 (3.13) | 88 (4.44) |
| ~5 years | 1081 | 6 (0.56) | 2 (0.19) | 17 (1.57) | 25 (2.31) |
| ~6 years | 798 | 3 (0.38) | 2 (0.25) | 17 (2.13) | 22 (2.76) |
| ~7 years | 750 | 4 (0.53) | 1 (0.13) | 8 (1.07) | 13 (1.73) |
| ~8 years | 635 | 2 (0.31) | 1 (0.16) | 9 (1.42) | 12 (1.89) |
| ~9 years | 513 | 1 (0.19) | 0 (0) | 5 (0.97) | 6 (1.17) |
| ~10 years | 421 | 0 (0) | 0 (0) | 7 (1.66) | 7 (1.66) |
| ~11 years | 288 | 0 (0) | 0 (0) | 1 (0.35) | 1 (0.35) |
| ~12 years | 230 | 0 (0) | 0 (0) | 2 (0.87) | 2 (0.87) |
| >12 years | 323 | 2 (0.62) | 0 (0) | 6 (1.86) | 8 (2.48) |
| Total | 25,773 | 261 (1.01) | 28 (0.11) | 1388 (5.39) | 1675 (6.50) |
HPIV: Human parainfluenza virus.
Seasonal distribution of human parainfluenza viruses
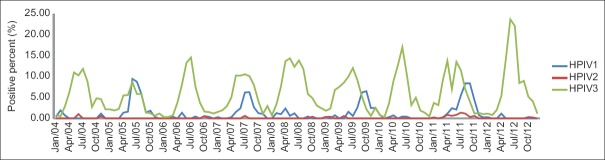
During the period of January 2004 through December 2012 in the study, HPIV3 was the predominant type in each of the years with various frequencies (2.92%, 69/2364 to 6.77%, 178/2630) from year to year [Figure 1], and there is an increasing HPIV3 frequency from 2005 to 2008 (2.92%, 69/2364 to 6.77%, 178/2630), then decreasing from 2008 to 2010 (6.77%, 178/2630 to 5.00%, 168/3363), followed with another increasing (5.00%, 168/3363 to 7.34%, 281/3826) from 2010 to 2012, which form trends from trough to peak, then to trough. The increase from trough to peak varied from 2.34% to 3.85%. HPIV1 was the second one with a higher frequency from 1.51% (43/2843) to 2.43% (97/3990) in every other year, including 2005, 2007, 2009, and 2011. HPIV2 was the one with the lowest frequency in each of the years [Figure 1].
Figure 1.

Human parainfluenza virus 1–3 detected in each of the calendar years during 2004 to 2012.
Among these years, the activity of HPIV3 usually increased at the junction of spring and summer (from May to June in each of the years), then reached the peak in July 2004, 2006, 2007, 2008, and 2009, or June 2005, 2010, 2011, and 2012 with frequencies varying from 8.66% (11/127) to 23.69% (59/249), and decreased at the junction of summer and autumn (from August to September in each of the years), then reached the trough in winter. However, there were other peaks in May 2008 and 2011, and in April 2011, respectively [Figure 2].
Figure 2.
Seasonality of human parainfluenza viruses: Proportion detected in each calendar month over every year during 2004 to 2012.
Although the seasonality of HPIV1 is similar to that of HPIV3 both of them with the peak in summer, the peak of HPIV1 appeared mainly in August or September in every other year, after that of HPIV3, except 2005, which was in June.
Only a few HPIV2 positive specimens were detected in each calendar month over every year during 2004 to 2012, no seasonality character of HPIV2 was shown in Figure 2.
Clinical diagnosis for children with human parainfluenza virus infections
More than 60% (17,068/25,773, 66.22%) of the pediatric patients involved in the study were diagnosed with pneumonia, and the clinical diagnosis for those HPIVs positive patients listed in Table 2 indicated that specimens from patients with URI had the highest HPIVs detection rate (12.49%, 157/1257), followed by patients diagnosed as bronchitis (11.13%, 276/2479), asthma (9.31%, 43/462), pneumonia (6.06%, 1034/17,068), and bronchiolitis (5.91%, 150/2536). No HPIV was detected from patients with the diagnosis of unexplained fever.
Table 2.
Clinical diagnosis of patients infected with HPIVs
| Diagnosis | n | HPIV1, n (%) | HPIV2, n (%) | HPIV3, n (%) | P* | HPIVs (1, 2, 3), n (%) |
|---|---|---|---|---|---|---|
| URI | 1257 | 47 (3.74) | 6 (0.48) | 104 (8.27) | <0.01 | 157 (12.49) |
| Bronchitis | 2479 | 53 (2.14) | 8 (0.32) | 215 (8.67) | <0.01 | 276 (11.13) |
| Bronchiolitis | 2536 | 12 (0.47) | 2 (0.08) | 136 (5.36) | <0.01 | 150 (5.91) |
| Pneumonia | 17,068 | 145 (0.85) | 12 (0.07) | 877 (5.14) | <0.01 | 1034 (6.06) |
| Asthma | 462 | 2 (0.43) | 0 (0) | 41 (8.87) | <0.01 | 43 (9.31) |
| Underlying diseases | 1506 | 2 (0.13) | 0 (0) | 15 (1.00) | <0.01 | 17 (1.13) |
| Fever | 465 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total | 25,773 | 261 (1.01) | 28 (0.11) | 1388 (5.39) | <0.01 | 1675 (6.50) |
*Two-tailed Chi-square test, testing the distribution of each illness or diagnosis between the three HPIV types. HPIV: Human parainfluenza virus; URI: Upper respiratory infection.
Compared to HPIV1 and 2, 3 was shown more positive specimens and higher positive rates in five of the six groups except group of fever (P < 0.01), especially from patients diagnosed as asthma (8.87%:0.43%:0), bronchitis (8.67%:2.14%:0.32%), and URI (8.27%:3.74%:0.48%). HPIV3 was detected most frequently from patients with asthma (8.87%, 41/462), followed by those with bronchitis (8.67%, 215/2479), URI (8.27%, 104/1257), bronchiolitis (5.36%, 136/2536), pneumonia (5.14%, 877/17,068), and ARIs with other underlying diseases (1.00%, 15/1506). HPIV1 was detected most frequently from patients with URI (3.74%, 47/1257), followed by those with bronchitis (2.14%, 53/2479), pneumonia (0.85%, 145/17,068), bronchiolitis (0.47%, 12/2536), asthma (0.43%, 2/462), and ARIs with other underlying disease (0.13%, 2/1506). The most predominant type of HPIVs detected from patients with severe lower respiratory infections (LRIs), such as pneumonia and bronchiolitis, was HPIV3.
Among the HPIV positive, 2 patients co-infected with HPIV1 and HPIV3 and 36 with other respiratory viruses. Taking together, 38 were detected with more than 2 viruses. Among them, 26 (68.42%, 26/38) were diagnosed with pneumonia, 5 (13.16%, 5/38) with bronchiolitis, 5 (13.16%, 5/38) with bronchitis, and 2 with URI (5.26%, 2/38) which indicated that most of the co-infected were with LRIs. Furthermore, 9 of these 38 (23.68%, 9/38) were diagnosed with the underlying disease [Table 2].
DISCUSSION
HPIV is one of the important etiological viruses of ARIs in infants and young children in Beijing, which has been determined previously by virus isolation and/or multiplex reverse transcription-polymerase chain reaction, respectively.[12,16] However, those data were based on a relatively short term, so more data covering a longer time during 2004–2012 were accumulated in this study to disclose the regional HPIV infection pattern in Beijing, China.
In this study, it was revealed that HPIV3 is the predominant type among HPIVs1–3 (P < 0.01). In central and South America during 2006 through 2010, the HPIV type most frequently isolated was HPIV3.[10] In Thai, children with lower respiratory tract infection from 2010 to 2013, Ruampunpong et al. reported that 4.8% of the samples were positive for HPIV, among which 0.5%, 2.5%, and 1.5% were positive for HPIV1, 3, and 4, respectively, and none were positive for HPIV2.[17] Yano et al. reported that compared with other HPIV types, the prevalence rates of HPIV3 were the highest in Mie Prefecture in Japan,[18] while Mizuta et al. isolated more HPIV3 (3.4%) strains than HPIV1 (1.8%) and HPIV2 (0.9%) between 2002 and 2011 at pediatric clinics in Yamagata, Japan.[11] In Guangzhou, China, 3.7% were positive for HPIV, including 2.1% for HPIV3, 1.2% for HPIV1, 0.4% for HPIV2, and 0.2% for HPIV4.[14] The data demonstrated HPIV3 the predominant type among HPIVs in various regions worldwide.
The age distribution in this hospital-based study indicated that children younger than 3 years old were more likely to be infected by HPIVs, and HPIV3 infection among HPIVs was more common than HPIV1 and 2 in all age groups, especially in those under 3 years of age with the highest frequency (8.63%) in those 6 months to 1-year old. HPIV1 infection was more common in children younger than 4 years old with the higher frequencies in age groups of 1–2 years (1.63%), 2–3 years (1.54%), and 6–12 months (1.52%). Although HPIV2 was the lowest detected one among HPIV1–3, it is easier to find HPIV2 infection in children younger than 8 years. It is generally accepted that HPIV3, as well as RSV infection, are common in infants and young children, whereas HPIV1 and 2 infections tend to be common in older persons.[11] However, the age distribution of both HPIV1 and 3 infections following the similar trend peak at 6 months to 3 years of age in the study. Only a few HPIV2 positive cases were shown in the study, so it is difficult to confirm if the age distribution trend of HPIV2 in children younger than 8 years old is different from HPIV1 and 3.
Compared to the data from a short period, which had suggested that no regular seasonal distribution of HPIV infection was found, except that HPIV1 and 3 were more common than HPIV2 and 4,[12,16] this study revealed the seasonality of HPIV activity based on the data from specimens collected during a period of nearly 10 years. The data of seasonal distribution of HPIVs during January 2004 to December 2012 indicated that HPIV3 was the dominant type in each year with different frequencies from year to year, which formed trends from trough to peak, then to trough. The circulation of HPIV1 infection was shown very clearly in Figure 1, which revealed a peak of HPIV1 infection in every odd year in the study, such as 2005, 2007, 2009, and 2011.
The peak of HPIV1 appeared mainly in August or September in every other year, after that of HPIV3, except that in June 2005 in the study. Although Mizuta et al. also conducted a long-term investigation (during 2002 to 2011) on HPIV infection; they found no clear seasonality of HPIV1 infections, and in Japan, including the NESID system, has indicated a seasonal pattern in HPIV1 infections. These data support the conclusion that HPIV infections follow both endemic and epidemic patterns, and the seasonality of HPIVs varies depending on regions. In this study, HPIV3 infection was shown mainly in May to July, mostly with one peak in July or June in each of the years, occasionally with a second peaks in May 2008 or April 2011, which is consistent with the ideas of Mizuta et al. that HPIV3 infection was grouped in clear annual seasonal outbreaks, mainly between May and July.[11] However, no seasonality character of HPIV2 was shown in the study because of the low frequency, which is contrary to the conclusion that HPIV2 infections commonly occurred in the autumn-winter season every 2 years in Japan.[11] More data should be accumulated to reveal the seasonal character of HPIV2.
The data of HIPVs’ clinical characteristics based on the hospitalized patients indicated that HPIV infection was detected not only in patients diagnosed as URI, but also in those diagnosed as LRI, including bronchitis, bronchiolitis, and pneumonia. HPIV3 is the dominant type among HPIV1–3 in six disease groups (P < 0.01), especially in the group of asthma. Because no monoclonal antibody against HPIV4 is available so far, the relationship of HPIV4 with ARIs in children could not be included in this study in which antigen detection was used.
In conclusion, HPIV is one of the important viral causes of ARIs in infants and young children in Beijing based on the data from a long-term study covering almost 10 years. Children younger than 3 years old are more susceptible to HPIVs infection. Among HPIVs1–3, HPIV3 is the dominant type in all these years and in all disease groups. And, this study revealed clearly the seasonal character of HPIV1 with a peak in every odd year and HPIV3 with trends from trough to peak, then to trough.
Financial support and sponsorship
This study was supported by the grants from the Beijing Municipal Science and Technology Commission (No. Z111107056811041) and the Beijing Municipal Health Bureau “Advanced Personnel Training Program” (No. 2011-3-068).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Karron RA, Collins PL. Fields Virology. 5th ed. Philadepia, PA 19106, USA: Knipe DM, Lippincott Williams and Wilkins; 2007. Parainfluenza viruses; pp. 1497–152. [Google Scholar]
- 2.Lau SK, Li KS, Chau KY, So LY, Lee RA, Lau YL, et al. Clinical and molecular epidemiology of human parainfluenza virus 4 infections in Hong Kong: Subtype 4B as common as subtype 4A. J Clin Microbiol. 2009;47:1549–52. doi: 10.1128/JCM.00047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuta K, Saitoh M, Kobayashi M, Tsukagoshi H, Aoki Y, Ikeda T, et al. Detailed genetic analysis of hemagglutinin-neuraminidase glycoprotein gene in human parainfluenza virus type 1 isolates from patients with acute respiratory infection between 2002 and 2009 in Yamagata prefecture, Japan. Virol J. 2011;8:533. doi: 10.1186/1743-422X-8-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizuta K, Tsukagoshi H, Ikeda T, Aoki Y, Abiko C, Itagaki T, et al. Molecular evolution of the haemagglutinin-neuraminidase gene in human parainfluenza virus type 3 isolates from children with acute respiratory illness in Yamagata prefecture, Japan. J Med Microbiol. 2014;63(Pt 4):570–7. doi: 10.1099/jmm.0.068189-0. [DOI] [PubMed] [Google Scholar]
- 5.Almajhdi FN. Hemagglutinin-neuraminidase gene sequence-based reclassification of human parainfluenza virus 3 variants. Intervirology. 2015;58:35–40. doi: 10.1159/000369208. [DOI] [PubMed] [Google Scholar]
- 6.Billaud G, Morfin F, Vabret A, Boucher A, Gillet Y, Crassard N, et al. Human parainfluenza virus type 4 infections: A report of 20 cases from 1998 to 2002. J Clin Virol. 2005;34:48–51. doi: 10.1016/j.jcv.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Berman S. Epidemiology of acute respiratory infections in children of developing countries. Rev Infect Dis. 1991;13(Suppl 6):S454–62. doi: 10.1093/clinids/13.supplement_6.s454. [DOI] [PubMed] [Google Scholar]
- 8.Laguna-Torres VA, Gómez J, Ocaña V, Aguilar P, Saldarriaga T, Chavez E, et al. Influenza-like illness sentinel surveillance in Peru. PLoS One. 2009;4:e6118. doi: 10.1371/journal.pone.0006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comach G, Teneza-Mora N, Kochel TJ, Espino C, Sierra G, Camacho DE, et al. Sentinel surveillance of influenza-like illness in two hospitals in Maracay, Venezuela: 2006-2010. PLoS One. 2012;7:e44511. doi: 10.1371/journal.pone.0044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villaran MV, García J, Gomez J, Arango AE, Gonzales M, Chicaiza W, et al. Human parainfluenza virus in patients with influenza-like illness from Central and South America during 2006-2010. Influenza Other Respir Viruses. 2014;8:217–27. doi: 10.1111/irv.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuta K, Abiko C, Aoki Y, Ikeda T, Itagaki T, Katsushima F, et al. Epidemiology of parainfluenza virus types 1, 2 and 3 infections based on virus isolation between 2002 and 2011 in Yamagata, Japan. Microbiol Immunol. 2012;56:855–8. doi: 10.1111/j.1348-0421.2012.00507.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Zhao LQ, Deng J, Zhu RN, Qian Y. Parainfluenza virus infections in pediatric patients with acute respiratory infections in Beijing during 2001-2003 (in Chinese) Chin J Epidemol. 2006;27:44–6. [PubMed] [Google Scholar]
- 13.Huang Z, Dong L, Chen X, Zhang H, Zhou X, Luo Y, et al. Epidemiologic features of parainfluenza virus infection in children in Wenzhou Area (in Chinese) J Appl Clinic Pediatr. 2006;21:1066–7. [Google Scholar]
- 14.Liu WK, Liu Q, Chen DH, Liang HX, Chen XK, Huang WB, et al. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect Dis. 2013;13:28. doi: 10.1186/1471-2334-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, Jiang Z. Zhu Futang's Practical Pediatrics. 7th ed. Beijing: People's Medical Publishing House; 2002. Respiratory infection; pp. 1167–215. [Google Scholar]
- 16.Zhao L, Qian Y, Wang F, Zhu R, Deng J. Human parainfluenza virus infections in infants and young children with acute respiratory infections in Beijing (in Chinese) Chin J Pediatr. 2007;45:91–5. [PubMed] [Google Scholar]
- 17.Ruampunpong H, Payungporn S, Samransamruajkit R, Pratheepamornkul T, Theamboonlers A, Poovorawan Y. Human parainfluenza virus infection in Thai children with lower respiratory tract infection from 2010 to 2013. Southeast Asian J Trop Med Public Health. 2014;45:610–21. [PubMed] [Google Scholar]
- 18.Yano T, Fukuta M, Maeda C, Akachi S, Matsuno Y, Yamadera M, et al. Epidemiological investigation and seroprevalence of human parainfluenza virus in Mie Prefecture in Japan during 2009-2013. Jpn J Infect Dis. 2014;67:506–8. [PubMed] [Google Scholar]