Abstract
Background:
Both uniportal and triportal thoracoscopic lobectomy and sublobectomy are feasible for early-stage non-small cell lung cancer (NSCLC). The aim of this study was to compare the perioperative outcomes of uniportal and triportal thoracoscopic lobectomy and sublobectomy for early-stage NSCLC.
Methods:
A total of 405 patients with lung lesions underwent thoracoscopic lobectomy or sublobectomy through a uniportal or triportal procedure in approximately 7-month period (From November 2014 to May 2015). A propensity-matched analysis, incorporating preoperative variables, was used to compare the short-term outcomes of patients who received uniportal or triportal thoracoscopic lobectomy and sublobectomy.
Results:
Fifty-eight patients underwent uniportal and 347 patients underwent triportal pulmonary resection. The conversion rate for uniportal and triportal procedure was 3.4% (2/58) and 2.3% (8/347), respectively. The complication rate for uniportal and triportal procedure was 10.3% and 9.5%, respectively. There was no perioperative death in either group. Most patients had early-stage NSCLC in both groups (uniportal: 45/47, 96%; triportal: 313/343, 91%). Propensity score-matching analysis demonstrated no significant differences in operation time, intraoperative blood loss, numbers of dissected lymph nodes, number of stations of lymph node dissected, duration of chest tube, and complication rate between uniportal and triportal group for early-stage NSCLC. However, the duration of postoperative hospitalization was longer in the uniportal group (6.83 ± 4.17 vs. 5.42 ± 1.86 d, P = 0.036) compared with the triportal group.
Conclusions:
Uniportal thoracoscopic lobectomy and sublobectomy is safe and feasible, with comparable short-term outcomes with triportal thoracoscopic pulmonary resection. Uniportal lobectomy and sublobectomy lead to similar cure rate as triportal lobectomy and sublobectomy for early NSCLC.
Keywords: Early-stage Nonsmall Cell Lung Cancer, Lobectomy, Sublobectomy, Uniportal, Video-assisted Thoracoscopic Surgery
INTRODUCTION
The incidence and mortality of lung cancer ranked first among men worldwide and was the second cause of cancer death among women.[1] In China, lung cancer is the leading cause of cancer death whether in urban or rural, for both men and women.[2] Thus, lung cancer is a serious malignant disease affecting public health both in China and worldwide.
For now, video-assisted thoracic surgery (VATS) lobectomy with systematic lymph node dissection has been a widespread standard procedure for early-stage nonsmall cell lung cancer (NSCLC) in the last decade, with decreased postoperative morbidities, shortened hospital length of stay, and comparable 5-year survival.[3,4] In recent years, sublobectomy was regarded as an alternative procedure for highly selected early-stage NSCLC, with comparable local relapse rate and nonsignificant difference of 5-year survival in uncontrolled trials,[5,6] although two meta-analyses demonstrated no significant benefits of VATS sublobectomy over VATS lobectomy for early-stage NSCLC regarding short-term and long-term outcomes.[7,8] The results of 5-year survival rate from two ongoing trials will answer whether VATS sublobectomy is a better procedure for early-stage NSCLC as compared to VATS lobectomy.[9,10]
Besides the extension of resection, the number of incisions is also an important aspect for the thoracic surgeon when performing VATS pulmonary resection. Uniportal was first introduced as a procedure for the diagnosis of the nature of pulmonary lesions in 2005.[11] Later, uniportal thoracoscopic technique was used for more complicated thoracic procedures such as lobectomy, pneumonectomy, and bronchoplasty.[12,13,14,15,16] When compared with multiple incisions with VATS pulmonary resection, the uniportal procedure has the advantage of the trend for reduced usage of analgesics, shortened hospitalization, minimal inflammations with a comparable short-term outcome.[12,17,18,19]
In China, single-utility port VATS pulmonary resection was first reported in 2010.[20] Subsequently, the single-operation hole VATS pulmonary resection was reported in 2014.[21] However, to our knowledge, no real uniportal VATS pulmonary resection was reported in China so far. Therefore, the aim of this study was to assess the uniportal procedure in patients with NSCLC and report the initial experiences of uniportal VATS pulmonary resection in our cancer hospital.
METHODS
This is a prospective study which was performed in the Department of Thoracic Surgery in Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College between November 2014 and May 2015. This study was approved by the Institutional Review Board at Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College, and all patients provided written informed consents before operation. The primary endpoint of this study was to demonstrate the feasibility of uniportal VATS pulmonary resection, the short-term outcomes using this new procedure were compared with triportal VATS pulmonary resection.
Fifty-eight uniportal VATS pulmonary resection procedures were accomplished based on the techniques reported by Gonzalez-Rivas et al.[14] and our previous experiences with triportal VATS pulmonary resection.[22] At the same time, 347 patients receiving triportal VATS pulmonary resection were used as controls.
Variables studied in each patient include age, sex, comorbidities (hypertension, coronary heart disease, and chronic obstructive pulmonary disease), current smoker, forced expiratory volume in 1 s (FEV1), tumor size and position, type and duration of operation, pathological stage, histologic type, number of lymph nodes retrieved, duration of chest tube, hospital length of stay, and postoperative complications.
Surgical procedures
All surgical procedures were performed under general anesthesia with double-lumen intubation. Patients were placed in the full lateral decubitus position, and the operator and thoracoscopic assistant stood at the anterior side of the patient.
The details of triportal VATS pulmonary resection procedures were reported previously.[22] Generally, one port for viewing was done at the seventh intercostal spaces on the middle axillary line, and two ports for working on the anterior axillary line and posterior axillary line, respectively, on which the intercostal spaces in detail according to the location of lesion by computed tomography. Thoracoscopic segmentectomy began with identification and ligation of the segmental vein. Subsequently, the bronchus or artery was ligated, depending on the segment resected. The segmental pulmonary veins, arteries, and bronchi were dissected by electrocautery and stapled by endoscopic stapler separately. We used reventilation to confirm the intersegmental plane according to the inflation-deflation line and divided it by an endoscopic stapler. The intraoperative frozen section must be used for examination of the station 10 and station 11–12 lymph nodes and resection margins after completion of segmentectomy. If a tumor was located on the edge of the segment, or the resection margin was inadequate on frozen section intraoperatively, or the station 10 or station 11–12 lymph nodes were metastatic, a multiple segmentectomy or lobectomy should be available.
Uniportal VATS pulmonary resection procedures were performed as follows. The incision, about 3–5 cm long, is performed at the fifth or sixth intercostal space at the middle axillary line. In most cases, the camera was placed on the posterior side of the incision, and other working instruments were placed on the anterior side. Both the operator and the thoracoscopic assistant stand at the anterior side of the patient. When the endostapler is applied, the camera's position must be changed to accommodate the stapler. All pulmonary vessels and bronchus in the resected lobe or sublobe were basically sectioned with the use of endoscopic staplers. Usually, the bronchus is resected at the last stage of lobectomy or sublobectomy. The specimen was put in the bag under the thoracoscopic assistance and was removed through the incision protector.[14]
Hilar or mediastinal lymph node dissection was performed for all patients. The procedure of dissection was selected based on the guidelines and previously reported techniques for VATS lobectomy.[22] Lung cancer staging was carried out according to AJCC 2009 cancer staging.[23]
Statistical analysis
The SPSS software package 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were presented as mean value ± standard deviation (SD) for continuous variables, and percentages for dichotomous variables. Continuous variables were analyzed using the t-test or nonparametric test, and categorical variables were analyzed using the chi-square test. The significant level was set at a P < 0.05.
RESULTS
From November 2014 to May 2015, a total of 405 patients received VATS pulmonary resection. We attempted 58 VATS pulmonary resections using the uniportal approach. Except 2 patients underwent conversion to open thoracotomy because of intraoperative pulmonary artery bleeding, 56 patients underwent uniportal thoracoscopic pulmonary resection with a conversion rate of 3.4%. Of 58 patients who underwent uniportal VATS pulmonary resection, 45 patients had adenocarcinomas, 2 patients had squamous cell carcinomas, 2 patients had metastatic tumors, and 9 patients had benign lesions including 1 harmatoma, 5 chronic inflammatory lesions, and 3 granulomas. Total 96% (45/47) of patients in uniportal group had early-stage NSCLC. Three hundred and forty-seven patients underwent triportal pulmonary resection with a conversion rate of 2.3% (8/347). Except 2 benign lesions and 2 carcinoid tumors, there are 343 NSCLC patients undergoing resection. And 91% (313/343) patients had early-stage NSCLC.
The demographic, preoperative characteristics, operative details, and postoperative complications of 405 patients are described in Tables 1 and 2. No significant difference was found in operative time, intraoperative blood loss, duration of chest tube, and complication rate. There was no surgical mortality in either of the two groups. However, less number of dissected lymph nodes (7.02 ± 8.60 vs. 13.34 ± 9.26, P < 0.001) and less number of stations of lymph node dissected (4 ± 1 vs. 6 ± 1, P < 0.001) were seen in the uniportal group compared with triportal group.
Table 1.
Clinical characteristics of patients before surgery
| Characteristics | All patients | Propensity–matched patients | ||||
|---|---|---|---|---|---|---|
| Uniportal (n = 58) | Triportal (n = 347) | P | Uniportal (n = 47) | Triportal (n = 47) | P | |
| Age (years) | 55.75 ± 12.22 | 59.63 ± 9.96 | 0.011 | 56.67 ± 11.62 | 60.77 ± 11.04 | 0.092 |
| Gender (male), n (%) | 32 (55.2) | 175 (50.4) | 0.504 | 25 (53.2) | 14 (29.8) | 0.021 |
| Current smoker, n (%) | 22 (37.9) | 166 (47.8) | 0.161 | 16 (34.0) | 19 (40.4) | 0.522 |
| Comorbidity, n (%) | ||||||
| Diabetic mellitus | 6 (10.3) | 35 (10.1) | 0.952 | 4 (8.5) | 5 (10.6) | 0.726 |
| Hypertension | 14 (24.1) | 117 (33.7) | 0.149 | 9 (19.1) | 12 (25.5) | 0.458 |
| Coronary heart disease | 9 (15.5) | 34 (9.8) | 0.191 | 9 (19.1) | 6 (12.8) | 0.398 |
| Histologic type, n (%) | <0.001 | 0.239 | ||||
| Adenocarcinoma | 45 (77.6) | 308 (88.8) | 45 (95.7) | 42 (89.4) | ||
| Squamous cell carcinoma | 2 (3.4) | 35 (10.1) | 2 (4.3) | 5 (109) | ||
| Carcinoid cancer | 0 (0) | 2 (0.6) | ||||
| Metastatic tumors | 2 (3.4) | 0 (0) | – | – | ||
| Others | 9 (15.5) | 2 (0.6) | – | – | ||
| Harmotoma | 1 (1.7) | 0 (0) | ||||
| Chronic inflammation | 5 (8.6) | 1 (0.3) | ||||
| Granuloma | 3 (5.2) | 1 (0.3) | ||||
| FEV1 (L) | 2.42 ± 0.61 | 2.37 ± 0.72 | 0.632 | 2.39 ± 0.60 | 2.41 ± 0.59 | 0.871 |
| Tumor size (cm) | 2.24 ± 1.57 | 2.20 ± 1.22 | 0.849 | 2.02 ± 1.30 | 1.62 ± 1.04 | 0.112 |
| Tumor location, n (%) | 0.489 | 0.696 | ||||
| LUL | 11 (19.0) | 94 (27.1) | 10 (21.3) | 14 (29.8) | ||
| LLL | 14 (24.1) | 57 (16.4) | 8 (17.0) | 6 (12.8) | ||
| RUL | 16 (27.6) | 106 (30.5) | 12 (25.5) | 15 (31.9) | ||
| RML | 5 (8.6) | 23 (6.6) | 5 (10.6) | 6 (6.4) | ||
| RLL | 12 (20.7) | 67 (19.3) | 12 (25.5) | 9 (19.1) | ||
| Operative procedure, n (%) | <0.001 | 0.261 | ||||
| Lobectomy | 31 (53.4) | 271 (78.1) | 28 (59.6) | 21 (44.7) | ||
| Wedge resection | 18 (31.0) | 54 (15.6) | 11 (23.4) | 18 (38.3) | ||
| Segmentectomy | 9 (15.5) | 22 (6.3) | 8 (17.0) | 8 (17.0) | ||
| Staging, n (%) | 0.151 | 0.392 | ||||
| Tis | 9 (19.1) | 50 (14.6) | 9 (19.1) | 15 (31.9) | ||
| I | 27 (57.4) | 229 (66.8) | 27 (57.4) | 26 (55.3) | ||
| II | 9 (19.1) | 34 (9.9) | 9 (19.1) | 5 (10.6) | ||
| III | 2 (4.3) | 30 (8.7) | 2 (4.3) | 1 (2.1) | ||
FEV1: Forced expiratory volume in 1 s; LUL: Left upper lobe; LLL: Left lower lobe; RUL: Right upper lobe; RML: Right middle lobe; RLL: Right lower lobe.
Table 2.
Short–term outcomes of patients after thoracoscopic lobectomy or sublobectomy
| Perioperative data | All patients | Propensity-matched patients | ||||
|---|---|---|---|---|---|---|
| Uniportal (n = 58) | Triportal (n = 347) | P | Uniportal (n = 47) | Triportal (n = 47) | P | |
| Operative time (min) | 138.83 ± 63.63 | 135.62 ± 55.51 | 0.701 | 144.95 ± 65.81 | 130.91 ± 46.88 | 0.237 |
| Blood loss (ml) | 73.58 ± 51.52 | 74.08 ± 64.53 | 0.958 | 79.76 ± 56.37 | 72.77 ± 28.49 | 0.450 |
| Lymph nodes retrieved | 7.02 ± 8.60 | 13.34 ± 9.26 | <0.001 | 7.83 ± 7.86 | 7.81 ± 7.99 | 0.987 |
| Number of lymph nodes stations | 4 ± 1 | 6 ± 1 | <0.001 | 5 ± 1 | 5 ± 2 | 1.000 |
| Conversion to thoracotomy, n (%) | 2 (3.4) | 8 (2.3) | 0.950 | 1 (2.1) | 1 (2.1) | 1.000 |
| Postoperative hospital stay (day) | 6.54 ± 3.84 | 6.29 ± 2.59 | 0.547 | 6.83 ± 4.17 | 5.42 ± 1.86 | 0.036 |
| Duration of chest tube (day) | 5.02 ± 1.92 | 5.25 ± 2.16 | 0.471 | 5.17 ± 2.09 | 4.56 ± 1.71 | 0.125 |
| Overall complication, n (%) | 6 (10.3) | 33 (9.5) | 0.842 | 4 (8.5) | 5 (10.6) | 1.000 |
| Pulmonary complications, n (%) | 3 (5.1) | 14 (4.0) | 0.963 | 2 (4.2) | 2 (4.2) | 1.000 |
| Atelectasis | 0 (0) | 4 (1.2) | 0 (0) | 1 (2.1) | ||
| Pneumonia | 2 (3.4) | 5 (1.4) | 1 (2.1) | 1 (2.1) | ||
| Air leak >7 days | 1 (1.7) | 5 (1.4) | 1 (2.1) | 0 (0) | ||
| Nonpulmonary complications, n (%) | 3 (5.1) | 19 (5.5) | 0.827 | 2 (4.2) | 3 (6.3) | 1.000 |
| Reoperation | 0 (0) | 4 (1.2) | 0 (0) | 1 (2.1) | ||
| Atrial fibrillation | 3 (5.1) | 15 (4.3) | 2 (4.2) | 2 (4.2) | ||
In order to make propensity analysis, we excluded 11 patients with metastatic tumors and benign lesions, thus leaving 47 patients with lung cancer into the final analysis. The flowchart of this study is outlined in Figure 1. The two study groups were well matched with respect to age, forced expiratory volume in 1 s (FEV1) as a percentage of predicted, comorbidities including coronary heart disease and diabetic mellitus, tumor size and location, operative procedures, and staging. Most patients had early-stage NSCLC (45/47, 96% in uniportal group; 41/42, 98% in triportal group).
Figure 1.
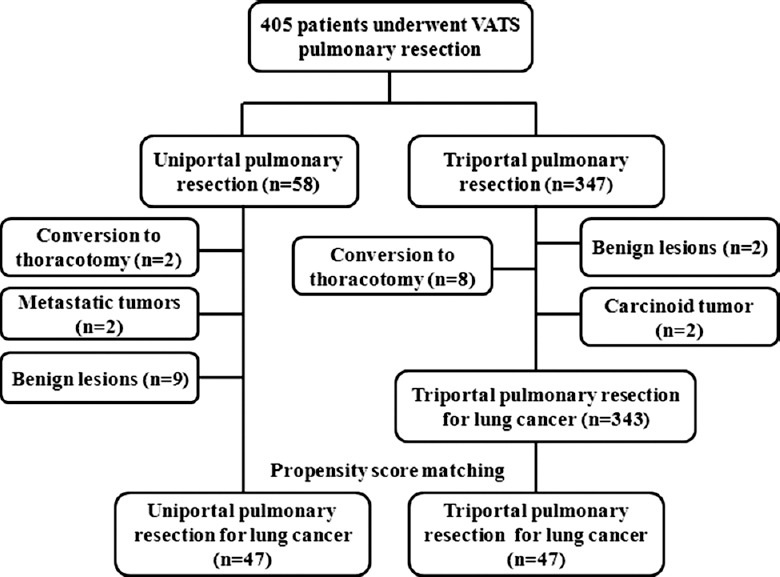
Flowchart of the study.
Analysis of the propensity-matched group for intraoperative and postoperative outcomes demonstrate that no significant differences were found in the conversion rate, operation time, intraoperative blood loss, numbers of dissected lymph nodes, number of stations of lymph node dissected, duration of chest tube, and complication rate between uniportal and triportal groups. However, postoperative hospital length of stay was significantly longer in the uniportal group (6.83 ± 4.17 days vs. 5.42 ± 1.86, P = 0.036) as compared with the triportal group.
DISCUSSION
In our study, we found that the short-term outcomes of uniportal VATS pulmonary resection were comparable to those in triportal VATS pulmonary resection.
Gonzalez-Rivas et al. described the first series of uniportal thoracoscopic lobectomy in 2013, and they concluded that uniportal thoracoscopic anatomic resection is a feasible and safe procedure with good perioperative results.[14] In this study, the uniportal VATS pulmonary resection is also shown to be feasible and safe based on intraoperative and postoperative variables, and this is true for pulmonary wedge resection or for anastomotic resections including lobectomy and segmentectomy. In our study, the mean operative time was 138.83 ± 63.63 min, which is comparable, but slightly better than that reported by Gonzalez-Rivas et al. (154.1 ± 46 min).[14] The conversion rate in this study is 3.4%, which is also in the range of values being reported so far.[14,18] Mean intraoperative blood loss was 74 ml, which is also comparable to that reported by Wang et al.[18]
The number of lymph nodes retrieved was less in the uniportal VATS group than in the triportal VATS group in unmatched analysis, because 16% (9/58) of patients were benign, under which lymph node resections were not necessary. However, after adjusting for preoperative variables, no significant differences were found on intraoperative and postoperative outcomes including operative time, volume of blood loss, and number of lymph nodes retrieved between uniportal and triportal groups. And in the propensity-matched analysis in our study, most patients (96%) had early-stage NSCLC. The mean number of lymph nodes retrieved in the uniportal group was 7.83 ± 7.86, which is comparable to 7.81 ± 7.99 in the triportal group. Wang et al. reported an increased number of lymph nodes retrieved in the single incision group than in the multiple incisions (two or three incisions) group.[18] Therefore, uniportal VATS has the potential to achieve a comparable oncologic cure rate with multiple incisions VATS.
In this study, there are 18 pulmonary wedge resections in the uniportal group. Rocco et al. reported their 10-year experience on 644 patients who underwent uniportal thoracoscopic pulmonary wedge resections in 2013, although uniportal thoracoscopic anatomic lobectomy or segmentectomy was not reported in that series.[24] However, uniportal VATS is still a useful procedure for diagnostic purposes and to resect small pulmonary nodules.
Postoperative hospital length of stay was longer in the uniportal group than in the triportal group in our study. Poor wound healing after removal of chest tube and resuture of wound may be attributed to this result. In the uniportal group, we did not place preset stitches as we did in the triportal group, which explained poor wound healing after the chest tube removed. We had placed preset stitches as we did in the triportal group after we found this unsatisfying outcome. We believe that the rate of poor wound healing will be reduced significantly.
We believe that the beneficial effects of uniportal lobectomy and sublobectomy lie in two aspects. The first is that it may decrease postoperative inflammatory reaction.[19] The second is that it can bring good cosmetic effect for patients without compromising oncologic cure rate with traditional triportal lobectomy and sublobectomy for early-stage NSCLC.[19]
There are several limitations in our study. First, the number of patients who underwent uniportal VATS pulmonary resection was relatively small. This was a prospective observational study, and propensity matching analysis was conducted which might have improved the reliability of these results. Second, the results were from one medical center, which limit the generalization of the conclusion. Third, our study did not include the analysis of postoperative pain or long-term survival outcomes. Therefore, the result of this study needs further validation in a randomized controlled clinical trial with a large number of patients.
In conclusion, uniportal VATS pulmonary resection is safe and feasible, with comparable short-term outcomes with triportal VATS pulmonary resection. Uniportal pulmonary resection has the potential to achieve comparable oncologic cure rate with triportal pulmonary resection for early-stage NSCLC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011 (in Chinese) Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higuchi M, Yaginuma H, Yonechi A, Kanno R, Ohishi A, Suzuki H, et al. Long-term outcomes after video-assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non-small cell lung cancer. J Cardiothorac Surg. 2014;9:88. doi: 10.1186/1749-8090-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nwogu CE, D’Cunha J, Pang H, Gu L, Wang X, Richards WG, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance) Ann Thorac Surg. 2015;99:399–405. doi: 10.1016/j.athoracsur.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong C, Fang W, Mao T, Yao F, Chen W, Hu D. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg. 2012;94:362–7. doi: 10.1016/j.athoracsur.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 6.Carr SR, Schuchert MJ, Pennathur A, Wilson DO, Siegfried JM, Luketich JD, et al. Impact of tumor size on outcomes after anatomic lung resection for stage 1A non-small cell lung cancer based on the current staging system. J Thorac Cardiovasc Surg. 2012;143:390–7. doi: 10.1016/j.jtcvs.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Huang C, Liu H, Chen Y, Li S. Sublobectomy versus lobectomy for stage IA (T1a) non-small-cell lung cancer: A meta-analysis study. World J Surg Oncol. 2014;12:138. doi: 10.1186/1477-7819-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Sun Y, Wang R, Ye T, Zhang Y, Chen H. Meta-analysis of lobectomy, segmentectomy, and wedge resection for stage I non-small cell lung cancer. J Surg Oncol. 2015;111:334–40. doi: 10.1002/jso.23800. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, Saji H, Nakajima R, Okada M, Asamura H, Shibata T, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L) Jpn J Clin Oncol. 2010;40:271–4. doi: 10.1093/jjco/hyp156. [DOI] [PubMed] [Google Scholar]
- 10.Comparison of Different Types of Surgery in Treating Patients with Stage IA Non-Small Cell Lung Cancer. [Last accessed on 2015 Aug 28]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00499330 .
- 11.Rocco G, Khalil M, Jutley R. Uniportal video-assisted thoracoscopic surgery wedge lung biopsy in the diagnosis of interstitial lung diseases. J Thorac Cardiovasc Surg. 2005;129:947–8. doi: 10.1016/j.jtcvs.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez D, de la Torre M, Paradela M, Fernandez R, Delgado M, Garcia J, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg. 2011;40:e21–8. doi: 10.1016/j.ejcts.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Rivas D, de la Torre M, Fernandez R, Garcia J. Video: Single-incision video-assisted thoracoscopic right pneumonectomy. Surg Endosc. 2012;26:2078–9. doi: 10.1007/s00464-011-2127-x. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Rivas D, Paradela M, Fernandez R, Delgado M, Fieira E, Mendez L, et al. Uniportal video-assisted thoracoscopic lobectomy: Two years of experience. Ann Thorac Surg. 2013;95:426–32. doi: 10.1016/j.athoracsur.2012.10.070. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Rivas D, Delgado M, Fieira E, Mendez L. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg. 2013;17:889–91. doi: 10.1093/icvts/ivt340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Rivas D, Delgado M, Fieira E, Fernandez R. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg. 2014;3:E2. doi: 10.3978/j.issn.2225-319X.2014.03.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang BY, Tu CC, Liu CY, Shih CS, Liu CC. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg. 2013;96:977–82. doi: 10.1016/j.athoracsur.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Wang BY, Liu CY, Hsu PK, Shih CS, Liu CC. Single-incision versus multiple-incision thoracoscopic lobectomy and segmentectomy: A propensity-matched analysis. Ann Surg. 2015;261:793–9. doi: 10.1097/SLA.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 19.Ng CS. Uniportal VATS in Asia. J Thorac Dis. 2013;5(Suppl 3):S221–5. doi: 10.3978/j.issn.2072-1439.2013.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu X, Xue Z, Zhang L, Hou X, Ma K. Primary report of lobectomy with single utility port complete video-assisted thoracoscopic surgery (in Chinese) Chin J Lung Cancer. 2010;13:19–21. doi: 10.3779/j.issn.1009-3419.2010.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu C, Ma H, Ni B, He J, Li C, Ding C, et al. Analysis of single-operation-hole thoracoscopic lobectomy in 113 clinical cases (in Chinese) Chin J Lung Cancer. 2014;17:424–7. doi: 10.3779/j.issn.1009-3419.2014.05.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu JW, Chen GY, Sun KL, Wang DW, Zhang BH, Li N, et al. Application of video-assisted thoracic surgery in the standard operation for thoracic tumors. Cancer Biol Med. 2013;10:28–35. doi: 10.7497/j.issn.2095-3941.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DB, Compton CC, Fritz AG, Greene FL, Trotti A. 7th ed. New York: Springer-Verlag; 2010. AJCC Cancer Staging Handbook; pp. 299–323. [Google Scholar]
- 24.Rocco G, Martucci N, La Manna C, Jones DR, De Luca G, La Rocca A, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg. 2013;96:434–8. doi: 10.1016/j.athoracsur.2013.04.044. [DOI] [PubMed] [Google Scholar]


