Abstract
Background:
Previous data are controversial about the association of renal artery stenosis (RAS) with clinical outcome in patients with heart failure. Definition of RAS in previous studies might not be appropriate. By definition of RAS with renal duplex sonography, we investigated the association of RAS with clinical outcome in patients with heart failure.
Methods:
In this retrospective study, we identified 164 patients with heart failure (New York Heart Association classification ≥II; left ventricular ejection fraction <50%) who had received renal duplex sonography during hospital stay. RAS was defined as renal-aortic ratio ≥3.5 or a peak systolic velocity ≥200 cm/s (or both), or occlusion of the renal artery. Categorical data of patients were compared using the Chi-square test or Fisher's exact test. Cox proportional hazards regression modeling technique was used to investigate the prognostic significance of possible predictors.
Results:
Finally, 143 patients were enrolled. Median follow-up time was 32 months (1–53 months). Twenty-two patients were diagnosed as RAS by renal duplex sonography, including 13 unilateral RAS (3 left RAS, 10 right RAS) and 9 bilateral RAS. There were more all-cause mortality and cardiovascular death in patients with RAS than patients without RAS. By multivariate analysis, RAS was a significant predictor for all-cause death and cardiovascular death (hazard ratio [HR] = 4.155, 95% confidence interval [CI]: 1.546–11.164, P = 0.005; and HR = 3.483, 95% CI: 1.200–10.104, P = 0.022, respectively). As for composite endpoint events, including death, nonfatal myocardial infarction, ischemic stroke or intracranial hemorrhage, rehospitalization for cardiac failure, and renal replacement therapy, only angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker was significant predictor. RAS was not a significant predictor for composite endpoint events.
Conclusions:
Our data suggested that RAS is associated with a poorer clinical outcome in patients with heart failure.
Keywords: Atherosclerosis, Cardiac Dysfunction, Heart Failure, Renal Artery Stenosis
INTRODUCTION
It is presumed that presence of renal artery stenosis (RAS) confers a worse outcome for patients with heart failure.[1] Possible mechanisms include activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, volume retention associated with renal ischemia, which are important contributors in the development of heart failure.[1,2] The study of de Silva et al. reported that RAS was associated with increased morbidity and mortality in patients with chronic heart failure during a 3-year follow-up.[3] However, a later published study did not validate this association.[4] In these studies, significant RAS was defined by diameter stenosis of >50% using contrast-enhanced magnetic resonance angiography.
We think that definition of RAS by diameter stenosis of >50% does not correspond to a severe RAS, which might be associated with poor clinical outcome. Our recent published data showed that RAS >70% helps to predict major adverse cardiac events after acute myocardial infarction, whereas RAS >50% does not.[5] Conlon et al.[6] reported a significant decrease in 4-year survival in patients with RAS undergoing coronary angiography and found that increasing severity of RAS has an incremental detriment on survival probability. These data suggested that severity of RAS, rather RAS alone, helps to predict the risk of adverse event.
Duplex sonography can discriminate ≥60% RAS from <60% RAS accurately.[7] We presumed that RAS definition by duplex sonography could help to predict poor outcome in patients with heart failure, so we investigated this presumption in this study.
METHODS
Study population
Data came from Renal Artery Stenosis in patients with HEart Failure (RASHEF) database. Patients in RASHEF data were retrieved from DHC-Picture Archiving and Communications System/Radiology Information System (PACS/RIS system) in Beijing Anzhen Hospital, Capital Medical University. In this DHC-PACS/RIS system, from January 2010 to June 2012, renal duplex sonography was performed in 2075 hospitalized patients, including 1925 patients with echocardiography performed during hospital stay. Renal duplex sonography was performed in: (1) Patients with hypertension; (2) Patients with azotemia, unexplained renal failure, or worsening renal function after administration of angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker (ACEI/ARB) agent; (3) Patients with sudden, unexplained pulmonary edema; and (4) Patients with multivessel coronary artery disease.
In these 1925 patients, there were 169 patients diagnosed as heart failure. Definition of heart failure in this study was: Stage II, III, or IV (according to the New York Heart Association [NYHA] classification) heart failure and left ventricular eject fraction <0.50 by echocardiography. Renal duplex sonography in 2 of the 169 patients was technically inadequate for interpretation. In remaining 167 patients, 98 patients were diagnosed as ischemic heart failure, and 69 patients were diagnosed as nonischemic heart failure. Definition of ischemic heart failure in this study was: Stage II, III, or IV (according to the NYHA classification) heart failure due to coronary artery disease and left ventricular eject fraction <0.50 by echocardiography. Ethical approval was granted by the Ethics Committee of Beijing Anzhen Hospital. In the 98 patients with ischemic heart failure, 3 patients with RAS received renal artery stent implantation and were not included in this study. Finally, 95 ischemic heart failure patients and 69 nonischemic heart failure patients were included in this study.
Renal duplex sonography
Patients were studied in the anterior, lateral decubitus, and prone position to visualize all portions of the renal artery. Renal duplex sonography was performed with Philips iU22G4 ultrasound system (USA) or GE Logiq E9 ultrasound system (USA). RAS was defined by duplex scanning as having a renal aortic ratio of ≥3.5, a peak renal artery systolic velocity of ≥200 cm/s, or a renal artery occlusion. This criterion can discriminate ≥60% atherosclerotic RAS (ARAS) from <60% RAS accurately.[7]
Follow-up
Patients were contacted by telephone, at outpatient department and/or via MedTrak System of Beijing Anzhen Hospital. Composite endpoints of major adverse events include all-cause death and cardiovascular death, nonfatal myocardial infarction, ischemic stroke or intracranial hemorrhage, rehospitalization for cardiac failure, renal replacement therapy. Composite endpoints events free survival was termed as the interval between the first hospitalization and the first occurrence of any major adverse events. Myocardial infarction referred to type 1 or type 4b myocardial infarction.[8]
Statistical analysis
Continuous data were shown as mean ± standard deviation (SD) or median (range). Categorical data were compared using the Chi-square test or Fisher's exact test. We used Cox proportional hazards regression modeling technique to investigate the prognostic significance of possible predictors. All reported P values were two-tailed. Results were considered to be statistically significant if P < 0.05. Data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
Initially, 95 patients with ischemic heart failure and 69 patients without ischemic heart failure were included in this study.
Baseline characteristics of the study population are summarized in Tables 1 and 2. Data were presented as non-RAS patients or RAS patients. Initially, 164 patients were involved. However, 10 patients with ischemic heart failure and 11 patients with nonischemic heart failure were not contacted successfully during follow-up and were not included in the analysis. These patients could not be contacted by telephone, or at the outpatient department. Finally, 143 patients were included in the analysis, with 85 patients with ischemic heart failure and 58 patients with nonischemic heart failure. In the 58 nonischemic heart failure patients, heart failure was caused by hypertension in 39 patients, renal failure in 1 patient, congenital heart disease in 1 patient, valvular heart disease in 8 patients, dilated cardiomyopathy in 9 patients. Median follow-up time was 32 months (1–53 months) for study population; and median follow-up time was 33 months (1–53 months) for non-RAS patients and 22 months (1–53 months) for RAS patients. Twenty-two patients were diagnosed as RAS by renal duplex sonography, including 13 unilateral RAS (3 left RAS, 10 right RAS) and 9 bilateral RAS. Patients with renal artery stent implantation were not included.
Table 1.
Baseline characteristics of patients with heart failure
| Characteristics | Heart failure | Ischemic heart failure | Nonischemic heart failure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 143) | NonRAS (n = 121) | RAS (n = 22) | Total (n = 85) | NonRAS (n = 70) | RAS (n = 15) | Total (n = 58) | NonRAS (n = 51) | RAS (n = 7) | |
| Male, n (%) | 106 (74.1) | 92 (76.0) | 14 (63.6) | 63 (74.1) | 54 (77.1) | 9 (60.0) | 43 (74.1) | 38 (74.5) | 5 (71.4) |
| Age (years), mean ± SD | 60.7 ± 11.4 | 59.5 ± 11.5 | 67.1 ± 8.7 | 63.3 ± 10.7 | 62.1 ± 10.8 | 68.5 ± 9.2 | 56.9 ± 11.4 | 55.8 ± 11.5 | 64.3 ± 7.4 |
| Hypertension, n (%) | 104 (72.7) | 85 (70.2) | 19 (86.4) | 62 (72.9) | 49 (70.0) | 13 (86.7) | 42 (72.4) | 36 (70.6) | 6 (85.7) |
| Prior stroke, n (%) | 21 (14.7) | 15 (12.4) | 6 (27.3) | 16 (18.8) | 10 (14.3) | 6 (40) | 5 (8.6) | 5 (9.8) | 0 (0.0) |
| Diabetes, n (%) | 46 (32.2) | 36 (29.8) | 10 (45.5) | 34 (40.0) | 27 (38.6) | 7 (46.7) | 12 (20.7) | 9 (17.6) | 3 (42.9) |
| Smoking, n (%) | 67 (46.9) | 58 (47.9) | 9 (40.9) | 43 (50.6) | 36 (51.4) | 7 (46.7) | 24 (41.4) | 22 (43.1) | 2 (28.6) |
| Height (cm), mean ± SD (n) | 168.5 ± 7.6 (112) | 168.5 ± 7.3 (96) | 169.0 ± 9.4 (16) | 167.9 ± 6.9 (62) | 167.5 ± 6.0 (51) | 169.3 ± 9.1 (11) | 169.4 ± 8.6 (50) | 169.5 ± 8.5 (45) | 168.4 ± 10.9 (5) |
| Weight (kg), mean ± SD (n) | 74.4 ± 14.8 (126) | 74.3 ± 15.1 (108) | 74.8 ± 13.3 (18) | 71.2 ± 11.7 (73) | 71.0 ± 11.9 (61) | 72.5 ± 11.0 (12) | 78.8 ± 17.5 (53) | 78.7 ± 17.8 (47) | 79.5 ± 17.2 (6) |
| Hemoglobin (g/L), mean ± SD | 127.0 ± 18.9 | 128.0 ± 18.0 | 121.1 ± 22.8 | 126.2 ± 16.0 | 127.4 ± 14.4 | 120.1 ± 21.8 | 128.2 ± 22.6 | 128.8 ± 22.2 | 123.3 ± 26.5 |
| Serum creatinine (μmol/L), mean ± SD | 143.1 ± 128.5 | 137.5 ± 130.0 | 174.3 ± 117.8 | 124.8 ± 107.4 | 116.3 ± 110.8 | 164.1 ± 81.8 | 170.0 ± 151.2 | 166.4 ± 148.7 | 196.3 ± 179.2 |
| eGFR (ml·min−1·1.73 m−2), mean ± SD | 63.5 ± 29.7 | 66.8 ± 28.5 | 45.4 ± 30.0 | 67.2 ± 28.5 | 71.6 ± 25.4 | 46.4 ± 33.9 | 58.2 ± 30.8 | 60.3 ± 31.4 | 43.3 ± 21.5 |
| Triglyceride (mmol/L), mean ± SD | 1.86 ± 1.19 | 1.85 ± 1.16 | 1.90 ± 1.36 | 1.79 ± 1.08 | 1.83 ± 1.10 | 1.58 ± 0.98 | 1.97 ± 1.34 | 1.88 ± 1.26 | 2.70 ± 1.89 |
| LDL-C (mmol/L), mean ± SD | 2.67 ± 0.89 | 2.62 ± 0.87 | 2.95 ± 0.95 | 2.60 ± 0.98 | 2.49 ± 0.97 | 3.11 ± 0.91 | 2.77 ± 0.72 | 2.79 ± 0.69 | 2.57 ± 1.00 |
eGFR was calculated by CKD-EPI equation. SD: Standard deviation; LDL-C: Low-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate; RAS: Renal artery stenosis.
Table 2.
Baseline characteristics of patients with heart failure
| Characteristics | Heart failure | Ischemic heart failure | Nonischemic heart failure | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 143) | NonRAS (n = 121) | RAS (n = 22) | Total (n = 85) | NonRAS (n = 70) | RAS (n = 15) | Total (n = 58) | NonRAS (n = 51) | RAS (n = 7) | ||
| Anterior wall infarction, n (%) | 24 (16.8) | 20 (16.5) | 4 (18.2) | 24 (28.2) | 20 (28.6) | 4 (26.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Inferior wall infarction, n (%) | 26 (18.2) | 21 (17.4) | 5 (22.7) | 26 (30.6) | 21 (30.0) | 5 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Non-ST elevation myocardial | 21 (14.7) | 18 (14.9) | 3 (13.6) | 21 (24.7) | 18 (25.7) | 3 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| infarction, n (%) | ||||||||||
| NYHA classification | 2.4 ± 0.7 | 2.4 ± 0.6 | 2.4 ± 0.8 | 2.47 ± 0.7 | 2.50 ± 0.7 | 2.33 ± 0.7 | 2.40 ± 0.7 | 2.37 ± 0.6 | 2.57 ± 1.0 | |
| Left ventricular eject fraction (%) | 43.4 ± 8.3 | 43.1 ± 8.5 | 44.5 ± 7.0 | 42.7 ± 7.1 | 42.6 ± 7.0 | 43.0 ± 7.8 | 44.3 ± 9.8 | 43.8 ± 10.3 | 47.9 ± 3.1 | |
| LVEDD (mm) | 59.8 ± 5.0 | 59.6 ± 4.8 | 60.7 ± 6.1 | 59.3 ± 4.9 | 58.9 ± 4.5 | 61.1 ± 6.2 | 60.5 ± 5.1 | 60.6 ± 5.0 | 59.9 ± 6.3 | |
| Medications on discharge, n (%) | ||||||||||
| ACEI/ARB | 73 (51.0) | 63 (52.1) | 10 (45.5) | 32 (37.6) | 26 (31.4) | 6 (40.0) | 41 (70.7) | 37 (72.5) | 4 (57.1) | |
| Beta-receptor blocker | 96 (67.1) | 83 (68.6) | 13 (59.1) | 60 (70.6) | 51 (72.9) | 9 (60.0) | 36 (62.1) | 32 (62.7) | 4 (57.1) | |
| Nitrites | 69 (48.3) | 55 (45.5) | 14 (63.6) | 55 (64.7) | 43 (61.4) | 12 (80.0) | 14 (24.1) | 12 (23.5) | 2 (28.6) | |
| Duretics | 64 (44.8) | 56 (46.3) | 8 (36.4) | 32 (37.6) | 27 (38.6) | 5 (33.3) | 32 (55.2) | 29 (56.9) | 3 (42.9) | |
| Spironolactone | 10 (7.0) | 9 (7.4) | 1 (4.5) | 7 (8.2) | 6 (8.6) | 1 (6.7) | 3 (5.2) | 3 (5.9) | 0 (0.0) | |
| Aspirin | 84 (58.7) | 70 (57.9) | 14 (63.6) | 65 (76.5) | 54 (77.1) | 11 (72.2) | 19 (32.8) | 16 (31.4) | 3 (42.9) | |
| Clopidogrel | 56 (39.2) | 44 (36.4) | 12 (54.5) | 53 (62.4) | 42 (60.0) | 11 (73.3) | 3 (5.2) | 2 (3.9) | 1 (14.3) | |
NYHA: New York Heart Association; ACEI/ARB: Angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker; LVEDD: Left ventricular end diastolic diameter; RAS: Renal artery stenosis.
Major adverse events
All-cause mortality and cardiovascular mortality
There were 23 deaths in this study, including 1 myocardial infarction, 5 heart failure, 1 cardiac arrest, 1 end-stage renal failure in 8 ischemic heart failure patients with RAS; 3 myocardial infarction, 7 heart failure, 1 cardiac arrest, 1 intracranial hemorrhage in 12 ischemic heart failure patients without RAS; 1 end-stage renal failure in 1 nonischemic heart failure with RAS; and 2 heart failure in 2 nonischemic heart failure without RAS. Gender, age, hypertension, prior stroke, diabetes mellitus, smoking, left ventricular eject fraction, left ventricular end-diastolic diameter, hemoglobin, serum creatinine, estimated glomerular filtration rate (eGFR),[9] triglyceride, low-density lipoprotein cholesterol, ACEI/ARB, beta-receptor blocker, and RAS were assumed to be possible predictors for all-cause mortality, cardiovascular death, and composite endpoints.
By univariate analysis, gender, diabetes mellitus, left ventricular eject fraction, hemoglobin, ACEI/ARB, and RAS were significant predictors for all-cause mortality; and diabetes mellitus, left ventricular eject fraction, hemoglobin, ACEI/ARB, and RAS were significant predictors for cardiovascular death [Table 3]. However, by multivariate analysis, only diabetes mellitus, left ventricular eject fraction, ACEI/ARB, and RAS were significant predictors for all-cause mortality and cardiovascular death [Table 4].
Table 3.
Univariate analysis of possible predictors for all-cause mortality, cardiovascular death and composite endpoint events
| Items | All-cause mortality | Cardiovascular death | Composite endpoint events | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | |
| Male | 0.034 | 2.442 | 1.069–5.574 | 0.050 | 2.374 | 0.999–5.641 | 0.052 | 1.73 | 0.995–3.010 |
| Age | 0.100 | 1.033 | 0.994–1.074 | 0.185 | 1.028 | 0.987–1.070 | 0.006 | 1.035 | 1.010–1.061 |
| Hypertension | 0.846 | 0.916 | 0.376–2.228 | 0.991 | 1.005 | 0.390–2.594 | 0.126 | 1.673 | 0.865–3.236 |
| Prior stroke | 0.269 | 1.748 | 0.649–4.710 | 0.481 | 1.478 | 0.498–4.397 | 0.002 | 2.591 | 1.412–4.754 |
| Diabetes mellitus | 0.003 | 3.525 | 1.525–8.146 | 0.012 | 3.010 | 1.268–7.146 | 0.017 | 1.904 | 1.124–3.226 |
| Smoking | 0.226 | 0.588 | 0.249–1.390 | 0.200 | 0.552 | 0.222–1.370 | 0.443 | 0.813 | 0.479–1.380 |
| Left ventricular eject fraction | 0.049 | 0.964 | 0.930–1.000 | 0.041 | 0.962 | 0.927–0.998 | 0.255 | 0.984 | 0.958–1.012 |
| LVEDD | 0.650 | 1.019 | 0.940–1.104 | 0.475 | 1.030 | 0.950–1.117 | 0.261 | 1.029 | 0.979–1.082 |
| Hemoglobin | 0.013 | 0.972 | 0.950–0.994 | 0.034 | 0.975 | 0.952–0.998 | 0.000 | 0.973 | 0.958–0.988 |
| Serum creatinine | 0.789 | 1.000 | 0.997–1.003 | 0.959 | 1.000 | 0.997–1.003 | 0.003 | 1.002 | 1.001–1.004 |
| eGFR | 0.174 | 0.990 | 0.977–1.004 | 0.397 | 0.994 | 0.979–1.008 | 0.000 | 0.983 | 0.975–0.992 |
| Triglyceride | 0.373 | 0.819 | 0.528–1.271 | 0.352 | 0.799 | 0.498–1.282 | 0.469 | 1.081 | 0.875–1.337 |
| LDL-C | 0.839 | 1.048 | 0.666–1.649 | 0.649 | 0.891 | 0.543–1.463 | 0.141 | 0.790 | 0.578–1.081 |
| ACEI/ARB | 0.010 | 0.295 | 0.116–0.749 | 0.009 | 0.261 | 0.096–0.713 | 0.001 | 0.386 | 0.222–0.673 |
| Beta-receptor blocker | 0.484 | 1.395 | 0.550–3.538 | 0.668 | 1.230 | 0.477–3.171 | 0.316 | 1.345 | 0.753–2.403 |
| RAS | 0.000 | 4.494 | 1.939–10.414 | 0.008 | 3.458 | 1.391–8.594 | 0.001 | 2.759 | 1.523–4.999 |
eGFR was calculated by CKD-EPI equation. ACEI/ARB: Angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker; LVEDD: Left ventricular end diastolic diameter; RAS: Renal artery stenosis; eGFR: Estimated glomerular filtration rate; HR: Hazard ratio; CI: Confidence interval; LDL-C: Low-density lipoprotein cholesterol.
Table 4.
Multivariate analysis of possible predictors for all-cause mortality, cardiovascular death and composite endpoint events
| Items | All-cause mortality | Cardiovascular death | Composite endpoint events | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | ||||
| Male | 0.101 | 2.064 | 0.868 | 4.909 | 0.105 | 2.106 | 0.856 | 5.181 | 0.384 | 1.305 | 0.717 | 2.376 |
| Age | 0.744 | 1.008 | 0.963 | 1.054 | 0.848 | 1.005 | 0.959 | 1.052 | 0.280 | 1.016 | 0.987 | 1.045 |
| Prior stroke | 0.951 | 0.965 | 0.310 | 3.002 | 0.927 | 0.944 | 0.277 | 3.222 | 0.149 | 1.660 | 0.835 | 3.302 |
| Diabetes mellitus | 0.018 | 3.117 | 1.217 | 7.984 | 0.040 | 2.749 | 1.046 | 7.228 | 0.174 | 1.546 | 0.825 | 2.895 |
| Left ventricular eject fraction | 0.015 | 0.945 | 0.904 | 0.989 | 0.009 | 0.939 | 0.896 | 0.984 | 0.232 | 0.981 | 0.950 | 1.012 |
| Hemoglobin | 0.723 | 0.995 | 0.969 | 1.022 | 0.640 | 0.993 | 0.965 | 1.022 | 0.436 | 0.993 | 0.974 | 1.011 |
| eGFR | 0.346 | 1.007 | 0.992 | 1.022 | 0.245 | 1.009 | 0.994 | 1.025 | 0.264 | 0.994 | 0.985 | 1.004 |
| ACEI/ARB | 0.003 | 0.201 | 0.070 | 0.580 | 0.002 | 0.166 | 0.052 | 0.527 | 0.005 | 0.418 | 0.226 | 0.773 |
| RAS | 0.005 | 4.155 | 1.546 | 11.164 | 0.022 | 3.483 | 1.200 | 10.104 | 0.164 | 1.632 | 0.819 | 3.254 |
eGFR was calculated by CKD-EPI equation. ACEI/ARB: Angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker; LVEDD: Left ventricular end diastolic diameter; RAS: Renal artery stenosis; eGFR: Estimated glomerular filtration rate; HR: Hazard ratio; CI: Confidence interval.
Kaplan–Meier plot of the probability of freedom from all-cause mortality and cardiovascular death showed that event-free survival between the two groups was significantly different using log-rank test (Chi-square = 14.925, P < 0.01, Figure 1; Chi-square = 8.175, P = 0.004, Figure 2).
Figure 1.
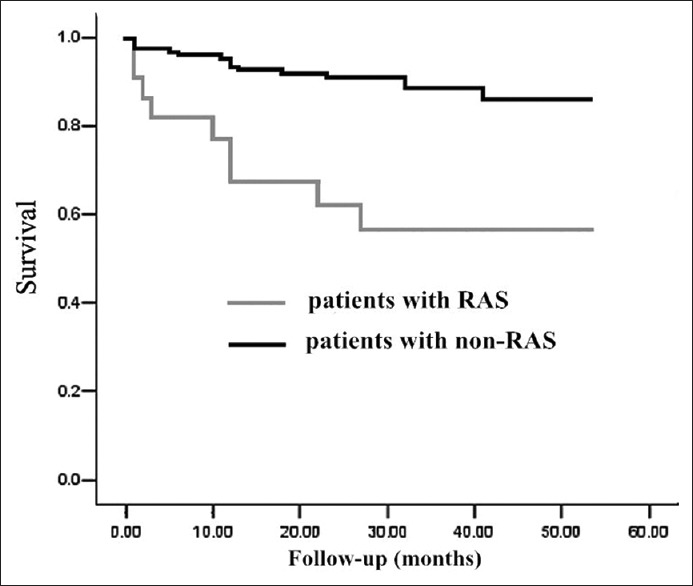
Kaplan-Meier plot of the probability of freedom from all-cause mortality. Difference between patients with renal artery stenosis (RAS) or non-RAS was statistically significant by log-rank test (P < 0.01). Patients’ number corresponded to different time points was 121, 116, 110, 74, 35, 4 for non-RAS group and 22, 16, 13, 8, 5, 2 for RAS group.
Figure 2.
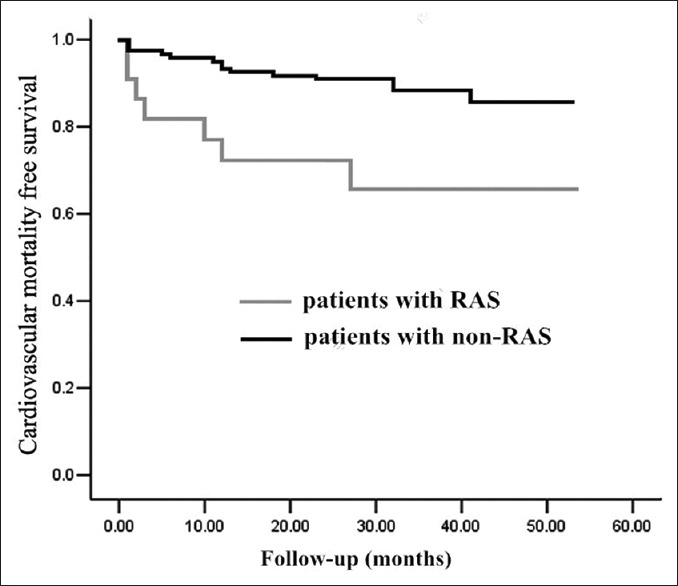
Kaplan–Meier plot of the probability of freedom from cardiovascular mortality. Difference between patients with renal artery stenosis (RAS) or non-RAS was statistical significant by log-rank test (P = 0.004). Patients’ number corresponded to different time points was 121, 116, 110, 74, 35, 4 for non-RAS group and 22, 16, 13, 8, 5, 2 for RAS group.
Nonfatal myocardial infarction
During follow-up, no patient developed nonfatal myocardial infarction.
Nonfatal stroke
There were seven non-fatal strokes in patients with no RAS and one nonfatal stroke in patients with RAS, and this difference was not significant between two groups by Fisher's exact test (P = 1.000). In patients with ischemic heart failure, there were six nonfatal strokes in patients with no RAS and no nonfatal stroke in patients with RAS. In patients with nonischemic heart failure, there was one nonfatal stroke in patients with no RAS and one nonfatal stroke in patients with RAS.
Rehospitalization
There were 23 first rehospitalizations for cardiac failure during follow-up, 5 in RAS patients and 18 in non-RAS patients. The difference between two groups was not statistically significant (P = 0.353). In patients with ischemic heart failure, there were 14 first rehospitalizations in patients with no RAS and 4 first rehospitalizations in patients with RAS. In patients with nonischemic heart failure, there were 4 first rehospitalizations in patients without RAS and one first rehospitalization in patients with RAS. A total number of rehospitalization for cardiac failure was 38 in this study population, 29 (mean 0.24) in patients with non-RAS and 9 (mean 0.41) in patients with RAS.
Renal replacement therapy
During follow-up, renal replacement therapy occurred in 14 patients. Two ischemic heart failure patients with non-RAS and 3 ischemic heart failure patients with RAS received hemodialysis therapy. One ischemic heart failure patient with non-RAS received kidney transplantation. Five nonischemic heart failure patients with non-RAS and 3 nonischemic heart failure patients with RAS received hemodialysis. Totally, there were 8 renal replacement therapies in 121 patients with non-RAS and 6 renal replacement therapies in 22 patients with RAS. The difference between two group was statistically significant (P = 0.003).
>Composite endpoint events
There were 56 patients, including 15 RAS patients and 41 non-RAS patients, who developed composite endpoint events during follow-up, and the difference between two groups was statistically significant (Chi-square = 7.808; P = 0.002). Age, prior stroke, diabetes mellitus, hemoglobin, serum creatinine, eGFR, ACEI/ARB, and RAS were significant predictors for composite endpoints by univariate analysis [Table 3]. By multivariate Cox regression analysis, only ACEI/ARB was a significant predictor for composite endpoint events [Table 4].
Medications on discharge are listed in Table 2. Anti-angiotensin therapy included ACEI and ARB. Ten patients with RAS received anti-angiotensin therapy and 63 patients with non-RAS received anti-angiotensin therapy. The difference between two groups was statistically significant (P = 0.646). One patient with bilateral RAS suffered progressive renal insufficiency after valsartan therapy, which was not reversible after terminating valsartan therapy.
DISCUSSION
In this study, we analyzed the association of RAS with clinical outcome in patients with heart failure. With the definition of RAS by renal duplex sonography, results suggested that, in patients with heart failure, RAS was associated with increased risk of all-cause death and cardiovascular death.
Previous studies had investigated the association of RAS and long-term outcome of a patient with heart failure and have achieved controversial results. One earlier study found that compared with 62 patients with non-RAS, defined by magnetic resonance angiography, 73 patients with RAS had prolonged hospital stay and a higher mortality.[3] They believed that RAS is a significant predictor for a poor long-term outcome. However, this association was not validated by a later study.[4] In this study, compared with 254 patients with non-RAS, defined by magnetic resonance angiography, 112 patients with RAS had more hospital admissions and more prolonged hospital stay because of vascular events and worse prognosis. However, the multivariable analysis did not show RAS could predict a worse outcome. These authors thought that RAS is just a bystander rather an independent predictor of worse outcome in patients with chronic heart failure.[4]
The reason underlying this controversy is uncertain. We think that a threshold of severity exists for RAS to affect the future outcome. Definition of RAS might have led to controversial results about RAS in previous studies. In the above studies, stenosis of >50% by magnetic resonance angiography was used to determine RAS.[3,4] We have shown that RAS >70% helps to predict major adverse cardiac events after acute myocardial infarction, whereas RAS >50% does not.[5] In our study, RAS was defined by renal duplex sonography, which was based on altered hemodynamic parameter of blood flow. Duplex sonography can discriminate ≥60% RAS from <60% RAS accurately.[7] Our results suggested that RAS is associated with a poor outcome. Patient with RAS was in 4.2-fold risk of death and 3.5-fold risk of cardiovascular death than patients with non-RAS. There were more renal replacement therapies in patients with RAS than patients without RAS. As for composite endpoint events, only ACEI/ARB was significant predictors. Definition of RAS may also help to explain why some studies failed to prove the benefits of renal revascularization, including Angioplasty and Stenting for Renal Artery Lesions study.[10,11]
Since RAS is associated with a poor outcome, it is worth to investigate whether renal revascularization is beneficial for patients with heart failure. Recently published Cardiovascular Outcomes in Renal Atherosclerotic Lesions study did not show any benefit of renal revascularization either.[12] Renal revascularization confers no significant benefit with respect to prevention of clinical events when added to comprehensive and multifactorial medical therapy for ARAS patients with hypertension or chronic kidney disease.[12] In this study, there were 12.0% (55) and 15.0% (472) patients with heart failure in stenting plus medical therapy group and medical therapy only group, respectively, but subgroup analysis of renal revascularization in heart failure patients was not published. Recent data about benefits of renal revascularization has been reported. Renal revascularization helps to improve heart failure control and reduce heart failure hospitalizations, which could be attributed to better blood pressure control and reintroduction of anti-angiotensin therapy.[13] Possible mechanisms might include ameliorated left ventricular remodeling after renal stent implantation, which is independent of blood pressure reduction in patients with hypertension.[14,15]
The limitations of this study were as follow: This was a retrospective study, and patients in our sample were not enrolled consecutively. Possible selection bias exists, which might have made our study undervalued.
In conclusion, our study suggested that RAS, defined by renal duplex sonography, was associated with a poor outcome in patients with heart failure. Future studies should address the benefits of renal revascularization in patients with heart failure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.de Silva R, Nikitin NP, Bhandari S, Nicholson A, Clark AL, Cleland JG. Atherosclerotic renovascular disease in chronic heart failure: Should we intervene? Eur Heart J. 2005;26:1596–605. doi: 10.1093/eurheartj/ehi304. [DOI] [PubMed] [Google Scholar]
- 2.Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis. The case for renal artery stenting for treatment of renal artery stenosis? Circulation. 2007;115:263–9. doi: 10.1161/CIRCULATIONAHA.106.619015. [DOI] [PubMed] [Google Scholar]
- 3.de Silva R, Loh H, Rigby AS, Nikitin NP, Witte KK, Goode K, et al. Epidemiology, associated factors, and prognostic outcomes of renal artery stenosis in chronic heart failure assessed by magnetic resonance angiography. Am J Cardiol. 2007;100:273–9. doi: 10.1016/j.amjcard.2007.02.098. [DOI] [PubMed] [Google Scholar]
- 4.Bourantas CV, Loh HP, Lukaschuk EI, Nicholson A, Mirsadraee S, Alamgir FM, et al. Renal artery stenosis: An innocent bystander or an independent predictor of worse outcome in patients with chronic heart failure? A magnetic resonance imaging study. Eur J Heart Fail. 2012;14:764–72. doi: 10.1093/eurjhf/hfs057. [DOI] [PubMed] [Google Scholar]
- 5.Zheng B, Liu J, Ma Q, Zhao D, Wang X, Zheng Z. Association of atherosclerotic renal artery stenosis with major adverse cardiovascular events after acute myocardial infarction. Chin Med J. 2014;127:618–22. [PubMed] [Google Scholar]
- 6.Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60:1490–7. doi: 10.1046/j.1523-1755.2001.00953.x. [DOI] [PubMed] [Google Scholar]
- 7.Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med. 1995;122:833–8. doi: 10.7326/0003-4819-122-11-199506010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita K, Tonelli M, Lloyd A, Levey AS, Coresh J, Hemmelgarn BR. Alberta Kidney Disease Network. Clinical risk implications of the CKD Epidemiology Collaboration (CKD-EPI) equation compared with the Modification of Diet in Renal Disease (MDRD) Study equation for estimated GFR. Am J Kidney Dis. 2012;60:241–9. doi: 10.1053/j.ajkd.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Zheng B, Yan HB, Liu RF, Cheng SJ, Wang J, Zhao HJ, et al. Is it necessary to stent renal artery stenosis patients before cardiopulmonary bypass procedures? Chin Med J. 2011;124:1453–7. [PubMed] [Google Scholar]
- 11.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, et al. ASTRAL Investigators. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–62. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 12.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane GC, Xu N, Mistrik E, Roubicek T, Stanson AW, Garovic VD. Renal artery revascularization improves heart failure control in patients with atherosclerotic renal artery stenosis. Nephrol Dial Transplant. 2010;25:813–20. doi: 10.1093/ndt/gfp393. [DOI] [PubMed] [Google Scholar]
- 14.Rzeznik D, Przewlocki T, Kablak-Ziembicka A, Kozanecki A, Roslawiecka A, Lach J, et al. Effect of renal artery revascularization on left ventricular hypertrophy, diastolic function, blood pressure, and the one-year outcome. J Vasc Surg. 2011;53:692–7. doi: 10.1016/j.jvs.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Zeller T, Rastan A, Schwarzwälder U, Müller C, Frank U, Bürgelin K, et al. Regression of left ventricular hypertrophy following stenting of renal artery stenosis. J Endovasc Ther. 2007;14:189–97. doi: 10.1177/152660280701400211. [DOI] [PubMed] [Google Scholar]


