Highlight
VvGuaS, a novel allele of the VvTPS24 gene, is responsible for the production of the rotundone precursor α-guaiene. Two specific polymorphisms distinguish VvGuaS from its non-guaiene-producing homologue VvPNSeInt.
Key words: Pinot Noir, rotundone, sesquiterpene synthase, sesquiterpenoids, Shiraz, wine aroma.
Abstract
Rotundone was initially identified as a grape-derived compound responsible for the peppery aroma of Shiraz wine varieties. It has subsequently been found in black and white pepper and several other spices. Because of its potent aroma, the molecular basis for rotundone formation is of particular relevance to grape and wine scientists and industry. We have identified and functionally characterized in planta a sesquiterpene synthase, VvGuaS, from developing grape berries, and have demonstrated that it produces the precursor of rotundone, α-guaiene, as its main product. The VvGuaS enzyme is a novel allele of the sesquiterpene synthase gene, VvTPS24, which has previously been reported to encode VvPNSeInt, an enzyme that produces a variety of selinene-type sesquiterpenes. This newly discovered VvTPS24 allele encodes an enzyme 99.5% identical to VvPNSeInt, with the differences comprising just 6 out of the 561 amino acid residues. Molecular modelling of the enzymes revealed that two of these residues, T414 and V530, are located in the active site of VvGuaS within 4 Å of the binding-site of the substrate, farnesyl pyrophosphate. Mutation of these two residues of VvGuaS into the corresponding polymorphisms in VvPNSeInt results in a complete functional conversion of one enzyme into the other, while mutation of each residue individually produces an intermediate change in the product profile. We have therefore demonstrated that VvGuaS, an enzyme responsible for production of the rotundone precursor, α-guaiene, is encoded by a novel allele of the previously characterized grapevine gene VvTPS24 and that two specific polymorphisms are responsible for functional differences between VvTPS24 alleles.
Introduction
Terpenoids are one of the most diverse and abundant classes of specialized metabolites in the plant kingdom and perform a variety of functions, including defence against insects and microbes (Pare and Tumlinson, 1999) or the attraction of pollinators (Martin et al., 2009). Many terpenoids are volatile and therefore have the potential to act as aroma compounds, a function that is particularly relevant in the cultivated grapevine Vitis vinifera L., owing to the use of its berries in wine making (Lund and Bohlmann, 2006; Dunlevy et al., 2009). There have been several studies into the effect of 10-carbon monoterpenoids, such as linalool, nerol, and geraniol, on the muscat aroma of white wine varieties (Sanchez-Palomo et al., 2005; Vilanova and Sieiro, 2006; Dziadas and Jelen, 2010), and into the genetic basis for monoterpene biosynthesis in grapes (Martin and Bohlmann, 2004; Battilana et al., 2011; Martin et al., 2012). In recent studies, the presence of 15-carbon sesquiterpenoids in grapes has been investigated (Coelho et al., 2006; Kalua and Boss, 2010; May and Wust, 2012; Matarese et al., 2013; Matarese et al., 2014), although their presence in wine and the individual and collective effect of sesquiterpenes on wine aroma are poorly understood (Robinson et al., 2014b).
In 2007, the sesquiterpene ylangene was identified as a ‘marker compound’ for the peppery aroma in Shiraz wine, although the chemical itself was not found to have a significant aroma (Parker et al., 2007). A different bicyclic sesquiterpene, rotundone, was subsequently identified as the compound responsible for the peppery aroma (Wood et al., 2008). Rotundone exhibits extremely low detection thresholds of 8ng/L in water and 16ng/L in wine (Siebert et al., 2008). This specialized plant metabolite is a stable sesquiterpenoid composed of a guaiene carbon skeleton with a single ketone group in the carbon 2 position. Its identification in Shiraz represented the first demonstration of a specific sesquiterpene directly linked to an aroma characteristic in wine. Given the potent effect of this single volatile metabolite, an understanding of the gene or genes responsible for rotundone biosynthesis in grapes will provide a platform to select for desired levels of rotundone in different grape varieties. This could include utilizing techniques such as marker-assisted selection or metabolic engineering for the purpose of producing wines with increased or less peppery character. Such options have previously been outlined with respect to grape monoterpene content following the discovery of a polymorphism within 1-deoxy-D-xylulose 5-phosphate synthase responsible for increased linalool, nerol, and geraniol content in berries of some varieties (Battilana et al., 2011).
The precise mechanism of rotundone biosynthesis is unknown. Based on its structure, the direct precursor is likely to be α-guaiene, which can be formed in a single step from farnesyl pyrophosphate (FPP) by a terpene synthase (TPS) (Kumeta and Ito, 2010). This hypothesis is further supported by recent evidence that α-guaiene can be converted to rotundone via a non-specific oxidation reaction mediated by a fungal laccase enzyme in the presence of chemical mediators (Schilling et al., 2013) or by direct oxidation (Huang et al., 2014). Furthermore, rotundone is found in plants that also contain α-guaiene and other guaiene-type sesquiterpenes (bicyclic compounds comprising 5-carbon and 7-carbon rings), including pepper (Piper spp.; Jirovetz et al., 2002), agarwood (Aquilaria spp.; Ishihara et al., 1991), and some Cyperus spp. (Ishihara et al., 1991; Olawore et al., 2006; Kumeta and Ito, 2010). α-Guaiene has been identified in numerous plants and in essential oils (Pandey et al., 2003; Wei and Shibamoto, 2007; Sundaresan et al., 2009; Pripdeevech et al., 2011) and has thus been more widely detected in comparison to rotundone. This may, however, be due to differences in concentration and ease of detection. Following the initial identification of rotundone in Shiraz grapes and wine, it has been found in a number of other red and white wine varieties, demonstrating that it is not unique to a single grapevine cultivar (Caputi et al., 2011; Mattivi et al., 2011; Matarese et al., 2014; Scarlett et al., 2014). Nevertheless, the absence of rotundone in many varieties could suggest that genes responsible for its biosynthesis, or for the biosynthesis of its precursor, may be absent or differentially expressed in some varieties.
In plants, TPSs are encoded by a multigene family with up to 150 members in some species (Chen et al., 2011). The TPS family has been relatively well defined in grapevine, with 69 full-length TPS genes identified on the published genomic scaffold based on an inbred Pinot Noir genome, PN40024 (Jaillon et al., 2007; Martin et al., 2010). These included 30 potential genes of the TPS-a subfamily, generally considered responsible for sesquiterpene biosynthesis, which were found to exist in two clusters on chromosomes 18 and 19. Of these, 13 of the encoded enzymes were functionally characterized by Martin et al. (2010) using Escherichia coli engineered to produce and accumulate the substrate FPP. Other grape-derived TPSs have previously been functionally characterized in other studies (Lucker et al., 2004; Martin et al., 2009; Martin et al., 2012; Matarese et al., 2014). While there is no clear candidate for the gene responsible for the biosynthesis of a rotundone precursor, a recombinantly expressed protein from Pinot Noir named VvPNSeInt, whose main products were selinene and intermedeol, also produced α-guaiene as a minor product (3.5%) (Martin et al., 2010).
In order to investigate the possibility that polymorphic differences in TPS genes could alter their function, we amplified a number of TPS cDNAs from developing grape berries of the Shiraz variety, a variety known to produce rotundone. Here we report the functional characterization and mutagenic analysis of one of the encoded enzymes by transient expression in Nicotiana benthamiana leaves. We demonstrate that a novel allele of VvTPS24, the gene expected to encode VvPNSeInt, actually encodes VvGuaS, an enzyme with predominantly α-guaiene synthase activity. Furthermore, we show that two specific nucleotide substitutions in the VvTPS24 gene can change its encoded product from VvGuaS into VvPNSeInt, and thus relate two grapevine polymorphisms with a physiologically relevant functional outcome.
Materials and Methods
Plant material and nucleic acid isolation and amplification
Total RNA was isolated from Vitis vinifera cv. Shiraz grown in the Nuriootpa Research vineyard, Barossa Valley, South Australia, and harvested at various times of the 2013 season. Harvested grapes were frozen immediately in liquid nitrogen and stored at −80°C until required. RNA extractions were carried out as described (Sweetman et al., 2012) and cDNA was synthesized using the iScript™ Select cDNA Synthesis Kit (Bio-Rad) as per the manufacturer’s instructions. VvTPS24 was amplified from cDNA using Phusion® High-Fidelity DNA Polymerase (NEB) with the primer pair 5′- CACCATGTCTGTTCCACTATCAGTCTCAG-3′ and 5′-TTACATTGGCACAGGATCTATG-3′ under standard PCR conditions. In the first instance, amplicons were ligated into pENTR/D-TOPO (Invitrogen) and at least three clones were sequenced (AGRF, Adelaide).
Site-directed mutagenesis
Mutations were carried out on cDNAs in the pENTR/D-TOPO vector using an adaptation of the QuikChange Site Directed Mutagenesis kit (Stratagene) essentially as described (Drew et al., 2011). In short, the complete T414S mutant was amplified, complete with plasmid, using the mutagenesis primer 5′-GCAACGCGCTGGTAAGC TCTGCCTGCTCTATG-3′ and its reverse complement pair (non-complementary nucleotide shown in bold). The V530M mutant was made with the 5′-CTGAATTTTAGCCGGATGA TGGACGTCTTGTACAA-3′ primer and its reverse complement pair. The double mutant T414S/V530M was created in a two-step process by carrying out the V530M mutation using the T414S mutant as a template. All products were confirmed via sequencing.
Homology modelling
The coordinates of 5-epi-aristolochene synthase (TEAS; Protein Data Bank accession 5EAT) (Starks et al., 1997) were used as a template structure for homology modelling of the VvGuaS sequence. This template structure included a structural analogue of FPP bound within the active site for determination of its proximity to residues forming the surface of the binding cavity. Homology modelling was performed using satisfaction of spatial restraints of all non-hydrogen atoms using MODELLER version 7.2 (Eswar et al., 2003). All figures were generated using PyMOL (DeLano Scientific LLC, San Francisco, CA, USA) and modelling of mutated amino acid residues was carried out within the VvGuaS model using the PyMOL residue mutation function.
Heterologous expression in Nicotiana benthamiana and solid-phase micro-extraction
Forward and reverse primers were designed with USER-overhangs to enable cloning into a USER-compatible version of the pEAQ-HT vector. USER cloning was performed as previously described (Nour-Eldin et al., 2006). The pEAQ-HT plasmid (kindly provided by George Lomonosonoff, John Innes Research Centre, Norwich, UK) harbouring the viral suppressor p19 was modified to harbour a USER cassette. N. benthamiana plants were grown from seeds at 24°C day/19°C night cycles for 5 weeks before infiltration. Transformation of Agrobacterium tumefaciens and subsequent expression in N. benthamiana were performed as described previously (Yang et al., 2012; Bach et al., 2014). A whole leaf from infiltrated N. benthamiana was inserted into a 20ml glass vial and the volatiles extracted at 60°C for 20min using a Supelco 57298-U, 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane StableFlex/SS (1cm) fibre (Supelco Sigma-Aldrich, Denmark). Fibres were reconditioned for 20min at 240°C. Analysis was performed in triplicate for each sample and N. benthamiana leaves expressing p19 from the pEAQ-HT vector were used as controls for comparison.
GC-MS method and data analysis
Samples were analysed on a Shimadzu GCMS‐QP2010 Plus using an Agilent HP-5ms Ultra Inert fused Silica capillary column of 29 m length × 0.25 mm diameter × 0.25 μm film thickness, inserted directly into the ion source of the MS (Simonsen et al., 2009; Drew et al., 2013). The pressure was kept at 16 kPa, giving a column flow of 1.25mL/min. The injection port temperature was set to 250°C, and lower injection port temperatures were investigated to confirm that a significant degree of thermal degradation did not occur (Andersen et al., 2015). The oven temperature was set to 60°C for 3min, and increased to 160°C at a rate of 7°C/min, before a further increase to 300°C at a rate of 50°C/min. This temperature was held for 5min and finally increased to 320°C at a rate of 50°C/min, where it was maintained for 3min. The carrier gas was H2 and the ionization electron energy was 70eV. The ion source temperature was 230°C with an interface temperature of 280°C. The total run time was 28.49min. A C7-C30 standard series (Sigma-Aldrich, Denmark) was used to calculate retention indices (I). All data were analysed using the Shimadzu software Lab Solutions, GCMS Solutions version 2.50 SU3. NIST and Wiley 2008 libraries were used in conjunction with the NIST Standard Reference Database for compound identification. Putative compound assignment was made based on a combination of retention index (RI) and mass spectrum similarity, and by comparison to authentic standards for α-guaiene, α-bulnesene, α-copaene, δ-cadinene, α-humulene, and β-caryophyllene.
Results
Identification of a novel Vitis vinifera guaiene synthase
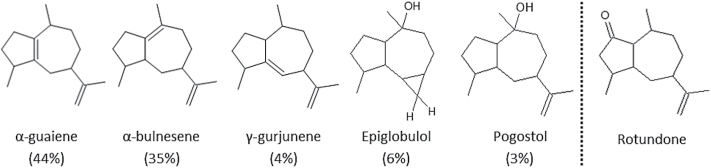
Recent work investigated the global expression of mRNA transcripts in Shiraz grapes and identified a number of potential members of the TPS-a subfamily expected to produce sesquiterpenes, and actively transcribed in developing Shiraz berries (Sweetman et al., 2012). The products of these enzymes included a number of monocyclic and bicyclic sesquiterpenes, some of which have previously been identified as products of grape enzymes (Martin et al., 2010). However, one of the TPSs we investigated, encoded by VvTPS24 (National Center for Biotechnology Information [NCBI] accession XM_002282452), was of particular interest owing to the high proportion of guaiene-type sesquiterpenes produced. The activity of the encoded enzyme was investigated through Agrobacterium-mediated transient expression of VvTPS24 cDNA into N. benthamiana leaves, followed by analysis of volatile products produced using solid phase micro-extraction (SPME) GC-MS. Comparison of RIs and mass spectra to those of authentic standards from appropriate GC-MS libraries enabled identification of the major products as α-guaiene (44%) and α-bulnesene (35%, also known as δ-guaiene). A number of minor products were also produced, putatively annotated as epiglobulol, γ-gurjunene, and pogostol, that also exhibit the characteristic guaiene-type 5,7 bicyclic carbon skeleton of the peppery aroma compound rotundone (Fig. 1). Based on this analysis, we named this VvTPS24 gene product VvGuaS, despite the expectation that it should encode the selinene-producing VvPNSeInt (Martin et al., 2010). GC-MS data for VvGuaS products are presented in the Supplementary data.
Fig. 1.
Chemical structures of the five major volatile products of VvGuaS expressed in N. benthamiana leaves and analysed via SPME-GCMS. Absolute stereochemistry is not specified. The proportion of each compound detected, as measured by percentage of TIC, is shown below each compound. Note the presence of the common 5,7 bicyclic ring in all structures. The structure of the peppery aroma compound, rotundone, is presented for comparison.
VvGuaS is a polymorphic variant of the VvTPS24 gene
The array of products detected following expression of this protein was somewhat unexpected given that VvPNSeInt, an enzyme apparently encoded by the VvTPS24 gene, was previously functionally characterized and found to produce a completely different array of sesquiterpene products. BLAST searches utilizing the nucleic acid sequence of our isolated cDNA, as well as the amino acid sequence of VvGuaS, demonstrated that it was 99.5% identical to a sesquiterpene synthase transcript (NCBI accession XM_002282452). It is on this sequence that the synthetic terpene synthase construct HM807406 was designed, which encoded the selinene synthase VvPNSeInt (Martin et al., 2010). No sequences from the NCBI reference sequence genome, based on the Pinot Noir-derived variety PN40024, matched our variant. However, contig VV78X107636.8 (NCBI accession AM459143), produced as part of the whole genome shotgun sequencing of Pinot Noir (Velasco et al., 2007), contained a predicted protein-encoding region that contained ambiguous nucleotide calls at four out of six positions corresponding to differences between VvGuaS and VvPNSeInt. Specifically, the VvGuaS and VvPNSeInt amino acid sequences differed at positions 403, 405, 414, 431, 499, and 530, and the predicted protein product of contig VV78X107636.8 showed ambiguous residues at all but the first two positions. The presence of these ambiguous base calls at these sites strongly indicates that the VvTPS24 gene is heterozygous and that alleles encoding both VvGuaS and VvPNSeInt exist in the Pinot Noir genome. Meanwhile, the absence of any sequence corresponding to VvGuaS in the NCBI reference genome suggests that it may have been bred to homozygosity during the development of the PN40024 inbred variety, with only the allele encoding VvPNSeInt remaining.
Structural comparison of VvGuaS and VvPNSeInt
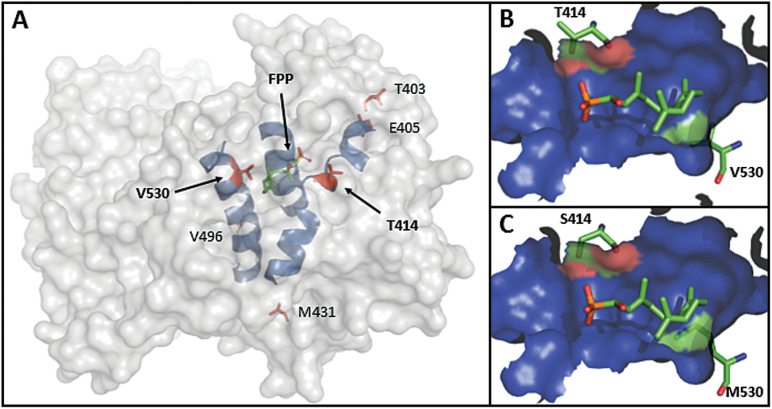
To investigate the reasons for the dramatic functional differences observed between these two enzymes, a comparison of the structural location of the amino acid difference between VvGuaS and VvPNSeInt was undertaken. Molecular modelling of the two enzymes based on the TEAS template structure revealed that two of the varying amino acid positions were directly located in the active site, proximal to the location of FPP binding and subsequent catalysis, while the other four amino acid differences were located more peripherally (Fig. 2A). The two polymorphisms within the active site of VvGuaS compared to VvPNSeInt corresponded to T414S and V530M substitutions. In both cases, modelling of the electrostatic surface of the active site of VvGuaS demonstrated that the T414 and V530 residues both contribute to the internal binding site of FPP (Fig. 2B, C) and are located on separate alpha-helices that contribute to the formation of the internal cavity comprising the FPP substrate-binding site. The estimated distances from the most proximal atoms of the side-chains of T414 and V530 to FPP in the molecular model of VvGuaS were approximately 4 Å. The remaining four residue differences between VvGuaS and VvPNSeInt, namely T403R, E405D, M431I, and V499F, were located a minimum of 10 Å from the FPP binding site and did not contribute to the molecular surface of the internal cavity (Fig. 2A).
Fig. 2.
A molecular model of the global VvGuaS protein structure based on the TEAS template. (A) A computed enzyme surface is shown as a transparent grey solid, three relevant helices of the secondary structure are visible in blue, and bound FPP, as located in TEAS, is shown centrally located in green (diphosphate group in red). The position of the six amino acid residues that differ between VvPNSeInt and VvGuaS is shown in red and labelled, with T414 and V530 indicated by an arrow. (B) Close-up view of part of the internal active site cavity of VvGuaS and (C) VvPNSeInt. The molecular surface of the internal cavity is shown predominantly in blue, with the FPP substrate and side-chains of amino acid residues 414 and 530 (VvGuaS numbering used) shown as sticks. The contributions of relevant amino acid residues to the formation of the cavity molecular surface are shown using the equivalent colours of the contributing atoms (carbons in green and oxygen in red).
It has previously been demonstrated that amino acid residues located near the site of FPP binding within enzymes of the sesquiterpene synthase family can have profound effects on the metabolites produced, with the observed effects decreasing with decreasing proximity (Greenhagen et al., 2006). To investigate whether the two amino acid residue polymorphisms located at the FPP binding site of VvGuaS could be responsible for the different products generated compared with the previously characterized isoform VvPNSeInt, we carried out site-directed mutagenesis of the VvTPS24 cDNA to produce versions with either individual (T414S and V530M) or double (T414S/V530M) mutations. Agrobacterium-mediated transient transformation of N. benthamiana leaves was carried out with these mutated versions of VvGuaS and the volatile metabolites were analysed via SPME GC-MS (Table 1).
Table 1.
Volatile metabolic products of VvGuaS expressed in N. benthamiana
| TIC% | |||||||
|---|---|---|---|---|---|---|---|
| Compound | RI | Ref RIb | WT | T414S | V530M | T414S/V530M | VvPNSeIntc |
| α-copaenea | 1373 | 1372–1376 | 2 | 3 | 1 | 1 | - |
| α-gurjunene | 1407 | 1402–1411 | 1 | 1 | 0 | 0 | - |
| α-guaiene a | 1437 | 1428–1437 | 44 | 41 | 24 | 5 | 3.5 |
| Unknown | 1440 | n/a | 0 | 0 | 0 | 1 | - |
| γ-gurjunene | 1478 | 1479 | 4 | 3 | trace | 0 | - |
| Selina-4(14),11-diene | 1482 | 1472–1488 | 0 | 4 | 24 | 40 | 34 |
| Epiglobulol | 1488 | 1463–1497 | 6 | 12 | 2 | 2 | - |
| α-selinene | 1492 | 1478–1497 | 0 | 7 | 16 | 23 | 14 |
| Unknown | 1498 | n/a | 5 | 5 | 4 | 4 | - |
| α-bulnesene a | 1506 | 1500–1515 | 35 | 18 | 12 | 0 | - |
| Epi-α-selinene | 1517 | 1518 | 1 | 4 | 8 | 12 | 15 |
| δ-cadinene a | 1524 | 1519–1530 | 0 | 0 | 3 | 2 | - |
| Pogostol | 1653 | 1637–1656 | 3 | 2 | 0 | 0 | - |
| Intermedeol | 1660 | 1654–1667 | 0 | 2 | 8 | 9 | 30 |
TIC percentages are semi-quantitative because they depend on each compound’s affinity for the SPME fibre. Compound identities reported based on comparison to authentic standards (a) or putatively annotated based on mass spectrum similarity (>90% as calculated by Shimadzu GCMS Solution 2.50 software against compounds in NIST08 and Wiley08 libraries) and RI within the range of reported references, except for epi-α-selinene and intermedeol, which are based on RI alone. b Ranges of reported retention indices for DB5 and similar columns as compiled in the NIST Standard Reference Database 69: NIST Chemistry WebBook. c Products previously reported for VvPNSeInt shown for comparison (Martin et al., 2010).
Effect of site-directed mutagenesis of selected VvGuaS residues
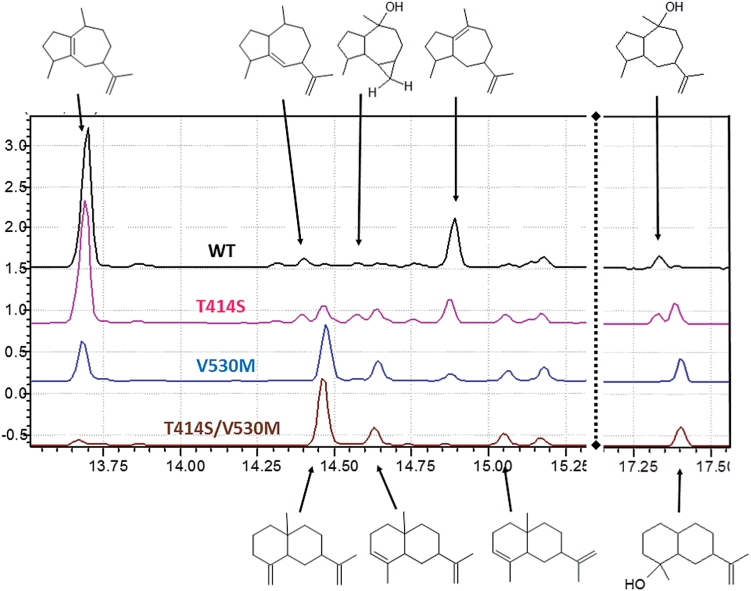
Compared with wild-type (WT) VvGuaS, the T414S mutant exhibited a relatively minor change in product profile, with the two major volatile metabolites continuing to be α-guaiene (41%) and α-bulnesene (18%). The relatively small decrease in the total proportion of α-bulnesene was replaced by the new products selina-4,11-diene (4%) and α-selinene (7%). These new products were observed at retention times where no trace sesquiterpene ions were visible in the WT VvGuaS enzyme. Additionally, the proportion of epi-α-selinene increased from 1% to 4% of the total ion chromatogram (TIC). Thus, the T414S mutant had 12 identifiable products, compared to nine for the WT enzyme, with each of the new products consisting of sesquiterpenes with a 6,6 bicyclic carbon backbone similar to products of VvPNSeInt (Table 1 and Supplementary data). In the case of VvGuaS-V530M, a more significant change in the range of products was observed, with the overall theme again being a shift towards the product profile reported for VvPNSeInt. The proportions of the 5,7 bicyclic α-guaiene (24%) and α-bulnesene (12%) significantly decreased compared to WT, and substantially higher amounts of the 6,6 bicyclic compounds putatively identified as selina-4,11-diene (24%), α-selinene (16%), and epi-α-selinene (8%) were produced. Pogostol (a hydroxy-guaiene) was no longer present, while a hydroxy-selinene compound, likely intermedeol, now comprised 8% of the TIC. Analysis of the double mutant VvGuaS-T414S/V530M demonstrated that its guaiene synthase activity was almost completely compromised, with only 5% of the volatile products comprising α-guaiene, and α-bulnesene not detectable. Instead, the major products were predominantly the 6,6 bicyclic sesquiterpenes selina-4,11-diene (40%), α-selinene (23%), epi-α-selinene (12%), and a hydroxylated selinene (9%) with the same reported RI as intermedeol.
Discussion
Following the widespread and relatively rapid domestication and cultivation of grapevine over the past several thousand years (This et al., 2006), there are now up to 10 000 acknowledged varieties of V. vinifera (Alleweldt and Dettweiler, 1994). While several hundred of these varieties are commonly used for the production of wine, much remains to be discovered concerning the precise content of aroma-active compounds in most of them. Nevertheless, it is apparent that white wine varieties tend to have a higher relative content of monoterpene compounds, while sesquiterpenes have so far been predominantly found in red wine varieties (Coelho et al., 2006; Vilanova and Sieiro, 2006; Coelho et al., 2007; Kalua and Boss, 2009, 2010; May and Wust, 2012; Matarese et al., 2013; Matarese et al., 2014; Robinson et al., 2014a). While this may, in part, be a result of the localization of sesquiterpene compounds in berry exocarp, and thus heavily influenced by the involvement of berry skin in the winemaking process, studies focusing on berries have shown similar results. This suggests that a complete molecular understanding of the enzymatic pathways involved in sesquiterpene biosynthesis in grapes could provide valuable information regarding the origins of biochemicals present in wine.
The bicyclic sesquiterpene rotundone, first identified in Shiraz wine, is an extraordinarily potent aroma compound with an extremely low detection threshold (Siebert et al., 2008; Wood et al., 2008). Conferring a distinct peppery or spicy note to wine, rotundone is also the first sesquiterpene conclusively shown to have a significant effect on wine character, and has been described as one of the most important aroma compounds in wine (Geffroy et al., 2014). Following its initial identification in Shiraz, a variety that is famous for its peppery character, rotundone has been found in several other cultivars, including Duras, Durif, Graciano, Grüner Veltliner, Schioppettino, and Vespolina (Caputi et al., 2011; Qian and Shellhammer, 2012; Geffroy et al., 2014). Owing to the importance of this compound as a chemical determinant of wine aroma, several studies have investigated the location and timing of rotundone biosynthesis in grapes, and the environmental factors and viticultural practices that may influence final concentrations. Rotundone has been found in exocarp and skin, but is absent from pulp or seeds (Caputi et al., 2011). Absolute levels were shown to increase late in berry ripening and were generally higher in cooler vintages (Caputi et al., 2011; Geffroy et al., 2014). These data add to physiological studies investigating the production of other grape specialized metabolites, whose results have demonstrated that their biosynthesis can be influenced by biotic factors such as insect attack (D’Onofrio et al., 2009), or abiotic factors such as light exposure, crop load, and nitrogen levels (Chapman et al., 2004; des Gachons et al., 2005; Chone et al., 2006).
Given the direct link between rotundone and the peppery aroma of wine, the elucidation of the metabolic pathway of its biosynthesis, and the enzymes responsible for catalytic steps within this pathway, would open up possibilities for investigating grapevine varieties for their potential to produce a pepper aroma. Additionally, techniques such as quantitative mRNA transcript analysis could enable the real-time monitoring of environmental or agronomic factors that affect the expression of the genes responsible. As a sesquiterpene ketone, the most likely biosynthetic pathway of rotundone is via a guaiene intermediate, that is, a bicyclic 5,7 ring carbon skeleton (see Fig. 1), that would be directly generated from FPP by a terpene synthase (Kumeta and Ito, 2010). Indeed, the oxidation of α-guaiene by a fungal laccase has been shown to result in the production of rotundone (Schilling et al., 2013), and rotundone accumulates over time in commercial samples of guaiene that are exposed to the environment (unpublished observation). Recently, Huang et al. (2014) demonstrated that rotundone is one of the main products of the auto-oxidation of α-guaiene. Their data suggest that α-guaiene can slowly oxidize to rotundone over a number of weeks at room temperature. This process is dramatically increased at temperatures of 40–50ºC (Huang et al., 2014). If spontaneous conversion of α-guaiene to rotundone plays a role physiologically, this could potentially explain the increased levels of rotundone in grapes that are harvested significantly later than usual (Geffroy et al., 2014; Davies et al., 2015). In contrast, two separate studies have indicated that increased temperature actually correlates with lower rotundone concentrations (Zhang et al., 2013; Scarlett et al., 2014). This suggests that small increases in ambient temperature do not have a significant effect on the rate of any non-enzymatic α-guaiene oxidation that may be occurring.
While it has been shown that α-guaiene can be oxidized to rotundone spontaneously (Huang et al., 2014), there is also the possibility that the final step in rotundone production is carried out enzymatically. The oxidation of sesquiterpene hydrocarbons into more specialized products has been well described in a number of plant species. This would likely be carried out by a member of the cytochrome P450 family. The oxidation of valencene into nootkatone is a specific example of a sesquiterpene hydrocarbon being converted to a ketone in a single step by a cytochrome P450 (Cankar et al., 2011). Numerous other examples exist of sesquiterpene oxidation being carried out by this family of enzymes, predominantly from the diverse CYP71 subfamily (Ro et al., 2006; Pickel et al., 2012). In summary, although there is currently no biochemical evidence that rotundone is enzymatically produced from α-guaiene in grapes, structurally similar compounds have been shown to be produced from the combined action of a terpene synthase and cytochrome P450 in other plants. Therefore, whether it is the non-enzymatic oxidation of α-guaiene that leads to the accumulation of rotundone in some wines, or whether an enzyme exists that can specifically catalyse the formation of rotundone from α-guaiene, is yet to be determined. Sweetman et al. (2012) previously reported that transcripts corresponding to VvTPS24 were present in Shiraz berries during veraison, but were not detected at significant levels at harvest. Although this work reported data from only a single season, if their results prove to be representative of VvGuaS expression, this could suggest that downstream modification of α-guaiene after veraison is crucial in determining rotundone levels.
The findings presented here demonstrate that a newly identified allele of the VvTPS24 gene encodes VvGuaS, a sesquiterpene synthase whose main product is the rotundone precursor α-guaiene (Fig. 3). We showed that as few as one or two polymorphisms in VvTPS24 determine whether it will produce α-guaiene (Fig. 3). A previously characterized enzyme, VvPNSeInt, also encoded by VvTPS24, was shown to produce selina-4,11-diene (34%) and intermedeol (30%), and only 3.5% α-guaiene (Martin et al., 2010). Both selina-4,11-diene and intermedeol differ structurally from the products of the allele identified in this report, in that they have a 6,6 bicyclic carbon skeleton rather than the 5,7 carbon skeleton of guaiene-type sesquiterpenes. Other than α-guaiene, the only product of VvGuaS that was also reported to be a product of VvPNSeInt was putatively identified as epi-α-selinene, which accounted for 1% of the TIC of VvGuaS compared with 15% for VvPNSeInt (Martin et al., 2010). These data suggest that although the enzymes encoded by the two VvTPS24 alleles share 99.5% identity at the amino acid level, they clearly have different catalytic functions. Furthermore, analysis of the available sequence data indicates that VvTPS24 is heterozygotic for the two alleles in the variety Pinot Noir, while the inbred variety PN40024 is likely homozygotic for VvPNSeInt.
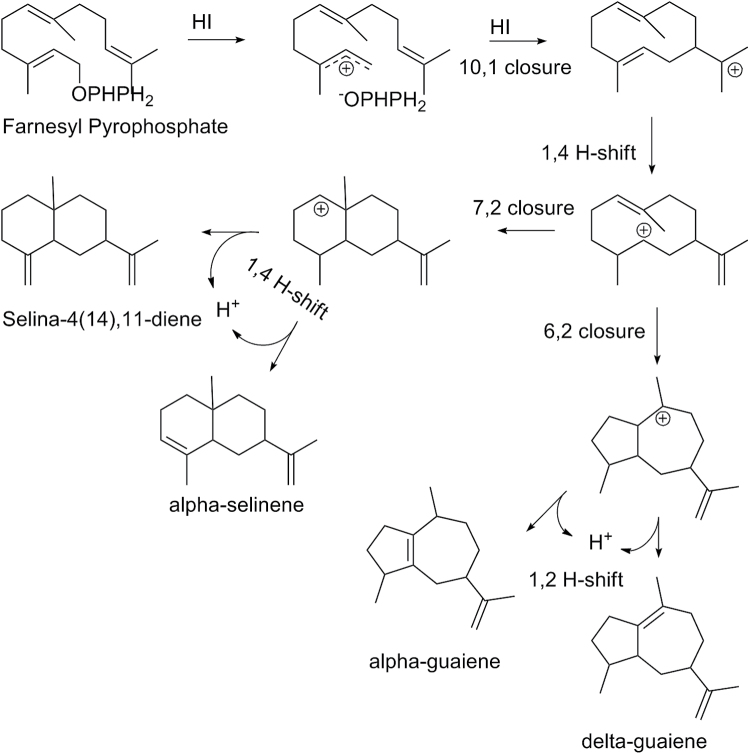
In VvGuaS, the double mutation of T414S and V530M, and to a lesser extent the V530M mutation alone, caused a change in the conformation of the active site that resulted in the formation of the second ring favouring a 7,2 closure rather than the 6,2 closure favoured by the WT VvGuaS enzyme (Fig. 4). The products of the double mutant therefore consisted predominantly of sesquiterpenes with a 6,6 bicyclic carbon skeleton, in contrast to WT VvGuaS, which produces a range of guaiene-like sesquiterpenes with a 5,7 bicyclic skeleton (Fig. 3). In other words, the VvGuaS-T414S/V530M double mutant enzyme produced a product profile equivalent to the alternative VvTPS24 allele, VvPNSeInt. This change in product profile was partially achieved with both of the single mutations T414S and V530M, with the latter having a greater effect. The four major products of the WT VvGuaS enzyme (α-guaiene, α-bulnesene, epiglobulol, and γ-gurjunene) were responsible for 89% of the TIC, while the major four products for the double mutant made up 84% of the TIC, indicating there was no significant decrease in catalytic specificity resulting from the functional change. Similarly, notwithstanding the semi-quantitative nature of SPME GC-MS, no significant differences were observed in the absolute quantities of products produced by the respective enzymes.
Fig. 4.
Scheme demonstrating the mechanism of carbocation formation and second ring closure catalysed by VvGuaS (right-hand side) and the T414S/V530M mutant or VvPNSeInt (left-hand side).
Fig. 3.
Relevant sections of GC-MS traces, shown as extracted ion chromatograms for marker ion 204, for WT VvGuaS (black), and mutant enzymes T414S (pink), V530M (blue), and T414S/V530M (brown). Major peaks corresponding to specific sesquiterpenes are indicated with black arrows. The vertical dotted line delineates the discontinuous chromatogram. Above are the structures of four major products of WT VvGuaS, comprising (from left to right) α-guaiene, γ-gurjunene, epiglobulol, α-bulnesene, and pogostol. Below are four of the major products of T414S/V530M, and also VvPNSeInt, comprising selina-4,11-diene, α-selinene, epi- α-selinene, and intermedeol.
Differences in the functionality exhibited by the two highly similar enzymes can potentially be explained by the existence of lone-pair electrons on the sulfur atom of methionine (present in VvPNSeInt), which could be involved in the stabilization of the carbocation intermediate. While the lone-pair on the sulfur is the most likely new interaction partner, differences to the preferred bond closure of the carbocation could be the result of steric changes associated with the relatively minor size differences between the substituted residues (Fig. 2). Although the crystal structure of the WT and mutant enzymes would be required to answer such mechanistic speculation, it has previously been reported that very minor changes to the active site of TPSs can have a significant impact on their products (Greenhagen et al., 2006; O’Maille et al., 2008). In the present study, we used protein-encoding sequences from two alleles of the same gene to direct our mutational analyses and demonstrated that a maximum of two specific residue changes were necessary and sufficient to change the nature of the second ring closure.
In summary, at least two alleles of the VvTPS24 gene exist in grapevine, both of which encode functional sesquiterpene synthases (VvGuaS and VvPNSeInt). Despite only minor differences in amino acid sequence, we demonstrated that one allele encodes an α-guaiene synthase (VvGuaS), in contrast to the previously investigated version that encodes a selinene synthase (VvPNSeInt). Furthermore, the functional differences between these enzymes can be traced back to two specific polymorphisms in the active site of the protein. The heterozygosity of Pinot Noir for both versions of this enzyme, as evidenced by the existence of contig VV78X107636.8, suggests that it is not only the presence of the α-guaiene-producing allele that is responsible for rotundone accumulation. Nevertheless, the fact that the VvGuaS-producing allele has been discovered in the Shiraz variety, famous for its peppery aroma, could suggest that further investigation into the expression of these two highly similar, but functionally distinct, alleles is warranted. The identification of a grapevine enzyme that produces α-guaiene as its main product is a vital step in determining the molecular basis for rotundone accumulation in grapes, and opens up the possibility for further studies into the factors that influence the peppery aroma of wine.
Supplementary Data
Supplementary materials are available at JXB online.
Supplementary data. Gas chromatogram and mass spectral data for VvGuaS products.
Acknowledgements
The authors would like to thank the reviewer of a previous version of this manuscript, who drew our attention to the presence of contig VV78X107636.8, thus demonstrating that VvTPS24 is heterozygous in Pinot Noir. The pEAQ-HT plasmid was kindly provided by George Lomonosonoff (John Innes Research Centre, Norwich, UK). CS was supported by grants from the Grape and Wine Research and Development Corporation, and the University of the Adelaide and Wine 2030 programme. DPD received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement 275422, which supported a Marie Curie International Outgoing Fellowship. The Danish Strategic Research Council Grant “SPOTLight” supported the work of TBA and HTS. The research work was supported by a grant from the VILLUM Research Center for Plant Plasticity, by the Center for Synthetic Biology “bioSYNergy” supported by the UCPH Excellence Programme for Interdisciplinary Research, and by an ERC Advanced Grant to BLM (ERC-2012-ADG_20120314, Project No: 323034).
References
- Alleweldt G, Dettweiler E. 1994. The genetic resources of Vitis: World list of grapevine collections . Siebeldingen: Bundesforschungsanstalt für Rebenzüchtung Geilweilerhof. [Google Scholar]
- Andersen TB, Cozzi F, Simonsen HT. 2015. Optimization of biochemical screening methods for volatile and unstable sesquiterpenoids using HS-SPME-GC-MS. Chromatography 2, 277–292. [Google Scholar]
- Bach S, Bassard J-E, Andersen-Ranberg J, Moeldrup M, Simonsen HT, Hamberger B. 2014. High throughput testing of terpenoid biosynthesis candidate genes using transient expression in Nicotiana benthamiana . In: Rodriguez-Concepcion M, ed. Plant Isoprenoids . New York: Springer. [DOI] [PubMed] [Google Scholar]
- Battilana J, Emanuelli F, Gambino G, Gribaudo I, Gasperi F, Boss PK, Grando MS. 2011. Functional effect of grapevine 1-deoxy-D-xylulose 5-phosphate synthase substitution K284N on Muscat flavour formation. Journal of Experimental Botany 62, 5497–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cankar K, van Houwelingen A, Bosch D, Sonke T, Bouwmeester H, Beekwilder J. 2011. A chicory cytochrome P450 mono-oxygenase CYP71AV8 for the oxidation of (+)-valencene. FEBS Letters 585, 178–182. [DOI] [PubMed] [Google Scholar]
- Caputi L, Carlin S, Ghiglieno I, Stefanini M, Valenti L, Vrhovsek U, Mattivi F. 2011. Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the ‘peppery’ character of wine. Journal of Agricultural and Food Chemistry 59, 5565–5571. [DOI] [PubMed] [Google Scholar]
- Chapman DM, Matthews MA, Guinard JX. 2004. Sensory attributes of cabernet sauvignon wines made from vines with different crop yields. American Journal of Enology and Viticulture 55, 325–334. [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E. 2011. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant Journal 66, 212–229. [DOI] [PubMed] [Google Scholar]
- Chone X, Lavigne-Cruege V, Tominaga T, Van Leeuwen C, Castagnede C, Saucier C, Dubourdieu D. 2006. Effect of vine nitrogen status on grape aromatic potential: flavor precursors (S-cysteine conjugates), glutathione and phenolic content in Vitis vinifera L. cv. Sauvignon blanc grape juice. Journal International Des Sciences De La Vigne Et Du Vin 40, 1–6. [Google Scholar]
- Coelho E, Rocha SM, Barros AS, Delgadillo I, Coimbra MA. 2007. Screening of variety- and pre-fermentation-related volatile compounds during ripening of white grapes to define their evolution profile. Analytica Chimica Acta 597, 257–264. [DOI] [PubMed] [Google Scholar]
- Coelho E, Rocha SM, Delgadillo I, Coimbra MA. 2006. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Analytica Chimica Acta 563, 204–214. [Google Scholar]
- D’Onofrio C, Cox A, Davies C, Boss PK. 2009. Induction of secondary metabolism in grape cell cultures by jasmonates. Functional Plant Biology 36, 323–338. [DOI] [PubMed] [Google Scholar]
- Davies C, Nicholson EL, Bottcher C, Burbidge CA, Bastian SEP, Harvey KE, Huang AC, Taylor DK, Boss PK. 2015. Shiraz wines made from grape berries (Vitis vinifera) delayed in ripening by plant growth regulator treatment have elevated rotundone concentrations and “pepper” flavor and aroma. Journal of Agricultural and Food Chemistry 63, 2137–2144. [DOI] [PubMed] [Google Scholar]
- des Gachons CP, Van Leeuwen C, Tominaga T, Soyer JP, Gaudillere JP, Dubourdieu D. 2005. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. Journal of the Science of Food and Agriculture 85, 73–85. [Google Scholar]
- Drew DP, Dueholm B, Weitzel C, Zhang Y, Sensen C, Simonsen HT. 2013. Transcriptome analysis of Thapsia laciniata Rouy provides insights into terpenoid biosynthesis and diversity in Apiaceae. International Journal of Molecular Sciences 14, 9080–9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew DP, Hrmova M, Lunde C, Jacobs AK, Tester M, Fincher GB. 2011. Structural and functional analyses of PpENA1 provide insights into cation binding by type IID P-type ATPases in lower plants and fungi. Biochimica et Biophysica Acta-Biomembranes 1808, 1483–1492. [DOI] [PubMed] [Google Scholar]
- Dunlevy JD, Kalua CM, Keyzers RA, Boss PK. 2009. The production of flavour and aroma compounds in grape berries. In: Roubelakios-Andelakis KA, ed. Grapevine Molecular Physiology and Biotechnology , Vol. 2 Dordrecht: Springer Netherlands. [Google Scholar]
- Dziadas M, Jelen HH. 2010. Analysis of terpenes in white wines using SPE-SPME-GC/MS approach. Analytica Chimica Acta 677, 43–49. [DOI] [PubMed] [Google Scholar]
- Eswar N, John B, Mirkovic N, et al. 2003. Tools for comparative protein structure modeling and analysis. Nucleic Acids Research 31, 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffroy O, Dufourcq T, Carcenac D, Siebert TE, Herderich MJ, Serrano E. 2014. Effect of ripeness and viticultural techniques on the rotundone concentration in red wine made from Vitis vinifera L. cv. Duras. Australian Journal of Grape and Wine Research. [Google Scholar]
- Greenhagen BT, O’Maille PE, Noel JP, Chappell J. 2006. Identifying and manipulating structural determinates linking catalytic specificities in terpene synthases. Proceedings of the National Academy of Science of the Unites States of America 103, 9826–9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A-C, Burrett S, Sefton MA, Taylor DK. 2014. Production of the pepper aroma compound, (-)-rotundone, by aerial oxidation of ɑ-guaiene. Journal of Agricultural and Food Chemistry 62, 10809–10815. [DOI] [PubMed] [Google Scholar]
- Ishihara M, Tsuneya T, Uneyama K. 1991. Guaiane sesquiterpenes from agarwood. Phytochemistry 30, 3343–3347. [Google Scholar]
- Jaillon O, Aury JM, Noel B, et al. 2007. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449, 463–467. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. 2002. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. Journal of Chromatography A 976, 265–275. [DOI] [PubMed] [Google Scholar]
- Kalua CM, Boss PK. 2009. Evolution of volatile compounds during the development of cabernet sauvignon grapes (Vitis vinifera L.). Journal of Agricultural and Food Chemistry 57, 3818–3830. [DOI] [PubMed] [Google Scholar]
- Kalua CM, Boss PK. 2010. Comparison of major volatile compounds from Riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest. Australian Journal of Grape and Wine Research 16, 337–348. [Google Scholar]
- Kumeta Y, Ito M. 2010. Characterization of delta-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Physiology 154, 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker J, Bowen P, Bohlmann J. 2004. Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (-)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65, 2649–2659. [DOI] [PubMed] [Google Scholar]
- Lund ST, Bohlmann J. 2006. The molecular basis for wine grape quality - a volatile subject. Science 311, 804–805. [DOI] [PubMed] [Google Scholar]
- Martin DM, Aubourg S, Schouwey MB, Daviet L, Schalk M, Toub O, Lund ST, Bohlmann J. 2010. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biology 10, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Bohlmann J. 2004. Identification of Vitis vinifera (-)-alpha-terpineol synthase by in silico screening of full-length cDNA ESTs and functional characterization of recombinant terpene synthase. Phytochemistry 65, 1223–1229. [DOI] [PubMed] [Google Scholar]
- Martin DM, Chiang A, Lund ST, Bohlmann J. 2012. Biosynthesis of wine aroma: transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewurztraminer grapes. Planta 236, 919–929. [DOI] [PubMed] [Google Scholar]
- Martin DM, Toub O, Chiang A, Lo BC, Ohse S, Lund ST, Bohlmann J. 2009. The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains. Proceedings of the National Academy of Sciences of the United States of America 106, 7245–7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese F, Cuzzola A, Scalabrelli G, D’Onofrio C. 2014. Expression of terpene synthase genes associated with the formation of volatiles in different organs of Vitis vinifera . Phytochemistry 105, 12–24. [DOI] [PubMed] [Google Scholar]
- Matarese F, Scalabrelli G, D’Onofrio C. 2013. Analysis of the expression of terpene synthase genes in relation to aroma content in two aromatic Vitis vinifera varieties. Functional Plant Biology 40, 552–565. [DOI] [PubMed] [Google Scholar]
- Mattivi F, Caputi L, Carlin S, Lanza T, Minozzi M, Nanni D, Valenti L, Vrhovsek U. 2011. Effective analysis of rotundone at below-threshold levels in red and white wines using solid-phase microextraction gas chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry 25, 483–488. [DOI] [PubMed] [Google Scholar]
- May B, Wust M. 2012. Temporal development of sesquiterpene hydrocarbon profiles of different grape varieties during ripening. Flavour and Fragrance Journal 27, 280–285. [Google Scholar]
- Nour-Eldin HH, Hansen BG, Norholm MH, Jensen JK, Halkier BA. 2006. Advancing uracil-excision based cloning towards an ideal technique for cloning PCR fragments. Nucleic Acids Research 34, e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Maille PE, Malone A, Dellas N, et al. 2008. Quantitative exploration of the catalytic landscape separating divergent plant sesquiterpene synthases. Nature Chemical Biology 4, 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olawore NO, Usman LA, Ogunwande IA, Adeleke KA. 2006. Constituents of rhizome essential oils of two types of Cyperus articulatus L. grown in Nigeria. Journal of Essential Oil Research 18, 604–606. [Google Scholar]
- Pandey AK, Rai MK, Acharya D. 2003. Chemical composition and antimycotic activity of the essential oils of corn mint (Mentha arvensis) and lemon grass (Cymbopogon flexuosus) against human pathogenic fungi. Pharmaceutical Biology 41, 421–425. [Google Scholar]
- Pare PW, Tumlinson JH. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiology 121, 325–332. [PMC free article] [PubMed] [Google Scholar]
- Parker M, Pollnitz AP, Cozzolino D, Francis IL, Herderich MJ. 2007. Identification and quantification of a marker compound for ‘Pepper’ aroma and flavor in shiraz grape berries by combination of chemometrics and gas chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry 55, 5948–5955. [DOI] [PubMed] [Google Scholar]
- Pickel B, Drew DP, Manczak T, Weitzel C, Simonsen HT, Ro DK. 2012. Identification and characterization of a kunzeaol synthase from Thapsia garganica: implications for the biosynthesis of the pharmaceutical thapsigargin. Biochemical Journal 448, 261–271. [DOI] [PubMed] [Google Scholar]
- Pripdeevech P, Khummueng W, Park SK. 2011. Identification of odor-active components of agarwood essential oils from Thailand by solid phase microextraction-GC/MS and GC-O. Journal of Essential Oil Research 23, 46–53. [Google Scholar]
- Qian M, Shellhammer T. 2012. Flavor chemistry of wine and other alcoholic beverages . Washington DC: American Chemical Society. [Google Scholar]
- Ro DK, Paradise EM, Ouellet M, et al. 2006. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE. 2014a. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. American Journal of Enology and Viticulture 65, 1–24. [Google Scholar]
- Robinson AL, Boss PK, Solomon PS, Trengrove RD, Heymann H, Ebeler SE. 2014b. Origins of grape and wine flavor. Part 1. Chemical components and viticultural impacts. American Journal of Enology and Viticulture , in press. [Google Scholar]
- Sanchez-Palomo E, Diaz-Maroto MC, Perez-Coello MS. 2005. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC-MS. Talanta 66, 1152–1157. [DOI] [PubMed] [Google Scholar]
- Scarlett NJ, Bramley RGV, Siebert TE. 2014. Within-vineyard variation in the ‘pepper’ compound rotundone is spatially structured and related to variation in the land underlying the vineyard. Australian Journal of Grape and Wine Research 20, 214–222. [Google Scholar]
- Schilling B, Granier T, Locher E. 2013. 1-Hydroxy-octahydroazulenes as fragrances. US Patent Application US13/703,761. Publication number 20130089904 A1. [Google Scholar]
- Siebert TE, Wood C, Elsey GM, Pollnitz AP. 2008. Determination of rotundone, the pepper aroma impact compound, in grapes and wine. Journal of Agricultural and Food Chemistry 56, 3745–3748. [DOI] [PubMed] [Google Scholar]
- Simonsen HT, Riedel C, Gade LB, Jebjerg CP, Guzman A, Mølgaard P. 2009. Chemical composition and antibacterial activity of the leaf essential oil of Baccharis magellanica (Lam.) Pers. and Baccharis elaeoides Remy from Chile. Journal of Essential Oil Research 21, 377–380. [Google Scholar]
- Starks CM, Back KW, Chappell J, Noel JP. 1997. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 277, 1815–1820. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Singh SR, Mishra AN, Shasany AK, Darokar MP, Kalra A, Naqvi AA. 2009. Composition and comparison of essential oils of Pogostemon cablin (Blanco) Benth. (Patchouli) and Pogostemon travancoricus Bedd. var. travancoricus . Journal of Essential Oil Research 21, 220–222. [Google Scholar]
- Sweetman C, Wong DC, Ford CM, Drew DP. 2012. Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics 13, 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This P, Lacombe T, Thomas MR. 2006. Historical origins and genetic diversity of wine grapes. Trends in Genetics 22, 511–519. [DOI] [PubMed] [Google Scholar]
- Velasco R, Zharkikh A, Troggio M, et al. 2007. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS One 2, e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilanova M, Sieiro C. 2006. Determination of free and bound terpene compounds in Albarino wine. Journal of Food Composition and Analysis 19, 694–697. [Google Scholar]
- Wei A, Shibamoto T. 2007. Antioxidant activities and volatile constituents of various essential oils. Journal of Agricultural and Food Chemistry 55, 1737–1742. [DOI] [PubMed] [Google Scholar]
- Wood C, Siebert TE, Parker M, et al. 2008. From wine to pepper: rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. Journal of Agricultural and Food Chemistry 56, 3738–3744. [DOI] [PubMed] [Google Scholar]
- Yang Z, Drew DP, Jorgensen B, et al. 2012. Engineering mammalian mucin-type O-glycosylation in plants. Journal of Biological Chemistry 287, 11911–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Scarlett NJ, Sheehan D, Barlow S, Krstic M, Herderich MJ, Howell K. 2013. Intrabunch variability of rotundone concentration in Vitis vinifera cv. Shiraz wine grapes at harvest. In: Robinson EMC, Godden PW, Johnson DL, eds. Australian Wine Industry Technical Conference , 13-18th July 2013. Adelaide, SA: Proceedings of the Fifteenth Australian Wine Industry Technical Conference. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.