Highlight
We unveil the impacts of a 14-year-long drought treatment on the leaf physiology of Quercus ilex showing a higher plasticity in photosynthetic and respiratory traits.
Key words: Carbon-use efficiency; day respiration; Jmax; mesophyll conductance; rainfall exclusion; Vc,max.
Abstract
Terrestrial carbon exchange is a key process of the global carbon cycle consisting of a delicate balance between photosynthetic carbon uptake and respiratory release. We have, however, a limited understanding how long-term decreases in precipitation induced by climate change affect the boundaries and mechanisms of photosynthesis and respiration. We examined the seasonality of photosynthetic and respiratory traits and evaluated the adaptive mechanism of the foliar carbon balance of Quercus ilex L. experiencing a long-term rainfall-exclusion experiment. Day respiration (R d) but not night respiration (R n) was generally higher in the drought treatment leading to an increased R d/R n ratio. The limitation of mesophyll conductance (g m) on photosynthesis was generally stronger than stomatal limitation (g s) in the drought treatment, reflected in a lower g m/g s ratio. The peak photosynthetic activity in the drought treatment occurred in an atypical favourable summer in parallel with lower R d/R n and higher g m/g s ratios. The plant carbon balance was thus strongly improved through: (i) higher photosynthetic rates induced by g m; and (ii) decreased carbon losses mediated by R d. Interestingly, photosynthetic potentials (V c,max, J max, and TPU) were not affected by the drought treatment, suggesting a dampening effect on the biochemical level in the long term. In summary, the trees experiencing a 14-year-long drought treatment adapted through higher plasticity in photosynthetic and respiratory traits, so that eventually the atypical favourable growth period was exploited more efficiently.
Introduction
Warmer and drier conditions are expected globally under current climate change scenarios and particularly in the Mediterranean region (Somot et al., 2008; Friend, 2010; IPCC, 2013). Seasonal reoccurring drought is the main natural environmental factor in the Mediterranean region limiting plant growth and yield (Specht, 1969; Di Castri, 1973). Projected water shortages are thus likely to intensify the limitations on plant productivity and forest growth. Several studies have already reported drought-induced forest impacts and diebacks in the Mediterranean region (Peñuelas et al., 2001; Martínez-Vilalta and Piñol, 2002; Raftoyannis et al., 2008; Allen et al., 2010; Carnicer et al., 2011; Matusick et al., 2013), as well as shifts in vegetation composition (Jump and Peñuelas, 2005; Anderegg et al., 2013). Seasonal summer drought limits plant growth and productivity most strongly through reductions in the plant carbon budget, which depends on the balance between photosynthesis and respiration (Flexas et al., 2006). Winter has been somehow overlooked, despite the importance of potential recovery and growth periods for the annual carbon budget, especially for evergreen vegetation (Sperlich et al., 2014, 2015). The Mediterranean region is characterized by a high variability in temperature and precipitation regimes, especially in mountainous areas such as the Prades Mountains in north-eastern Spain (Barbeta et al., 2013). Climate extremes combined with high interannual variability complicate the scaling of carbon dynamics from one year to another (Reynolds et al., 1996; Morales et al., 2005; Gulías et al., 2009). In fact, the modelling performance in Mediterranean-type ecosystems is particularly poor (Morales et al., 2005; Vargas et al., 2013) owing to under-represented soil–water patterns and our limited understanding of the effects of water stress on both carbon uptake and release (Hickler et al., 2009; Niinemets and Keenan, 2014).
The non-photorespiratory carbon release in leaves is called mitochondrial respiration—a central metabolic process that produces energy (ATP, NADPH) and carbon skeletons for cellular maintenance and growth. It also contributes to significant carbon losses—especially under stress conditions—altering the net carbon gain (van Oijen et al., 2010). Although the drought responses of Mediterranean vegetation have been investigated extensively, most studies concern photosynthetic responses (reviewed by Flexas et al., 2014), whereas respiratory responses in leaves have largely been neglected (Niinemets, 2014). Also, it is not clear how seasonality and other abiotic stressors affect the balance of night respiration (R n) and day respiration (R d) in the leaves. This is partly owing to measurement difficulties; R n can easily be measured by darkening the leaf, but R d is harder to obtain and is traditionally estimated from carbon-response curves with the Laisk method, from light-response curves with the Kok method, or with an amended version of the Kok method with chlorophyll fluorescence developed by Yin et al. (2009) (reviewed by Yin et al., 2011). Measurement constraints and lacking research priorities can account for the dearth of data on respiratory responses to abiotic stress, particularly drought (Atkin and Macherel, 2009; Heskel et al., 2014). Wright et al. (2006) provided evidence that irradiance, temperature, and precipitation affect respiration in a wide range of woody species around the world; Mediterranean species, however, were not covered. Catoni et al. (2013) recently provided evidence that temperature, and monthly rainfall to a lesser extent, could explain the seasonal variation of R d in several Mediterranean maquis species. Galmés et al. (2007) noted that the number of studies on plant respiration responses to drought is generally limited, but particularly so for Mediterranean species. This is surprising considering the obvious importance of water stress in the Mediterranean region. Seasonal acclimation of respiration is believed to be more important in sclerophyllic perennial leaves (Galmés et al., 2007; Zaragoza-Castells et al., 2007, 2008) than in plants with short-lived leaves (review by Atkin and Macherel, 2009). A better characterization of the respiratory responses to drought relative to carbon gain is vital for elucidating the overall effects on carbon exchange dynamics in water-limited environments. Rainfall-exclusion experiments in natural ecosystems are laborious and expensive but are highly valuable to simulate more realistically long-term drought. Some studies have addressed the photosynthetic limitations under long-term drought in natural ecosystems comprising stomatal, mesophyll, and biochemical components (Limousin et al., 2010; Martin-StPaul et al., 2012). To the best of our knowledge, the effects of long-term experimental drought on photosynthesis in parallel with night and day respiration has not been investigated so far on mature species in natural ecosystems.
Quercus ilex L. is one of the ‘flagship’ species for the Mediterranean Basin because it is a typical evergreen sclerophyllic tree extending over a large geographical range and forms the terminal point of secondary succession over vast areas in the Iberian Peninsula, including low and higher altitudes, and near-coastal sites with an oceanic climate, as well as inland sites with a semi-arid climate (Lookingbill and Zavala, 2000; Niinemets, 2015). However, reduced stem growth and higher mortality rates found for Q. ilex in response to drought (Barbeta et al., 2013) could decrease the distribution under predicted future drier conditions. Hence, Q. ilex is the ideal candidate to evaluate the seasonal acclimation of the foliar carbon balance in the long-term drought experiment of Prades (north-eastern Spain) where partial rainfall exclusion has been applied for the last 14 years, reducing soil moisture by an average of 13% (Ogaya et al., 2014; Barbeta et al., 2015).
We investigated the variations of foliar respiratory and photosynthetic traits of Q. ilex affected by seasonal changes in growth temperature and precipitation from winter to spring and summer. Furthermore, we studied the impact of long-term experimental drought on key limitations of photosynthesis comprising stomatal, mesophyllic, and biochemical components, as well as mitochondrial respiration during the day and night. Based on these parameters, we evaluated the response pattern of the foliar intrinsic water- and carbon-use efficiency (WUE i and CUE i, respectively) with respect to the simulated drought. Our aim was to improve our understanding of the boundaries and mechanisms of foliar respiration and photosynthesis in terms of seasonal acclimation and adaptation to drought. We provide here a set of needed parameters that potentially help to improve model simulation of ecosystem carbon fluxes.
Material and methods
Experimental site
The experimental site was situated in the Prades Mountains in southern Catalonia (north-eastern Spain; 41°21′N, 1°2′E) at 950 m above sea level on a 25% south-facing slope. Temperature, photosynthetically active radiation, air humidity, and precipitation have been monitored continuously with a meteorological station installed at the site. The climate is Mediterranean, with a mean annual rainfall of 609mm and a mean annual temperature of 12.2 °C (climate data from the meteorological station for 1999–2012). The soil is a Dystric Cambisol over Paleozoic schist with a depth of 35–90cm. The forest is characterized by a dense, multi-stemmed crown dominated by Q. ilex and Phillyrea latifolia L. with a maximum height of 6–10 m. The understorey is composed of Arbutus unedo L., Erica arborea L., Juniperus oxycedrus L., and Cistus albidus L. A long-term rainfall-exclusion experiment has been established and maintained in this forest since 1999 to simulate in situ projected decreases in precipitation in the Mediterranean region (Peñuelas et al., 2007). Four control and four treatment plots of 15×10 m were installed at the same altitude along the mountain slope. In the treatment plots, rain was partially excluded by PVC strips suspended 0.5–0.8 m above the soil (covering 30% of the soil surface). A ditch of 0.8 m in depth around the plots intercepted the runoff water from above the plots and conducted the water around to the bottom. The control plots received no treatment.
Sampling method
We conducted three seasonal field campaigns: winter (5–11 January 2013), spring (30 April–4 May 2013) and summer (24–29 July 2013) (Fig. 1). Eight twigs for each drought and control plot (two replicates for each plot) were cut with a pruning pull from the sun-exposed crowns of Q. ilex trees. We recut the twigs under water in the field, wrapped them in plastic bags to minimize transpiration, and transported them in water buckets to a nearby laboratory. The twigs were pre-conditioned overnight in the laboratory at room temperature (22–26 °C), freshly recut in the morning, and then kept in dim light [50–100 photosynthetic photon-flux density (PPFD) in µmol photons m−2 s−1]. Once the leaves showed open stomata [stomatal limitation (g s)>0.03mol H2O m−2 s−1] and a stable stomatal internal CO2 concentration (C i, µmol CO2 mol air−1), we started the response curves. In a few cases, the twigs were kept for one or two additional nights until gas exchange was sufficiently stable to conduct a light-response curve. We have adopted this method to overcome limitations that we often faced in the field such as: (i) accessibility of the branches of mature trees (canopy height between 6 and 10 m); (ii) limited ability of the instruments to reach the standard leaf temperature (T leaf) of 25 °C; and (iii) unpredictable plant responses such as closed stomata or patchy stomatal conductance (Mott and Buckley, 1998, 2000). With the pre-conditioned twigs, in contrast, we reached stable gas-exchange values that are required for conducting a noise-free light- or CO2-response curve and to estimate reliably the photosynthetic potentials (see Supplementary Fig. S1 at JXB online). The leaves remained fresh and functional for several days controlled by stomatal conductance and fluorescent signals. The cut twigs showed stable values of night respiration for several days (see Supplementary Fig. S2 at JXB online). This method works well on Mediterranean oak species including Q. ilex as shown in other studies (Haldimann and Feller, 2004; Niinemets et al., 2005; Sperlich et al., 2015). This method provided us with the opportunity to look at the potential physiological properties under standardized conditions that are representative for each season of a control group and a drought treatment and that are independent of short-term meteorological variability (e.g. cloud cover, extreme temperatures, chilly periods, rain events) and unpredictable plant responses.
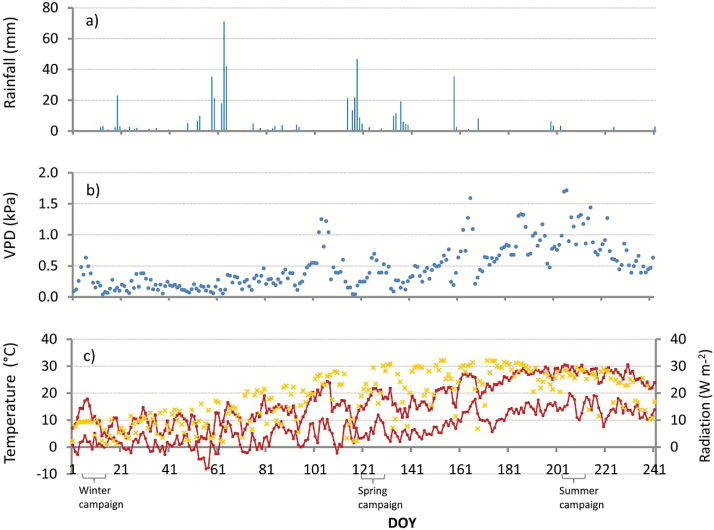
Fig. 1.
Environmental variables for the days of the year (DOY) from January to August 2013: rainfall (a), atmospheric vapour-pressure deficit (VPD) (b), and maximum and minimum temperatures (°C) (c) on the primary y-axes (red circles) and radiation (yellow crosses) on the secondary y-axes. The field campaigns are indicated.
Analyses of gas exchange and chlorophyll fluorescence
Gas exchange and chlorophyll fluorescence were measured with a Li-Cor LI-6400XT Portable Photosynthesis System equipped with a LI-6400-40 Leaf Chamber Fluorometer (Li-Cor, Lincoln, USA). Response curves of net assimilation versus PPFD were recorded in parallel with chlorophyll fluorescence measurements on mature, fully expanded leaves. In the summer campaign, we additionally conducted response curves of net assimilation versus CO2. Some of the Q. ilex leaves were too small to fill the leaf cuvette (2cm2), so the measured parameters were adjusted after the measurements. The leaves were prepared and acclimated prior to recording the response curves as described by Sperlich et al. (2014). First, we measured the maximum quantum yield of photosystem II (PSII; unitless) [F v/F m=(F m–F o)/F m] of a dark-adapted leaf (>30min) at ambient CO2 (C a of 400 µmol CO2 m−2 s−1) and T leaf of 25 °C. F o is the minimal fluorescence measured under darkness, and F m is the maximal fluorescence measured after a saturating light pulse. F v/F m describes the fraction of absorbed photons used in photochemistry under dark conditions and serves as the primary stress indicator of the photosystems. Typical values range between 0.74 and 0.85. Ratios of <0.80 are indicative of induced photoprotection (sustained energy dissipation), and ratios <0.74 are indicative of chronic photoinhibition (Björkman and Demmig, 1987; Maxwell and Johnson, 2000; Verhoeven, 2014). We then acclimated the leaf to saturating light conditions (PPFD of 1200 µmol photons m−2 s−1) and simultaneously recorded gas exchange and chlorophyll fluorescence parameters at ambient CO2 and T leaf as above: net assimilation rate (A net, µmol CO2 m−2 s−1), g s, C i, non-photochemical quenching (unitless) [NPQ=(F m – F m’)/F m’, where F m’ is the maximal fluorescence of a light-adapted leaf], and the effective quantum yield of PSII (unitless) [ΦPSII=(F m’ – F s)/F m’, where F s is the steady-state fluorescence of a light-adapted leaf].
We used the relationship of A net versus g s to estimate the foliar water-use efficiency (WUE i) which is defined as the amount of carbon gained per unit water used (Flexas et al., 2013).The electron-transport rate based on the effective quantum yield of PSII (J CF in µmol electron m−2 s−1) was calculated as
| (1) |
where ε is a scaling factor accounting for the partitioning of intercepted light between PSI and PSII. We assumed that light was equally distributed between the two photosystems (ε=0.5) (Bernacchi et al., 2002; Niinemets et al., 2005). The foliar absorbance (αL, unitless) was 0.932 for Q. ilex (Sperlich et al., 2014). J CF at ambient CO2 and saturating light was termed J amb.
Light experiments
Light-response curves (A/PPFD) were generated by automatically applying changes in the photosynthetically active radiation with the LI-6400XT light source at a leaf chamber internal concentration (C a) of 400 µmol CO2 mol air−1. To obtain precise responses at the low range of the light gradient for estimating the daily mitochondrial respiration by the Kok effect (Kok, 1948), we used the following PPFD sequence (in µmol photons m−2 s−1): 2500→2000→1500→1000→800→600→500→400→300→200→150→125→100→75→50→40→30→20→10→5→0. The minimum and maximum times between each light level for the generation of the A/PPFD curves were set to 1 and 2min, respectively. The rapid changes in light levels prevented the correct adjustment of T leaf. We fixed the Peltier-block temperature (T block) in the leaf cuvette, so that T leaf was 25 °C at the beginning of the A/PPFD curve. In the lower light levels where day respiration was estimated, T leaf had dropped on average by 0.8 °C (standard error ±0.0004). Day respiration (R d in µmol CO2 m−2 s−1) was estimated from the light-response curves with the method proposed by Yin et al. (2009) combining measurements of gas exchange and chlorophyll fluorescence. This method amended the Kok method (Kok, 1948) by substituting the A/PPFD relationship with A/(PPFD×ΦPSII/4) (see Yin et al., 2009, for details on the protocol). We estimated night respiration (R n in µmol CO2 m−2 s−1) after darkening the leaf for 20–30min, ensuring that all reaction centres had been closed (controlled with F o). R d and R n were then normalized to unity at 25 °C with an Arrhenius function [parameter=exp(c – ΔH a/RT k)]. T k is the leaf temperature (in Kelvin) and R is the molar gas constant (0.008314 kJ K−1 mol−1). The values of the scaling constant c (18.72, dimensionless) and energy of activation ΔH a (46.39 kJ mol−1) for leaf respiration were taken from Bernacchi et al. (2001).
Thereafter, we applied a correction of R d to account for the increase of C i with decreases of PPFD described by Kirschbaum and Farquhar (1987). In this method, the intercept of plots of photosynthetic electron transport to PPFD is minimized through the adjustment of R d via iteration (see Weerasinghe et al., 2014, for details on the protocol).
With R d from the light-response curves, we calculated the intrinsic carbon-use efficiency (CUE i) as proportion of carbon assimilated per carbon respired (Gifford, 2003), which served as a rough indicator for the foliar carbon balance (Pattison et al., 1998; Galmés et al., 2007):
| (2) |
CO2 experiments
The C a concentrations used to generate the CO2-response curves were 400→300→200→150→100→50→400→400→600→ 800→1200→2000 µmol CO2 mol air−1. T leaf was set to 25 °C. The saturating PPFD used was 1200 µmol photons m−2 s−1 based on light-response curves conducted prior to the measurements campaigns. The results of all light-response curves after the measurement campaign, however, indicated a saturating PPFD of 1500 µmol photons m−2 s−1. The minimum and maximum times for stabilizing A net, g s, and C i for each log were set to 4 and 6min, respectively. Diffusion leakage was corrected as described by Flexas et al. (2007).
Estimation of mesophyll conductance
We estimated g m (mol m−2 s−1 bar−1) using the variable-J method of Harley et al. (1992):
| (3) |
where Γ* is the CO2 concentration at which the photorespiratory efflux of CO2 equals the rate of photosynthetic CO2 (37.43 ppm at 25 °C). Γ* and its temperature response were taken from Bernacchi et al. (2002). The chloroplastic CO2 concentration (C c in µmol CO2 mol air−1) was determined as:
| (4) |
Photosynthesis model
The photosynthesis model of Farquhar et al. (1980) considers photosynthesis as the minimum of the potential rates of Rubisco activity (A c) and ribulose-1,5-bisphosphate (RuBP) regeneration (A j). A third limitation (A p) was implemented that considers the limitation by triose-phosphate use at high CO2 concentrations when the CO2 response shows a plateau or decrease (Sharkey, 1985). The model was further modified by replacing C i with C c for the chloroplast where the actual carboxylation takes place (reviewed by Flexas et al., 2008). As outlined above, we used the variable-J method for the C c calculation to create A/C c curves. The modelled assimilation rate A mod was then calculated by the minimum of these three potential rates from the A/C c curves:
| (5) |
where:
| (6) |
where V c,max (µmol CO2 m−2 s−1) is the maximum rate of Rubisco carboxylation, K c is the Michaelis–Menten constant of Rubisco for CO2, O is the partial pressure of O2 at Rubisco, and K o is the Michaelis–Menten constant of Rubisco for O2, taken from Bernacchi et al. (2002). The equation representing photosynthesis limited by RuBP regeneration is:
| (7) |
where J 1200 (in µmol electron m−2 s−1) is the rate of electron transport at a PPFD of 1200 µmol photons m−2 s−1 and saturating CO2. We assumed that J 1200 became J max under light and CO2 saturation when the maximum possible rate of electron transport was theoretically achieved, although we may have underestimated the true J max (Buckley and Diaz-Espejo, 2015). The limitation of triose-phosphate use was estimated as:
| (8) |
where TPU is the rate of triose-phosphate use at saturating CO2 concentrations, and αTPU is the proportion of glycerate not returned to the chloroplasts. Eqn 8 is from von Caemmerer (2000) after correcting a typographical error in the expression 3αTPU/2 to 3αTPU, as described by Gu et al. (2010). This equation fits the A/C c curve plateau at high CO2 when a further increase in C c does not produce any increase of A net anymore or, in some cases, even produces a decline of A net.
In addition to the A/C c curves, we replaced C c with C i in Eqns 6–8 and thus applied the above photosynthesis model to the traditional A/C i curve. We used an adequate set of kinetic constants from Bernacchi et al. (2001). We considered V c,max, J 1200, and TPU from the A/C c curve as the ‘true’ biochemical potential to drive photosynthesis whereas the parameters from the A/C i curve were the ‘apparent’ photosynthetic potential.
Statistical analyses
We estimated the true and apparent values of V c,max, J 1200, and TPU from Eqns 6–8 with the SOLVER Excel tool. SOLVER iteratively changes the parameters to minimize the sum of squares of the deviation of observed A net versus modelled A mod. We then performed further statistical analyses with R version 3.0.2 (http://www.r-project.org/). Differences in the parameters between control and drought plots were determined with Student’s t-test (P≤0.05). The normality of the data was tested with the Shapiro–Wilk test, and the data was normalized if not normally distributed. One-factorial ANOVA with season as the main factor was tested for seasonal differences in the parameters. Significant differences were determined at P≤0.05 with Tukey’s honestly significant difference test. Linear regression analyses were conducted to study the relationships among various leaf traits such as A net/g s, A net/g m, J 1200/V c,max, g m/g s, and R n /R d. We tested for differences in regression slopes and intercepts with analyses of co-variance (ANCOVAs).
Results
Environmental conditions over the sampling period
Frost events were frequent in winter and snowfall was also observed. The maximum temperatures during the day were on an average 4.9 °C (Table 1). The spring was humid with a precipitation comparable to that in winter (246 and 269mm, respectively) and was relatively cold (average of 12 °C) with occasional night frosts (Fig. 1). Spring together with winter accounted for nearly 80% of the annual average precipitation. The summer, in contrast, was dry and warm (total precipitation of 21mm and average temperature of 20.3 °C), with a vapour- pressure deficit (VPD) nearly twice as high as in spring (0.83 kPa) (Table 1). The partial rainfall exclusion reduced the soil water content (SWC) by a total of 13% from the beginning of the experiment in 1999 until the end of our measurement campaign in 2013. For the period of our measurement campaign, the SWC was on average 14% lower in the partial rainfall-exclusion plots compared with the control plots (Table 1). This difference was highest in spring, with a 24% lower SWC in the drought plots compared with the control plots.
Table 1.
Dates and days of the year (DOY) for each season in 2013 with mean temperature (T), total precipitation (Prec.), mean vapour-pressure deficit (VPD), mean radiation, and the percentage of the difference in the soil water content between the control and drought plots (ΔSWC)
| Season | Date | DOY | T (°C) | Prec. (mm) | VPD (kPa) | Radiation (W m−2) | ΔSWC (%) |
|---|---|---|---|---|---|---|---|
| Winter | 1 January–21 March 2013 | 1–79 | 4.9 | 269 | 0.20 | 9.1 | 5.3 |
| Spring | 22 March–21 June 2013 | 79–171 | 12.0 | 246 | 0.45 | 21.3 | 23.9 |
| Summer | 22 June–31 August 2013 | 172–242 | 20.3 | 21.8 | 0.83 | 25.0 | 7.7 |
| Total | 1 January–31 August 13 | 1–242 | 12.1 | 537 | 048 | 18.3 | 13.5 |
Seasonal changes in photosynthetic parameters
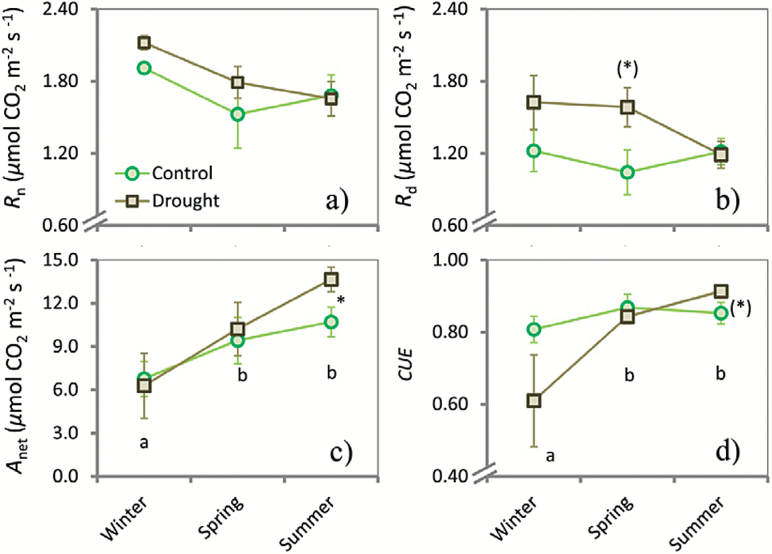
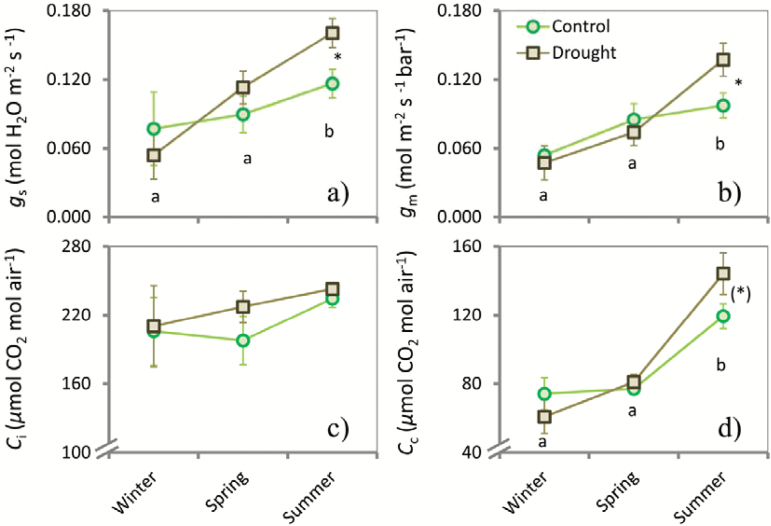
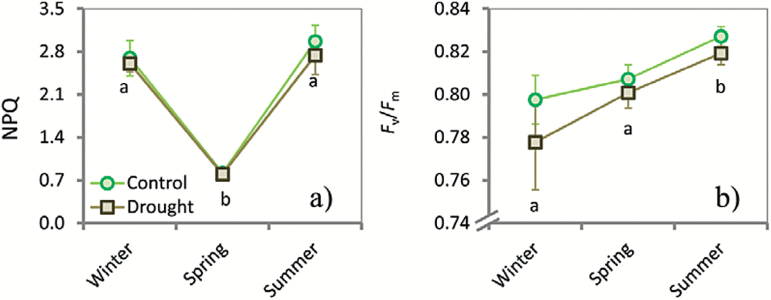
We analysed the seasonality of the photosynthetic parameters using the full dataset independent of treatment. Winter had a strong effect on several parameters with lower average values than in spring and summer, except for R n and C i (Table 2). A net, g s, g m, and F v/F m were significantly (P<0.05) and R d, C c, CUE i were marginally significantly (P<0.10) lower in winter than in either spring or summer (Figs 2 and 3). In summer, we found the highest mean values of A net, g s, g m, and C c, which were significantly different from those in spring and winter (Fig. 3). F v/F m was also highest in summer, demonstrating that the photosynthetic systems in spring had not yet fully recovered from the low winter temperatures but operated at peak efficiency in summer (Fig. 4b). NPQ is an indicator for photoinhibitory stress and dissipation of excess energy and was lowest in spring (significantly different from both winter and summer) (Fig. 4a). Neither ΦCO2 nor ΦPSII differed significantly between the seasons (Table 2 and Supplementary Fig. S3 at JXB online). The saturating PPFDs for A net and J cf were 1484 and 1552, respectively, and did not change seasonally.
Table 2.
Means (±standard errors) of a set of photosynthetic parameters and foliar traits for Q. ilex for the control group and the drought treatment in three seasonal campaigns (n=5–9)
| Variable | Control | Drought | ||||
|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Winter | Spring | Summer | |
| R n | 1.91±0.03 | 1.53±0.28 | 1.68±0.17 | 2.12±0.06 | 1.79±0.13 | 1.65±0.14 |
| R d | 1.22±0.17 | 1.04±0.19 | 1.21±0.11 | 1.62±0.22 | 1.58±0.16 | 1.19±0.11 |
| R d/R n | 0.64±0.09 | 0.69±0.03 | 0.74±0.05 | 0.77±0.11 | 0.88±0.03 | 0.73±0.06 |
| A net | 6.76±1.2 | 9.43±1.0 | 10.71±1.0 | 5.52±2.0 | 10.17±0.7 | 13.66±0.9 |
| g s | 0.077±0.032 | 0.090±0.016 | 0.116±0.012 | 0.054±0.021 | 0.113±0.009 | 0.161±0.013 |
| g m | 0.054±0.009 | 0.085±0.014 | 0.097±0.011 | 0.047±0.017 | 0.074±0.017 | 0.137±0.014 |
| C i | 206±30 | 198±21 | 234±8 | 210±20 | 227±8 | 243±6 |
| C c | 74±9 | 77±3 | 119±7 | 61±10 | 81±5 | 139±23 |
| NPQ | 2.70±0.29 | 0.82±0.02 | 2.97±0.26 | 2.61±0.14 | 0.80±0.02 | 2.74±0.31 |
| Fv/Fm | 0.80±0.011 | 0.81±0.007 | 0.83±0.005 | 0.78±0.022 | 0.80±0.007 | 0.82±0.005 |
| ΦCO2 | 0.0074±0.0020 | 0.0092±0.0009 | 0.0102±0.0014 | 0.0054±0.0014 | 0.0097±0.0008 | 0.0119±0.0018 |
| ΦPS2 | 0.215±0.045 | 0.250±0.024 | 0.206±0.029 | 0.220±0.009 | 0.273±0.021 | 0.218±0.030 |
| V c,max | 107±9 | 120±11 | ||||
| J max | 132±11 | 148±12 | ||||
| TPU | 9.4±1.2 | 7.6±1.3 | ||||
Fig. 2.
Line graphs depicting seasonal changes of night respiration (R n) (a), day respiration (R d) (b), net assimilation rate (A net) (c), and (d) carbon-use efficiency (CUE i) (d) for Q. ilex. Seasonal campaigns were conducted in winter, spring, and summer 2013. Asterisks and asterisks in brackets indicate significant (P<0.05) and marginally significant (P<0.1) differences between the control and drought plots for each season, respectively. Different lower-case letters indicate differences between seasons. Vertical bars indicate standard errors of the mean (n=59).
Fig. 3.
Line graphs depicting seasonal changes of stomatal conductance (g s) (a), mesophyll conductance (g m) (b), stomatal internal CO2 concentration (C i) (c), and chloroplastic CO2 concentration (C c) (d) in sunlit leaves of Q. ilex. Seasonal campaigns were conducted in winter, spring, and summer 2013. Asterisks and asterisks in brackets indicate significant (P<0.05) and marginally significant (P<0.1) differences between the control and drought plots for each season, respectively. Different lower-case letters indicate differences between seasons. Vertical bars indicate standard errors of the means (n=59).
Fig. 4.
Line graphs depicting seasonal changes of non-photochemical quenching (NPQ) (a) and maximum quantum efficiency of PSII (F v/F m) (b) for Q. ilex. Seasonal campaigns were conducted in winter, spring, and summer 2013. Different lower-case letters indicate differences between seasons. Vertical bars indicate standard errors of the means (n=59).
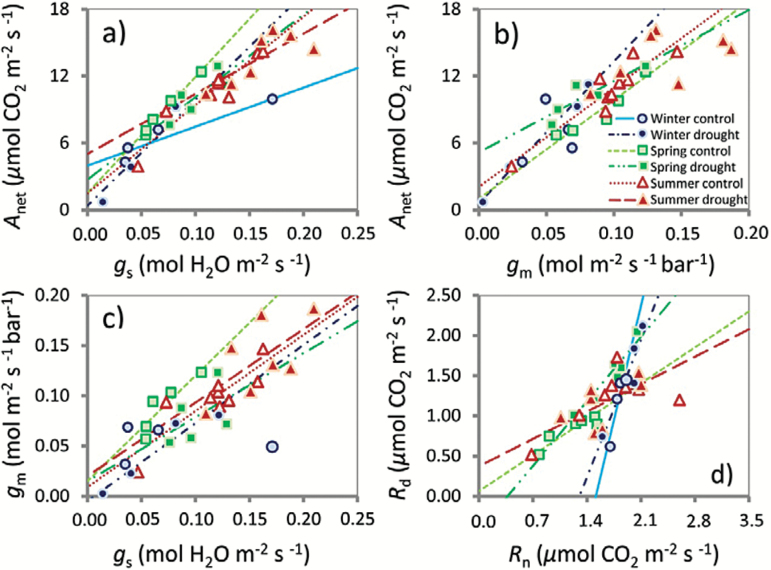
Several relationships were analysed with ANCOVAs to test whether seasonal changes in environmental conditions produced significant differences in slopes (Table 3). We analysed the relationship of A net/g s as an indicator for WUE i. The slope of this relationship for the control group was significantly gentler in winter compared with spring and summer, indicating a lower WUE i. For the relationship of A net/g m, we analysed the effect of the mesophyll internal CO2 diffusion on net carbon assimilation. This relationship had a significantly steeper slope in winter in comparison with summer in the drought group. The relationship of g m/g s unveils the relative contribution of stomatal and mesophyll diffusion limitation on the net carbon assimilation. The relationship of g m/g s was significantly steeper in the control plot in spring in comparison with summer. We analysed the relative importance of day and night mitochondrial respiration with the relationship of R d/R n. The slope was significantly steeper in winter compared with spring and summer in both the control and drought plots.
Table 3.
Regression equations and coefficients of determination (R2) for Anet/gs, Anet/gm, gm/gs, and Rd/Rn for Q. ilex in three sampling campaigns in the control and drought plots
P values indicate the significance of the differences between the slopes for the control and drought plots. Equations for non-significant relationships are not displayed (n=5–9).
| Variable | Campaign | Plot | Equation | R 2 | P |
|---|---|---|---|---|---|
| A net/g s | Total | Control | y=60.7x+3.68 | 0.72 | 0.417 |
| Drought | y=74.7x+1.92 | 0.88 | |||
| Winter 2013 | Control | y=36.1x+3.98 | 0.86 | 0.009 | |
| Drought | y=94.9x+0.39 | 0.92 | |||
| Spring 2013 | Control | y=104.1x+1.51 | 0.98 | 0.380 | |
| Drought | y=74.0x+2.71 | 0.68 | |||
| Summer 2013 | Control | y=79.1x+1.49 | 0.89 | 0.222 | |
| Drought | y=53.9x+5.01 | 0.64 | |||
| A net/g m | Total | Control | y=79.3x+2.61 | 0.77 | 0.513 |
| Drought | y=70.2x+4.00 | 0.75 | |||
| Winter 2013 | Control | 0.279 | |||
| Drought | y=115.1x+0.08 | 0.62 | |||
| Spring 2013 | Control | y=88.5x+1.01 | 0.92 | 0.521 | |
| Drought | y=63.8x+5.17 | 0.80 | |||
| Summer 2013 | Control | y=88.8x+2.07 | 0.85 | 0.040 | |
| Drought | y=30.5x+9.47 | 0.10 | |||
| g m/g s | Total | Control | y=0.254x+0.059 | 0.06 | 0.011 |
| Drought | y=0.757x+0.011 | 0.57 | |||
| Winter 2013 | – | ||||
| Drought | y=0.595x+0.017 | 0.56 | |||
| Spring 2013 | Control | y=1.051x+0.015 | 0.86 | 0.337 | |
| Drought | y=0.637x+0.015 | 0.27 | |||
| Summer 2013 | Control | y=0.758x+0.009 | 0.75 | 0.949 | |
| Drought | y=0.732x+0.020 | 0.30 | |||
| R d/R n | Total | Control | y=0.540x+0.263 | 0.59 | 0.0035 |
| Drought | y=0.980x 0.272 | 0.68 | |||
| Winter 2013 | Control | y=4.05x 6.14 | 0.78 | 0.279 | |
| Drought | y=1.036x 0.343 | 0.61 | |||
| Spring 2013 | Control | y =0.639 x+0.063 | 0.96 | 0.0126 | |
| Drought | y=1.147x 0.427 | 0.95 | |||
| Summer 2013 | – | ||||
| Drought | y=0.487x+0.373 | 0.38 |
Effect of experimental drought
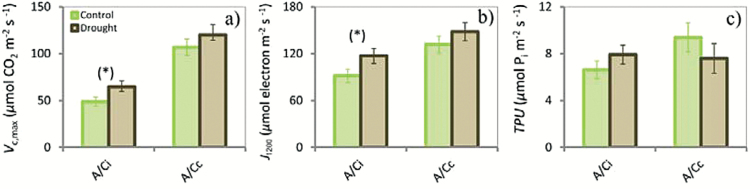
R d/R n for all seasons combined was significantly higher in the drought treatment (0.79±0.06) compared with the control plots (0.71±0.03). No other general trends were detected. In the respective seasons, however, we found significant effects of the drought treatment, with several parameters showing higher average values compared with the control group (Figs 2 and 3): A net, g s, and g m were significantly higher, and CUE i and C c were marginal significantly higher in summer, and R d was marginally significantly higher in spring. We conducted carbon-response curves in summer only (see Material and methods). J 1200, V c,max, and TPU were thus only available for the summer campaign. The drought treatment had no significant effect on these photosynthetic potentials when estimated from an A/C c curve (Fig. 5). Additionally, we estimated the apparent photosynthetic potential from A/C i curves. The drought treatment had a marginally significant effect on the apparent J 1200 and apparent V c,max with lower values in the control plot, but no effect on the apparent TPU (Fig. 5). A comparison of the photosynthetic potential from A/C i and A/C c curves indicated that the foliar internal diffusion limitation imposed by g m accounted on average for a 54% higher V c,max and a 30% higher J 1200 and a 29% higher TPU with regard to the apparent photosynthetic potential.
Fig. 5.
Bar graphs of maximum carboxylation rate (V c,max) (a), electron-transport rate at saturating light and CO2 (J 1200) (b), and triose-phosphate use (TPU) (c) estimated with CO2-response curves based on C i (A/C c) and C c (A/C c) in the control and drought plots for the summer campaign. Marginal significant differences (P<0.1) between the control and drought plots are indicated by asterisks in brackets. Vertical bars indicate standard errors of the means (control, n=7; drought, n=8).
The ANCOVAs in the respective seasons identified significant differences in slopes as a result of the experimental drought. The slope of A net/g s was significantly steeper in the control compared with the treatment group in the winter campaign, indicating a higher WUE i in the control group (Fig. 6). The slope of A net/g m was significantly steeper in the control group compared with the treatment group in the summer campaign (Fig. 6). The overall slope of g m/g s was significantly steeper in the control group compared with the treatment group when all seasons were combined (Fig. 6). The slope of R d/R n was significantly gentler in the control group compared with the treatment group in the spring campaign and when all seasons were combined. Neither season nor treatment significantly affected the slopes of A net/R d, A net/R n, J amb/ A net, and C c/C i (Supplementary Tables S1–S4 at JXB online).
Fig. 6.
Scatter plots and regression lines of stomatal conductance (g s) versus net assimilation rate (A net) (a), mesophyll conductance (g m) versus A net (b), g s versus g m (c) and night respiration (R n) versus day respiration (R d) (d) for each season and for control and drought plots. Only the regression lines for significant relationships (P<0.05) are displayed.
Discussion
The scaling of carbon dynamics from one year to another is particularly challenging in Mediterranean environments due to climate extremes combined with a high interannual variability (Reynolds et al., 1996; Morales et al., 2005; Gulías et al., 2009). We aimed to investigate the effect of seasonal changes in temperature and precipitation from winter to spring and summer on the photosynthetic and respiratory traits of a widely abundant Mediterranean tree species. However, abiotic stress under field conditions often hampers gas-exchange measurements due to deviations from the standard temperature (25 °C) or unpredictable plant responses, e.g. patchy stomatal conductance (Mott and Buckley, 1998, 2000), making it impossible to conduct response curves for the estimation of photosynthetic and respiratory parameters. Our data was thus obtained on cut twigs under standardized conditions in order to provide insights into the photosynthetic and respiratory potential independently of meteorological variability in the field. The cutting-twig method allowed us to analyse the long-term acclimation to the environmental conditions from which the twigs were derived and has been applied by other experts in the field (e.g. Epron and Dreyer, 1992; Niinemets et al., 1999, 2005; Laisk et al., 2002; Haldimann and Feller, 2004; Heskel et al., 2014; Dusenge et al., 2015). Our study thus provides a mechanistic description of seasonal changes in photosynthetic and respiratory processes under long-term drought that contributes to improve our understanding of the impacts of future climate change in Mediterranean-type ecosystems.
Effect of seasonality on photosynthetic and respiratory traits
We found that cold winter temperatures had a stronger negative impact on the leaf physiology of Q. ilex than summer drought. The standardized A net under controlled conditions (see Material and methods) was approximately half the rate in winter compared with the peak found in summer, yet relatively high average winter values were reached (6.5±1.3) that were comparable to those reported in other studies (Gratani, 1996; Ogaya and Peñuelas, 2003). Both g m and g s reduced the CO2 concentration in the chloroplasts in winter compared with spring and summer. In winter, however, g m limited photosynthesis relatively more than g s. High water availability and low VPDs make the reduction of transpiratory water loss through stomatal closure less urgent in the winter period. There is some evidence that g m acts as a stronger regulator for photosynthesis in winter (Sperlich et al., 2014), although very few studies have examined the behaviour of g m under natural winter conditions. Low temperatures in winter hamper photosynthetic metabolism and enzymatic activities (e.g. Corcuera et al., 2004; Aranda et al., 2005), which may account for a concurrent downregulation of photosynthesis through g m, as our results indicated. This was paralleled by a drastic decrease in the foliar carbon-use efficiency. In winter, chilly or freezing temperatures often coincide with clear skies and relatively high solar irradiances. The imbalance created between light energy absorbed in photochemistry and light energy used in metabolism increases the susceptibility to photoinhibitory stress (Demmig-Adams and Adams, 1992). This imbalance is particularly problematic for the evergreen vegetation, and thermal acclimation to winter conditions is essential to survive these adverse conditions (Blumler, 1991; Öquist and Huner, 2003). As a response, thylakoid membranes are reorganized, reaction centres are closed, and antennal size is reduced in order to protect the photosynthetic apparatus against overexcitation by the incoming radiation (García-Plazaola et al., 1997; Huner et al., 1998; Ensminger et al., 2012; Verhoeven, 2014). The increased NPQ and decreased F v/F m found in our study are good proxies for these photoprotective processes in the thylakoid membranes, indicating an increased thermal dissipation of excess energy and a decreased photochemical efficiency (Maxwell and Johnson, 2000). Thus, we found that Q. ilex acclimated to the winter conditions with reoccurring night frosts, and exploited the winter period photosynthetically at the cost of lower assimilation rates and a lower carbon-use efficiency (see also Hurry et al., 2000; Dolman et al., 2002; Sperlich et al., 2014). We underline the fact that winter acclimation and exploitation can be essential for Mediterranean evergreen tree species to recover from stressful summer periods and to achieve a positive annual carbon balance.
Notably lower NPQs in spring indicate that the photosystems experienced the least amount of photochemical stress in this period. This is because the spring in our study was particularly cool and wet and was characterized by a low VPD. The high NPQ in winter and summer, in contrast, reflects strong photoprotection against photoinhibitory stress due to the temperature extremes. However, the photoprotective mechanisms seemed to be effective: the optimal light intensity for net assimilation and the electron transport (approximately 1500 µmol photons m−2 s−1 for both) and the effective quantum yield of PSII (ΦPSII) (Supplementary Fig. S3) did not change between the seasons.
The assimilation rates and the carbon-use efficiency increased from winter to spring, although it was not until summer that the peak photosynthetic activity was reached. The elevated F v/F m underlines the fact that the photosynthetic apparatus fully recovered its maximum photochemical efficiency in summer. This contrasts with a very low total precipitation measured during the summer (22mm). However, Q. ilex can benefit from water reserves in deep soil layers and also in rock fractures (Barbeta et al., 2015), which also explains its water-spending behaviour during drier periods (Sánchez-Costa et al., 2015). It is known that Q. ilex develops a profound root system with a lignotuber that can make up as much as half of the total tree biomass (Canadell et al., 1999), which is vital to withstand abiotic stress periods or disturbances. The precipitation in winter and spring together nearly reached the annual mean, so that deep soil water reserves are likely to have been yet filled in summer. High water availability in combination with high summer temperatures can account for the high photosynthetic activity in a potential water stress period. The replenishment of soil water reserves early in the growing season is critical to endure seasonal summer droughts in Mediterranean trees (Sperlich et al., 2015). Pinto et al. (2014) also found the highest sap flow rates of Quercus suber L. in summer because its roots had access to the groundwater.
Effect of rainfall exclusion on photosynthetic and respiratory traits
Drought experiments with rainfall exclusion under natural conditions can serve as valuable real-time model simulations for scenarios of future climate change. Unfortunately, long-term experiments over several years are costly and laborious and thus are particularly scarce. The rainfall exclusion in Prades, maintained since 1999, has reduced soil moisture by 13% with respect to ambient conditions and is probably the longest continuous-drought experiment worldwide (Wu et al., 2011; Ogaya et al., 2014).
Plants face a trade-off under water stress: the closure of the stomates reduces transpiratory water loss but at the same time constrains CO2 diffusion to the chloroplasts. Besides g s, g m can act as a second leaf internal valve regulating the gas exchange through carbonic anhydrase and aquaporins and can thus help to prevent dehydration of vacuoles and cells (Terashima and Ono, 2002; Lopez et al., 2013; Perez-Martin et al., 2014). When chronic water stress begins to deplete stores of non-structural carbohydrates, plants are particularly reliant on photosynthetic products for refinement, repair, and protective actions (Niinemets et al., 2009). We have provided novel evidence that g m not only imposes an additional leaf internal resistance to gas exchange but can also facilitate the CO2 diffusion to the chloroplasts in comparison with a stronger control by stomata under drought. This was reflected by a comparatively higher g m that increased the g m/g s ratio under long-term drought (see also Galmés et al., 2013). Our results are supported by recent findings obtained in Q. ilex and Pinus halepensis leaves showing a higher leaf internal CO2 conductance in parallel with a stricter stomatal control under severe drought (Sperlich et al., 2015).
In addition to the importance of the diffusive capacity of stomata and mesophyll for the foliar carbon balance, this balance also depends strongly on the relationship of photosynthesis with respiration. However, the extent to which R n or R d are affected by water scarcity is highly uncertain. We found that R d was approximately 74% of R n and that the long-term rainfall-exclusion experiment increased the ratio of R d/R n (0.79±0.04) compared with the control plot (0.71±0.03) due to a higher R d. Some studies found increased foliar respiration under severe water stress (Ghashghaie et al., 2001), but reductions were also reported (Flexas et al., 2006). The leaf may exert an acclimation of the respiratory metabolism through R d because the demands for energy (ATP and NADPH) for synthesis of sucrose and carbon skeletons in the cytoplasm are higher under stressful conditions (Flexas et al., 2006; Zaragoza-Castells et al., 2007). R d provides the basis for building up heat-stabilizing components such as heat-shock proteins or biogenic volatile organic compounds protecting the plant against detrimental effects (Tcherkez and Ribas-Carbó, 2012). Higher photoinhibitory stress can thus increase the respiratory metabolic activity expressed as a higher protein turnover at a given overall protein content (Niinemets, 2014; Weerasinghe et al., 2014). This might explain the generally higher values of R d that we found in the drought treatment. A lower stress level would evidently lead to a lower demand for energy and carbon skeletons and hence to a lower protein turnover. We found an indication for this in the effective photoprotective mechanism and lower rates of R d in the summer campaign characterized by favourable conditions. In contrast to the results of Zaragoza-Castells et al. (2007), in our study only R d but not R n acclimated seasonally. The higher R d in the drought treatment decreased significantly and coincided with the lower values of the control group, which remained unaffected throughout the seasons. This decrease of R d in the drought treatment in summer—paralleled by higher rates of photosynthesis—significantly decreased R d/R n and increased the foliar carbon-use efficiency. Griffin and Turnbull (2013) showed that a decreased R d/R n can be explained by a suppressed light-saturated rate of oxygenation in photorespiration. Although we did not measure photorespiration directly, our data showed increased g m and thus elevated CO2 concentrations in the chloroplasts (increase of 35% from spring to summer) (Fig. 3), which would benefit carboxylation over oxygenation. Overall, we found that R d—as the key player for the foliar carbon balance in Q. ilex—was the most responsive to seasons or treatment effects.
We found that the drought treatment had no significant effect on J 1200, V c,max, or TPU in the summer campaign. Our results emphasize that the increased photosynthetic activity in the drought treatment in summer was not attributed to a higher potential in the biochemistry of photosynthesis, but rather than to an increased diffusive capacity of both g s and g m. Interestingly, analysis of the apparent J 1200 and apparent V c,max (derived from A/C i curves) identified marginally significant higher values in the rainfall-exclusion plot. This shows that the A/C c method is more appropriate and that the traditional fitting method based on A/C i curves may lead to confounding effects—as also shown in grapevines by Flexas et al. (2006). The foliar internal diffusion limitation imposed by g m accounted on average for a 54% higher V c,max, a 30% higher J max, and a 29% higher TPU of the apparent photosynthetic potential. Similar values were reported for nearly 130 C3 species in a recent study by Sun et al. (2014).
We postulate firstly, that the summer period provided counterintuitively favourable conditions (as discussed above), and secondly, that the trees in the drought treatment acclimated most efficiently the balance between energy supply versus energy consumption in this period. Recent findings underscore the high plasticity of Q. ilex in response to seasonal changes in temperature or soil water compared with other Mediterranean species (Sperlich et al., 2015). The rainfall-exclusion experiment in Prades was shown to result in a higher stem mortality (Barbeta et al., 2013) and in a reduced leaf area in Q. ilex (Ogaya and Peñuelas, 2006), while increasing leaf mass per area (data not shown). With fewer leaves and lower total leaf area, the concentration of biochemical resources per leaf would increase. This might contribute to explaining the partly higher photosynthetic rates and carbon-use efficiency in the drought treatment. Sperlich et al. (2015) also found higher photosynthetic potentials in crowns that suffered a reduced total leaf area after a severe drought.
In this study, we examined the seasonality of photosynthetic and respiratory traits and evaluated the adaptive mechanism in response to reduced soil water under partial rainfall exclusion. A high climatic variability in the Mediterranean region can lead to counterintuitive effects, with the peak photosynthetic activity in summer, which is usually characterized by a high level of abiotic stress. The trees experiencing a 14-year-long drought treatment adapted through a higher plasticity in photosynthetic traits, so that eventually an atypical favourable growth period in summer was exploited more efficiently, with g m and R d as the determining parameters. Drought-induced growth declines may be attenuated in the long-term through morphological and physiological acclimation to drought (Leuzinger et al., 2011; Barbeta et al., 2013). Fewer leaves in the drought treatment were compensated by higher net photosynthetic rates. The similarity of photosynthetic potentials in the treatment and control plots suggests that there is also a dampening effect on the biochemical level.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Four exemplary samples of carbon-response curves conducted at leaves of (i) twigs attached to the tree (field), (ii) after cutting and pre-conditioning the twigs under dim light in water in the lab for one night (day 1) and (iii) two nights (day 2).
Supplementary Fig. S2. Bar chart depicting the evolution of night respiration (R n) of Q. ilex directly after cutting the twig and after darkening for 30min (field), and at day 1, 2, and 3 after being pre-conditioned under dim light in water in the laboratory.
Supplementary Fig S3. Line graphs depicting seasonal changes of (a) quantum yield of CO2 (ΦCO2) and (b) effective quantum yield of PSII (ΦPSII) for Q. ilex.
Supplementary Fig. S4. Scatter plots and regression lines of maximum carboxylation rate (V c,max) versus maximum rate of electron transport (J max) derived from (a) A/C c and (b) A/C i response curves for control and drought plots in summer 2013.
Supplementary Table S1. Regression equations and coefficients of determination (R 2) for A net/R d for Q. ilex in three sampling campaigns in control and drought plots.
Supplementary Table S2. Regression equations and coefficients of determination (R 2) for A net/R n for Q. ilex in three sampling campaigns in control and drought plots.
Supplementary Table S3. Regression equations and coefficients of determination (R 2) for J amb/A net for Q. ilex in three sampling campaigns in control and drought plots.
Supplementary Table S4. Regression equations and coefficients of determination (R 2) for C c/C i for Q. ilex in three sampling campaigns in control and drought plots.
Acknowledgements
The research was funded by the European Community’s Seventh Framework Programme GREENCYCLESII (FP7 2007–2013) under grant agreements n° 238366 and by the Ministerio de Economica y Competividad under grant agreement n° CGL2010590-C02-01 (MED_FORESTREAM project) and nº CSD2008-00040 (Consolider-Ingenio MONTES project). AB, RO and JP acknowledge funding from the Spanish Government g1-3rant CGL2013-48074-P, the Catalan Government project SGR 2014–274, and the European Research Council Synergy grant ERC-SyG-610028 IMBALANCE-P. M.
References
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH (Ted) 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259, 660–684. [Google Scholar]
- Anderegg WRL, Kane JM, Anderegg LDL. 2013. Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change 3, 30–36. [Google Scholar]
- Aranda I, Castro L, Alía R, Pardos JA, Gil L. 2005. Low temperature during winter elicits differential responses among populations of the Mediterranean evergreen cork oak (Quercus suber). Tree Physiology 25, 1085–1090. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Macherel D. 2009. The crucial role of plant mitochondria in orchestrating drought tolerance. Annals of botany 103, 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeta A, Mejía-Chang M, Ogaya R, Voltas J, Dawson TE, Peñuelas J. 2015. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Global Change Biology 21, 1213–1225. [DOI] [PubMed] [Google Scholar]
- Barbeta A, Ogaya R, Peñuelas J. 2013. Dampening effects of long-term experimental drought on growth and mortality rates of a Holm oak forest. Global Change Biology 19, 1–12. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, Caemmerer S Von, Long SP. 2002. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiology 130, 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C, Portis Jr a R, Long SP. 2001. Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell and Environment 24, 253–259. [Google Scholar]
- Björkman O, Demmig B. 1987. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170, 489–504. [DOI] [PubMed] [Google Scholar]
- Blumler MA. 1991. Winter-deciduous versus evergreen habit in Mediterranean regions : a model. In: USDA forest service general technical report PSW-126 , 194–197. [Google Scholar]
- Buckley TN, Diaz-Espejo A. 2015. Reporting estimates of maximum potential electron transport rate. New Phytologist 205, 14–17. [DOI] [PubMed] [Google Scholar]
- Canadell J, Djema A, López B, Lloret F, Sabaté S, Siscart D, Gracia CA. 1999. Structure and dynamics of the root system. In: Rodà F, Retana J, Gracia CA, Bellot J, eds. Ecology of Mediterranean evergreen oak forests . Berlin/Heidelberg: Springer, 47–59. [Google Scholar]
- Carnicer J, Coll M, Ninyerola M, Pons X, Sánchez G, Peñuelas J. 2011. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proceedings of the National Academy of Sciences, USA 108, 1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castri F. 1973. Climatographical comparison between Chile and the western coast of North America. In: Di Castri F, Mooney HA, eds. Mediterranean-type ecosystems: origin and structure . Berlin: Springer, 21–36. [Google Scholar]
- Catoni R, Varone L, Gratani L. 2013. Variations in leaf respiration across different seasons for Mediterranean evergreen species. Photosynthetica 51, 295–304. [Google Scholar]
- Corcuera L, Morales F, Abadia A, Gil-Pelegrin E. 2004. The effect of low temperatures on the photosynthetic apparatus of Quercus ilex subsp. ballota at its lower and upper altitudinal limits in the Iberian peninsula and during a single freezing-thawing cycle. Trees 19, 99–108. [Google Scholar]
- Demmig-Adams B, Adams WW. 1992. Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology 43, 599–626. [Google Scholar]
- Dolman AJ, Moors EJ, Elbers JA. 2002. The carbon uptake of a mid latitude pine forest growing on sandy soil. Agricultural and Forest Meteorology 111, 157–170. [Google Scholar]
- Dusenge ME, Wallin G, Gårdesten J, Niyonzima F, Adolfsson L, Nsabimana D, Uddling J. 2015. Photosynthetic capacity of tropical montane tree species in relation to leaf nutrients, successional strategy and growth temperature. Oecologia 177, 1183–1194. [DOI] [PubMed] [Google Scholar]
- Ensminger I, Berninger F, Streb P. 2012. Response of photosynthesis to low temperature. In: Flexas J, Loreto F, Medrano H, eds. Terrestrial photosynthesis in a changing environment—a molecular, physiological and ecological approach . Cambridge: Cambridge University Press, 272–289. [Google Scholar]
- Epron D, Dreyer E. 1992. Effects of severe dehydration on leaf photosynthesis in Quercus petruea (Matt.) Liebl.: photosystem II efficiency, photochemical and nonphotochemical fluorescence quenching and electrolyte leakage. Tree Physiology 10, 273–284. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthesis CO2 assimilation in leaves of C3 species. Planta 149, 78–90. [DOI] [PubMed] [Google Scholar]
- Flexas J, Bota J, Galmés J, Medrano H, Ribas-carbo M. 2006. Keeping a positive carbon balance under adverse conditions : responses of photosynthesis and respiration to water stress. Physiologia Plantarum 127, 343–352. [Google Scholar]
- Flexas J, Díaz-Espejo A, Berry JA, Cifre J, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbó M. 2007. Analysis of leakage in IRGA’s leaf chambers of open gas exchange systems: quantification and its effects in photosynthesis parameterization. Journal of Experimental Botany 58, 1533–1543. [DOI] [PubMed] [Google Scholar]
- Flexas J, Diaz-Espejo A, Gago J, Gallé A, Galmés J, Gulías J, Medrano H. 2014. Photosynthetic limitations in Mediterranean plants: a review. Environmental and Experimental Botany 103, 12–23. [Google Scholar]
- Flexas J, Niinemets U, Gallé A, et al. 2013. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynthesis research 117, 45–59. [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. 2008. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell and Environment 31, 602–621. [DOI] [PubMed] [Google Scholar]
- Friend AD. 2010. Terrestrial plant production and climate change. Journal of Experimental Botany 61, 1293–1309. [DOI] [PubMed] [Google Scholar]
- Galmés J, Perdomo JA, Flexas J, Whitney SM. 2013. Photosynthetic characterization of Rubisco transplantomic lines reveals alterations on photochemistry and mesophyll conductance. Photosynthesis Research 115, 153–166. [DOI] [PubMed] [Google Scholar]
- Galmés J, Ribas-Carbó M, Medrano H, Flexas J. 2007. Response of leaf respiration to water stress in Mediterranean species with different growth forms. Journal of Arid Environments 68, 206–222. [Google Scholar]
- García-Plazaola JI, Faria T, Abadia J, Abadia A, Chaves MM, Pereira JS. 1997. Seasonal changes in xanthophyll composition and photosynthesis of cork oak (Quercus suber L.) leaves under Mediterranean climate. Journal of Experimental Botany 48, 1667–1674. [Google Scholar]
- Ghashghaie J, Duranceau M, Badeck FW, Cornic G, Adeline MT, Deleens E. 2001. 13C of CO2 respired in the dark in relation to 13C of leaf metabolites: comparison between Nicotiana sylvestris and Helianthus annuus under drought. Plant, Cell and Environment 24, 505–515. [Google Scholar]
- Gifford RM. 2003. Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Functional Plant Biology 30, 171. [DOI] [PubMed] [Google Scholar]
- Gratani L. 1996. Leaf and shoot growth dynamics of Quercus ilex L. Acta Oecologica 17, 17–27. [Google Scholar]
- Griffin KL, Turnbull MH. 2013. Light saturated RuBP oxygenation by Rubisco is a robust predictor of light inhibition of respiration in Triticum aestivum L. Plant Biology 15, 769–775. [DOI] [PubMed] [Google Scholar]
- Gu L, Pallardy SG, Tu K, Law BE, Wullschleger SD. 2010. Reliable estimation of biochemical parameters from C₃ leaf photosynthesis-intercellular carbon dioxide response curves. Plant, Cell and Environment 33, 1852–74. [DOI] [PubMed] [Google Scholar]
- Gulías J, Cifre J, Jonasson S, Medrano H, Flexas J. 2009. Seasonal and inter-annual variations of gas exchange in thirteen woody species along a climatic gradient in the Mediterranean island of Mallorca. Flora—Morphology, Distribution, Functional Ecology of Plants 204, 169–181. [Google Scholar]
- Haldimann P, Feller U. 2004. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5- bisphosphate carboxylase /oxygenase. Plant, Cell and Environment 27, 1169–1183. [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2 . Plant Physiology 98, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heskel MA, Bitterman D, Atkin OK, Turnbull MH, Griffin KL. 2014. Seasonality of foliar respiration in two dominant plant species from the Arctic tundra: response to long-term warming and short-term temperature variability. Functional Plant Biology 41, 287–300. [DOI] [PubMed] [Google Scholar]
- Hickler T, Fronzek S, Araújo MB, Schweiger O, Thuiller W, Sykes MT. 2009. An ecosystem model-based estimate of changes in water availability differs from water proxies that are commonly used in species distribution models. Global Ecology and Biogeography 18, 304–313. [Google Scholar]
- Huner NPA, Öquist G, Sarhan F. 1998. Energy balance and acclimation to light and cold. Trends in Plant Science 3, 224–230. [Google Scholar]
- Hurry V, Strand a, Furbank R, Stitt M. 2000. The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana . The Plant Journal 24, 383–96. [DOI] [PubMed] [Google Scholar]
- IPCC. . 2013. Summary for policymakers. In: T.F S., Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, eds. Climate change 2013: the physical science basis . Cambridge, UK, and New York: Cambridge University Press. [Google Scholar]
- Jump AS, Peñuelas J. 2005. Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters 8, 1010–1020. [DOI] [PubMed] [Google Scholar]
- Kirschbaum MU, Farquhar GD. 1987. Investigation of the CO2 dependence of quantum yield and respiration in Eucalyptus pauciflora . Plant Physiology 83, 1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B. 1948. A critical consideration of the quantum yield of Chlorella-photosynthesis. Enzymologia 13, 1–56. [Google Scholar]
- Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E. 2002. A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus. Plant, Cell and Environment 25, 923–943. [Google Scholar]
- Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Körner C. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends in Ecology and Evolution 26, 236–241. [DOI] [PubMed] [Google Scholar]
- Limousin J-M, Misson L, Lavoir A-V, Martin NK, Rambal S. 2010. Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant, Cell and Environment 33, 863–875. [DOI] [PubMed] [Google Scholar]
- Lookingbill TR, Zavala MA. 2000. Spatial pattern of Quercus ilex and Quercus pubescens recruitment in Pinus halepensis dominated woodlands. Journal of Vegetation Science 11, 607–612. [Google Scholar]
- Lopez D, Venisse J-S, Fumanal B, Chaumont F, Guillot E, Daniels MJ, Cochard H, Julien J-L, Gousset-Dupont A. 2013. Aquaporins and leaf hydraulics: poplar sheds new light. Plant and Cell Physiology 54, 1963–1975. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Piñol J. 2002. Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. Forest Ecology and Management 161, 247–256. [Google Scholar]
- Martin-StPaul NK, Limousin J-M, Rodríguez-Calcerrada J, Ruffault J, Rambal S, Matthew LG, Misson L. 2012. Photosynthetic sensitivity to drought varies among populations of Quercus ilex along a rainfall gradient. Functional Ecology 39, 25–37. [DOI] [PubMed] [Google Scholar]
- Matusick G, Ruthrof KX, Brouwers NC, Dell B, Hardy GSJ. 2013. Sudden forest canopy collapse corresponding with extreme drought and heat in a Mediterranean-type eucalypt forest in southwestern Australia. European Journal of Forest Research 132, 497–510. [Google Scholar]
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 51, 659–668. [DOI] [PubMed] [Google Scholar]
- Morales P, Sykes MT, Prentice IC, et al. 2005. Comparing and evaluating process-based ecosystem model predictions of carbon and water fluxes in major European forest biomes. Global Change Biology 11, 2211–2233. [DOI] [PubMed] [Google Scholar]
- Mott KA, Buckley TN. 1998. Stomatal heterogeneity. Journal of Experimental Botany 49, 407–417. [Google Scholar]
- Mott KA, Buckley TN. 2000. Patchy stomatal conductance: emergent collective behaviour of stomata. Trends in Plant Science 1385, 258–262. [DOI] [PubMed] [Google Scholar]
- Niinemets U. 2014. Improving modeling of the ‘dark part’ of canopy carbon gain. Tree Physiology 34, 557–563. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 2015. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex . New Phytologist 205, 79–96. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T. 2005. Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant, Cell and Environment 28, 1552–1566. [Google Scholar]
- Niinemets U, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. 2009. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. Journal of Experimental Botany 60, 2249–70. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Keenan T. 2014. Photosynthetic responses to stress in Mediterranean evergreens: mechanisms and models. Environmental and Experimental Botany 103, 24–41. [Google Scholar]
- Niinemets Ü, Oja V, Kull O. 1999. Shape of leaf photosynthetic electron transport versus temperature response curve is not constant along canopy light gradients in temperate deciduous trees. Plant, Cell and Environment 22, 1497–1513. [Google Scholar]
- Ogaya R, Llusià J, Barbeta A, Asensio D, Liu D, Alessio GA, Peñuelas J. 2014. Foliar CO2 in a holm oak forest subjected to 15 years of climate change simulation. Plant Science 226, 101–7. [DOI] [PubMed] [Google Scholar]
- Ogaya R, Peñuelas J. 2003. Comparative seasonal gas exchange and chlorophyll fluorescence of two dominant woody species in a Holm Oak Forest. Flora 198, 132–141. [Google Scholar]
- Ogaya R, Peñuelas J. 2006. Contrasting foliar responses to drought in Quercus ilex and Phillyrea latifolia . Biologia Plantarum 50, 373–382. [Google Scholar]
- van Oijen M, Schapendonk A, Höglind M. 2010. On the relative magnitudes of photosynthesis, respiration, growth and carbon storage in vegetation. Annals of Botany 105, 793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öquist G, Huner NPA. 2003. Photosynthesis of overwintering evergreen plants. Annual Review of Plant Biology 54, 329–55. [DOI] [PubMed] [Google Scholar]
- Pattison RR, Goldstein G, Ares A. 1998. Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecología 117, 449–459. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Lloret F, Montoya R. 2001. Severe drought effects on Mediterranean woody flora in Spain. Forest Science 47, 214–218. [Google Scholar]
- Peñuelas J, Ogaya R, Boada M, Jump AS. 2007. Migration, invasion and decline: changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 30, 829–837. [Google Scholar]
- Perez-Martin A, Michelazzo C, Torres-Ruiz JM, Flexas J, Fernández JE, Sebastiani L, Diaz-Espejo A. 2014. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: correlation with gene expression of carbonic anhydrase and aquaporins. Journal of Experimental Botany 65, 3143–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto CA, Nadezhdina N, David JS, Kurz-Besson C, Caldeira MC, Henriques MO, Monteiro FG, Pereira JS, David TS. 2014. Transpiration in Quercus suber trees under shallow water table conditions: the role of soil and groundwater. Hydrological Processes 28, 6067–6079. [Google Scholar]
- Raftoyannis Y, Spanos I, Radoglou K. 2008. The decline of Greek fir (Abies cephalonica Loudon): relationships with root condition. Plant Biosystems 142, 386–390. [Google Scholar]
- Reynolds JF, Kemp PR, Acock B, Chen J, Moorhead DL. 1996. Progress, limitations and challenges in modeling the effects of elevated CO2 on plants and ecosystems. In: Koch GW, Mooney HA, eds. Carbon dioxide and terrestrial ecosystems . San Diego: Academic Press, 347–380. [Google Scholar]
- Sánchez-Costa E, Poyatos R, Sabaté S. 2015. Contrasting growth and water use strategies in four co-occurring Mediterranean tree species revealed by concurrent measurements of sap flow and stem diameter variations. Agricultural and Forest Meteorology 207, 24–37. [Google Scholar]
- Sharkey TD. 1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Botanical Review 51, 53–105. [Google Scholar]
- Somot S, Sevault F, Déqué M, Crépon M. 2008. 21st Century climate change scenario for the Mediterranean using a coupled atmosphere–ocean regional climate model. Global and Planetary Change 63, 112–126. [Google Scholar]
- Specht RL. 1969. A comparison of the sclerophyllous vegetation characteristics of Mediterranean type climates in France, California, and southern Australia. I. Structure, morphology, and succession. Australian Journal of Botany 17, 277–292. [Google Scholar]
- Sperlich D, Chang CT, Peñuelas J, Gracia C, Sabaté S. 2014. Foliar photochemical processes and carbon metabolism under favourable and adverse winter conditions in a Mediterranean mixed forest, Catalonia (Spain). Biogeosciences 11, 5657–5674. [Google Scholar]
- Sperlich D, Chang CT, Peñuelas J, Gracia C, Sabaté S. 2015. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiology 35, 501–520. [DOI] [PubMed] [Google Scholar]
- Sun Y, Gu L, Dickinson RE, et al. 2014. Asymmetrical effects of mesophyll conductance on fundamental photosynthetic parameters and their relationships estimated from leaf gas exchange measurements. Plant, Cell and Environment 37, 978–994. [DOI] [PubMed] [Google Scholar]
- Terashima I, Ono K. 2002. Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant and Cell Physiology 43, 70–8. [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Ribas-Carbo M. 2012. Interactions between photosynthesis and day respiration. In: Flexas J, Loreto F, Medrano H, eds. Terrestrial photosynthesis in a changing environment . Cambridge, UK: Cambridge University Press, 41–53. [Google Scholar]
- Vargas R, Sonnentag O, Abramowitz G, et al. 2013. Drought influences the accuracy of simulated ecosystem fluxes : a model-data meta-analysis for Mediterranean oak woodlands. Ecosystems 16, 749–764. [Google Scholar]
- Verhoeven A. 2014. Sustained energy dissipation in winter evergreens. New Phytologist 201, 57–65. [Google Scholar]
- von Caemmerer S. 2000. Biochemical Models of Leaf Photosynthesis. Techniques in Plant Science Nr.2 . Collingwood: CSIRO Publishing Australia. [Google Scholar]
- Weerasinghe LK, Creek D, Crous KY, Xiang S, Liddell MJ, Turnbull MH, Atkin OK. 2014. Canopy position affects the relationships between leaf respiration and associated traits in a tropical rainforest in Far North Queensland. Tree Physiology 34, 564–584. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Atkin OK, Lusk CH, Tjoelker MG, Westoby M. 2006. Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: evidence from comparisons across 20 sites. New Phytologist 169, 309–319. [DOI] [PubMed] [Google Scholar]
- Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate B a. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Global Change Biology 17, 927–942. [Google Scholar]
- Yin X, Struik PC, Romero P, Harbinson J, Evers JB, Van Der Putten PEL, Vos J. 2009. Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical C photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant, Cell and Environment 32, 448–64. [DOI] [PubMed] [Google Scholar]
- Yin X, Sun Z, Struik PC, Gu J. 2011. Evaluating a new method to estimate the rate of leaf respiration in the light by analysis of combined gas exchange and chlorophyll fluorescence measurements. Journal of Experimental Botany 62, 3489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza-Castells J, Sánchez-Gómez D, Hartley IP, Matesanz S, Valladares F, Lloyd J, Atkin OK. 2008. Climate-dependent variations in leaf respiration in a dry-land, low productivity Mediterranean forest: the importance of acclimation in both high-light and shaded habitats. Functional Ecology 22, 172–184. [Google Scholar]
- Zaragoza-Castells J, Sánchez-Gómez D, Valladares F, Hurry V, Atkin OK. 2007. Does growth irradiance affect temperature dependence and thermal acclimation of leaf respiration? Insights from a Mediterranean tree with long-lived leaves. Plant, Cell and Environment 30, 820–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.