Highlight
CYP71BE5 from grapevine was identified as a sesquiterpene oxidase capable of transforming α-guaiene to (−)-rotundone, responsible for the characteristic spicy aroma in wines.
Key words: Cytochrome P450, grapevine, guaiene, rotundone, sesquiterpene oxidase, Vitis vinifera, wine.
Abstract
(−)-Rotundone is a potent odorant molecule with a characteristic spicy aroma existing in various plants including grapevines (Vitis vinifera). It is considered to be a significant compound in wines and grapes because of its low sensory threshold and aroma properties. (−)-Rotundone was first identified in red wine made from the grape cultivar Syrah and here we report the identification of VvSTO2 as a α-guaiene 2-oxidase which can transform α-guaiene to (−)-rotundone in the grape cultivar Syrah. It is a cytochrome P450 (CYP) enzyme belonging to the CYP 71BE subfamily, which overlaps with the very large CYP71D family and, to the best of our knowledge, this is the first functional characterization of an enzyme from this family. VvSTO2 was expressed at a higher level in the Syrah grape exocarp (skin) in accord with the localization of (−)-rotundone accumulation in grape berries. α-Guaiene was also detected in the Syrah grape exocarp at an extremely high concentration. These findings suggest that (−)-rotundone accumulation is regulated by the VvSTO2 expression along with the availability of α-guaiene as a precursor. VvSTO2 expression during grape maturation was considerably higher in Syrah grape exocarp compared to Merlot grape exocarp, consistent with the patterns of α-guaiene and (−)-rotundone accumulation. On the basis of these findings, we propose that VvSTO2 may be a key enzyme in the biosynthesis of (−)-rotundone in grapevines by acting as a α-guaiene 2-oxidase.
Introduction
Wine flavor is complex, involving hundreds of volatile molecules. Among them, only a few molecules have been identified as valuable contributors to wine aroma, such as monoterpenoids (Rapp and Mandery, 1986), C13 norisoprenoids (Winterhalter and Rouseff, 2002), volatile sulfur compounds (Darriet et al., 1995; Tominaga et al., 1996, 1998a) and methoxypyrazines (Allen et al., 1991). These compounds mainly originate from the secondary metabolites produced in grapevines (Vitis vinifera). Some of them exist as odorless forms, such as cysteine (Tominaga et al., 1998b; Thibon et al., 2010), glutathione (Peyrot Des Gachons et al., 2002) and glycoside conjugates (Park et al., 1991) and can be released by chemical and enzymatic reactions during wine making and aging. In addition, the relative concentrations of these compounds in grape berries depend on the grape variety and are further influenced by diverse factors such as environmental conditions and the timing of harvest (Styger et al., 2011). Finally, the combinations of these compounds can be expressed by wine makers as the attractive characteristics of each wine, reflecting the grape variety and terroir.
(−)-Rotundone, an oxygenated sesquiterpene, has been identified as a molecule responsible for the spicy aroma in various plants including grapes and a large number of important herbs and spices such as black and white pepper, oregano, basil, thyme, marjoram and rosemary (Wood et al., 2008). Since this molecule is an extremely potent aroma compound with a low sensory threshold (16ng l-1 in red wine, 8ng l-1 in water), even trace levels of (−)-rotundone can give a pleasant peppery aroma to various foods and beverages, including wine. (−)-Rotundone has been found in several grape varieties, particularly Syrah (regionally called Shiraz), Mourvèdre, and Durif from Australia (Wood et al., 2008; Herderich et al., 2012), and Schioppettino, Vespolina, and Grüner Veltliner from Europe (Caputi et al., 2011; Mattivi et al., 2011). It has been also detected in several tissues, especially the exocarp, stem and leaf tissues (Capone et al., 2012). Furthermore, the accumulation of (−)-rotundone during grape maturation is influenced by diverse environmental factors, such as low atmospheric temperatures (Caputi et al., 2011; Herderich et al., 2012), soil properties and topography (Scarlett et al., 2014), and soil moisture from irrigation (Geffroy et al., 2014). A recent study showed that a grape surface temperature exceeding 25°C negatively affects the concentration of (−)-rotundone in Syrah grapes (Zhang et al., 2015). Thus, (−)-rotundone is increasingly the focus of much interest as an aroma. However, the mechanism of its biosynthesis in plants is still unclear.
α-Guaiene is a sesquiterpene hydrocarbon found in oil extracts from various plants (Kapadia et al., 1967; Rakotonirainy et al., 1997; Pino et al., 2001) including grapevines (Schreier et al., 1976; Coelho et al., 2006). This compound is a potential precursor of (−)-rotundone, as they have common structures, namely, the unique five- and seven-membered rings. Therefore, (−)-rotundone may be synthesized by the oxidation of α-guaiene at position carbon-2(C-2). The chemical formation of (−)-rotundone by the aerial oxidation of α-guaiene via 2-hydroperoxyguaiene has been proposed (Huang et al., 2014) and (2R)-rotundol and (2S)-rotundol are reported intermediates. (Huang et al., 2015a, b). This type of chemical formation may be one of the pathways to synthesize (−)-rotundone. However, further investigation is required to explain the mechanisms of the formation of rotundone in grapevines in terms of oxidation by the enzymes.
Cytochrome P450 (CYP) enzymes play critical roles in oxidative reactions during the biosynthesis of various natural compounds in plants including terpenoids. Several CYPs, for example the (+)-δ-cadinene-8-hydroxylase CYP706B1 (Luo et al., 2001), the premnaspirodiene oxygenase CYP71D55 (Takahashi et al., 2007), the α-humulene 10-hydroxylase CYP71BA1 (Yu et al., 2011), the santalene/bergamotene oxidase CYP76F39v1, the bergamotene oxidase CYP76F37v1 (Diaz-Chavez et al., 2013) and the (+)-valencene oxidase CYP71AV8 (Cankar et al., 2011), have been reported to catalyze the oxidation of sesquiterpenes in several plants. However, plant CYPs that can transform α-guaiene to (−)-rotundone have never been reported.
Here, we identify the α-guaiene 2-oxidase VvSTO2, which is capable of transforming α-guaiene to (−)-rotundone, from the grapevine cultivar Syrah. It is a cytochrome P450 belonging to the CYP71BE subfamily. Finally, we propose that VvSTO2 is one of the key enzymes involved in the pathway of (−)-rotundone biosynthesis in grapevines.
Materials and methods
Plant materials and chemicals
Grapevines, Vitis vinifera, were grown in Nagano Prefecture (lat. 36º20′35′′N; long. 138º18′5′′E; 640 m above sea level) in Japan. The cultivars Syrah and Merlot were harvested in the growing season of 2012/13.
(−)-Rotundone and deuterated 2H5-rotundone were prepared as previously described (Takase et al., 2015). α-Guaiene was synthesized from guaiol by a dehydration procedure with thionyl chloride as the middle step of (−)-rotundone synthesis, and purified by thin layer chromatography using Merck silica gel 60F-254 plates (0.25mm, precoated). α-Guaiene [purity, 90% by gas chromatography-mass spectrometry (GC-MS) analysis] was identified by nuclear magnetic resonance (NMR) spectroscopy as described previously (Ito et al., 2005). β-Nootkatol was synthesized by the reduction of nootkatone as previously reported (Takahashi et al., 2007) with slight modification and identified by NMR spectroscopy. 1H NMR (400 MHz) and 13C NMR (100MHz) spectra were acquired with a Bruker AVANCE 400 spectrometer and measurements were conducted in deuterated chloroform (CDCl3) at ambient temperature. Chemical shifts were recorded in parts per million (ppm). All chemicals required for the synthesis, CDCl3 for NMR, HPLC-grade ethanol, HPLC-grade methanol, n-hexane, n-pentane, ethyl acetate, (−)-α-cedrene and (+)-valencene, were purchased from Sigma-Aldrich Japan (Osaka, Japan). Milli-Q water was obtained from a Milli-Q purification system (Merck Millipore, Tokyo, Japan).
Isolation of CYPs from V. vinifera cv. Syrah
Total RNA was extracted from the Syrah grape exocarp by the cetyltrimethylammonium bromide (CTAB) method (Chang et al., 1993), treated with an RNase-Free DNase set (Qiagen, Valencia, CA, USA) and purified with an RNeasy Plant Mini kit (Qiagen) in accordance with the provided protocol. cDNA was synthesized using SuperScript III reverse transcriptase (Life Technologies Inc., Rockville, MD, USA). To obtain the full-length cDNA, polymerase chain reaction (PCR) was performed using Phusion High-Fidelity DNA Polymerase (New England BioLabs Inc., Ipswich, MA, USA) with cDNA as the template. Gene-specific primers were designed with the information in the 12-fold coverage genome sequence assembly of the grapevine cultivar Pinot Noir PN40024 (NCBI BioProject, accession: PRJNA33471) for the target gene, primer 1 and 2 originating from XM_010646246 for VvSTO2, primer 3 and 4 originating from XM_010654905 for VvSTO4, and primer 5 and 6 originating from XM_010657579 and NC_012016 for VvSTO6, as listed in Supplementary Table S1 at JXB online. PCR products of ~1.5kb were subcloned into the pT7 Blue vector via TA cloning using a DNA ligation kit (Takara Bio Inc., Japan) and confirmed by sequencing.
Heterologous expression of CYPs in yeasts
To generate C-terminal fusion proteins with the V5 epitope, cDNA fragments with each stop codon removed were amplified by PCR using primer 7 and 8 for VvSTO2, primer 9 and 10 for VvSTO4, and primer 11 and 12 for VvSTO6 (Supplementary Table S1), and cloned into the pYES2.1/V5-His-TOPO® vector via a pYES2.1 TOPO® TA expression kit (Invitrogen) in accordance with the provided protocol. The resulting construct and the empty pYES2 vector (Invitrogen) as a control were transformed to the BJ2168 yeast strain (MATa, prc1-407, prb1-1122, pep4-3, leu2, trp1, ura3-52, gal2; Nippon Gene) as previously described (Gietz and Schiestl, 2007).
Preparation of recombinant CYP proteins
Transformed yeast cells were grown from single colonies and incubated overnight at 30ºC with shaking in 50ml of SD medium without uracil containing 2% glucose. Yeast cells were collected by centrifugation and transferred in 250ml of SD medium without uracil containing 2% galactose at a starting OD600 of 0.4 to induce protein expression. After incubation overnight at 30ºC with shaking, the cells were pelleted by centrifugation. Recombinant proteins were extracted from yeast cells by the enzymatic breaking method (Pompon et al., 1996) with slight modifications. Microsomes were isolated by ultracentrifugation at 100000×g for 60min and protein concentration was determined by the Bradford method (Coomassie Plus Protein Assay, Thermo Scientific, Rockford, IL, USA). The prepared proteins (10 μg per lane) were separated by SDS-PAGE on 10% (w/v) slab gels (e-PAGEL, ATTO Co., Tokyo, Japan) and transferred to a PVDF membrane (Amersham). Recombinant proteins were detected by western blotting with a mouse monoclonal anti-V5-HRP antibody (Invitrogen) and an ECL Prime Western Blotting Detection Reagent (Amersham).
In vitro enzyme assay
Standard enzyme assays were performed in a total volume of 200 μl containing 50mM Tris-HCl, pH 7.5, 1mM NADPH, 200 μg of microsomal proteins and 100 μM substrates. The mixture was then incubated at 30ºC for 2h. The reaction products were extracted with the same volume (200 μl) of ethyl acetate. The organic phase was evaporated by N2 purging and directly subjected to GC-MS analysis. For determination of kinetic properties, an enzyme reaction was performed in triplicate at each substrate concentration from 2.6–130 μM of α-guaiene or from 2–100 μM of (+)-valencene with 200 μg of microsomal protein at 30ºC for 30min. Enzyme assays adding α-guaiene and (+)-valencene simultaneously at the same concentrations of 80 μM were performed in triplicate with 200 μg of microsomal protein at 30ºC for 30min, to detect whether these substrates interfere with each enzyme reaction of VvSTO2. The reaction was terminated by adding 200 μl of methanol and 10ng μl-1 (−)-α-cedrene as an internal standard. The resulting reaction products were diluted up to 2ml with Milli-Q and subjected to stir bar sorptive extraction (SBSE) and GC-MS analysis. Other potential substrates were purchased from Sigma-Aldrich Japan (Osaka, Japan) and tested. The kinetic properties were calculated from the Hanes-Woolf plot.
GC-MS analysis
The in vitro reaction products extracted directly using ethyl acetate were analyzed on an Agilent 6890 Series GC system coupled to an Agilent 5973 insert mass selective detector (MSD). The reaction products were separated on a BP20 column (50 m × 0.22mm × 0.25 μm; Trajan Scientific and Medical, Melbourne, Australia) with helium carrier gas at a constant flow rate of 1.1ml min-1. The GC oven temperature was programmed from 40ºC (held for 2min) to 130ºC at 15ºC min-1 and then increased to 260ºC (held for 5min) at 10ºC min-1. MS measurements were performed in the scan mode using electron ionization at 70eV. The MS transfer line was held at 260ºC and the scan range was set from m/z 40 to 300.
Stir bar sorptive extraction (SBSE)
SBSE was performed as previously described (Takase et al., 2015). A stir bar coated with 24 μl of polydimethylsiloxane (PDMS, Twister, GERSTEL GmbH, Mülheim an der Ruhr, Germany) was conditioned for 60min at 300ºC in a flow of nitrogen. The collected grape samples were transferred to 10ml headspace vials, and the vials were sealed with a screw cap after the addition of a stir bar. SBSE was performed at room temperature for 60min by stirring at 1500rpm. The stir bar was washed with Milli-Q water, dried with lint-free paper and introduced to a glass thermal desorption liner. The glass liner was placed in a thermal desorption unit. The stir bar was reconditioned by soaking in methanol:dichloromethane (1:1, v/v) for 24h, dried at room temperature and stored carefully until the next use.
GC-MS analysis using SBSE method
The in vitro reaction products obtained by SBSE were analyzed on an Agilent 7890A Series GC system equipped with an Agilent 5975C inert XL MSD with a Triple-Axis Detector, a thermal desorption unit (TDU, GERSTEL GmbH) and a programmed temperature vaporizing injector (CIS4, GESTER GmbH). For thermal desorption, the TDU was programmed from 40ºC (held for 0.5min) to 300ºC (held for 3min) at 200ºC min-1 with 50ml min-1 desorption flow. The transfer capillary temperature was fixed at 300ºC. Desorbed compounds were trapped at 10ºC on a Tenax TA packed liner in the CIS4. After desorption, the CIS4 was programmed from 10ºC to 250ºC (held for 1min) at 720ºC min-1 to inject trapped compounds onto the column in the splitless mode for a splitless time of 2min. The reaction products were separated on a BP20 column (50 m × 0.22mm × 0.25 μm; Trajan Scientific and Medical, Melbourne, Australia) and measured using the same conditions as described for ‘GC-MS analysis’ above.
GC-MS/MS analysis using SBSE method
The samples collected from grape tissues by SBSE were analyzed on an Agilent 7890A Series GC system coupled to an Agilent 7000 GC/MS Triple Quad system and equipped with a TDU and a CIS4 system. For thermal desorption, the TDU and CIS4 were programed under the same conditions as for GC-MS analysis, as described above. Compounds were separated on a BP20 column (50 m × 0.22mm × 0.25 μm; Trajan Scientific and Medical) with helium carrier gas at a constant flow rate of 1.1, and the GC oven temperature was programmed from 40ºC (held for 2min) to 130ºC at 15ºC min-1, then increased to 160ºC at 2ºC min-1, and further increased to 260ºC (held for 5min) at 5ºC min-1. The GC/MS Triple Quad system was operated in the electron impact (EI) ionization mode at 70eV, the nitrogen flow in the collision cell was 1.5ml min-1 and the helium quench flow was 2.25ml min-1. MS measurements were performed in the multiple reaction monitoring (MRM) mode. The transitions m/z 204 to 147 for α-guaiene, m/z 218 to 163 for (−)-rotundone and m/z 223 to 166 for 2H5-rotundone as an internal standard were selected. Collision energies for α-guaiene, (−)-rotundone, and 2H5-rotundone were 3, 8 and 7eV, respectively.
Quantitative real-time RT-PCR analysis
Total RNA was extracted from the grape exocarp and mesocarp by the CTAB method (Chang et al., 1993), treated with the RNase-Free DNase Set (Qiagen) and purified with the RNeasy Plant Mini Kit (Qiagen) in accordance with the provided protocol. Quantitative real-time RT-PCR was performed with a One-Step SYBR PrimeScript PLUS RT-PCR kit (Takara-Bio Inc.) using an ABI Prism 7300 real-time PCR system (Life Technologies Inc.) in accordance with the provided protocol. The specific primers for VvSTO2 (primer 13 and 14) and for 18S rRNA (GenBank accession no. AF207053, primer 15 and 16) (Supplementary Table S1) were designed using Primer Express 1.0 software (Life Technologies Inc.). Quantitative real-time RT-PCR was performed in technical triplicate and all samples were normalized to the data of 18S rRNA as an internal control.
Extractions of α-guaiene and (−)-rotundone from grape tissues
α-Guaiene and (−)-rotundone were extracted as previously described (Takase et al., 2015) with slight modification. Grape berry tissue was frozen and then pulverized in liquid nitrogen using a mixer mill (MM 400, Retsch, Haan, Germany). After adding 50 μl of 2H5-rotundone (50 μg l-1) as the internal standard, the powder (1g, fresh weight) was extracted with 5ml of n-pentane:ethyl acetate (9:1, v/v) by shaking for 1h in a shaker at room temperature. The extract was centrifuged at 3000rpm for 10min. The organic solvent was carefully removed by nitrogen gas purging. The residue was dissolved in 500 μl of ethanol, to which 4.5ml of aqueous tartrate buffer (pH 3.2) was added thereafter. The prepared grape samples were subjected to SBSE and GC-MS/MS analysis.
Phylogenetic analysis
Phylogenetic analysis was performed using the entire predicted amino acid sequences of V. vinifera putative CYP71BE family proteins and related terpene-modifying P450 proteins from the GenBank database. Sequence alignments were generated on the basis of comparison of the amino acid sequences using the ClustalW program (Thompson et al., 1994) with the following values: 10 for gap opening penalty and 0.1 for gap extension penalty in pairwise alignment; 10 for gap opening penalty and 0.2 for gap penalty in multiple alignment; Gonnet for protein weight matrix; available residue-specific penalties; available hydrophilic penalties; 4 for gap separation distance; and 30% delay divergent cutoff. These alignments were adopted to construct neighbor-joining phylogenetic trees using MEGA 6.06 with the scope of all selected taxa, amino acid substitution type, Poisson model, uniform rates, homogeneous pattern among lineages and complete deletion for gaps or missing data treatment (Tamura et al., 2013). The scale bar of 0.1 indicates a 10% change and each number shown next to the branches is the number of replicate trees in which the related taxa clustered in the bootstrap test with 1000 replicates.
Results
Isolation of putative sesquiterpene oxidase genes from V. vinifera cv. Syrah
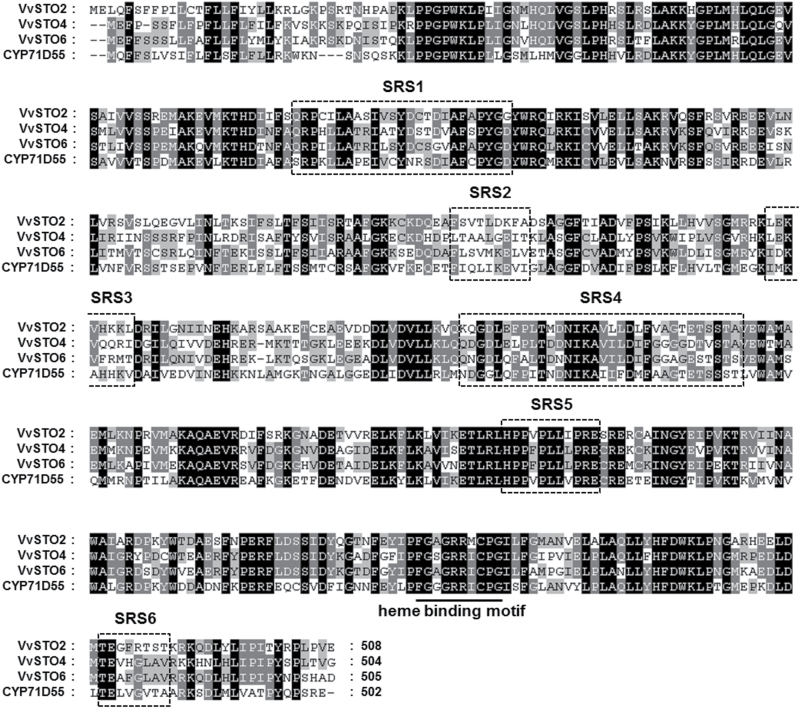
The premnaspirodiene oxygenase CYP71D55 can catalyze the successive reactions leading to the generation of solavetivone via the oxidation of premnaspirodiene at position C-2. It can also oxidize (+)-valancene at position C-2 to transform β-nootkatol (Takahashi et al., 2007). Furthermore, it is a cytochrome P450 within the CYP71D subfamily. In particular, the successive oxidations of premnaspirodiene by CYP71D55 seem to be similar to our target reaction. Therefore, to identify putative sesquiterpene oxidases responsible for the oxidation of α-guaiene in grapevines, six CYP71D55-like genes (more than 50% identity to CYP71D55) were identified as candidates by homology sequencing with the premnaspirodiene oxygenase CYP71D55 from the 12-fold coverage genome sequence assembly of the grapevine cultivar Pinot Noir PN40024 (NCBI BioProject, Accession: PRJNA33471) (Jansen et al., 2006; Jaillon et al., 2007; Goremykin et al., 2009). Comparison with the V. vinifera cytochrome P450s listed on the Cytochrome P450 Homepage (Nelson, 2009) suggests that these genes belong to the CYP71BE family. Its family overlaps with the very large CYP71D family, according to the information from the P450 nomenclature committee (care of Dr. Nelson). They were named V. vinifera sesquiterpene oxidases, VvSTO1 to VvSTO6, and corresponded to each reference sequence (RefSeq) from the 12-fold coverage grapevine genome sequence assembly listed in Supplementary Table S2. Among six uncharacterized candidate genes, three genes – VvSTO2, VvSTO4 and VvSTO6 – could be successfully isolated from the Syrah grape exocarp by PCR using specific primers. Three other genes could not be isolated in this study. The isolated cDNAs VvSTO2, VvSTO4 and VvSTO6 had the ORFs of 1527, 1515 and 1518 base pairs, and encoded the predicted proteins of 508, 504 and 505 amino acids, respectively. The alignment of their predicted amino acid sequences and CYP71D55 showed that they possessed the highly conserved motifs among the eukaryotic P450s, such as the heme-binding motif FxxGxRxCxG (Chapple, 1998; Ralston et al., 2001) and the putative substrate recognition sites (SRSs) originally described by Gotoh (Gotoh, 1992) (Fig. 1). VvSTO2, VvSTO4 and VvSTO6 showed 53–58% sequence identity with CYP71D55 at the protein sequence level. These putative V. vinifera P450s, VvSTO2, VvSTO4 and VvSTO6, were subsequently assigned to CYP71BE5, CYP71BE1 and CYP71BE10 by the P450 nomenclature committee (Nelson, 2009), and their respective DDBJ accession numbers are LC055499, LC055500 and LC055501.
Fig. 1.
Amino acid sequence alignment of VvSTO2, VvSTO4, VvSTO6 and CYP71D55. Multiple sequence alignment was performed using ClustalW and visualized by the software Genedoc. Six of the putative SRS regions defined by Gotoh (Gotoh, 1992) are outlined and the heme-binding motifs are underlined and annotated. The black and gray shades indicate similar amino acids, respectively.
Functional identification of V. vinifera cytochrome P450s by in vitro enzyme assay
To demonstrate the enzymatic function of the isolated genes, the yeast strain BJ2168 was transformed with the expression plasmid containing each ORF. VvSTO2, VvSTO4 and VvSTO6 expressions were confirmed by western blot analysis using an anti-V5-HRP antibody (Supplementary Fig. S1). The predicted molecular mass of VvSTO2, VvSTO4 and VvSTO6 fusion proteins with the V5 epitope were 61.4, 60.9 and 61.1kDa, respectively. For each fusion protein, the apparent band was detected in accord with the position of its predicted molecular mass.
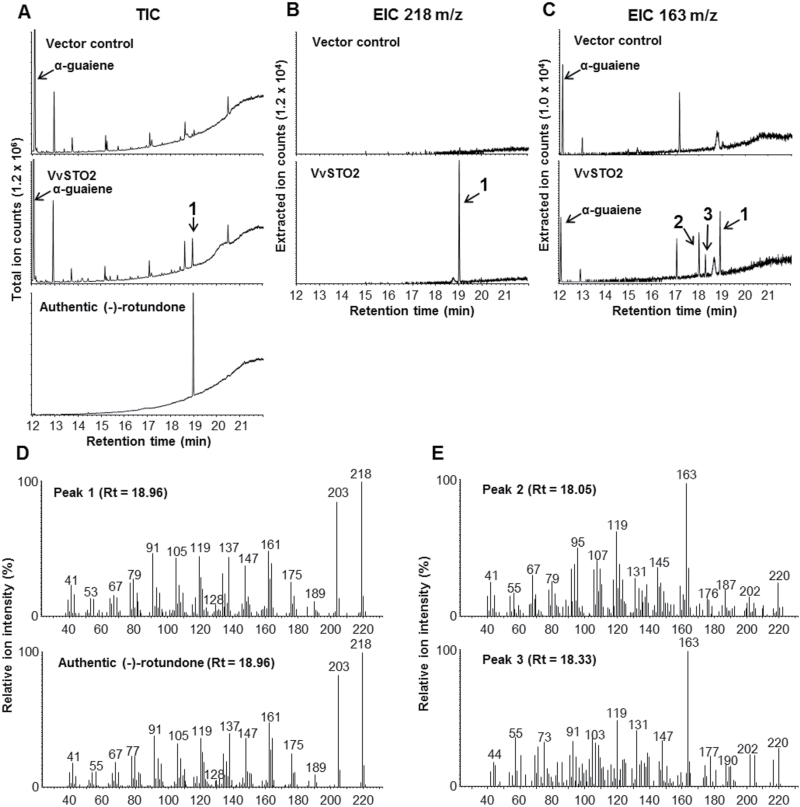
The microsomes from yeast cells expressing each of the candidate P450s were assayed in vitro with α-guaiene as the substrate and the reaction product was analyzed by GC-MS. No product was detected in assays using the microsomes from yeast cells containing an empty pYES vector (Fig. 2A). In contrast, the formation of a major reaction product by VvSTO2 catalysis (peak 1) was detected (Fig. 2A). The reaction product of peak 1 by VvSTO2 catalysis was apparently shown also in the extracted-ion chromatogram (EIC) of m/z 218 which was the molecular weight of (−)-rotundone (Fig. 2B). Furthermore, the EIC of m/z 163 showed two small peaks in addition to peak 1 (Fig. 2C). The retention time (Rt) and the mass spectrum of peak 1 matched those of authentic (−)-rotundone (Fig. 2A–D). Interestingly, the mass spectra of peaks 2 and 3 were identical to those of (2R)-rotundol and (2S)-rotundol (Fig. 2E), respectively (Huang et al., 2015a). Additionally, VvSTO4 and VvSTO6 produced several reaction products with α-guaiene as a substrate (Supplementary Fig. S2). Their unknown reaction products were presumed to correspond to mono-hydroxylated sesquiterpenes on the basis of a parent ion of 220 or 222 m/z in GC-MS analysis and were not identified. However, the mass spectra of these unknown compounds were apparently different from those of (2R)-rotundol and (2S)-rotundol (Huang et al., 2015a). The absence of spontaneous oxidative reaction of α-guaiene to (−)-rotundone was confirmed in our in vitro assay through performing numerous repetitions with the microsomes from yeast cells containing an empty pYES vector, VvSTO4 or VvSTO6 for 2 to 48h.
Fig. 2.
GC-MS analysis of enzymatic reaction products by in vitro assay of α-guaiene with VvSTO2. (A) Total ion chromatograms (TICs) of incubation mixtures resulting from in vitro assay of α-guaiene with vector control and recombinant VvSTO2, and of authentic (−)-rotundone. (B) Extracted ion chromatograms (EICs) at m/z 218 and (C) m/z 163 corresponding to each TIC of the same incubation mixtures resulting from in vitro assay of α-guaiene. (D) Mass spectra of peak 1 and authentic (−)-rotundone. (E) Mass spectra of peaks 2 and 3. The mass spectra of peak 2 and 3 were identical to those of (2R)-rotundol and (2S)-rotundol, respectively (Huang et al., 2015a). Corresponding chromatograms were shown at the same scale as indicated in the vertical axis.
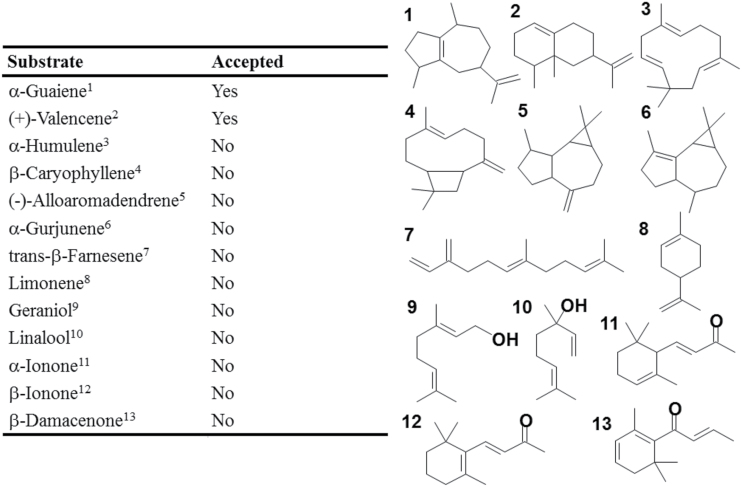
Substrate specificity and kinetic properties of the VvSTO2 protein
The VvSTO2 protein was analyzed further for substrate specificity and kinetics, because it demonstrated the capability for rotundone synthesis from α-guaiene. The enzyme activity of the VvSTO2 protein was examined using other sesquiterpenes and monoterpenes as potential substrates, as listed in Fig. 3. The microsomes containing the VvSTO2 protein could use (+)-valencene as the substrate as well as α-guaiene. On the other hand, the VvSTO2 protein did not accept other sesquiterpenes, monoterpenes or C13 norisoprenoids as a substrate. As a result, β-nootkatol accumulated, as previously reported for the function of the CYP71D55 protein (Takahashi et al., 2007) (Supplementary Fig. S3). However, other reaction products such as α-nootkatol and nootkatone were not detected.
Fig. 3.
Substrate specificity of VvSTO2, with left-hand panel indicating whether VvSTO2 accepted each compound as a substrate. The right-hand side of the panel shows the structures of the corresponding numbered substrates.
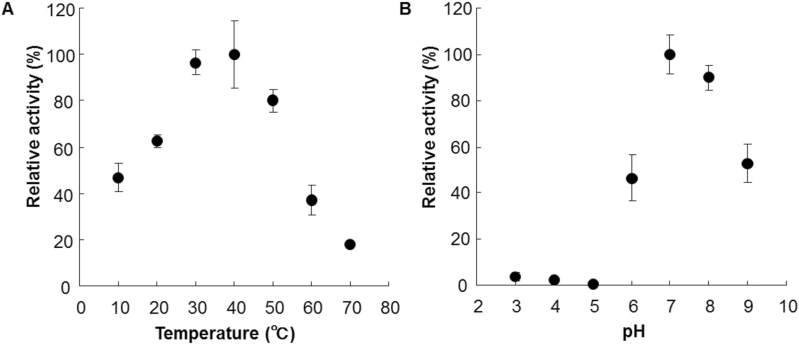
The apparent Km values for the transformations of α-guaiene to (−)-rotundone and (+)-valencene to β-nootkatol were calculated from the Hanes-Woolf plot to be 30 μM and 35 μM, respectively (Supplementary Fig. S4). These low Km values are similar to those (7.4±1.2 μM to 52±2 μM) for other sesquiterpene oxidases that can transform (+)-valencene to β-nootkatol (Gavira et al., 2013). As a result of the enzyme assays adding α-guaiene and (+)-valencene simultaneously, the production rates of (−)-rotundone and β-nootkatol were 82.7% and 73.3%, respectively, compared to the enzyme assays which added each substrate independently. These results suggest that α-guaiene and (+)-valencene can interfere with each enzyme reaction of VvSTO2, but the interference is relatively low. For transforming α-guaiene to (−)-rotundone, the optimum temperature was in the range from 30–40ºC and the optimum pH was in the range from 7.0–8.0 (Fig. 4).
Fig. 4.
Enzymatic characterization of recombinant VvSTO2. (A) Temperature profile of VvSTO2 activity. (B) pH dependence of VvSTO2 activity. The buffers were citrate buffer (pH 3, 4, 5, 6), Tris-HCl buffer (pH 7, 8, 9). VvSTO2 activity was measured with α-guaiene as a substrate. Values are the mean ± standard deviation of three technical replicates.
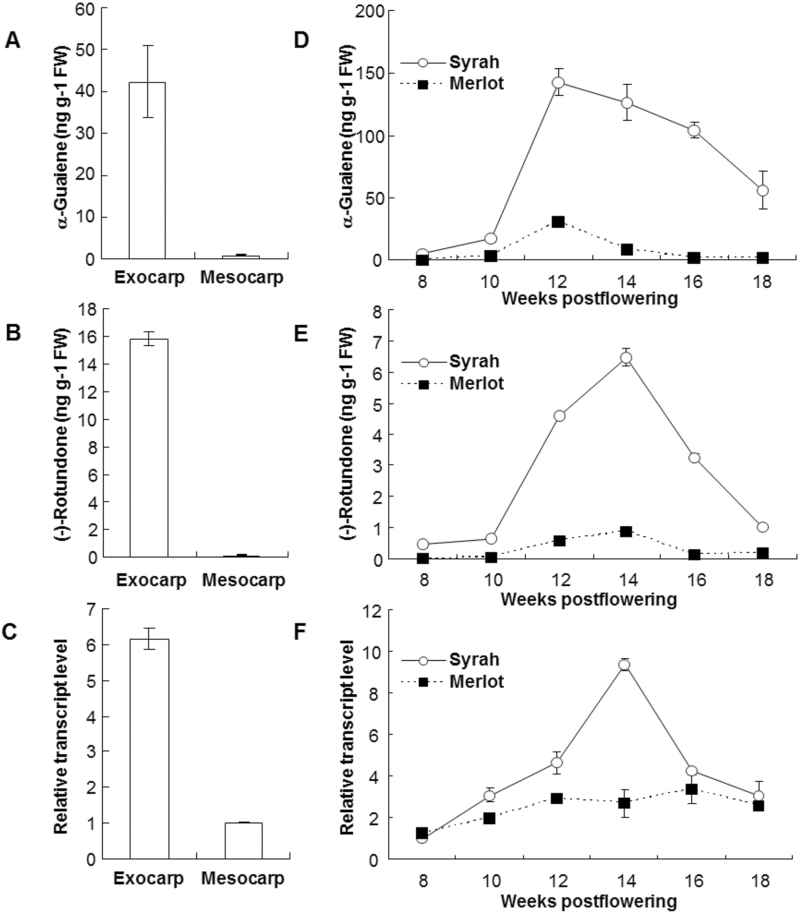
Patterns of the accumulation of α-guaiene, (−)-rotundone, and VvSTO2 mRNA in grape berries
The concentrations of α-guaiene and (−)-rotundone in grape berries were analyzed by GC-MS/MS using the SBSE method and VvSTO2 transcript levels were quantified by real-time RT-PCR analysis. A previous report showed that (−)-rotundone is localized in the grape berry exocarp (Caputi et al., 2011); however, the localization of α-guaiene was not clear. The tissue specificity of VvSTO2 transcript levels and the concentration of α-guaiene and (−)-rotundone in various tissues of grape berries were investigated using mature Syrah grapes. The concentrations of α-guaiene and (−)-rotundone were higher in the exocarp than in mesocarp (flesh) (Fig. 5A, B). Consistent with this, the VvSTO2 transcript levels in the exocarp were much higher than those in the mesocarp (Fig. 5C). These findings further support the observation that sesquiterpene biosynthesis and accumulation in grape berries is restricted to the exocarp (May et al., 2013). Furthermore, the patterns of the accumulation of α-guaiene and (−)-rotundone and VvSTO2 transcript levels in the exocarp during the grape maturation between 8 and 18 weeks postflowering were investigated, comparing the high-rotundone cultivar Syrah with the low-rotundone cultivar Merlot. At the same time, the general fruit components such as the total soluble solid and the titratable acidity during grape maturation were analyzed (Supplementary Fig. S5). The accumulation of α-guaiene in the Syrah grape exocarp reached a maximum at 12 weeks postflowering and then decreased (Fig. 5D). Interestingly, the accumulation of (−)-rotundone reached the maximum at 14 weeks postflowering, which is 2 weeks later than the accumulation of α-guaiene, and then decreased progressively (Fig. 5E). These results support the hypothesis that the biosynthesis of (−)-rotundone occurs following the accumulation of α-guaiene. In contrast, the concentrations of α-guaiene and (−)-rotundone in the Merlot grape exocarp was always much lower than those in the Syrah grape exocarp during grape maturation (Fig. 5D, E). Moreover, the patterns of VvSTO2 transcript levels in the Syrah and Merlot grape exocarps were completely different and consistent with the patterns of (−)-rotundone accumulation during grape maturation (Fig. 5F). Taken together, these findings suggest that VvSTO2 is involved in the biosynthesis of (−)-rotundone in grape berries.
Fig. 5.
Patterns of α-guaiene and (−)-rotundone accumulation with relative transcript levels of VvSTO2 mRNA in grape berries. (A) Concentration of α-guaiene, (B) concentration of (−)-rotundone and (C) relative transcript levels of VvSTO2 mRNA in each tissue of Syrah grape berries. (D) Concentration of α-guaiene, (E) concentration of (−)-rotundone and (F) relative transcript levels of VvSTO2 mRNA in Syrah and Merlot grape exocarp during grape maturation after flowering. The transcipt levels of VvSTO2 mRNA were determined by the quantitative real-time RT-PCR. All samples were normalized using 18 rRNA as an internal control. The transcript levels in the mesocarp for the experiment in panel C and in the Syrah grape exocarp at 8 weeks postflowering for the experiment in panel F were set to 1 to calculate the relative transcript levels of VvSTO2 mRNA, respectively. Values are the mean ± standard deviation of three technical replicates. FW, fresh weight.
Discussion
(−)-Rotundone is as one of the most important compounds significantly contributing to the spicy characteristic notably in wines and grapes, since its first identification in the red wine made from the grape cultivar Syrah in 2008 (Wood et al., 2008). Although the factors affecting the accumulation of (−)-rotundone and its chemical formation via the oxidation of α-guaiene have been reported (Huang et al., 2014), the mechanism of (−)-rotundone biosynthesis in plants has thus far remained unclear.
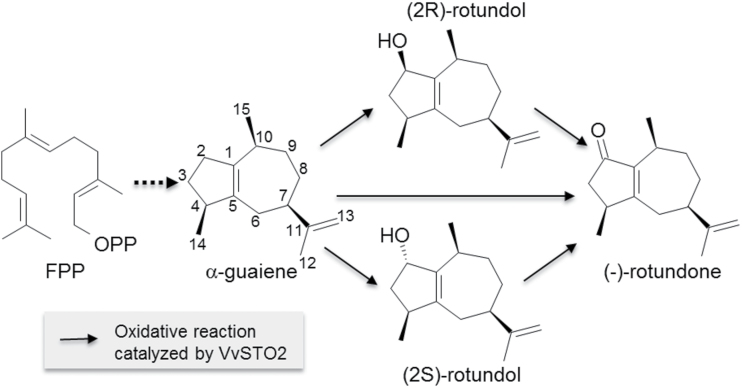
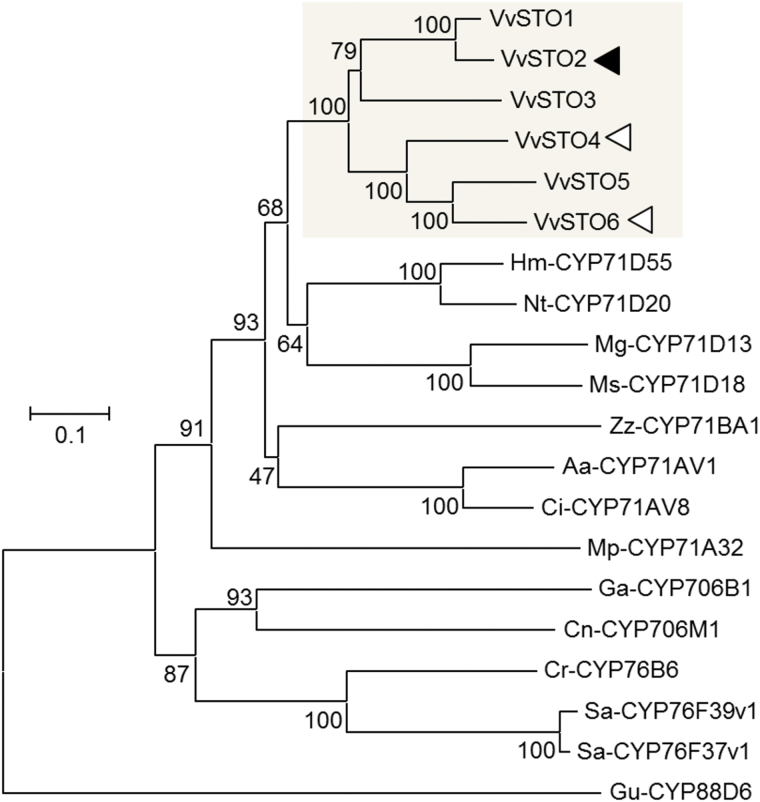
To identify the key enzyme involved in the biosynthesis of (−)-rotundone, we performed screening with the homology sequence of characterized sesquiterpene oxidase from the 12-fold coverage genome sequence assembly of the grapevine cultivar Pinot Noir PN40024 (Jansen et al., 2006; Jaillon et al., 2007; Goremykin et al., 2009). In this study, we identified a novel P450 enzyme, the α-guaiene 2-oxidase VvSTO2, from the grapevine cultivar Syrah, which can oxidize α-guaiene at position C-2, leading to the biosynthesis of (−)-rotundone (Fig. 6). VvSTO2, assigned as CYP71BE5, belongs to the CYP71BE subfamily of the CYP71 family within the CYP71 clan of enzymes. Moreover, the CYP71BE subfamily overlaps with the very large CYP71D subfamily. To the best of our knowledge, this study is the first to functionally characterize CYP71BE5 in this enzyme family. The CYP71 clan accounts for more than 50% of all plant CYPs. Although a huge diversity of their functions has been reported, including monoterpene and sesquiterpene oxidation in plant terpenoid metabolism (Nelson and Werck-Reichhart, 2011), plant CYPs capable of catalyzing α-guaiene to (−)-rotundone have never been functionally characterized. Phylogenetic analysis showed that VvSTO2 was closely related to other terpene-modifying CYPs, mostly enzymes belonging to the CYP71 family (Fig. 7).
Fig. 6.
Proposed pathway for biosynthesis of (−)-rotundone. FPP, farnesyl diphosphate; OPP, diphosphate in the structure of FPP.
Fig. 7.
Phylogenetic tree of V. vinifera CYP71BE proteins and related terpene-modifying CYPs from various plants including Artemisia annua (Aa), Catharanthus roseus (Cr), Cichorium intybus (Ci), C. nootkatensis (Cn), Gossypium arboreum (Ga), H. muticus (Hm), Mentha × gracilis (Mg), Mentha × piperita (Mp), Mentha spicata (Ms), N. tabacum (Nt), Santalum album (Sa), Zingiber zerumbet (Zz). The neighbor-joining tree was generated using ClustalW and MEGA5. The numbers indicate the bootstrap value (%) from 1000 replications. The scale bar shows the amino acid substitution ratio. Gu-CYP88D6, Glycyrrhiza uralensis β-amyrin-11-oxidase (AB433179), was used as the outgroup. V. vinifera CYP71BE proteins identified in this study are shown in the gray box. The closed arrowhead indicates VvSTO2 having the activity of rotundone synthesis and the open arrowheads indicate the CYPs isolated, incapable of producing (−)-rotundone in this study.
Several sesquiterpene oxidases that can catalyze a multistep oxidation of sesquiterpene in plants have been reported in previous studies. For example, the amorphadiene oxidase CYP71AV1 from Artemisia annua catalyzes a three-step oxidation of amorpha-4,11-diene to artemisinic acid (Ro et al., 2006), the 5-epi-aristolochene-1,3-dihydroxylase CYP71D20 from Nicotiana tabacum catalyzes a two-step oxidation of 5-epi-aristolochene to capsidiol (Takahashi et al., 2005), the premnaspirodiene oxygenase CYP71D55 from Hyoscyamus muticus catalyzes a two-step oxidation of premnaspirodiene to solavetivone (Takahashi et al., 2007), and the valencene oxidase CYP706M1 from Cupressus nootkatensis catalyzes a two-step oxidation of (+)-valencene to (+)-nootkatone (Cankar et al., 2014). These reactions showed coincidentally the existence of some intermediate compounds such as mono-hydroxylated sesquiterpene. Similarly, VvSTO2 generated (−)-rotundone as the major reaction product with α-guaiene, and presumably (2R)-rotundol and (2S)-rotundol as the intermediate compounds to synthesize (−)-rotundone were found in vitro enzyme assays, although their peaks were at trace levels. These results suggest that this enzyme is involved in the enzymatic oxidation of α-guaiene to (−)-rotundone and can catalyze a one-step oxidation of α-guaiene to (−)-rotundone or a two-step oxidation via a rapid second oxidation from (2R)-rotundol and (2S)-rotundol to (−)-rotundone (Fig. 6). To verify our proposed biosynthesis pathway of (−)-rotundone, further investigation using the synthetic standards of (2R)-rotundol and (2S)-rotundol is required.
The α-guaiene 2-oxidase VvSTO2 could oxidize (+)-valencene at position C-2 to β-nootkatol as well as α-guaiene at position C-2 to (−)-rotundone (Supplementary Fig. S3). On the other hand, VvSTO2 did not accept other sesquiterpenes, monoterpenes or C13 norisoprenoids as substrates (Fig. 3). These findings suggest that α-guaiene 2-oxidase possesses a relatively narrow substrate specificity for α-guaiene and (+)-valencene. VvSTO2 exhibited high affinity for α-guaiene and (+)-valencene substrates with low Km values. Both Km values of VvSTO2 ranged at the same levels as those of other sesquiterpene oxidases capable of transforming (+)-valencene to β-nootkatol (Gavira et al., 2013). Therefore, VvSTO2 might have the ability to act like α-guaiene 2-oxidase and/or (+)-valencene oxidase in grapevine. However, neither (+)-valencene nor β-nootkatol were identified in the Syrah and Merlot grape exocarps in this study. This might indicate that (+)-valencene and β-nootkatol are either contained at extremely low concentrations or are not contained in the grape exocarp. To the best of our knowledge, neither compound has been reported from the grape exocarp previously. These facts suggest that VvSTO2 plays a role as α-guaiene 2-oxidase rather than as (+)-valencene oxidase in the grape exocarp. In addition, regarding the regio-position of sesquiterpene that undergoes the oxidation by a CYP, the position C-2 of premnaspirodiene, the positions C-1, 2 and 3 of 5-epi-aristolochene, the positions C-2 and 12 of (+)-valencene, the position C-12 of germacrene A, the position C-12 of amorpha-4,11-diene and the position C-8 of α-humulene were reported (Takahashi et al., 2005, 2007; Cankar et al., 2011; Yu et al., 2011). The oxidation by VvSTO2 at the regio-position C-2 of α-guaiene was firstly observed. Furthermore, VvSTO4 and VvSTO6 also showed the capacity to oxidize α-guaiene to as yet unknown compounds including two compounds produced in common by both enzymes such as the peak 4 and 5 (Supplementary Fig. S3), but (−)-rotundone, (2R)-rotundol and (2S)-rotundol were not produced by them. These particular similarities of enzyme properties of VvSTO4 and VvSTO6 may be due to their amino acid sequence identity (71%). These observations suggest that VvSTO2 has unique regio-specificity compared with other V. vinifera CYP71BE proteins. VvSTO4 and VvSTO6 showed 61–62% amino acid sequence identity with VvSTO2, therefore the unique modification of α-guaiene to produce (−)-rotundone by VvSTO2 may be characterized by the difference in amino acid residues. In previous studies, the site-specific mutation of plant CYPs showed that residues such as those within SRS regions may affect the substrate selectivity, reaction product specificity, kinetic properties and regio-specificity (Kahn et al., 2001; Komori et al., 2013; Takahashi et al., 2005, 2007). To fully understand the substrate specificity and regio-specificity of VvSTO2, further functional and structural characterization by a site-specific mutation study is required.
Quantitative real-time RT-PCR analyses showed that VvSTO2 transcript levels in the exocarp were higher than those in the mesocarp in accordance with the localization of (−)-rotundone in grape berries (Fig. 5A–C). Additionally, α-guaiene was also detected in the exocarp at an extremely high concentration. These findings suggest that the accumulation of (−)-rotundone is regulated by the expression of VvSTO2 as well as the biosynthesis of α-guaiene.
VvSTO2 expression in the Syrah grape exocarp during grape maturation showed a similar pattern to the accumulation of (−)-rotundone and α-guaiene (Fig. 5D–F). Additionally, VvSTO2 expression during grape maturation was considerably higher in Syrah grape exocarp compared to Merlot grape exocarp, consistent with the patterns of α-guaiene and (−)-rotundone accumulation. Thereby, it may explain why (−)-rotundone concentration of Syrah grape was higher than that of Merlot grape. These functional analyses findings of VvSTO2 suggest that it plays a critical role as a α-guaiene 2-oxidase in the biosynthesis of (−)-rotundone in grapevines and that the accumulation of (−)-rotundone is also controlled by the biosynthesis of α-guaiene. Chemical oxidation of α-guaiene to (−)-rotundone may occur non-enzymatically according to previous reports (Huang et al., 2014), although it was not observed in our in vitro assay. These facts suggest that the enzymatic conversion of α-guaiene is more rapid than the non-enzymatic conversion in solution. Therefore it is more likely that the biosynthesis of (−)-rotundone from α-guaiene occurs enzymatically in grapevines, although some chemical oxidation cannot be ruled out. To fully determine the function of VvSTO2 in vivo in grapevine, transient expression assays such as over-expression studies of VvSTO2 in grapevine tissues not containing (−)-rotundone or containing it at low concentration, if necessary by feeding α-guaiene substrate, and knock-down studies of VvSTO2 in the high-rotundone cultivar Syrah, are required.
Sesquiterpenes are biosynthesized primarily via the mevalonate pathway operating in the cytosol and 2-C-methyl-D-erythriol-4-phosphate operating in plastids, and various sesquiterpene synthases contribute to the diversification of sesquiterpenes in plants (Chen et al., 2011). Several sesquiterpene synthases such as (−)-valencene synthase and (−)-germacrene D synthase have been isolated from grapevines (Lucker et al., 2004; Martin et al., 2009). Moreover, 69 putatively functional terpene synthase (TPS) genes, including 13 characterized sesquiterpene synthase genes belonging to the TPS-a family, were identified from the 12-fold coverage genome sequence assembly of the grapevine cultivar Pinot Noir (Martin et al., 2010). However, sesquiterpene synthase responsible for α-guaiene synthesis was not identified in grapevines, although a few enzymes producing α-guaiene as one of the major products were found in agarwood and patchouli (Faraldos et al., 2010; Kumeta and Ito, 2010). Recent studies have proposed that genes such as TPS and CYP involved in terpene biosynthetic pathways are organized as metabolic gene clusters and are located adjacent to each other in Arabidopsis (Field and Osbourn, 2008; Field et al., 2011), oat (Mugford et al., 2013) and rice (Shimura et al., 2007; Wilderman et al., 2004). Similarly, in eudicots and monocots, the unique patterns of TPS and CYP gene assembly were observed, indicating that TPS genes primarily pair with CYP71 clan genes, notably with the members of the CYP71 family (Boutanaev et al., 2015). Interestingly, VvSTO2 is clustered with two other putative CYP71BEs (VvSTO1 and VvSTO3; 90% and 66% identity with VvSTO2 at the amino acid sequence level, respectively) in a length of 36kb on chromosome 19. In addition, ten putative sesquiterpene synthases were also located on chromosome 19 (Martin et al., 2010), although their locations were relatively far from VvSTO2, in lengths ranging from 2.8 to 6.9Mb. The genetic organization may give an important clue to the identity of novel enzymes such as α-guaiene synthase and homologs of VvSTO2, relative to the biosynthesis of (−)-rotundone in grapevines.
Here, we successfully identified the α-guaiene 2-oxidase VvSTO2, which produces (−)-rotundone from α-guaiene via the (−)-rotundone biosynthetic pathway. VvSTO2 expression analysis gave strong evidence for VvSTO2 as a key enzyme in the biosynthesis of (−)-rotundone of grape berries. Moreover, we demonstrated that the accumulation of (−)-rotundone is regulated by the biosynthesis of α-guaiene as its precursor in grapevines. Currently, the elucidation of environmental factors and regional characteristics that affect the accumulation of (−)-rotundone is in progress. However, it is extremely difficult to evaluate and to obtain evidence for the effect of each factor due to the lack of information on the target gene in the biosynthesis of (−)-rotundone. The present study’s discoveries might contribute to a more profound understanding of the mechanism of (−)-rotundone biosynthesis in grapevines and, by providing target markers as a useful tool, could promote investigations that elucidate the mechanism by which the regional character, that is terroir, affects the accumulation of (−)-rotundone. Additionally, while (−)-rotundone is found in various important herbs and spices such as black and white pepper, oregano, basil, thyme, marjoram and rosemary (Wood et al., 2008), the α-guaiene 2-oxidase CYP71BE5 capable of synthesizing (−)-rotundone is isolated from only grapevines in this study. The genetic information of CYP71BE5 could serve as a powerful tool to isolate the common functional CYPs from other plants.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Specific primers used in this work.
Table S2. Putative CYP71BE family genes from the 12-fold coverage genome sequence assembly of the grapevine cultivar Pinot Noir PN40024.
Figure S1. Western blot analyses of recombinant V. vinifera P450s.
Figure S2. GC-MS analysis (total ion chromatogram) of enzymatic reaction products using α-guaiene with recombinant VvSTO4 and VvSTO6.
Figure S3. GC-MS analysis (total ion chromatogram) of enzymatic reaction products using (+)-valencene with VvSTO2.
Figure S4. Hanes-Woolf plots of recombinant VvSTO2 for α-guaiene and (+)-valencene.
Figure S5. Analysis of general fruit components during grape maturation.
Acknowledgments
The authors thank Dr Naoyuki Umemoto (Kirin Company, Ltd.), Dr Takuji Ooyama (Faculty of Life and Environmental Sciences, University of Yamanashi) for valuable discussion and Dr David Nelson (Department of Molecular Sciences, University of Tennessee) for helpful suggestions about P450 nomenclature and classification.
References
- Allen MS, Lacey MJ, Harris RL, Brown WV. 1991. Contribution of methoxypyrazines to Sauvignon blanc wine aroma. American Journal of Enology and Viticulture 42, 109–112. [Google Scholar]
- Boutanaev AM, Moses T, Zi J, Nelson DR, Mugford ST, Peters RJ, Osbourn A. 2015. Investigation of terpene diversification across multiple sequenced plant genomes. Proceedings of the National Academy of Sciences of the United States of America 112, E81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cankar K, van Houwelingen A, Bosch D, Sonke T, Bouwmeester H, Beekwilder J. 2011. A chicory cytochrome P450 mono-oxygenase CYP71AV8 for the oxidation of (+)-valencene. FEBS Letters 585, 178–182. [DOI] [PubMed] [Google Scholar]
- Cankar K, van Houwelingen A, Goedbloed M, Renirie R, de Jong RM, Bouwmeester H, Bosch D, Sonke T, Beekwilder J. 2014. Valencene oxidase CYP706M1 from Alaska cedar (Callitropsis nootkatensis). FEBS Letters 588, 1001–1007. [DOI] [PubMed] [Google Scholar]
- Capone DL, Jeffery DW, Sefton MA. 2012. Vineyard and fermentation studies to elucidate the origin of 1,8-cineole in Australian red wine. Journal of Agricultural and Food Chemistry 60, 2281–2287. [DOI] [PubMed] [Google Scholar]
- Caputi L, Carlin S, Ghiglieno I, Stefanini M, Valenti L, Vrhovsek U, Mattivi F. 2011. Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the ‘peppery’ character of wine. Journal of Agricultural and Food Chemistry 59, 5565–5571. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter 11, 113–116. [Google Scholar]
- Chapple C. 1998. Molecular-genetic analysis of plant cytochrome P450-dependent monooxygenases. Annual Review of Plant Physiology and Plant Molecular Biology 49, 311–343. [DOI] [PubMed] [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E. 2011. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant Journal 66, 212–229. [DOI] [PubMed] [Google Scholar]
- Coelho E, Rocha S, Delgadillo I, Coimbra MA. 2006. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Analytica Chimica Acta 563, 204–214. [Google Scholar]
- Darriet P, Tominaga T, Lavigne V, Boidron J-N, Dubourdieu D. 1995. Identification of a powerful aromatic component of Vitis vinifera L. var. Sauvignon wines: 4-mercapto-4-methylpentan-2-one. Flavour and Fragrance Journal 10, 385–392. [Google Scholar]
- Diaz-Chavez ML, Moniodis J, Madilao LL, Jancsik S, Keeling CI, Barbour EL, Ghisalberti EL, Plummer JA, Jones CG, Bohlmann J. 2013. Biosynthesis of Sandalwood Oil: Santalum album CYP76F cytochromes P450 produce santalols and bergamotol. PLoS One 8, e75053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraldos JA, Wu S, Chappell J, Coates RM. 2010. Doubly deuterium-labeled patchouli alcohol from cyclization of singly labeled [2-(2)H(1)]farnesyl diphosphate catalyzed by recombinant patchoulol synthase. Journal of the American Chemical Society 132, 2998–3008. [DOI] [PubMed] [Google Scholar]
- Field B, Fiston-Lavier AS, Kemen A, Geisler K, Quesneville H, Osbourn AE. 2011. Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proceedings of the National Academy of Sciences, USA 108, 16116–16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field B, Osbourn AE. 2008. Metabolic diversification – independent assembly of operon-like gene clusters in different plants. Science 320, 543–547. [DOI] [PubMed] [Google Scholar]
- Gavira C, Hofer R, Lesot A, Lambert F, Zucca J, Werck-Reichhart D. 2013. Challenges and pitfalls of P450-dependent (+)-valencene bioconversion by Saccharomyces cerevisiae. Metabolic Engineering 18, 25–35. [DOI] [PubMed] [Google Scholar]
- Geffroy O, Dufourcq T, Carcenac D, Siebert T, Herderich M, Serrano E. 2014. Effect of ripeness and viticultural techniques on the rotundone concentration in red wine made from Vitis vinifera L. cv. Duras. Australian Journal of Grape and Wine Research 20, 401–408. [Google Scholar]
- Gietz RD, Schiestl RH. 2007. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nature Protocols 2, 31–34. [DOI] [PubMed] [Google Scholar]
- Goremykin VV, Salamini F, Velasco R, Viola R. 2009. Mitochondrial DNA of Vitis vinifera and the issue of rampant horizontal gene transfer. Molecular Biology and Evolution 26, 99–110. [DOI] [PubMed] [Google Scholar]
- Gotoh O. 1992. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. Journal of Biological Chemistry 267, 83–90. [PubMed] [Google Scholar]
- Herderich MJ, Siebert TE, Parker M, Capone DL, Jeffery DW, Osidacz P, Francis IL. 2012. Spice up your life: analysis of key aroma compounds in shiraz. In: Flavor Chemistry of Wine and Other Alcoholic Beverages. American Chemical Society, 3–13. [Google Scholar]
- Huang AC, Burrett S, Sefton MA, Taylor DK. 2014. Production of the pepper aroma compound, (−)-rotundone, by aerial oxidation of alpha-guaiene. Journal of Agricultural and Food Chemistry 62, 10809–10815. [DOI] [PubMed] [Google Scholar]
- Huang AC, Sefton MA, Sumby CJ, Tiekink ERT, Taylor DK. 2015a Mechanistic studies on the autoxidation of α-guaiene: structural diversity of the sesquiterpenoid downstream products. Journal of Natural Products 78, 131–145. [DOI] [PubMed] [Google Scholar]
- Huang AC, Sefton MA, Taylor DK. 2015b . Comparison of the formation of peppery and woody sesquiterpenes derived from alpha-guaiene and alpha-bulnesene under aerial oxidative conditions. Journal of Agricultural and Food Chemistry 63, 1932–1938. [DOI] [PubMed] [Google Scholar]
- Ito M, Okimoto K, Yagura T, Honda G, Kiuchi F, Shimada Y. 2005. Induction of sesquiterpenoid production by methyl jasmonate in Aquilaria sinensis cell suspension culture. Journal of Essential Oil Research 17, 175–180. [Google Scholar]
- Jaillon O, Aury JM, Noel B, et al 2007. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449, 463–467. [DOI] [PubMed] [Google Scholar]
- Jansen RK, Kaittanis C, Saski C, Lee SB, Tomkins J, Alverson AJ, Daniell H. 2006. Phylogenetic analyses of Vitis (Vitaceae) based on complete chloroplast genome sequences: effects of taxon sampling and phylogenetic methods on resolving relationships among rosids. BMC Evolutionary Biology 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Le Bouquin R, Pinot F, Benveniste I, Durst F. 2001. A conservative amino acid substitution alters the regiospecificity of CYP94A2, a fatty acid hydroxylase from the plant Vicia sativa . Archives of Biochemistry and Biophysics 391, 180–187. [DOI] [PubMed] [Google Scholar]
- Kapadia VH, Naik VG, Wadia MS, Dev S. 1967. Sesquiterpenoids from the essential oil of. Tetrahedron Letters 8, 4661–4667. [Google Scholar]
- Komori A, Suzuki M, Seki H, Nishizawa T, Meyer JJ, Shimizu H, Yokoyama S, Muranaka T. 2013. Comparative functional analysis of CYP71AV1 natural variants reveals an important residue for the successive oxidation of amorpha-4,11-diene. FEBS Letters 587, 278–284. [DOI] [PubMed] [Google Scholar]
- Kumeta Y, Ito M. 2010. Characterization of delta-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Physiology 154, 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucker J, Bowen P, Bohlmann J. 2004. Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (−)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65, 2649–2659. [DOI] [PubMed] [Google Scholar]
- Luo P, Wang YH, Wang GD, Essenberg M, Chen XY. 2001. Molecular cloning and functional identification of (+)-delta-cadinene-8-hydroxylase, a cytochrome P450 mono-oxygenase (CYP706B1) of cotton sesquiterpene biosynthesis. Plant Journal 28, 95–104. [DOI] [PubMed] [Google Scholar]
- Martin DM, Aubourg S, Schouwey MB, Daviet L, Schalk M, Toub O, Lund ST, Bohlmann J. 2010. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biology 10, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Toub O, Chiang A, Lo BC, Ohse S, Lund ST, Bohlmann J. 2009. The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains. Proceedings of the National Academy of Sciences, USA 106, 7245–7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattivi F, Caputi L, Carlin S, Lanza T, Minozzi M, Nanni D, Valenti L, Vrhovsek U. 2011. Effective analysis of rotundone at below-threshold levels in red and white wines using solid-phase microextraction gas chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry 25, 483–488. [DOI] [PubMed] [Google Scholar]
- May B, Lange BM, Wust M. 2013. Biosynthesis of sesquiterpenes in grape berry exocarp of Vitis vinifera L.: evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry 95, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford ST, Louveau T, Melton R, Qi X, Bakht S, Hill L, Tsurushima T, Honkanen S, Rosser SJ, Lomonossoff GP, Osbourn A. 2013. Modularity of plant metabolic gene clusters: a trio of linked genes that are collectively required for acylation of triterpenes in oat. Plant Cell 25, 1078–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D, Werck-Reichhart D. 2011. A P450-centric view of plant evolution. Plant Journal 66, 194–211. [DOI] [PubMed] [Google Scholar]
- Nelson DR. 2009. The cytochrome p450 homepage. Human Genomics 4, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Morrison JC, Adams DO, Noble AC. 1991. Distribution of free and glycosidically bound monoterpenes in the skin and mesocarp of Muscat of Alexandria grapes during development. Journal of Agricultural and Food Chemistry 39, 514–518. [Google Scholar]
- Peyrot Des Gachons C, Tominaga T, Dubourdieu D. 2002. Sulfur aroma precursor present in S-glutathione conjugate form: identification of S-3-(hexan-1-ol)-glutathione in must from Vitis vinifera L. cv. Sauvignon Blanc. Journal of Agricultural and Food Chemistry 50, 4076–4079. [DOI] [PubMed] [Google Scholar]
- Pino JA, Rosado A, Fuentes V. 2001. Essential oil of rose-scented geranium (Pelargonium sp.) from Cuba. Journal of Essential Oil Research 13, 21–22. [Google Scholar]
- Pompon D, Louerat B, Bronine A, Urban P. 1996. Yeast expression of animal and plant P450s in optimized redox environments. Methods in Enzymology 272, 51–64. [DOI] [PubMed] [Google Scholar]
- Rakotonirainy O, Gaydou EM, Faure R, Bombarda I. 1997. Sesquiterpenes from patchouli (Pogostemon cablin) essential oil: assignment of the proton and carbon-13 NMR spectra. Journal of Essential Oil Research 9, 321–327. [Google Scholar]
- Ralston L, Kwon ST, Schoenbeck M, Ralston J, Schenk DJ, Coates RM, Chappell J. 2001. Cloning, heterologous expression, and functional characterization of 5-epi-aristolochene-1,3-dihydroxylase from tobacco (Nicotiana tabacum). Archives of Biochemistry and Biophysics 393, 222–235. [DOI] [PubMed] [Google Scholar]
- Rapp A, Mandery H. 1986. Wine aroma. Experientia 42, 873–884. [Google Scholar]
- Ro DK, Paradise EM, Ouellet M, et al 2006. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440, 940–943. [DOI] [PubMed] [Google Scholar]
- Scarlett NJ, Bramley RGV, Siebert TE. 2014. Within-vineyard variation in the ‘pepper’ compound rotundone is spatially structured and related to variation in the land underlying the vineyard. Australian Journal of Grape and Wine Research 20, 214–222. [Google Scholar]
- Schreier P, Drawert F, Junker A. 1976. Identification of volatile constituents from grapes. Journal of Agricultural and Food Chemistry 24, 331–336. [Google Scholar]
- Shimura K, Okada A, Okada K, et al 2007. Identification of a biosynthetic gene cluster in rice for momilactones. Journal of Biological Chemistry 282, 34013–34018. [DOI] [PubMed] [Google Scholar]
- Styger G, Prior B, Bauer FF. 2011. Wine flavor and aroma. Journal of Industrial Microbiology and Biotechnology 38, 1145–1159. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yeo YS, Zhao Y, O’Maille PE, Greenhagen BT, Noel JP, Coates RM, Chappell J. 2007. Functional characterization of premnaspirodiene oxygenase, a cytochrome P450 catalyzing regio- and stereo-specific hydroxylations of diverse sesquiterpene substrates. Journal of Biological Chemistry 282, 31744–31754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Zhao Y, O’Maille PE, Greenhagen BT, Noel JP, Coates RM, Chappell J. 2005. Kinetic and molecular analysis of 5-epiaristolochene 1,3-dihydroxylase, a cytochrome P450 enzyme catalyzing successive hydroxylations of sesquiterpenes. Journal of Biological Chemistry 280, 3686–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase H, Sasaki K, Shinmori H, Shinohara A, Mochizuki C, Kobayashi H, Saito H, Matsuo H, Suzuki S, Takata R. 2015. Analysis of rotundone in japanese syrah grapes and wines using SBSE with heart-cutting two-dimensional GC/MS. American Journal of Enology and Viticulture 66, 398–402. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibon C, Shinkaruk S, Jourdes M, Bennetau B, Dubourdieu D, Tominaga T. 2010. Aromatic potential of botrytized white wine grapes: identification and quantification of new cysteine-S-conjugate flavor precursors. Analytica Chimica Acta 660, 190–196. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Darriet P, Dubourdieu D. 1996. Identification of 3-mercaptohexyl acetate in Sauvignon wine, a powerful aromatic compound exhibiting box-tree odor. Vitis 35, 207–210. [Google Scholar]
- Tominaga T, Furrer A, Henry R, Dubourdieu D. 1998a . Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon Blanc wines. Flavour and Fragrance Journal 13, 159–162. [Google Scholar]
- Tominaga T, Peyrot des Gachons C, Dubourdieu D. 1998b . A new type of flavor precursors in Vitis vinifera L. cv. Sauvignon Blanc: S-cysteine conjugates. Journal of Agricultural and Food Chemistry 46, 5215–5219. [DOI] [PubMed] [Google Scholar]
- Wilderman PR, Xu M, Jin Y, Coates RM, Peters RJ. 2004. Identification of syn-pimara-7,15-diene synthase reveals functional clustering of terpene synthases involved in rice phytoalexin/allelochemical biosynthesis. Plant Physiology 135, 2098–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhalter P, Rouseff R. 2002. Carotenoid-derived aroma compounds: an introduction. In: Winterhalter P, Rouseff R, eds. Carotenoid-Derived Aroma Compounds. Washington, DC: American Chemical Society, 1–19. [Google Scholar]
- Wood C, Siebert TE, Parker M, et al 2008. From wine to pepper: rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. Journal of Agricultural and Food Chemistry 56, 3738–3744. [DOI] [PubMed] [Google Scholar]
- Yu F, Okamoto S, Harada H, Yamasaki K, Misawa N, Utsumi R. 2011. Zingiber zerumbet CYP71BA1 catalyzes the conversion of alpha-humulene to 8-hydroxy-alpha-humulene in zerumbone biosynthesis. Cellular and Molecular Life Sciences 68, 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Barlow S, Krstic M, Herderich MJ, Fuentes S, Howell K. 2015. Within-vineyard, within-vine and within-bunch variability of rotundone concentration in berries of Vitis vinifera L. cv. Shiraz. Journal of Agricultural and Food Chemistry 63, 4276–4283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.