Highlight
Aluminium-induced nitric oxide production enhances the aluminium sensitivity of wheat by decreasing pectin methylation of root cell-wall pectin, resulting in greater aluminium binding in root cell walls.
Key words: Aluminium, cell wall, nitric oxide, pectin, pectin methylation, pectin methylesterase.
Abstract
Nitric oxide (NO) is an important bioactive molecule involved in cell wall metabolism, which has been recognized as a major target of aluminium (Al) toxicity. We have investigated the effects of Al-induced NO production on cell wall composition and the subsequent Al-binding capacity in roots of an Al-sensitive cultivar of wheat (Triticum aestivum L. cv. Yang-5). We found that Al exposure induced NO accumulation in the root tips. Eliminating NO production with an NO scavenger (cPTIO) significantly alleviated the Al-induced inhibition of root growth and thus reduced Al accumulation. Elimination of NO, however, did not significantly affect malate efflux or rhizosphere pH changes under Al exposure. Levels of cell wall polysaccharides (pectin, hemicelluloses 1, and hemicelluloses 2) and pectin methylesterase activity, as well as pectin demethylation in the root apex, significantly increased under Al treatment. Exogenous cPTIO application significantly decreased pectin methylesterase activity and increased the degree of methylation of pectin in the root cell wall, thus decreasing the Al-binding capacity of pectin. These results suggest that the Al-induced enhanced production of NO decreases cell wall pectin methylation, thus increasing the Al-binding capacity of pectin and negatively regulating Al tolerance in wheat.
Received 12 June 2015; Revised 16 October 2015; Accepted 2 November 2015
Introduction
Aluminium (Al) toxicity is a major factor limiting crop productivity in acidic soils, which account for around 30% of the world’s arable land and approximately 50% of the world’s potentially arable land (Kochian et al., 2004; Ma, 2007; Kochian et al., 2015). Furthermore, up to 60% of the acidic soils in the world are in developing countries, where food production is critical (Kochian et al., 2005; Liu et al., 2014). Although it has been shown that the strong binding affinity of Al to cell components can alter a series of physiological and biochemical processes, disrupt cytoskeleton dynamics, destruct plasma membrane integrity, and distort calcium-dependent signal cascades, the underlying physiological and molecular mechanisms of Al-induced root growth inhibition are still not well understood (Kochian, 1995; Matsumoto, 2000; Rengel and Zhang, 2003; Ma, 2007).
Accumulating evidence suggests that the cell wall plays pivotal roles in the perception and manifestation of Al toxicity in plants (Horst et al., 2010; Kochian et al., 2015). The cell wall is the first point of contact when plant roots are exposed to Al, and serves as a major pool for the metal. For instance, approximately 85% of the Al taken up by Zea mays (maize) roots accumulated in the cell wall (Wang et al., 2004), and more than 77% of total Al was located in the cell wall of root apices in Triticum aestivum (wheat; Ma et al., 2004). Al bound to the cell wall negatively affects wall structure and function by increasing the rigidity and reducing cell expansion and mechanical extensibility, thus inhibiting root elongation (Van et al., 1994; Tabuchi and Matsumoto, 2001; Ma et al., 2004; Yang et al., 2010). The major Al binding site in cell walls is generally the pectic polysaccharides, because their negatively charged carboxylic groups have a high affinity for Al (Chang et al., 1999; Schmohl and Horst, 2000). Al binds preferentially to unmethylated pectin, catalysed by the activity of pectin methylesterase (PME). Recent studies suggest that cell wall hemicellulose metabolism is also susceptible to Al stress (Zhu et al., 2012; Zhu et al., 2014). Although the function and alteration of cell wall polysaccharides in Al-stressed roots of different plant species have been well documented (Eticha et al., 2005; Horst et al., 2010), the signals involved in the regulatory cascade leading to the modification of cell wall polysaccharide composition are still not well understood.
Nitric oxide (NO), a redox-active signalling molecule, is an important endogenous signalling molecule in regulating synthesis of the cell wall (Correa-Aragunde et al., 2008; Xiong et al., 2009; Zhang et al., 2011; Ye et al., 2015). For example, NO affected the cellulose content in roots of Solanum lycopersicum (tomato) in a dose-dependent manner (Correa-Aragunde et al., 2008), and Xiong et al. (2009) found that an exogenous supply of NO increased the pectin and hemicellulose contents of root cell walls in Oryza sativa (rice). The responses and adaptations of plants to the stress of metals, including Al, have previously been associated with NO (Illéš et al., 2006; Tian et al., 2007; Xu et al., 2010; González et al., 2012; Leterrier et al., 2012; Sun et al., 2014). Several studies have found that disturbing the homeostasis of endogenous NO interferes with physiological processes preventing Al from entering the roots (Wang and Yang, 2005; Wang et al., 2010; Zhou et al., 2012). However, the corresponding physiological roles and molecular mechanisms of NO in increasing or decreasing Al accumulation in the root apex under Al toxicity remain elusive. Considering the role of the cell wall in Al toxicity and Al absorption, it is possible that NO may interfere with cell wall properties to affect their capacity to bind with Al. Here, we have investigated the effects of Al-induced NO production on cell wall composition and the subsequent Al-binding capacity of the cell wall in roots of wheat.
Materials and methods
Plant materials and treatment
Seeds of wheat (Triticum aestivum L. cv. Yang-5) were surface-sterilized with 1% (v/v) NaClO solution for 20min, and then rinsed thoroughly with deionized water. The seeds were germinated in the dark before being grown in 2.5L of 0.5mM CaCl2 solution (pH 4.3) in a growth chamber under a 12h/25°C day and 12h/22°C night regime, with a light intensity of 300 μmol m−2 s−1, and a relative humidity of 70%. The solution was renewed daily.
After 3 days of pre-treatment, uniform seedlings were transferred to 0.5mM CaCl2 (pH 4.3) that contained either 30 μM or 0 μM AlCl3 for another 24h. For experiments with NO scavenger treatment, 3-day-old seedlings were placed in a 0.5mM CaCl2 (pH 4.3) solution with 30 μM AlCl3 spiked with 30 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (cPTIO) for 24h. The concentration used in this study was based on preliminary experiments from which the maximum induced responses were obtained.
Evaluation of Al resistance in wheat
Root elongation and plasma membrane integrity in wheat roots were determined after 24 h of treatment. Root length was measured before and after treatments. Relative root elongation was calculated as the percentage elongation of the root under the various treatments as compared with the Al-free control. The plasma membrane integrity was evaluated using Evans Blue uptake (Yamamoto et al., 2001).
Determination of NO content
The endogenous levels of NO in roots were visualized using the fluorescent probe diaminofluorescein-FM diacetate (DAF-FM DA) and epifluorescence microscopy (Sun et al., 2014; Xu et al., 2015). Briefly, root tips (0–10mm) were loaded with 10 μM DAF-FM DA in 20mM HEPES-NaOH buffer (pH 7.4) for 20min, washed three times with fresh buffer, and observed under an epifluorescence microscope. Fluorescence intensity was measured with the open source software Image-J (http://rsb.info.nih.gov/ij/). NO production was expressed as root fluorescence density.
Collection of root exudates and organic acid assays
After treatment, root exudates were collected and purified according to Zheng et al. (2005). Briefly, collected exudates were first passed through a cation exchange column filled with 5g of Amberlite IR-120B (H+ form) resin, and then through an anion exchange column filled with 2g of Dowex 1X8 resin (100–200 mesh, formate form). Organic acids retained on the anion exchange resin were eluted with 15mL of 1M HCl, and the eluent was concentrated to dryness using a rotary evaporator at 40°C. The residue was re-dissolved in 1mL Milli-Q water and filtered (0.2 μm) before analysis. The concentration of malate was analysed by HPLC (Agilent 1100, USA). The mobile phase was 0.5% KH2PO4 (pH 2.0) at a flow rate of 1mL min−1 and the detection wavelength was at 220nm.
Scanning electron microscope-energy dispersive X-ray microanalysis
Six root apexes (0–10mm) were excised and fixed in 2.5% (v/v) glutaraldehyde in 0.2M sodium phosphate (NaH2PO4/Na2HPO4) buffer (pH 7.2) overnight, and post-fixed in 1% (w/v) OsO4 for 2h. The specimen was dehydrated in a graded ethanol series (30–100%; v/v), followed by a mixture of alcohol and isoamyl acetate (v:v = 1:1) for about 30min, and then transferred to pure isoamyl acetate overnight. The sample was dried in a Hitachi Model HCP-2 critical point dryer with liquid CO2. Root samples were observed under a Hitachi S-3400 SEM with an energy-dispersive X-ray spectrometer (EDS).
Cell wall extraction and polysaccharide measurement
Cell wall materials were extracted according to Yang et al. (2011). Frozen root apexes (0–10mm) samples were thoroughly homogenized with 75% ethanol. The homogenate was kept undisturbed in ice-water for 20min. The homogenate was then centrifuged at 8000g for 10min at 4°C, and the pellets were washed for 20min each with acetone, methanol:chloroform mixture (1:1, v/v), and methanol. The supernatant was discarded and the pellet was freeze-dried.
Cell wall materials were fractionated into three fractions: pectin, hemicellulose 1 (HC1), and hemicellulose 2 (HC2). The pectin fraction was extracted twice by 0.5% (NH4)2C2O (ammonium oxalate) buffer containing 0.1% NaBH4 (pH 4) in a boiling water bath for 1h. Pellets were subsequently subjected to triple extractions with 4% KOH containing 0.1% NaBH4 at room temperature for a total of 24h, followed by extraction with 24% KOH containing 0.1% NaBH4. The pooled supernatants from the 4% and 24% KOH extractions thus yielded the HC1 and HC2 fractions, respectively. The uronic acid content in each cell wall fraction was assayed. Galacturonic acid (GalA) was used as a calibration standard and the root pectin, HC1, and HC2 contents were expressed as GalA equivalents.
Al content measurement
Total Al content in root apexes (0–10mm) was analysed according to Osawa and Matsumoto (2001). Briefly, excised root apices were digested with 10mL of 2M HCl. The samples were digested for at least 24h with occasional shaking.
The apoplastic and symplastic Al fractions in the root tips were collected according to the method described by Yu et al. (1999) and modified by Wang et al. (2004). Briefly, freshly excised 1-cm root tips from 20 seedlings were arranged in a filter unit (Ultrafree-MC, 0.45 μm; Millipore, Bedford, MA, USA) with the cut ends facing down. The water free-space fluid (WFSF) was collected by centrifugation at 3000g at 4°C for 15min. After collecting the WFSF, the root tips were frozen at −20°C. The symplastic 1 fraction was recovered from the frozen-thawed samples by centrifugation at 3000g at 4°C for 15min. The residue was washed with 70% ethanol twice, and the combined supernatant represented the symplastic 2 fraction. The residual cell wall material was then immersed in 2M HCl for at least 24h with occasional vortexing.
The Al content in pectin was determined according to Yang et al. (2011). In order to avoid the chelation of Al by oxalate, cell wall material (50mg) underwent extraction for pectin twice for 1h using hot water, which showed an extraction efficiency similar to ammonium oxalate buffer (Yang et al., 2011). The pellet (cell wall without pectin) was immersed in 2M HCl for at least 24h with occasional vortexing. The Al content of the pectin fraction was calculated by subtracting the Al content of the cell wall without pectin from the Al content of the cell wall.
The Al concentrations in the above extracts were determined on an Agilent 7500A ICP-MS (Agilent, Palo Alto, CA, USA). Al accumulation in root apexes was also detected by hematoxylin staining as described by Yamamoto et al. (2001).
Determination of degree of methylation of pectin
Cell wall material from wheat root apexes (0–10mm) was prepared the same way as for pectin determination. Ten millilitres of 1M KOH were added to aliquots of the pectin fraction to give 15mL of pectin solutions. The pectin hydrolysates were neutralized with dilute H3PO4 to pH 7.5 and adjusted to 20mL with ultrapure water. Hydrolysed pectin samples (1mL) were mixed with 1mL alcohol oxidase (1 units mL−1) and incubated at 25°C for 15min. Then, 2mL of fluoral-P (0.02M 2,4-pentanedione in 2.0M ammonium acetate and 0.05M acetic acid) was added and vortexed. The mixtures were incubated at 60°C for 15min and then cooled to room temperature. Methanol that was released from the cell wall material was measured by fluorometry (Klavons and Bennett, 1986).
Immunofluorescence
Immunofluorescence localization of cell wall pectin was performed using specific monoclonal antibodies according to Yang et al. (2008). After treatment, fresh roots were cut into thin cross-sections with a freezing microtome (SLEE MTC, Germany) from root zone 1 to 3mm behind the apex, and directly fixed in 4% paraformaldehyde in 50mM PIPES, 5mM MgSO4, and 5mM EGTA, pH 6.9. After 2h of fixation at room temperature, the samples were washed repeatedly with phosphate-buffered saline (PBS, pH 7.4) and blocked with 0.2% bovine serum albumin in PBS for 30min. Then the samples were incubated for 2h with the monoclonal antibodies JIM5 (specifically labels low methylesterified pectin) and JIM7 (specifically labels high methylesterified pectin), diluted 1:10 in PBS, followed by incubation with goat anti-rat IgG (whole molecule) fluorescein isothiocyanate conjugate. Finally, the samples were diluted 1:50 in PBS and left for 2h at 37°C. Samples were washed briefly with PBS three times and imaged.
PME activity assay
For extraction of PME, root apexes (0–10mm) were homogenized and suspended in 1M NaCl solution (pH 6.0). Extracts were centrifuged at 23 000g for 10min at 4°C and the supernatant was collected. PME activity was measured according to Anthon and Barrett (2004). An incubation solution was prepared, with 100 μL of 200mM PBS containing 0.64mg mL−1 of pectin, 10 μL of alcohol oxidase at 0.001 units μL−1 , and 50 μL of the PME sample. Samples were incubated for 10min at 30°C, and then 200 μL of a 0.5M NaOH solution containing 5mg mL−1 Purpald was added. After incubation at 30°C for 30min, 550 μL of water was added to give a final volume of 1.0mL. The absorbance at 550nm was measured with a spectrophotometer (Lambda 35; PerkinElmer, Waltham, MA, USA).
Characterization of TaALMT1 expression
The expression of TaALMT1 was determined by real-time quantitative reverse transcription PCR (qRT-PCR). Briefly, total RNA was extracted from 100mg of fresh-weight wheat seedling root apexes (0–10mm) using Trizol reagent according to the manufacturer’s protocol (Life Technologies, Rockville, MD, USA). One microgram of total RNA from each sample was reverse-transcribed into first-strand cDNA with a PrimeScript II 1st Strand cDNA Synthesis Kit (Takara, Dalian, Liaoning, China) according to the manufacturer’s protocol. The first-strand cDNA was used for SYBR Green-monitored qRT-PCR (Takara). The qRT-PCR analysis was performed using the MJ Opticon™ 2 Real-Time PCR System (MJ Research). The primer pairs used for real-time PCR analysis for TaALMT1 were those used by Tian et al. (2014), i.e. 5′-AAGAGCGTCCTTAATTCG-3′ and 5′-CCTTACATGATAGCTCAGGG-3′, and for the housekeeping gene TaActin were 5′-CTATCCTTCGTTTGGACCTT-3′ and 5′-GCGAGCTTCTCCTTTATGT-3′. The expression of TaALMT1 was calculated from the relative expression levels of TaALMT1 and the expression levels of the reference gene TaActin using arbitrary units. Three biological and three technological repeats were performed in RT-PCR. The relative expression level was analysed by the comparative CT method.
Statistical analysis
All data were statistically analysed using the SPSS package (version 11.0; SPSS Inc., Chicago, IL, USA); ANOVA was performed on the data sets, and the mean and SD of each treatment as well as least significant difference (LSD; P < 0.05 and P < 0.01) for each set of corresponding data were calculated. The figures were drawn using the software Origin 8.0 (Origin Lab Corporation, Northampton, MA, USA).
Results
Effect of Al and cPTIO on NO production, root elongation, and Evans Blue uptake
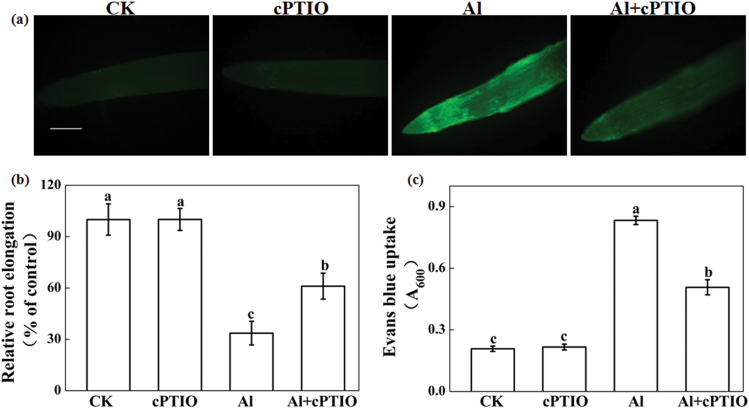
After treatment with 30 μM Al for 24h, a higher concentration of NO was observed in root tips of wheat (Fig. 1a), similar to that observed in our previous study (Sun et al., 2014). In root tips treated with Al plus cPTIO, an NO scavenger, significantly decreased NO content was noted when compared with those treated with Al alone (Fig. 1a). Application of cPTIO significantly alleviated Al-induced root inhibition (Fig. 1b) and Evans Blue uptake (Fig. 1c). Root elongation of wheat under treatment of Al + cPTIO was 61% of the control values, but only 34% of the control values for the Al treatment alone. Similarly, cPTIO application significantly reduced Evans Blue uptake induced by Al stress. In addition, Al treatment strongly induced callose production, which is a typical indicator of Al phytotoxicity. cPTIO application significantly reduced Al-induced callose deposition (see Supplementary Fig. S1 at JXB online).
Fig. 1.
Effect of the NO scavenger cPTIO on NO content, root elongation, and Evans Blue uptake of wheat seedling roots with or without Al exposure. Three-day-old seedlings were treated with 30 μM Al and with or without 30 μM cPTIO for 24h. (a) Detection of NO fluorescence using DAF-FM DA staining and fluorescence microscopy (n = 10). Scale bar, 1mm. (b) Root elongation was expressed relative to root elongation in control solutions of 0.5mM CaCl2, pH 4.3. Means ± SD (n = 20). (c) Seedling 10-mm root tips collected after 24h treatment were used to determine Evans Blue uptake. CK, 0.5mM CaCl2; cPTIO, 0.5mM CaCl2 + 30 μM cPTIO; Al, 0.5mM CaCl2 + 30 μM AlCl3; Al + cPTIO, 0.5mM CaCl2 + 30 μM AlCl3 + 30 μM cPTIO. Means ± SD (n = 3). Different letters indicate significant differences (P < 0.05) among the treatments.
Effect of cPTIO on Al accumulation in root apexes
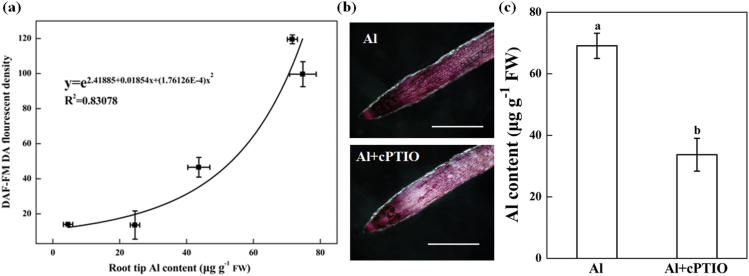
NO generation was positively correlated with root tip Al accumulation (P < 0.05; Fig. 2a). cPTIO treatment of the roots in the presence of Al reduced the Al content of the root tips as demonstrated by staining of root apices with hematoxylin (Fig. 2b) and quantification of Al content (Fig. 2c).
Fig. 2.
Relationship (P < 0.05) between Al accumulation and NO production. Three-day-old wheat seedlings were treated with 30 μM Al and with or without 30 μM cPTIO and 10-mm root tips were collected after 3, 6, 12, and 24h. (a) Correlation analysis of Al concentrations and NO production after 3, 6, 12, and 24h Al exposure. (b) Histochemical detection of Al accumulation by hematoxylin staining in the root apices after 24h of Al treatment. Scale bar, 0.5cm. (c) Al concentrations in root apices after 24h Al exposure.
Effect of Al and cPTIO on malate efflux and root surface pH changes
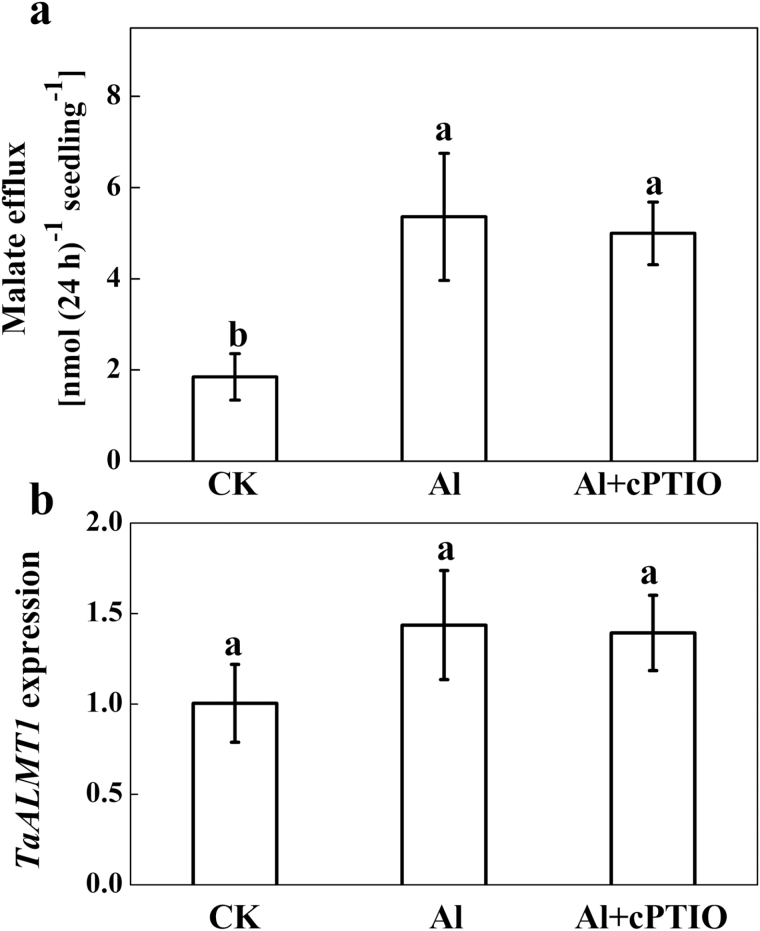
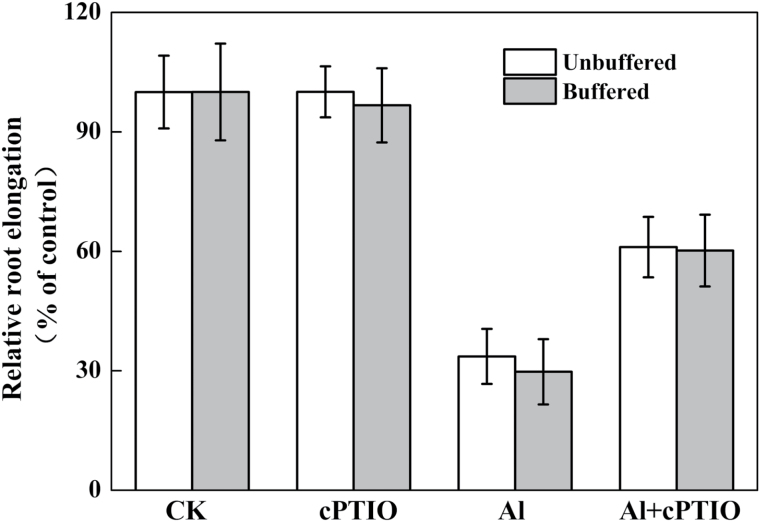
To determine whether the decreased Al accumulation after cPTIO treatment was due to Al-induced malate secretion in wheat as demonstrated by Delhaize et al. (1993), malate was quantified in the root exudates (Fig. 3a). Al treatment enhanced malate efflux, but treatment with cPTIO had no effect on Al-induced malate efflux. However, the slightly, but not significantly, enhanced expression of TaALMT1 after exposure to Al was not responsive to cPTIO application (Fig. 3b). These results indicate that the decreased Al accumulation in root apices after cPTIO treatment did not result from an increased malate efflux. Furthermore, the effect of Al and cPTIO on root elongation was independent of the buffering of the solution at pH 4.3 with MES according to Zhu et al. (2013) (Fig. 4).
Fig. 3.
Effect of the NO scavenger cPTIO on Al-induced root malate exudation and TaALMT1 expression in roots. Three-day-old seedlings were exposed to a 30 μM Al solution containing 0 or 30 μM cPTIO for 24h. (a) Root exudates were collected after 24h exposure and malate was analysed by HPLC. (b) Root apices (0–10mm) were collected. The relative expression of TaALMT1in 10-mm root apices was determined by qRT-PCR. Means ± SD (n = 3) with different letters are significantly different at P < 0.05.
Fig. 4.
Effect of the NO scavenger cPTIO on Al-induced root growth inhibition. Three-day-old seedlings were exposed to 0.5mM CaCl2 solution containing 0 or 30 μM Al with or without 10mM MES in the presence or absence of 30 μM cPTIO for 24h. The pH was adjusted to 4.3. Means ± SD (n = 20).
Effect of Al and NO scavenger on cell wall composition
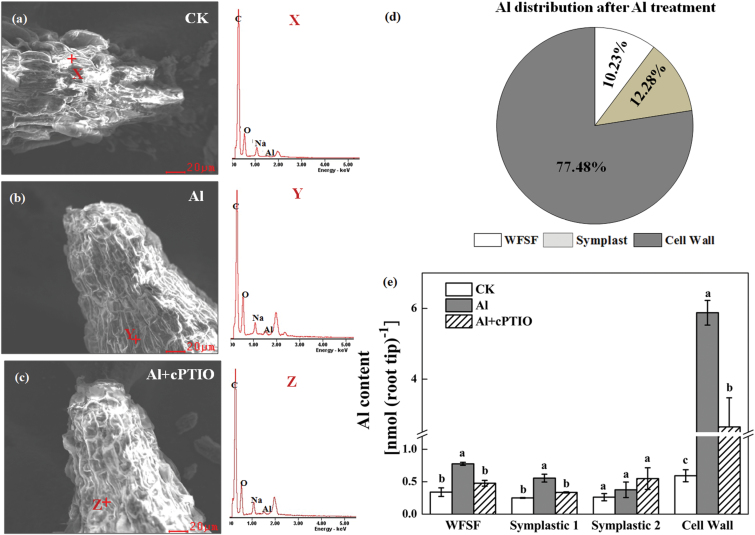
Based on the SEM-EDS images shown in Fig. 5a–c, carbon and oxygen were the most abundant elements within the cell wall and Al was absorbed alongside oxygen, which indicates that Al absorption may be determined by the oxygen-containing functional groups within the surface of the root tips. Furthermore, the EDS spectrum data confirmed that cPTIO treatment significantly decreased Al content in roots of wheat under Al stress (Fig. 5b, c). Because determining the total Al content of root tips does not reveal the cellular distribution, the Al content in different fractions of the apical 1-cm root tips could not be determined (Fig. 5d, e). Under Al treatment, only a little Al was found in the symplastic fraction; however, more than 75% of the Al taken up by wheat roots accumulated in the cell wall (Fig. 5d). Application of the NO scavenger cPTIO significantly decreased cell wall Al content (Fig. 5e).
Fig. 5.
SEM-EDS spectra and Al compartmentation in wheat root apices (10mm). Representative images of SEM and EDS spectra without Al (a), with Al (b), and with Al and cPTIO treatment (c) are given. Relative distribution of Al (d) and Al contents (e) of cell walls, symplast, and WFSF. Wheat seedlings were exposed to 0.5mM CaCl2 solution containing 30 μM Al with or without 30 μM cPTIO for 24h. The relative distribution of Al was only calculated for roots treated with Al alone. Data are means ± SD (n = 3). Means with different letters are significantly different at P < 0.05.
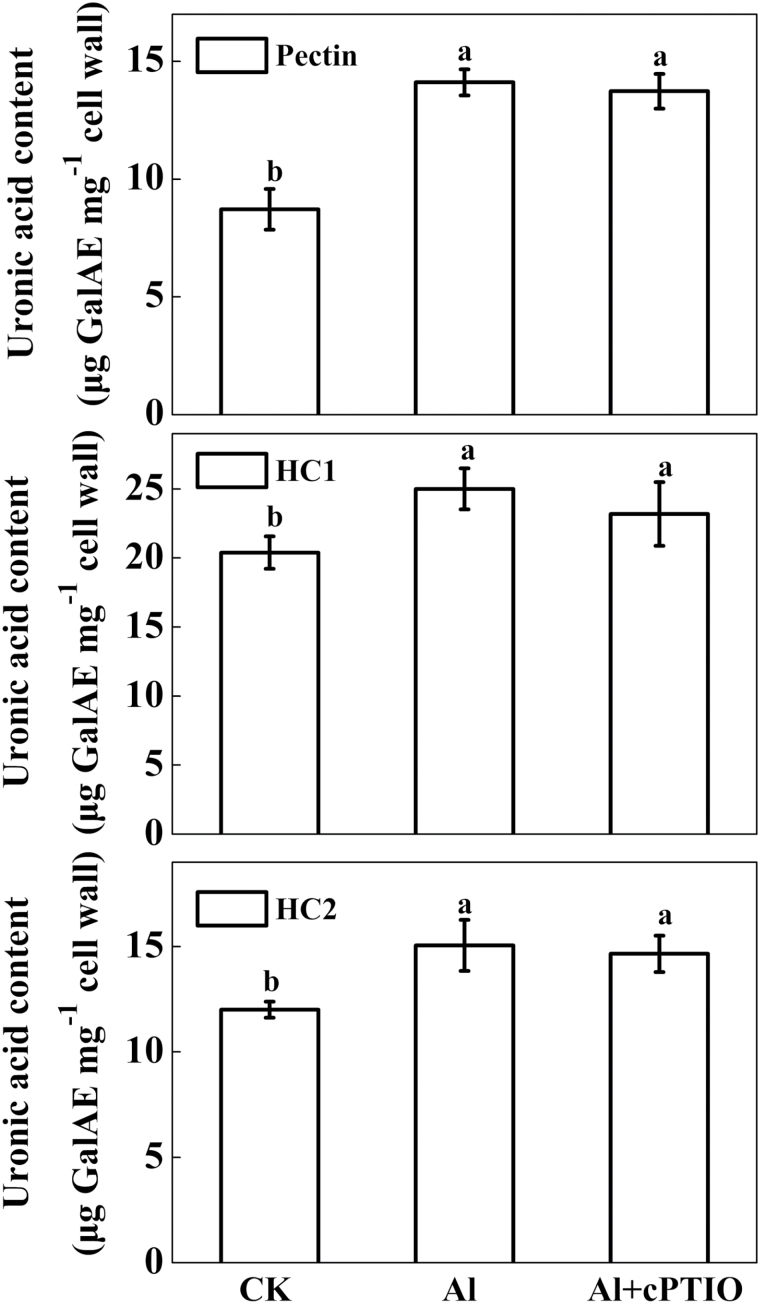
The uronic acid content of the cell wall polysaccharides pectin, HC1, and HC2 significantly increased under Al treatment compared with the control without an Al supply, independent of treatment with the NO scavenger cPTIO (Fig. 6).
Fig. 6.
Uronic acid content of cell wall fractions extracted from the root apex of wheat. Three-day-old seedlings were exposed to 0.5mM CaCl2 solution containing 0 or 30 μM Al with or without 30 μM cPTIO for 24h. Cell wall polysaccharides in 10-mm root apices were fractionated into pectin, HC1, and HC2 before measurement of uronic acid content, expressed as galacturonic acid equivalents (GalAE). Means ± SD (n = 3). Bars with different letters are significantly different at P < 0.05.
Effect of Al and NO scavenger on pectin methylation
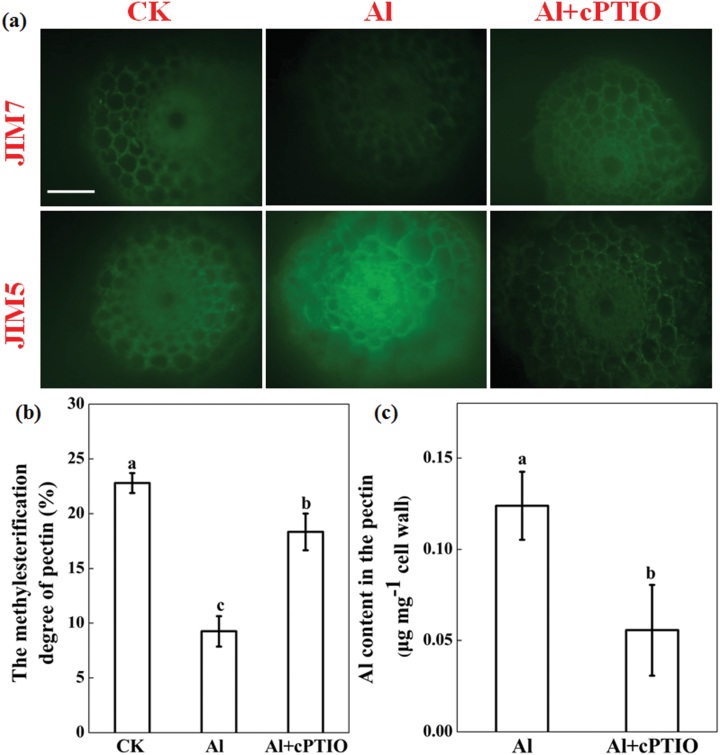
The monoclonal antibodies JIM5 (specifically labels low methylesterified pectin) and JIM7 (specifically labels high methylesterified pectin) were used for immunofluorescence localization of cell wall pectin. As shown in Fig. 7a, Al treatment led to decreased fluorescence of JIM7, but increased fluorescence of JIM5. Contrary to Al treatment, cPTIO application increased JIM7 fluorescence and decreased JIM5 fluorescence. The degree of pectin methylation in Al-treated seedlings decreased to 40% of that without Al treatment, and treatment with cPTIO greatly restored this Al-induced decrease of pectin methylation (Fig. 7b). Depletion of endogenous NO by cPTIO strongly reduced the amount of Al in the cell wall pectin fraction compared to Al treatment alone. However, there was no difference in Al accumulation in cell wall HC1 between the wheat seedlings treated with Al and those treated with Al + cPTIO (see Supplementary Fig. S3 at JXB online). These results suggest that the greater Al accumulation in the cell wall could be attributed to Al-induced increased demethylation of pectin by NO.
Fig. 7.
Effect of Al and cPTIO on the degree of pectin methylation and cell wall pectin Al content. Three-day-old wheat seedlings were treated with 0.5mM CaCl2 solution containing 0 or 30 μM Al with or without 30 μM cPTIO for 24h. (a) Immunolocalization of high methylesterified pectin (JIM7) and low methylesterified pectin (JIM5) in cross-sections of 10-mm root apices. Scale bars, 50 μm. (b) Degree of methylation of cell wall pectin extracted for root apices. (c) Al content of cell wall pectin. Means ± SD (n = 3). Means with different letters are significantly different at P < 0.05.
Effect of Al and NO scavenger on PME activity
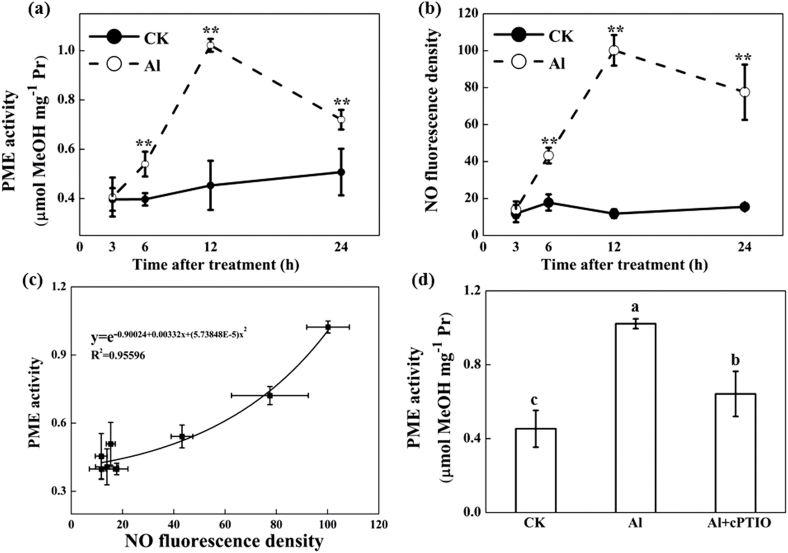
Al treatments resulted in a significant increase in PME activity after 6h of treatment in comparison with no Al treatment, which peaked at 12h (Fig. 8a). A similar pattern was observed in NO production by labelling endogenous NO using DAF-FM DA (Fig. 8b). Correlation analysis suggested that NO generation was positively correlated with PME activity (Fig. 8c). cPTIO application significantly decreased PME activity induced by Al in the root tips (Fig. 8d).
Fig. 8.
Effect of Al and cPTIO on PME activity and NO content in 10-mm root apices. Three-day-old wheat seedlings were treated with 0.5mM CaCl2 solution containing 0 or 30 μM Al with or without 30 μM cPTIO. PME activity (a), NO production (b), and the relationship between NO production and PME activity (c) after 3, 6, 12, and 24h treatment with or without Al. (d) PME activity after 24h treatment with and without Al and cPTIO. For quantification of NO production, root tips were loaded with 10 μM DAF-FM DA in 20mM HEPES-NaOH buffer (pH 7.4) and NO fluorescence was imaged after 20min. Images were analysed with Image-J and NO production was expressed as root fluorescence density. Means ± SD (n = 10). ** in (a) and (b) indicate significant difference between Al and control treatments at P < 0.01.
Discussion
Stress-induced NO may be endogenously produced and plays specific roles in plant responses to stress depending on the time and intensity of the NO produced (Floryszak-Wieczorek et al., 2007; Puyaubert and Baudouin, 2014; Sun et al., 2014). In addition to being a signalling molecule, it has been suggested that NO could also promote cytotoxic actions when produced at higher concentrations under stress conditions (Valderrama et al., 2007; Besson-Bard et al., 2008; Leterrier et al., 2012). Our previous studies suggested that an early NO burst at 3h plays an important role in Al resistance in root tips of Al-tolerant wheat genotype Jian-864 by modulating an enhanced antioxidant defence to adapt to Al stress (Sun et al., 2014; Sun et al., 2015), whereas the lack of NO accumulation at 3h but an extremely high NO concentration after 12h was noted in root tips of the Al-sensitive genotype Yang-5 (Sun et al., 2014). The possible mechanisms involved in Al-induced high NO and its association with Al sensitivity in roots of Yang-5, however, are unknown. Results from the present study with the genotype Yang-5 show that Al significantly increased NO production and strongly impaired the root elongation and plasma membrane integrity of root tips, which was significantly reverted after treatment with the NO scavenger cPTIO (Fig. 1). These results, similar to those reported by Chen et al. (2014) on Medicago sativa (alfalfa), wheat, rice, and tomato, suggest that Al-induced high NO production contributes to Al sensitivity in root elongation in wheat.
Al accumulation in the root apex is in many cases directly related to Al toxicity (Ma, 2007; Liu et al., 2014; Kochian et al., 2015). Our previous study showed that the Al content of the root apex was significantly higher in the sensitive wheat genotype Yang-5 compared to the Al-tolerant genotype Jian-864 (Sun et al., 2014), indicating that differing Al accumulation in the root apex might cause the variation in Al sensitivity between Yang-5 and Jian-864. In this study, we found that NO concentration was positively correlated with root tip Al accumulation. Elimination of NO by cPTIO significantly decreased Al accumulation in the root apex of wheat (Fig. 2). It is therefore possible that NO regulates Al accumulation in root tips of wheat, and subsequently increases the sensitivity of the wheat cultivar Yang-5 to Al stress. It is well documented that (i) Al-activated malate efflux (Delhaize et al., 1993; Tian et al., 2014) and (ii) alkalinization of the rhizosphere (Wang et al., 2006) play important roles in excluding Al from wheat roots. These mechanisms, however, do not account for our finding that cPTIO prevented Al from entering wheat roots by eliminating NO. First, the Al-sensitive wheat genotype Yang-5 secreted small amounts of malate after Al exposure, and the cPTIO-treated plants secreted as much malate during the Al treatment as those treated with Al alone (Fig. 3). Wang and Yang (2005) also reported that the application of exogenous NO to the culture medium failed to induced exudation of additional organic acids as compared with Al treatment alone. Second, by buffering the solutions to pH 4.3 with MES, we excluded the involvement of alkalinization of the rhizosphere in the alleviation of Al rhizotoxicity by cPTIO (Fig. 4). These results suggest that neither malate efflux nor changes in rhizosphere pH were responsible for the reduced Al accumulation occurring after NO elimination by cPTIO.
Our investigation also suggests that decreased Al accumulation in wheat roots by cPTIO did not result from decreased cell wall polysaccharide content. The cell wall is the major site of Al accumulation and plays pivotal roles in the manifestation and perception of Al toxicity in plants (Horst et al., 2010; Kochian et al., 2015). It has been reported that pectic polysaccharides and hemicelluloses are the two major components to bind Al in the cell wall (Eticha et al., 2005; Yang et al., 2008; Horst et al., 2010; Yu et al., 2015). The binding of Al may change cell wall structure, making it more rigid, and reducing cell expansion and mechanical extensibility, thus inhibiting root elongation (Ma et al., 2004; Kochian et al., 2005; Ma, 2007; Kopittke et al., 2015). In this study, we found that Al exposure induced a significant increase of uronic acid content in cell fractions, including pectin and hemicelluloses (Fig. 6). In addition, a high percentage (>70%) of the total Al accumulated by wheat roots was tightly bound to cell walls (Fig. 5d). Furthermore, a direct measurement of Al content in different cell wall components revealed that Al accumulated in the cell wall mainly in the pectin and HC1 fractions (see Supplementary Fig. S2 at JXB online), which was consistent with previous results found in a number of other plant species, such as wheat, maize, and rice (Tabuchi and Matsumoto, 2001; Eticha et al., 2005; Yang et al., 2008). It has been reported that pre-treatment of rice with exogenous NO decreased root cell wall pectin and hemicellulose content, and thus the binding of Al in root cell walls, and alleviated Al-induced inhibition of root elongation (Zhang et al., 2011). However, in the present study, depletion of Al-induced endogenous NO had no effect on cell wall polysaccharide content (Fig. 6), indicating that overall content was not involved in the decreased accumulation of Al in wheat roots. It is possible that the discrepancy between the studies may be attributed to different NO concentrations, because NO affects cell wall components in a dose-dependent manner (Correa-Aragunde et al., 2008).
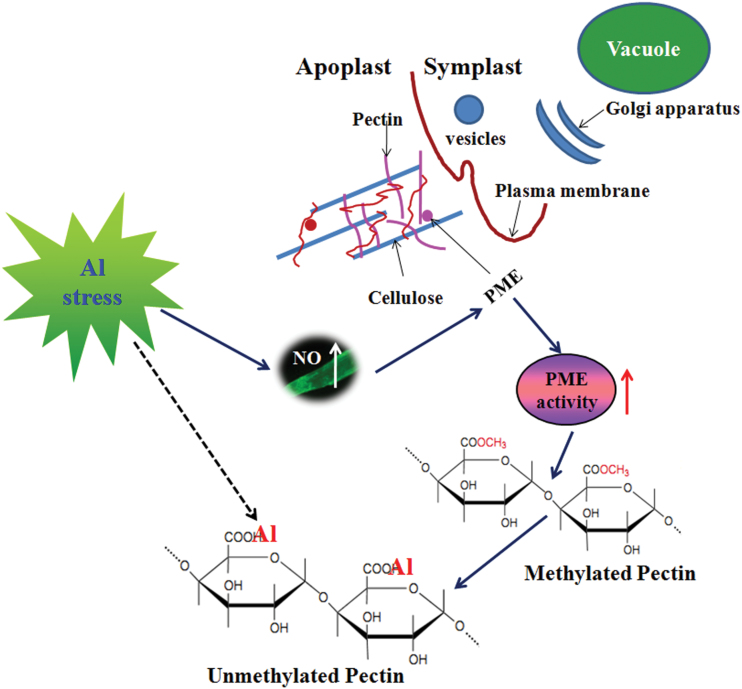
Depletion of endogenous NO acts to decrease Al accumulation in the cell wall, mainly by modulating the enhanced methylation of pectin. Pectic polysaccharides and hemicelluloses, in particular xyloglucan, in the walls are the two major components to bind Al in the wall (Zhu et al., 2014). Depletion of Al-induced endogenous NO by cPTIO significantly decreased pectin Al accumulation but had no effect on Al accumulation in HC1 (see Supplementary Fig. S3 at JXB online), indicating that endogenous NO affects the Al-binding capacity of the cell wall, probably by interfering with pectin structure but not HC1. Pectins are secreted into the wall as highly methylated and then undergo partial apoplastic demethylation processes through the action of PME, resulting in the exposure of free pectic carboxylic groups, which could serve as binding sites for Al in the cell wall (Zhu et al., 2014). Schmohl and Horst (2000) reported that the Al sensitivity of maize cell suspension cultures was negatively related to the degree of pectin methylation. In the present study, Al exposure significantly increased PME activity (Fig. 8a). Using immunofluorescence localization of pectin with two types of antibodies, the spatial distribution of both low-methylesterified and high-methylesterified pectin was determined. The low-methylesterified pectin was significantly enhanced in the epidermis and vascular tissues in Yang-5 root tips after Al exposure (Fig. 7a), and this was consistent with previous observations on an Al-sensitive rice cultivar (Yang et al., 2008). These results support the hypothesis that Al decreases the methylation level of pectin, and consequently results in higher Al binding in the cell wall, which is in agreement with results reported in maize and rice (Eticha et al., 2005; Yang et al., 2008). However, visualization of cells under the microscope indicated a much higher intensity of low-methylesterified pectin in the vascular regions after Al exposure as compared with the controls, although in Al excluders like wheat Al is not readily transported into the central cylinder. This could be due to the systemic effect of Al on NO formation. In agreement with the immunofluorescence localization of pectin, depletion of endogenous NO resulted in significant decreases in PME activity (Fig. 8c), leading to a significantly increased degree of methylation of pectin and less Al binding in pectin (Fig. 7). These results suggest that the decreased accumulation of Al in root tips after cPTIO treatment was the result of a decreased degree of cell wall pectin demethylation. Results from reinforcing NO production in the root tips of Vigna umbellata (rice bean) under Al stress provided additional evidence that increased NO enhances PME activity and pectin demethylation, and ultimately increases the accumulation of Al in cell wall (Zhou et al., 2012). Based on the results of our study, we propose a model to illustrate the linkage of Al-induced NO on pectin methylation as well as to Al sensitivity (Fig. 9). Al-induced increased endogenous NO accumulation removes methyl groups from pectin by activating PME, and causes enhanced Al binding to unmethylated carboxyl groups that have a high affinity for Al.
Fig. 9.
Schematic illustration of a proposed model illustrating the linkage of Al-induced NO production on pectin methylation as well as on Al binding to pectin. Al-enhanced NO production increases apoplastic PME activity, which decreases the methylation of cell wall pectin, thus increasing Al-binding capacity.
In summary, our study reveals a negative role of Al-induced NO production in response to Al stress in roots of the Al-sensitive T. aestivum genotype Yang-5. The mechanistic basis of the process is presumably through increased NO-regulated PME activity and thus decreased pectin methylation of the cell wall. Consequently, Al is able to target unmethylated pectin more easily and more Al is bound in the cell walls in this plant species.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Effects of cPTIO on the Al-induced callose production in root apexes of Yang-5.
Fig. S2. Al contents in different cell wall components.
Fig. S3. Effect of cPTIO on Al accumulation of different cell wall polysaccharides in roots of Yang-5.
Acknowledgements
We are grateful to Prof. Dr. Tingqiang Li (Zhejiang University, China) for kindly donating the specific pectin monoclonal antibodies JIM5 and JIM7. This work was financially supported by the National Basic Research Programme (973 Programme) of China (No. 2013CB127403), National Natural Science Foundation of China (31272237, 30771292), and the Foundation for University PhD Granting Discipline of the Ministry of Education (20120101110130) and IPNI.
Glossary
Abbreviations:
- Al,
Aluminium;
- NO,
Nitric oxide;
- cPTIO,
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide;
- DAF-FM DA,
Diaminofluorescein-FM diacetate;
- PME,
Pectin methylesterase;
- HC1,
Hemicellulose 1;
- HC2,
Hemicellulose 2;
- GalA,
Galacturonic acid.
References
- Anthon GE, Barrett DM. 2004. Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. Application to the assay of pectin methylesterase. Journal of Agricultural and Food Chemistry 52, 3749–3753. [DOI] [PubMed] [Google Scholar]
- Besson-Bard A, Pugin A, Wendehenne D. 2008. New insights into nitric oxide signaling in plants. Annual Review of Plant Biology 59, 21–39. [DOI] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H. 1999. Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant, Cell and Environment 22, 1009–1017. [Google Scholar]
- Chen M, Cui W, Zhu K, Xie Y, Zhang C, Shen W. 2014. Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. Journal of Hazardous Materials 267, 40–47. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Lombardo C, Lamattina L. 2008. Nitric oxide: an active nitrogen molecule that modulates cellulose synthesis in tomato roots. New Phytologist 179, 386–396. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. 1993. Aluminum tolerance in wheat (Triticum aestivum L.)(II. Aluminum-stimulated excretion of malic acid from root apices). Plant Physiology 103, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ. 2005. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell and Environment 28, 1410–1420. [Google Scholar]
- Floryszak-Wieczorek J, Arasimowicz M, Milczarek G, Jelen H, Jackowiak H. 2007. Only an early nitric oxide burst and the following wave of secondary nitric oxide generation enhanced effective defence responses of pelargonium to a necrotrophic pathogen. New Phytologist 175, 718–730. [DOI] [PubMed] [Google Scholar]
- González A, de los Ángeles Cabrera M, Henríquez MJ, Contreras RA, Morales B, Moenne A. 2012. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiology 158, 1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ, Wang Y, Eticha D. 2010. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany 106, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illéš P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovečka M. 2006. Aluminium toxicity in plants: internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. Journal of Experimental Botany 57, 4201–4213. [DOI] [PubMed] [Google Scholar]
- Klavons JA, Bennett RD. 1986. Determination of methanol using alcohol oxidase and its application to methyl ester content of pectins. Journal of Agricultural and Food Chemistry 34, 597–599. [Google Scholar]
- Kochian L, Piñeros M, Liu J, Magalhaes J. 2015. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annual Review of Plant Biology 66, 23.1–23.28. [DOI] [PubMed] [Google Scholar]
- Kochian LV. 1995. Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Biology 46, 237–260. [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology 55, 459–493. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Pineros MA, Hoekenga OA. 2005. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant and Soil 274, 175–195. [Google Scholar]
- Kopittke PM, Moore KL, Lombi E, Gianoncelli A, Ferguson BJ, Blamey FPC, Menzies NW, Nicholson TM, McKenna BA, Wang P. 2015. Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiology 167, 1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier M, Airaki M, Palma JM, Chaki M, Barroso JB, Corpas FJ. 2012. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis . Environmental Pollution 166, 136–143. [DOI] [PubMed] [Google Scholar]
- Liu J, Piñeros MA, Kochian LV. 2014. The role of aluminum sensing and signaling in plant aluminum resistance. Journal of Integrative Plant Biology 56, 221–230. [DOI] [PubMed] [Google Scholar]
- Ma JF. 2007. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. International Review of Cytology 264, 225–252. [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen R, Nagao S, Tanimoto E. 2004. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant and Cell Physiology 45, 583–589. [DOI] [PubMed] [Google Scholar]
- Matsumoto H. 2000. Cell biology of aluminum toxicity and tolerance in higher plants. International Review of Cytology 200, 1–46. [DOI] [PubMed] [Google Scholar]
- Osawa H, Matsumoto H. 2001. Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiology 126, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyaubert J, Baudouin E. 2014. New clues for a cold case: nitric oxide response to low temperature. Plant, Cell and Environment 37, 2623–2630. [DOI] [PubMed] [Google Scholar]
- Rengel Z, Zhang WH. 2003. Role of dynamics of intracellular calcium in aluminium‐toxicity syndrome. New Phytologist 159, 295–314. [DOI] [PubMed] [Google Scholar]
- Schmohl N, Horst W. 2000. Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant, Cell and Environment 23, 735–742. [Google Scholar]
- Sun C, Liu L, Yu Y, Liu W, Lu L, Jin C, Lin X. 2015. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. Journal of Integrative Plant Biology 57, 550–561. [DOI] [PubMed] [Google Scholar]
- Sun C, Lu L, Liu L, Liu W, Yu Y, Liu X, Hu Y, Jin C, Lin X. 2014. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytologist 201, 1240–1250. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Matsumoto H. 2001. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminum-induced growth inhibition. Physiologia Plantarum 112, 353–358. [DOI] [PubMed] [Google Scholar]
- Tian QY, Sun DH, Zhao MG, Zhang WH. 2007. Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos . New Phytologist 174, 322–331. [DOI] [PubMed] [Google Scholar]
- Tian Q, Zhang X, Ramesh S, Gilliham M, Tyerman SD, Zhang WH. 2014. Ethylene negatively regulates aluminium-induced malate efflux from wheat roots and tobacco cells transformedwith TaALMT1 . Journal of Experimental Botany 65, 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderrama R, Corpas FJ, Carreras A, Fernández-Ocaña A, Chaki M, Luque F, Gómez-Rodríguez MV, Colmenero-Varea P, del Río LA, Barroso JB. 2007. Nitrosative stress in plants. FEBS Letters 581, 453–461. [DOI] [PubMed] [Google Scholar]
- Van HL, Kuraishi S, Sakurai N. 1994. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiology 106, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Huang JJ, Bi YR. 2010. Nitrate reductase-dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Science 179, 281–288. [Google Scholar]
- Wang P, Bi S, Ma L, Han W. 2006. Aluminum tolerance of two wheat cultivars (Brevor and Atlas66) in relation to their rhizosphere pH and organic acids exuded from roots. Journal of Agricultural and Food Chemistry 54, 10033–10039. [DOI] [PubMed] [Google Scholar]
- Wang YS, Yang ZM. 2005. Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant and Cell Physiology 46, 1915–1923. [DOI] [PubMed] [Google Scholar]
- Wang Y, Stass A, Horst WJ. 2004. Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiology 136, 3762–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, An L, Lu H, Zhu C. 2009. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230, 755–765. [DOI] [PubMed] [Google Scholar]
- Xu J, Yin H, Li Y, Liu X. 2010. Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum . Plant Physiology 154, 1319–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SS, Zu Lin S, Lai ZX. 2015. Cadmium impairs iron homeostasis in Arabidopsis thaliana by increasing the polysaccharide contents and the iron-binding capacity of root cell walls. Plant and Soil 392, 71–85. [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H. 2001. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiology 125, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ. 2008. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology 146, 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhu XF, Peng YX, Zheng C, Li GX, Liu Y, Shi YZ, Zheng SJ. 2011. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiology 155, 1885–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Eticha D, Rao IM, Horst WJ. 2010. Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.). Journal of Experimental Botany 61, 3245–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye YQ, Jin CW, Fan SK, Mao QQ, Sun CL, Yu Y, Lin XY. 2015. Elevation of NO production increases Fe immobilization in the Fe-deficiency roots apoplast by decreasing pectin methylation of cell wall. Scientific Reports 5, 10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Jin C, Sun C, Wang J, Ye Y, Lu L, Lin X. 2015. Elevation of arginine decarboxylase-dependent putrescine production enhances aluminum tolerance by decreasing aluminum retention in root cell walls of wheat. Journal of Hazardous Materials 299, 280–288. [DOI] [PubMed] [Google Scholar]
- Yu Q, Tang C, Chen Z, Kuo J. 1999. Extraction of apoplastic sap from plant roots by centrifugation. New Phytologist 143, 299–304. [Google Scholar]
- Zhang Z, Wang H, Wang X, Bi Y. 2011. Nitric oxide enhances aluminum tolerance by affecting cell wall polysaccharides in rice roots. Plant Cell Reports 30, 1701–1711. [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Yang JL, He YF, Yu XH, Zhang L, You JF, Shen RF, Matsumoto H. 2005. Immobilization of aluminum with phosphorus in roots is associated with high aluminum resistance in buckwheat. Plant Physiology 138, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu XY, Chen LQ, Yang JL, Zheng SJ. 2012. Nitric oxide exacerbates Al-induced inhibition of root elongation in rice bean by affecting cell wall and plasma membrane properties. Phytochemistry 76, 46–51. [DOI] [PubMed] [Google Scholar]
- Zhu XF, Lei GJ, Wang ZW, Shi YZ, Braam J, Li GX, Zheng SJ. 2013. Coordination between apoplastic and symplastic detoxification confers plant aluminum resistance. Plant Physiology 162, 1947–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Shi YZ, Lei GJ, Fry SC, Zhang BC, Zhou YH, Braam J, Jiang T, Xu XY, Mao CZ. 2012. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. The Plant Cell Online 24, 4731–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Sun Y, Zhang BC, Mansoori N, Wan JX, Liu Y, Wang ZW, Shi YZ, Zhou YH, Zheng SJ. 2014. TRICHOME BIREFRINGENCE-LIKE27 affects aluminum sensitivity by modulating the O-acetylation of xyloglucan and xluminum-binding capacity in Arabidopsis. Plant Physiology 166, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.