Abstract
BACKGROUND
Metachromatic leukodystrophy (MLD) is an autosomal recessive disorder caused by deficiency in arylsulfatase A activity, leading to accumulation of sulfatide substrates. Diagnostic and monitoring procedures include demonstration of reduced arylsulfatase A activity in peripheral blood leukocytes or detection of sulfatides in urine. However, the development of a screening test is challenging because of instability of the enzyme in dried blood spots (DBS), the widespread occurrence of pseudodeficiency alleles, and the lack of available urine samples from newborn screening programs.
METHODS
We measured individual sulfatide profiles in DBS and dried urine spots (DUS) from MLD patients with LC-MS/MS to identify markers with the discriminatory power to differentiate affected individuals from controls. We also developed a method for converting all sulfatide molecular species into a single species, allowing quantification in positive-ion mode upon derivatization.
RESULTS
In DBS from MLD patients, we found up to 23.2-fold and 5.1-fold differences in total sulfatide concentrations for early- and late-onset MLD, respectively, compared with controls and pseudodeficiencies. Corresponding DUS revealed up to 164-fold and 78-fold differences for early- and late-onset MLD patient samples compared with controls. The use of sulfatides converted to a single species simplified the analysis and increased detection sensitivity in positive-ion mode, providing a second option for sulfatide analysis.
CONCLUSIONS
This study of sulfatides in DBS and DUS suggests the feasibility of the mass spectrometry method for newborn screening of MLD and sets the stage for a larger-scale newborn screening pilot study.
Metachromatic leukodystrophy (MLD),7 caused by deficiency of arylsulfatase A (EC 3.1.6.8), is a severe clinical condition categorized into infantile, late infantile, juvenile, and adult subtypes according to age of onset. Newborn screening (NBS) of MLD by direct measurement of arylsulfatase A activity or protein abundance is not likely to be feasible because of a severe pseudodeficiency problem (1) and relative instability of the enzyme in dried blood spots (DBS) (2). Sulfatides, the natural substrates for arylsulfatase A, have been shown to be highly increased in urine from MLD patients compared with healthy individuals (3–7). However, NBS programs typically use DBS, and dried urine samples (DUS) are usually not available. Recent studies have shown that sulfatides are increased in DBS from MLD patients (8, 9), but the discriminatory power of sulfatide concentrations in DBS between MLD patients and controls is less pronounced than in DUS, raising questions about the potential for NBS. The interest in screening for MLD is timely owing to new treatment options being investigated in the clinic (10).
Sulfatides occur as an ensemble of molecular species caused by variation in the structure of the fatty acyl chain attached to the sphingosine amino group (3). In the study reported here, we developed and optimized new tandem mass spectrometry (MS/MS) methods to detect sulfatides in DBS and DUS as disease markers of MLD. The first method covers the entire panel of sulfatide molecular species with ultra–high-performance liquid chromatography (UHPLC) and MS/MS in negative ion mode without the need to derivatize the analyte. The second method involves the enzymatic conversion of all sulfatide molecular species into a single species (lysosulfatide), after hydrolysis of fatty acid. Lysosulfatide is then converted into a derivative that can be readily detected by UHPLC-MS/MS in positive-ion mode. We compared these methods by analyzing DBS and DUS from ≤ 100 control samples and 14 MLD patients with various ages of onset of clinical symptoms.
Materials and Methods
All studies with human samples were carried out with institutional review board approval at the University of Washington. DUS and DBS from MLD patients were collected and provided by the MLD Foundation (http://www.mldfoundation.org/). We obtained DBS from MLD pseudodeficient patients from the National Referral Laboratory, Genetics and Molecular Pathology, South Australia Pathology, Women’s and Children’s Hospital, Adelaide, Australia. Anonymized control DUS and DBS from healthy newborns were obtained from the Centre Hospitalier Universitaire de Sherbrooke before being discarded. DBS from MLD patients were collected by puncturing the fingertip with a lancet and letting the blood drip onto Whatman 903 filter paper, which was air-dried for approximately 2 h and then mailed at ambient temperature over a few days to the University of Washington. DUS from MLD patients were collected on Whatmann-GE 903 paper disks (70 mm), which were allowed to air-dry for approximately 2 h at room temperature, then placed in a zip-lock plastic bag for shipment at ambient temperature. After receipt, DBS and DUS were stored at −20 °C in a closed jar with desiccant. DBS were manually punched with a 3-mm-diameter perforator, and a single 3-mm punch was used for analysis in microtiter plate format. DUS were punched with a 3- or 10-mm-diameter perforator, and a single 10-mm punch or two 3-mm punches were used for sulfatide analysis. C17:0, C24:0, C16:0-d5, and C18:0-d5 sulfatides were obtained as described (8).
SULFATIDE EXTRACTION AND SAMPLE PROCESSING
To extract sulfatides from DBS and DUS, 30 μL water was added to a 3-mm punch in a deep (1-mL) 96-well plate and incubated for 2 h (37 °C with orbital shaking). We added 300 μL methanol, pipetted the mixture up and down approximately 10 times, and centrifuged the plate for 5 min at 2000g at room temperature. A portion of the supernatant (200 μL) was transferred to a shallow (350-μL) 96-well plate and directly used for LC-MS/MS analysis. We used the same protocol for a 10-mm DUS punch, except with 50 μL water and 500 μL methanol.
ENZYMATIC CONVERSION AND DERIVATIZATION OF SULFATIDES
DBS were incubated in a deep (1-mL) 96-well plate for 16 h (37 °C with orbital shaking) in 30 μL of 50 mmol/L sodium acetate buffer, pH 6.0, containing 0.8% taurodeoxycholate and 2 mU sphingolipid ceramide N-deacylase (SCDase) (cat. no. S2563, Sigma-Aldrich). The reaction was quenched with 300 μL methanol, and 200 μL was transferred to a shallow (350 μL) 96-well plate. For direct lysosulfatide analysis, samples were transferred and subjected to LC-MS/MS analysis. For the analysis of lysosulfatide derivative, the methanol was evaporated under a stream of nitrogen, and 50 μL of 5 mmol/L succinyl ester reagent (see Supplementary Material, which accompanies the online version of this article at http://www.clinchem.org/content/vol62/issue1) in ethanol was added to each well. The plate was sealed with a silicone mat and incubated for 2 h at 37 °C with orbital shaking. Methanol (150 μL) was added to each well, and the sample was subjected to LC-MS/MS analysis.
UHPLC-MS/MS ANALYSIS
For UHPLC separation, we used an Acquity system equipped with an analytical column, HSS T3 C18 (50 × 2.1 mm, 1.8 μm), and a guard column, VanGuard HSS T3 (5 × 2.1 mm, 1.8 μm), all from Waters Corp. The UHPLC solvents were water/acetonitrile (50:50) with 0.1% formic acid (solvent A) and 2-propanol/acetonitrile (80:20) with 0.1% formic acid (solvent B). The elution solvents were prepared from LC-MS Optima grade acetonitrile ≥99.9%, water, 2-propanol, methanol ≥99.9%, and formic acid approximately 98% (Fisher). The gradient elution program started with 50% solvent B, 0–1 min to 95% solvent B (linear), 1–1.5 min to 100% solvent B (linear), 1.5–2 min to 100% solvent B (hold), and 2–2.5 min back to 50% solvent B for re-equilibration. Total injection-to-injection time was 2.5 min, and the injection volume was 10 μL. A triple-quadrupole tandem mass spectrometer (Xevo TQ, Waters Corp.) was used in negative- and positive-ion detection modes. Details for MS/MS methods are provided in online Supplementary Tables 5 and 6. An external calibration curve was acquired for each sample batch with C17:0 sulfatide standard (Avanti Polar Lipids). The use of internal standardization with C17:0 was not suitable because of the isobaric molecular mass with C16:1-OH sulfatide and coelution of isobaric sulfatides at UHPLC gradient conditions. We assumed 3.1 μL blood or urine in a 3-mm punch to calculate concentrations. Representative UHPLC–multiple reaction monitoring (MRM) traces are provided as Supplementary Fig. 1.
Results
OPTIMIZED EXTRACTION AND ANALYSIS OF SULFATIDES
We tested several protocols for extraction of sulfatides from DBS and DUS (see online Supplementary Table 1). The optimized method consisted of rehydrating the DBS or DUS by incubation with aqueous solution followed by adding an extraction solvent (methanol, isopropanol, or chloroform). For aqueous solutions, we examined pure water and 0.8% Triton X-100 in water. We also compared previously reported protocols that used buffer followed by chloroform/methanol extraction (9) or direct ethyl acetate extraction (8). We found that water incubation followed by methanol or isopropanol resulted in optimal extraction yields for all sulfatides (see online Supplementary Table 2), whereas other extraction protocols showed poorer yields. The repeated extraction into methanol resulted in only approximately 5% of sulfatides in the second extract (see online Supplementary Table 3). The extraction efficiency was measured after adding C17:0; C18:0, and C24:0 sulfatides on filter paper (with or without urine or blood) and comparing the UHPLC-MS/MS response to that obtained by injecting the same amount of sulfatide in a neat standard solution without processing.
We also optimized the UHPLC mobile-phase composition with the respect to the MS/MS response for native sulfatide species in positive- and negative-ion detection modes. We replaced mobile-phase additive (5 mmol/L ammonium formate) previously used by Barcenas et al. (8) with 0.1% formic acid and observed a 100% increase in negative-ion response and a modest 10%–20% increase in positive-ion response. This was arguably due to improved UHPLC peak shape caused by the reduction of silanol activity by 0.1% formic acid. Next, we optimized the organic modifiers, substituting protic solvent (methanol) with aprotic solvent (acetonitrile), which resulted in a substantial 10-fold signal gain in the negative-ion response. The overall approximate 20-fold MS/MS signal improvement compared with previously published methods (8, 9) was critical to attain analytical sensitivity for sulfatide analysis in DBS. The optimized UHPLC mobile phase composed of water, acetonitrile, isopropanol, and 0.1% formic acid additive was further used in sulfatide studies for both negative- and positiveion modes and also for detection of lysosulfatide and a lysosulfatide derivative.
Calibration curve QC studies are summarized in online Supplementary Table 4. The UHPLC-MS/MS responses for the test species (C16:0-d5, C18:0-d5, C17:0, and C24:0) as a function of the amount injected showed acceptable linearity and reproducibility. Repeating the analysis in the presence of DBS extract showed a matrix MS/MS suppression of approximately 40%. The effects of this suppression were minimized by the use of sulfatide internal standards in all UHPLC-MS/MS runs.
SULFATIDE PROFILES IN DBS
Previous reports have shown that the profile of sulfatide molecular species varies vastly depending on the tissue type (11). Therefore we covered a panel of 19 sulfatide species in extracted DBS and DUS from MLD patients and healthy newborns to obtain a comprehensive profile. Data about the MLD status of the patients are given in online Supplementary Table 7. We detected a total of 15 sulfatide species in DBS from MLD patients (Fig. 1A). As reported previously (8, 9), we confirmed C16:0, C16:0-OH, and C16:1-OH sulfatides to be the most abundant species (Fig. 1A) in DBS. However, we detected other minor sulfatide species that have shown a notable increase in DBS from MLD patients, such as C18:0, C18: 0-OH, C22–0, C24:0, C24:1, C24:0-OH, and C24:1-OH, some of which were not previously reported as increased in MLD whole-blood samples. Detailed information on all monitored sulfatide species and their concentrations in all DBS are given in online Supplementary Table 9, and a brief overview of mean values can be found in Table 1. In summary, the mean total sulfatide concentrations (sum of all detected species) were 9.5- and 3.3-fold higher in patients with severe and mild forms of MLD, respectively, compared with control samples (0.215 μg/mL). There was no overlap between groups, and given <20% CVs routinely achieved with single reaction monitoring (SRM) MS/MS technology (7), the difference in sulfatide concentration was sufficient to unambiguously detect MLD patients and stratify them on the basis of severity. Additionally, we found the most discriminatory MLD markers to be C16:1-OH and C16: 0-OH, with 23.2- and 13.1-fold mean increases, respectively, in the infantile form of MLD, and 5.1- and 4.6-fold increases in the juvenile/adult form of MLD. The minor sulfatide species showed severe MLD-to-control ratios of 2.0–12.5. On the basis of the analysis of 50 newborn DBS from nonaffected patients, we set the reference range to 0–0.37 μg/mL (0–95th percentile). This range is expected to be adjusted after a pilot study of approximately 150 000 DBS (see Discussion).
Fig. 1.
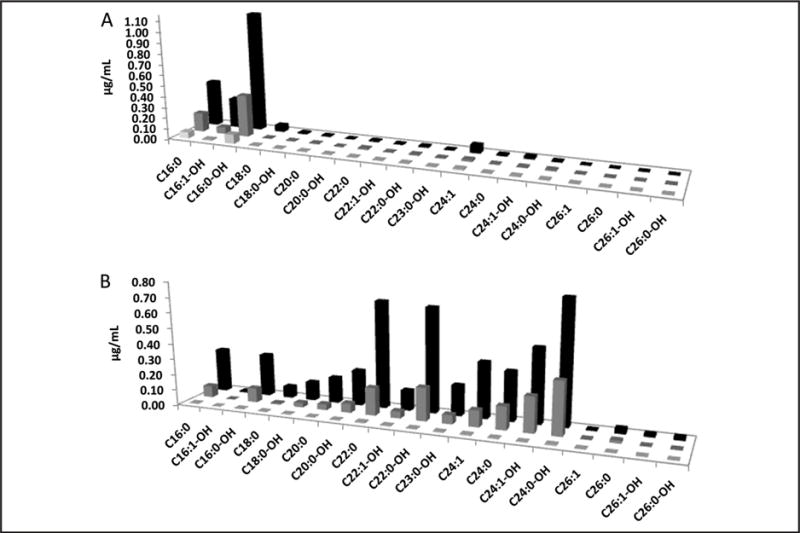
Concentrations of 19 sulfatides in blood or urine (μg/mL) detected in DBS (A) and DUS (B) from late infantile MLD patients (n = 11, black), juvenile/adult MLD patients (n = 3, medium gray), and controls (n = 50 for DBS and n = 104 for DUS, light gray).
Table 1.
Mean concentration of sulfatides detected in DBS and DUS from diagnosed MLD patients and healthy individuals.
| Sulfatide | DBS | DUS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control, μg/mL | MLD (early) | MLD (late) | Control, μg/mL | MLD (early) | MLD (late) | |||||
| μg/mL | Ratioa | μg/mL | Ratioa | μg/mL | Ratioa | μg/mL | Ratioa | |||
| C-16-0 | 0.057 | 0.422 | 7.4 | 0.174 | 3.1 | 0.004 | 0.281 | 66.1 | 0.073 | 17.3 |
| C-16-1-OH | 0.012 | 0.276 | 23.2 | 0.061 | 5.1 | NDb | 0.009 | ND | ND | ND |
| C-16-0-OH | 0.085 | 1.111 | 13.1 | 0.387 | 4.6 | 0.003 | 0.274 | 106.8 | 0.091 | 35.3 |
| C-18-0 | 0.005 | 0.062 | 12.5 | 0.005 | 0.9 | 0.002 | 0.074 | 44.7 | 0.009 | 5.21 |
| C-18-0-OH | 0.004 | 0.013 | 3.7 | 0.004 | 1.1 | 0.003 | 0.119 | 45.1 | 0.028 | 10.5 |
| C-20-0 | 0.005 | 0.008 | 1.8 | 0.006 | 1.3 | 0.003 | 0.165 | 55.1 | 0.036 | 12.1 |
| C-20-0-OH | 0.004 | 0.004 | 0.9 | ND | ND | 0.002 | 0.228 | 94.1 | 0.059 | 24.2 |
| C-22-0 | 0.005 | 0.011 | 2.4 | 0.012 | 2.5 | 0.007 | 0.692 | 101.7 | 0.177 | 26 |
| C-22-1-OH | 0.004 | 0.006 | 1.6 | 0.004 | 0.9 | 0.004 | 0.129 | 32.5 | 0.043 | 10.9 |
| C-22-0-OH | 0.006 | 0.011 | 1.8 | 0.009 | 1.5 | 0.005 | 0.676 | 137.3 | 0.212 | 43 |
| C-23-0-OH | 0.004 | 0.006 | 1.6 | 0.004 | 1.2 | 0.003 | 0.196 | 59.9 | 0.058 | 17.6 |
| C-24-1 | 0.011 | 0.074 | 6.5 | 0.016 | 1.4 | 0.006 | 0.360 | 57.9 | 0.105 | 17 |
| C-24-0 | 0.004 | 0.012 | 3.1 | 0.004 | 0.9 | 0.005 | 0.317 | 67.5 | 0.148 | 31.6 |
| C-24-1-OH | 0.006 | 0.022 | 3.7 | 0.007 | 1.1 | 0.003 | 0.479 | 163.9 | 0.227 | 77.6 |
| C-24-0-OH | 0.004 | 0.008 | 2.0 | 0.010 | 2.3 | 0.007 | 0.792 | 119.1 | 0.337 | 50.6 |
| C-26-1 | ND | ND | ND | ND | ND | 0.002 | 0.006 | 3.5 | 0.004 | 2.08 |
| C-26-0 | ND | ND | ND | ND | ND | ND | 0.033 | ND | 0.016 | ND |
| C-26-1-OH | ND | ND | ND | ND | ND | ND | 0.018 | ND | 0.003 | ND |
| C-26-0-OH | ND | ND | ND | ND | ND | ND | 0.020 | ND | 0.004 | ND |
| Total | 0.215 | 2.048 | 9.5 | 0.702 | 3.3 | 0.058 | 4.866 | 84.2 | 1.628 | 28.2 |
MLD-to-control ratio.
ND, not detectable.
All 14 MLD patients showed markedly increased total sulfatides compared with controls (Fig. 2A shows the mean total sulfatide values and SDs for all patients in each group). Sulfatide concentrations among MLD patients fell into 2 distinct groups: 3 patients with intermediate concentrations of sulfatides (3.3-fold increase compared with controls) and 11 with high concentrations of sulfatides (9.5-fold increase compared with controls) (Fig. 3). The 3 patients in the lower-concentration group showed MLD symptoms at a later age (8–22 years) compared with earlier onset of symptoms in the higher-concentrations group (11–26 months). Remarkably, despite C18:0 being a minor sulfatide species in terms of abundance, it appears to have discriminatory power for the severity of the disease. C18:0 sulfatide was present at low concentrations in all controls and also in 3 late-onset MLD samples, but it was 8- to 20-fold increased in all 11 early-onset MLD samples (Fig. 2B). In contrast, other low-abundance sulfatides such as C24:1 (Fig. 2B) were correlated with total sulfatide concentration, e.g., increased in all late- and early-onset MLD patients above the concentrations in control samples.
Fig. 2.
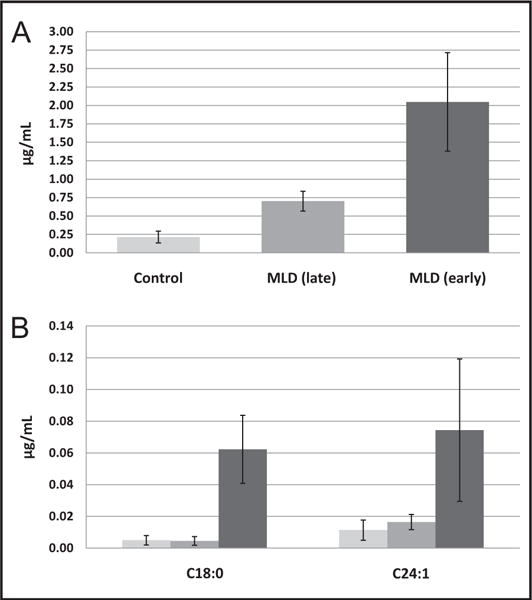
Mean total sulfatide concentrations in blood (μg/mL) across patient groups (A) and mean concentrations of C18:0 and C24:1 sulfatides (B) in DBS from MLD patients and healthy controls.
Error bars show SDs.
Fig. 3.
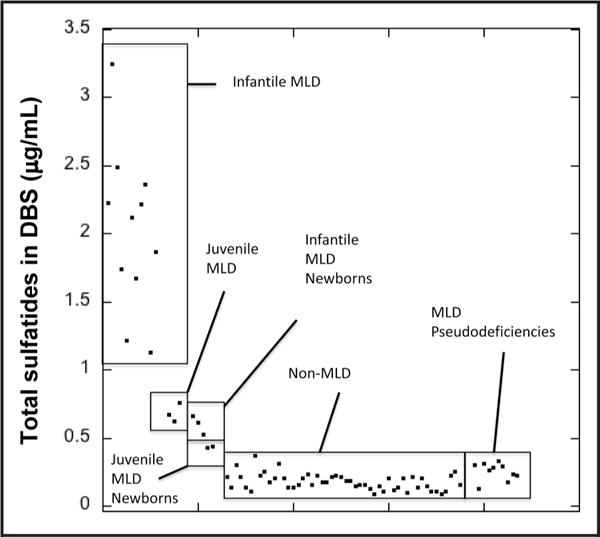
Total sulfatide concentrations in blood (μg/mL) in patients already diagnosed with MLD (n = 14), non-MLD newborns (n = 50), patients with MLD pseudodeficiencies (n = 10), newborn patients who developed infantile MLD (n = 3), and newborn patients who developed juvenile MLD (n = 2).
We also quantified the sulfatides in 12 DBS from individuals with MLD pseudodeficiency, i.e., low plasma arylsulfatase A enzymatic activity but no increased urinary sulfatides or symptoms of MLD (see online Supplementary Table 8 for a description of these samples). The results in online Supplementary Table 9 show essentially no increase in sulfatides compared with control DBS.
The results in Fig. 3 and online Supplementary Table 10 show sulfatide concentrations in newborn DBS from patients who went on to receive a diagnosis of MLD. These were obtained as stored DBS from the California state newborn screening laboratory. Information about the patients is provided in online Supplementary Table 7. As shown in Fig. 3, total sulfatides in the 3 newborns who developed late infantile MLD were increased above all values for the 50 non-affected newborns; the mean value was 3-fold above the mean total sulfatide value for the nonaffected DBS. The 2 newborns who developed juvenile MLD had sulfatides above all values for the 50 nonaffected newborns; the mean value was 2.1-fold above the mean total sulfatide value for the nonaffected DBS.
SULFATIDE PROFILES IN DUS
The application of the UHPLC-MS/MS method to analysis of extracted DUS resulted in the detection of 19 sulfatide species. In agreement with a previous study (7), the major sulfatide species in DUS were C24:0-OH, C22:0, and C22:0-OH, with MLD-to-control ratios of 120, 102, and 86, respectively. The mean total sulfatide concentration for the sum of 19 detected molecular species in late infantile MLD patients was 4.87 μg/mL, range 0.42–17.24 μg/mL. In juvenile/adult MLD forms, the mean was 1.63 μg/mL, range 0.90–3.04 μg/mL, and the concentration in controls was 0.058 μg/mL, ranging from undetectable to 0.179 μg/mL (Fig. 1B; detailed data for all species and all DUS in online Supplementary Table 11). Despite the substantial variance in total sulfatide concentrations among individuals within the same group, we were able to unambiguously differentiate MLD patients from controls. However, unlike with DBS, we were unable to stratify MLD patients with infantile and juvenile/adult forms (see online Supplementary Fig. 2). The mean total sulfatide concentrations in DUS showed approximately 10-fold larger ratios between late infantile MLD and juvenile/adult vs control (84.3 and 28.2, respectively) compared with DBS (9.5 and 3.3). However, this did not translate to a larger potential to distinguish the groups because of a broad distribution of sulfatide concentrations within the groups. For example, the total sulfatide concentration in control DUS spanned about 3 orders of magnitude, whereas in DBS the range was just 4-fold. On the basis of the analysis of 100 newborn DUS from nonaffected patients, we set the reference range at 0 – 0.123 μg/mL (0–95th percentile).
The finding of appropriate urine volume-normalizing parameters to evaluate urinary lipids is a longstanding challenge (12). Although normalization with the concentration of creatinine is commonly used for soluble analytes, it was proven inefficient in the case of ceramides (12). The isoform profile number has been proposed as a new MLD biomarker (7), which is the combined signal of the major isoforms relative to the intrinsic C18:0 sulfatide reference. The C18:0 isoform was chosen as the least-variable parameter of the total sulfatide profile among controls and MLD patients. However, we found that the C18:0 isoform was increased in DUS from MLD patients compared with controls, analogous to the other isoforms, and therefore we were unable to use the isoform profile number. Instead, we attempted to normalize on the concentration of creatinine and sphingomyelin (SM) (13). We analyzed DUS in triplicate, summed the SRM response of all sulfatide isoforms, and normalized to the raw response of creatinine or SM and creatinine:d3-creatinine or SM:d31-SM ratios. Nevertheless, none of the normalizations were effective to reduce triplicate CV (see online Supplementary Table 12) or result in more substantial differentiation between MLD patients and control groups (see online Supplementary Table 13).
ALTERNATIVE UHPLC-MS/MS METHODS FOR SULFATIDE ANALYSIS
We explored alternative methods to analyze sulfatides in the positive-ion mode and possible ways to simplify the analysis. The previous section demonstrated the feasibility of reliable discrimination between MLD patients and healthy controls on the basis of sulfatide profile and abundance in DBS and DUS. However, a panel of sulfatides was monitored in negative-ion mode, which may limit multiplexing capabilities and complicate data processing. To allow for improved MS/MS analysis in the positive-ion detection mode and simpler data interpretation, we developed a protocol converting all sulfatide species present in the sample to a single molecular species. The 2-step protocol consisted of (a) enzymatic conversion to the lysosulfatide and (b) derivatization with a succinyl ester reagent (Scheme 1). The reagent we synthetized in our laboratory introduces highly basic tertiary amine functionalities into the lysosulfatide, forming a stable derivative that is more suitable for MS/MS positive-ion detection mode than underivatized lysosulfatide. For the enzymatic conversion, we used a bacterial enzyme SCDase from Pseudomonas sp. that hydrolyses the N-acyl linkage between fatty acids and sphingosine bases in the ceramide moiety of sphingolipids. We ran the enzymatic reaction in ammonium acetate/taurodeoxycholate at pH 6.0, corresponding to the optimum SCDase pH for hydrolytic activity (14). We optimized the amount of SCDase per assay (see online Supplementary Table 14). Under these conditions, the hydrolysis yields in DBS samples from MLD patients were >92% (see online Supplementary Table 15) on the basis of conversion of C16:0-OH, C16:1-OH, and C24:1, the predominant sulfatides in DBS. Because sulfatide profiles vary among MLD-affected individuals (7), monitoring a panel of sulfatide isoforms is likely to be necessary for reliable discrimination between MLD patients and unaffected individuals.
Scheme 1. Protocol to enhance sensitivity of sulfatide positive-ion detection in DBS.
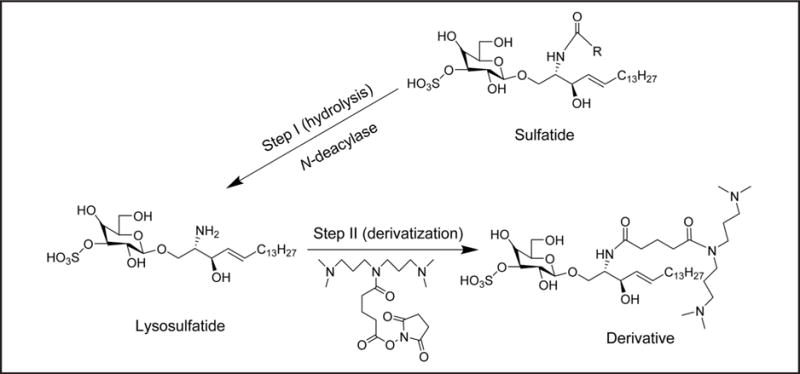
Step I: all sulfatide species are enzymatically converted to a single molecular species (lysosulfatide); step II: the lysosulfatide is reacted with the indicated reagent to improve detection sensitivity in positive-ion mode.
Our results show that lysosulfatide gives an approximate 6.5- and 10-fold lower response in negative- and positive-ion detection mode, respectively, compared with total sulfatide response in negative-ion mode (see online Supplementary Table 16). Therefore, we derivatized with a succinyl ester reagent to introduce a net positive charge into the lysosulfatide molecule to improve sensitivity (Scheme 1). Derivatization was nearly quantitative after 2.5 h of reaction time, and the response in positive-ion mode was improved 7-fold compared with underivatized lysosulfatide.
The discriminatory power of assays on the basis of determination of (a) total sulfatides or (b) lysosulfatide in negative-ion mode and (c) lysosulfatide or (d) lysosulfatide derivative in positive-ion mode is compared in online Supplementary Fig. 3. In summary, all 4 assays were able to differentiate controls from late infantile and juvenile/adult MLD patients; nevertheless, the separation was somewhat better in case of (a) and (d) because of stronger response with better ion statistics.
Discussion
Our initial study of sulfatides in DBS and DUS measured by UHPLC-MS/MS (8) suggested that DUS but not DBS gave sufficient separation between MLD-affected and nonaffected individuals to be of use for NBS. An independent study that used DBS showed a somewhat improved separation (9) but probably not sufficient for NBS. In the current study, we fully optimized the extraction of sulfatides from DBS and DUS and improved the signal-to-noise ratio for sulfatide detection by UHPLC-MS/MS. These factors contribute to a difference in sulfatide concentrations by MLD-affected and nonaffected individuals that is greatly improved relative to previous studies and suggest that it will be possible to use DBS for NBS of MLD.
With DBS, we observed a good correlation in the concentration of sulfatides and the age of onset of MLD (although given the small number of MLD samples, this correlation may not hold in larger sample sets). This was not the case with DUS, although all MLD patients had higher total sulfatides than the non-MLD controls. DUS presents a challenge compared with DBS in that data should be normalized to account for differential urine volume. For water-soluble analytes, this is typically done by measuring creatinine, which is produced from muscle at a constant rate, but this process is not appropriate for water-insoluble analytes such as sulfatide (12). A better normalization marker may be SM as a biomarker for cellular membranes, since sulfatides presumably reside in membrane fragments in urine; however, normalization of our DUS data to SM did not provide a better correlation of the sulfatide concentrations and the age of onset of MLD. The fact that the sulfatide molecular species distributions in DBS and DUS are dramatically different (this study and (8, 9)) strongly implies that urinary and blood sulfatides are not in rapid exchange, which in turn suggests that they arise from different tissue sources. The reason that the sulfatide/age-of-onset correlation is strong in DBS but not in DUS remains obscure. It is possible that multiple factors contribute to transport of sulfatides from renal tissue into urine, and these factors are not understood.
Our initial thoughts about conversion of sulfatide molecular species to a single species were that all analytes would funnel into a single species that could be detected by flow injection MS/MS, obviating the need for UHPLC. However, given the relatively low concentrations of sulfatides in DBS and DUS, flow injection with tandem MS/MS is probably not sufficient for analytical specificity, and the use of UHPLC seems to be required. Fortunately, the inject-to-inject time is sufficiently short to allow for high-throughput applications such as NBS. We are currently testing whether low-pressure LC with isocratic solvent can replace the UHPLC gradient/solvent configuration. Our ability to convert sulfatides to a derivative that can be analyzed in positive-ion MS/MS is expected to be valuable for multiplexing NBS of MLD with other diseases, including a panel of lysosomal storage diseases for which we have developed highly robust NBS assays in positive-ion mode (15). UHPLC-MS/MS in positive-ion mode can be used to simultaneously monitor the activity of several enzymes and a panel of biomarkers; for example, psychosine for Krabbe disease (16, 17), lyso-Gb3 for Fabry disease (18), glucosylsphingosine for Gaucher disease (19), and sulfatides for MLD. This will be possible with inject-to-inject times that are short enough for NBS.
Supplementary Material
Acknowledgments
Employment or Leadership: T. Suhr, MLD Foundation; F. Turecek, University of Washington.
Consultant or Advisory Role: M. Gelb, Perkin Elmer.
Stock Ownership: T. Suhr, Shire PLC and Genzyme (Sanofi).
Honoraria: None declared.
Research Funding: BioMarin Corp., Genzyme Corp., PerkinElmer, Shire Corp., and Waters Corp. M. Gelb, National Institute of Diabetes and Digestive and Kidney Diseases, NIH (R01 DK067859).
Expert Testimony: None declared.
Patents: Z. Spacil, patent PCT/US2012/064205.
Role of Sponsor: The funding organizations played a direct role in the design of study, review and interpretation of data, and preparation and approval of manuscript.
Footnotes
Nonstandard abbreviations: MLD, metachromatic leukodystrophy; NBS, newborn screening; DBS, dried blood spots; DUS, dried urine samples; MS/MS, tandem mass spectrometry; UHPLC, ultra–high-performance liquid chromatography; SCDase, sphingolipid ceramide N-deacylase; MRM, multiple reaction monitoring; SM, sphingomyelin.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
References
- 1.Fluharty AL, Meek WE, Kihara H. Pseudo arylsulfatase A deficiency: evidence for a structurally altered enzyme. Biochem Biophys Res Commun. 1983;112:191–7. doi: 10.1016/0006-291x(83)91815-6. [DOI] [PubMed] [Google Scholar]
- 2.Tan MAF, Dean Caroline J, Hopwood JJ, Meikle PJ. Diagnosis of metachromatic leukodystrophy by immune quantification of arylsulphatase A protein and activity in dried blood spots. Clin Chem. 2008;54:1924–5. doi: 10.1373/clinchem.2008.108456. [DOI] [PubMed] [Google Scholar]
- 3.Nishio H, Kodama S, Matsuo T. Analysis of fatty acids and sphingosines from urinary sulfatides in a patient with metachromatic leukodystrophy by gas chromatography-mass spectrometry. Brain Dev. 1985;7:614–21. doi: 10.1016/s0387-7604(85)80010-3. [DOI] [PubMed] [Google Scholar]
- 4.Natowicz MR, Prence EM, Chaturvedi P, Newburg DS. Urine sulfatides and the diagnosis of metachromatic leukodystrophy. Clin Chem. 1996;42:232–8. [PubMed] [Google Scholar]
- 5.Whitfield PD, Sharp PC, Johnson DW, Nelson P, Meikle PJ. Characterization of urinary sulfatides in metachromatic leukodystrophy using electrospray ionization-tandem mass spectrometry. Mol Genet Metab. 2001;73:30–7. doi: 10.1006/mgme.2001.3165. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Colsch B, Alonso C, Baumann N, Tabet JC, Mallet JM, et al. Synthetic sulfogalactosylceramide (sulfatide) and its use for the mass spectrometric quantitative urinary determination in metachromatic leukodystrophies. Glycoconj J. 2008;25:147–55. doi: 10.1007/s10719-007-9067-7. [DOI] [PubMed] [Google Scholar]
- 7.Kuchaø L, Asfaw B, Poupětová H, Honzíková J, Tureček F, Ledvinová J. Direct tandem mass spectrometric profiling of sulfatides in dry urinary samples for screening of metachromatic leukodystrophy. Clin Chim Acta. 2013;425:153–9. doi: 10.1016/j.cca.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barcenas M, Suhr TR, Scott CR, Turecek F, Gelb MH. Quantification of sulfatides in dried blood and urine spots from metachromatic leukodystrophy patients by liquid chromatography/electrospray tandem mass spectrometry. Clin Chim Acta. 2013;433C:39–43. doi: 10.1016/j.cca.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han M, Jun S-H, Song SH, Park H-D, Park KU, Song J. Ultra-performance liquid chromatography/tandem mass spectrometry for determination of sulfatides in dried blood spots from patients with metachromatic leukodystrophy. Rapid Commun Mass Spectrom. 2014;28:587–94. doi: 10.1002/rcm.6823. [DOI] [PubMed] [Google Scholar]
- 10.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 11.Berná L, Asfaw B, Conzelmann E. Determination of urinary sulfatides and other lipids by combination of reversed-phase and thin-layer chromatographies. Anal Biochem. 1999;311:304–11. doi: 10.1006/abio.1999.4002. [DOI] [PubMed] [Google Scholar]
- 12.Forni S, Fu X, Schiffmann R, Sweetman L. Falsely elevated urinary Gb3 (globotriaosylceramide, CTH, GL3) Mol Genet Metab. 2009;97:91. doi: 10.1016/j.ymgme.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Auray-Blais C, Millington DS, Barr C, Young SP, Mills K, Clarke JTR. Gb3/creatinine biomarkers for Fabry disease: issues to consider. Mol Genet Metab. 2009;97:237. doi: 10.1016/j.ymgme.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kurita T, Izu H, Sano M, Ito M, Kato I. Enhancement of hydrolytic activity of sphingolipid ceramide N-deacylase in the aqueous-organic biphasic system. J Lipid Res. 2000;41:846–51. [PubMed] [Google Scholar]
- 15.Spacil Z, Tatipaka H, Barcenas M, Scott CR, Turecek F, Gelb MH. High-throughput assay of 9 lysosomal enzymes for newborn screening. Clin Chem. 2013;59:502–11. doi: 10.1373/clinchem.2012.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turgeon CT, Orsini JJ, Sanders KA, Magera MJ, Langan TJ, Escolar M, et al. Measurement of psychosine in dried blood spots: a possible improvement to newborn screening programs for Krabbe disease. J Inherit Metab Dis. 2015;38:923–9. doi: 10.1007/s10545-015-9822-z. [DOI] [PubMed] [Google Scholar]
- 17.Chuang WL, Pacheco J, Zhang XK, Martin MM, Biski CK, Keutzer JM, et al. Determination of psychosine concentration in dried blood spots from newborns that were identified via newborn screening to be at risk for Krabbe disease. Clin Chim Acta. 2013;419:73–6. doi: 10.1016/j.cca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Boutin M, Auray-Blais C. Metabolomic discovery of novel urinary galabiosylceramide analogs as Fabry disease biomarkers. J Am Soc Mass Spectrom. 2015;26:499–510. doi: 10.1007/s13361-014-1060-3. [DOI] [PubMed] [Google Scholar]
- 19.Rolfs A, Giese AK, Mascher D, Elstein D, Zimran A, Bottcher T, et al. Glucosylsphingosine is a highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non-Jewish, Caucasian cohort of Gaucher disease patients. PLOS One. 2013;8:e7932. doi: 10.1371/journal.pone.0079732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


