Abstract
Brain edema, due largely to astrocyte swelling, is an important clinical problem in patients with acute liver failure. While mechanisms underlying astrocyte swelling in this condition are not fully understood, ammonia and associated oxidative/nitrosative stress (ONS) appear to be involved. Mechanisms responsible for the increase in reactive oxygen/nitrogen species (RONS) and their role in ammonia-induced astrocyte swelling, however, are poorly understood. Recent studies have demonstrated a transient increase in intracellular Ca2+ in cultured astrocytes exposed to ammonia. As Ca2+ is a known inducer of RONS, we investigated potential mechanisms by which Ca2+ may be responsible for the production of RONS and cell swelling in cultured astrocytes after treatment with ammonia. Exposure of cultured astrocytes to ammonia (5 mM) increased the formation of free radicals, including nitric oxide, and such increase was significantly diminished by treatment with the Ca2+ chelator BAPTA-AM. We then examined the activity of Ca2+-dependent enzymes that are known to generate RONS and found that ammonia significantly increased the activities of NADPH oxidase (NOX), constitutive nitric oxide synthase (cNOS) and phospholipase A2 (PLA2) and such increases in activity were significantly diminished by BAPTA. Pretreatment of cultures with 7-nitroindazole, apocyanin and quinacrine, respective inhibitors of cNOS, NOX and PLA2, all significantly diminished RONS production. Additionally, treatment of cultures with BAPTA or with inhibitors of cNOS, NOX and PLA2 reduced ammonia-induced astrocyte swelling. These studies suggest that the ammonia-induced rise in intracellular Ca2+ activates free radical producing enzymes that ultimately contribute to the mechanism of astrocyte swelling.
Keywords: Ammonia, astrocyte swelling/brain edema, calcium, hepatic encephalopathy, NADPH-oxidase, nitric oxide synthase, oxidative/nitrosative stress, phospholipase A2
INTRODUCTION
Hepatic encephalopathy (HE) is the neurological disorder resulting from severe liver failure. In its acute form (acute liver failure, ALF) it occurs following massive liver necrosis due to viral hepatitis, acetaminophen toxicity, or exposure to other hepatotoxins. It is characterized by an abrupt onset of delirium, seizures and coma and it has a high mortality rate (Capocaccia and Angelico 1991). Elevated levels of ammonia are consistently found in patients with HE, and such rise in ammonia levels has been strongly implicated in disease pathogenesis (Hazell and Butterworth 1999). Astrocytes appear to represent the principal neural cells affected by ammonia toxicity (Norenberg 1996)
Brain edema and associated increase in intracranial pressure leading to brain herniation are important complications of ALF and are the leading cause of death in these patients (Blei 1991). Although the basis for the edema remains to be determined, swelling of astrocytes represents a major component of the edema (Martinez 1968, Traber et al. 1989, Kato et al. 1992). Swelling of cerebral cortical astrocytes has also been observed after ammonia infusion in animals (Takahashi et al. 1991), and following the application of ammonia to brain slices (Ganz et al. 1989) and to cultured astrocytes (Norenberg et al. 1991).
Oxidative/nitrosative stress (ONS) has been suggested to play a major role in ammonia neurotoxicity. Pathophysiological levels of ammonia were shown to increase reactive oxygen and nitrogen species (RONS) production in experimental hyperammonemia in vivo, as well as in cultured astrocytes (for review, see Norenberg 2003). Reduced levels of antioxidant enzymes and lipid peroxidation in brain were reported in experimental models of hyperammonemia (Kosenko et al. 1997). Additionally, protein carbonylation and protein tyrosine nitration have been documented in cultured astrocytes exposed to ammonia (Schliess et al. 2002, Norenberg et al. 2007, Widmer et al. 2007).
Oxidative stress is also known to result in astrocyte swelling. Free radicals have been shown to cause cell swelling in cultured astrocytes (Chan et al. 1989), as well as in brain slices (Chan et al. 1982). Similarly, nitrosative stress was shown to cause astrocyte swelling in brain slices and in cultured astrocytes (Zielinska et al. 2003, Jayakumar et al. 2006). Although ONS appears to be major factor in astrocyte swelling (Norenberg et al. 2005), the precise means by which ammonia generates RONS and the mechanisms by which RONS contribute to the ammonia-induced astrocyte swelling remain to be defined.
One of the earliest events known to occur in cultured astrocytes following ammonia exposure is a rise in intracellular Ca2+ ([Ca2+]i) (Schliess et al. 2002, Norenberg et al. 2003, Rose et al. 2006). Since Ca2+ is known to promote RONS production (Chinopoulos and Adam-Vizi 2006), we hypothesized that the ammonia-induced rise in [Ca2+]i might contribute to RONS production and thereby initiate a process leading to astrocyte swelling. One possible means by which Ca2+ may generate RONS is through the activation of various Ca2+-dependent enzymes. Among these include NADPH oxidase (NOX) (Suzuki et al. 1985), which forms superoxide (Nakamura et al. 1989); constitutive nitric oxide synthase (cNOS) (Mayer et al. 1990), which generates nitric oxide; and the cytosolic form of phospholipase A2 (cPLA2) (Kramer and Sharp 1997) whose product, arachidonic acid, is known to produce free radicals (Cocco et al. 1999).
The present study examined the role of [Ca2+]i and Ca2+-dependent enzymes (cNOS, NOX and cPLA2) in the mechanisms of RONS production and cell swelling following ammonia treatment of cultured astrocytes.
MATERIALS AND METHODS
Astrocyte cultures
Primary cultures of rat cortical astrocytes were prepared as described previously Ducis et al. (1990). For details, see supplemental materials.
Measurement of reactive oxygen/nitrogen species (RONS) production
RONS production was measured by the method of Thorburne and Juurlink (1996) using 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) with minor modifications (Murthy et al. 2001).
Nitric oxide synthase (NOS) activity
Activity of NOS was determined by the conversion of [3H]arginine to [3H]citrulline in the presence (total NOS activity) or absence of Ca2+ (iNOS activity) in the reaction mixture, using the method of Bredt and Snyder (1989). For details, see supplemental materials.
NADPH oxidase (NOX) activity
NOX activity was determined by measuring superoxide production employing a chemiluminiscence method (Jaimes et al. 2004). For details, see supplemental materials.
Phospholipase A2 (PLA2) activity
Activity of PLA2 was determined by the method of Poulsen et al. (2007). For details, see supplemental materials.
Cell volume measurement
Astrocyte cell volume was determined using 3-O-methyl-[3H]glucose (OMG, NEN Life sciences, Boston, MA) by the method of Kletzien et al. (1975), as modified for astrocyte cultures by Kimelberg (1987) and Norenberg et al. (1991). For details, see supplemental materials.
Statistical analysis
All experiments performed were repeated 3–5 times using cells derived from different batches of astrocyte cultures. The number of individual culture plates in each group was 5–7 for all experiments. Data of all experiments were subjected to ANOVA followed by Tukey’s pos hoc comparison. A value of p<0.05 was considered significant. Values are mean ± SEM.
RESULTS
Effect of BAPTA on ammonia-induced reactive oxygen/nitrogen species (RONS) generation
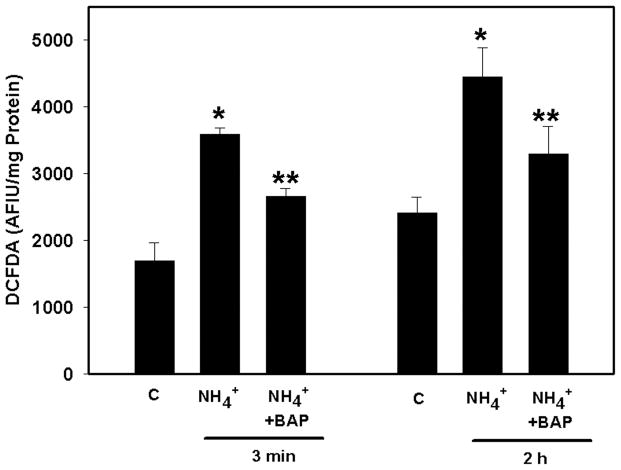
Treatment of cultures with ammonia (5 mM NH4Cl) increased RONS production (Figure 1). The concentration of ammonia used is relevant as similar concentrations are found in brains of animals with acute liver failure (Swain et al. 1992). This treatment results in a rapid decline in ammonia concentration to undetectable levels by 30–60 min (unpublished observations). At 3 min, ammonia increased RONS by 111.4% (p<0.05) and remained elevated for at least 2 h (84.07%) (p<0.05). Pretreatment (10 min) of cultures with BAPTA-AM (25 μM) significantly blocked ammonia-induced RONS production at 3 min (64.2%, p<0.05), 2 h (51.6%, p<0.05) (Figure 1), and >80% at 4 h (data not shown).
Figure 1.
Effect of the Ca2+ chelator BAPTA on ammonia-induced reactive oxygen/nitrogen species (RONS) production. Cultures were pretreated (15 min) with BAPTA/AM (25 μM), and then exposed to ammonia (5 mM NH4Cl). RONS production was determined by DCFDA fluorescence at 3 min and 2 h after ammonia treatment. Values are expressed as arbitrary fluorescence intensity units (AFIU) of DCFDA per mg cell protein. *p<0.01 vs. control; **p<0.05 vs. NH4+. C, control; BAP, BAPTA/AM.
Effect of ammonia on cNOS, NOX and PLA2 activities
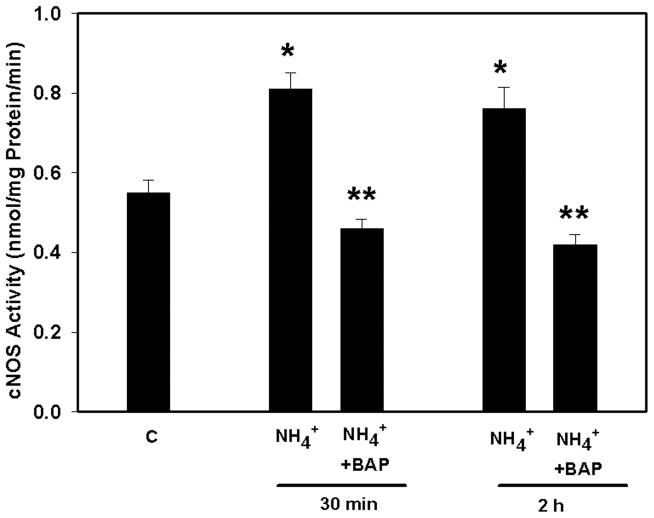
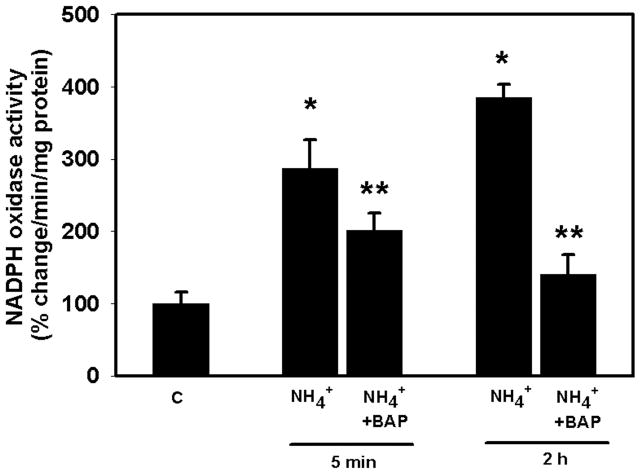
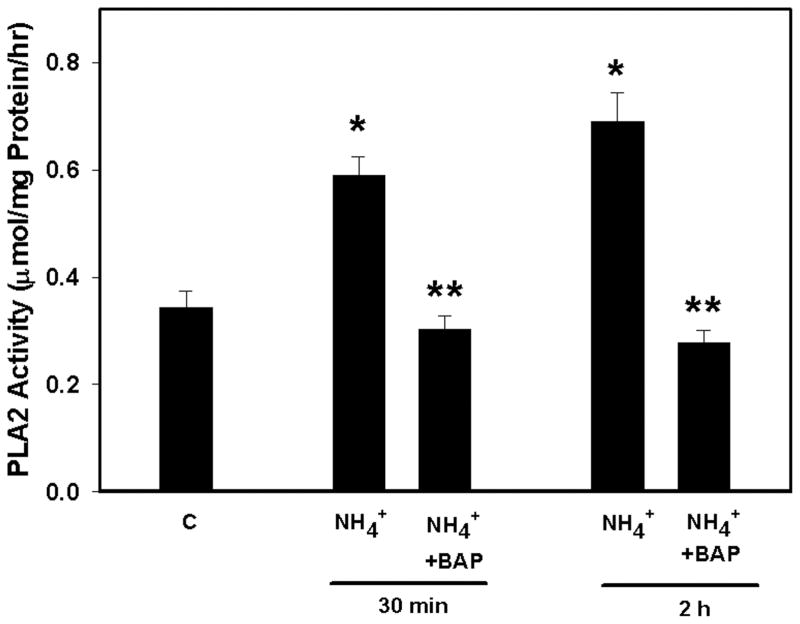
Ammonia increased cNOS activity by 40 and 45% (p<0.05) at 30 min and 2 h, respectively. Pretreatment (15 min) with BAPTA (25 μM) completely blocked the ammonia-induced increase in cNOS activity (Figure 2). Ammonia also significantly increased NOX activity (172.5%, p<0.01) as early as 5 min and such increase progressed to 268.9% (p<0.01) at 2 h. Pretreatment (15 min) of cultures with BAPTA partially diminished NOX activity at 5 min, whereas it was completely blocked at 2 h (Figure 3). Likewise, ammonia resulted in a 73% (p<0.01) increase in PLA2 activity by 30 min, which further increased at 2 h (103%; p<0.01). Pretreatment of cultures with BAPTA completely blocked PLA2 activity at both time points (Figure 4).
Figure 2.
Effect of BAPTA on ammonia-induced constitutive nitric oxide synthase (cNOS) activity. Ammonia (5 mM NH4Cl) increased cNOS activity at 30 min and 2 h. Pretreatment of cultures with BAPTA/AM (25 μM) completely blocked cNOS activity by ammonia. *p<0.01 vs. control; **p<0.01 vs. NH4.
Figure 3.
Effect of BAPTA on ammonia-induced NADPH oxidase (NOX) activity. Cultures were exposed to ammonia (5 mM NH4Cl), and NOX activity was determined. Ammonia significantly increased NOX activity at 5 min and 2 h. Pretreatment (15 min) of cultures with BAPTA significantly diminished NOX activity. *p<0.05 vs. control; **p<0.01 vs. NH4.
Figure 4.
Effect of BAPTA on ammonia-induced phospholipase A2 (PLA2) activity. Ammonia (5 mM NH4Cl) increased the activity of PLA2 at 30 min and 2h respectively. Pretreatment of cultures with BAPTA/AM (25 μM) completely blocked PLA2 activity. *p<0.01 vs. control; **p<0.01 vs. NH4 (NH4Cl).
Effect of inhibitors of cNOS, NOX and cPLA2 on ammonia-induced RONS generation
Treatment of cultures with ammonia significantly increased RONS production by 112% as early as 3 min, and remained elevated for at least 4 h. Pretreatment of cultures with 7-nitroindazole (7-NI, 50 μM), an inhibitor of cNOS, partially (63.9 %) blocked ammonia-induced RONS production at 3 min, whereas the activity was completely blocked at 2 and 4 h. Apocyanin, an inhibitor of NOX, did not block RONS production at 3 min, but completely blocked it at 2 and 4 h. Likewise, quinacrine, an inhibitor of PLA2, had no inhibitory effect on RONS production at 3 min; however, it partially blocked ammonia-induced RONS generation at 2 and 4 h (46%) (see Figure. S1, supplemental materials).
Effect of BAPTA on ammonia-induced astrocyte swelling
Astrocyte cultures were pretreated (15 min) with BAPTA (25 μM) and cell volume was measured 24 h after exposure to ammonia. Ammonia treatment resulted in a 55% (p<0.01) increase in cell volume which was almost completely blocked by BAPTA (90%, p<0.01) (see Figure. S2, supplemental materials).
Effect of inhibitors of cNOS, NOX and cPLA2 on ammonia-induced astrocyte swelling
Astrocyte cultures were pretreated (15 min) with 7-nitroindazole (7-NI), apocyanin (100–500 μM) or quinacrine (10 μM). Ammonia was added and cell volume was determined at 24 h. Treatment with 7-NI showed a 40% (p<0.05) blockade of astrocyte swelling, whereas apocyanin and quinacrine completely blocked the cell swelling (see Figure. S3, supplemental materials).
DISCUSSION
Brain edema is an important complication of acute ammonia neurotoxicity and ALF (for review, see Blei 1991). Astrocyte swelling (cytotoxic edema) appears to be the major component of brain edema in ALF (Martinez, 1968; Traber et al. 1989, Kato et al. 1992). While mechanisms mediating astrocyte swelling in ALF are not clear, we have recently shown that oxidative/nitrosative stress is involved in such swelling (Jayakumar et al. 2006). Factors responsible for the increase in RONS after ammonia treatment, however, remain poorly understood.
Several studies have shown that a transient rise in [Ca2+]i is one of the earliest events in cultured astrocytes exposed to ammonia (Schliess et al. 2002, Norenberg et al. 2003, Rose 2006). One consequence of elevated levels of [Ca2+]i is the formation of RONS (Chinopoulos and Adam-Vizi 2006). The present study demonstrates that astrocyte cultures exposed to ammonia increased RONS formation, and that such increase was significantly diminished by BAPTA, suggesting that a rise in [Ca2+]i was a major factor in the generation of RONS by ammonia.
While the mechanisms by which elevated levels of [Ca2+]i leads to RONS formation is not well known, several studies have shown that increased [Ca2+]i can activate NOX (Suzuki et al. 1985), and that such activation results in the production of superoxide and H2O2 (Nakamura et al. 1989). It has been previously shown that exposure of astrocyte cultures to ammonia stimulates the production of RONS (Murthy et al. 2001), and that RONS production was diminished by superoxide dismutase and catalase (Murthy et al. 2001), suggesting that among the free radicals generated by ammonia include superoxide and H2O2. The present study demonstrates that exposure of astrocyte cultures to ammonia significantly increased NOX activity and that treatment of cultures with the NOX inhibitor apocyanin significantly diminished ammonia-induced RONS production. This is in agreement with the findings of Reinehr et al. (2007) who reported increased levels of the NOX subunit p47(Phox) in astrocytes exposed to ammonia, and that the NOX inhibitor apocynin blocked RONS formation.
Another enzyme that can be activated by increased [Ca2+]i is cNOS (Mayer et al. 1990). Our study demonstrating that BAPTA significantly blocked ammonia-induced cNOS activity, indicate that the increase in [Ca2+]i also contributes to NO production. Additionally, astrocyte cultures exposed to 7-nitroindazole (7-NI), an inhibitor of cNOS completely blocked the ammonia-induced RONS formation. Consistent with these findings, various studies have shown that NOS activity, gene expression and NO levels are increased in experimental models of chronic HE (Rao et al. 1995; Master et al. 1999).
Increased [Ca2+]i is also known to activate PLA2 (Kramer and Sharp 1997). The mechanism by which PLA2 leads to the formation of RONS is likely through the increased production of arachidonic acid (AA). AA is known to produce various free radicals, including superoxide and hydrogen peroxide, presumably though AA’s inhibition of Complex I of the electron transport chain (Cocco et al. 1999). In the present study, we found that astrocyte cultures exposed to ammonia increased PLA2 activity and that BAPTA significantly blocked this activity. Additionally, astrocytes exposed to quinacrine, an inhibitor of PLA2, significantly diminished the ammonia-induced RONS generation.
The present study demonstrates that an early rise in [Ca2+]i appears to be responsible for the activation of cNOS, NOX and PLA2 following ammonia treatment, as this activation was blocked by BAPTA. Whether such effects of Ca2+ are direct or indirect, however, are not known. It is possible that a transient rise in [Ca2+]i could activate intracellular signaling pathways, which in turn, may stimulate the activities of these Ca2+-dependent enzymes. For example, cPLA2 has a binding domain for Ca2+ and its catalytic activity is significantly increased when this enzyme is phosphorylated by MAPKs (Hirabayashi and Shimizu 2000).
While our current data strongly supports the view that activation of Ca2+-dependent enzymes leading to ONS represents one mechanism for the ammonia-induced astrocyte swelling, the means by which Ca2+ and ONS results in such swelling is not completely understood. Excess Ca2+ is known to inhibit the mitochondrial electron transport chain leading to increased free radical production and associated ONS (Chinopoulos and Adam-Vizi 2006). It is also well known that ONS and arachidonic acid are important factors for the induction of the mPT (Scorrano et al. 2001), a phenomenon characterized by a sudden increase in the permeability of the inner mitochondrial membrane due to the opening of the permeability transition pore. This results in the collapse of inner mitochondrial membrane potential, leading to defective oxidative phosphorylation and free radical production (for reviews on the mPT, see (Zoratti and Szabo 1995). Induction of the mPT can lead to mitochondrial dysfunction and bioenergetic failure (Zoratti and Szabo 1995). Cell volume regulation is an energy-dependent process due to the operation of various ionic transport systems and extrusion of the osmotically active amino acids, all of which require energy (Kimelberg et al. 1993, Olson et al. 1992). Such mitochondrial dysfunction and associated disturbance in energy metabolism by ammonia, conceivably resulting from Ca2+, ONS and the mPT, may perturb astrocyte cell volume regulation and lead to cell swelling.
Consistent with the involvement of ionic transport systems and RONS in ammonia-induced astrocyte swelling, we have recently found that astrocyte cultures exposed to ammonia increased the activation of the Na-K-Cl cotransporter-1 (NKCC1) (Jayakumar et al. 2008), an ion transporter involved in cell volume regulation, Additionally, we found that oxidation and nitration of NKCC1 increased its activity. One may speculate that membrane proteins that are critically involved in the regulation of cell volume may become modified by oxidation/nitration, and that such protein modification may lead to their dysfunction and to cell swelling.
In summary, our study shows that an early increase in [Ca2+]i is an important factor in the mechanism of RONS production and cell swelling in astrocytes after exposure to ammonia. The increase in RONS, in part, appears to be a consequence of an ammonia-induced activation of certain Ca2+-dependent enzymes (cNOS, NOX and cPLA2). Inhibition of these enzymes, as well as treatment with BAPTA diminished RONS formation and cell swelling in cultured astrocytes. These studies highlight a critical role of Ca2+ in the generation of RONS, and in the development of the astrocyte swelling associated with ammonia neurotoxicity.
Supplementary Material
Effect of Ca2+-dependent enzyme blockers on ammonia-induced RONS production. All inhibitors were added 15 min before exposure to ammonia (5 mM NH4Cl) and RONS production was determined at 3 min and 2h after ammonia exposure. Values are expressed as arbitrary fluorescence intensity units (AFIU) of DCFDA per mg cell protein. *p<0.01 vs. control; **p<0.01 vs. NH4+ (NH4Cl). C, control; 7-NI, 7-nitroindazole, inhibitor of cNOS; APO, apocyanin, inhibitor of NADPH oxidase; QNC, quinacrine, inhibitor of PLA2.
Effect of BAPTA on ammonia-induced astrocyte cell volume. Cultures were pretreated with BAPTA/AM (25 μM) prior to exposure to ammonia (5 mM NH4Cl) and cell volume was determined 24 h after ammonia treatment. *p<0.01 vs. control; **p<0.01 vs. NH4+.
Effect of cNOS, NOX and PLA2 inhibitors on ammonia-induced astrocyte cell volume. All inhibitors were added 15 min before exposure to ammonia (5 mM NH4 Cl) and cell volume was determined 24 h later. *p<0.01 vs. control; **p<0.01 vs. NH4 (5 mM NH4Cl). C, control; 7-NI, 7-nitroindazole, inhibitor of cNOS; APO, apocyanin, inhibitor of NADPH oxidase; QNC (5 μM), quinacrine, inhibitor of PLA2.
Acknowledgments
This work was supported by the Department of Veterans Affairs and NIH Grant No. DK063311. ARJ is supported by the American Association for the Study of Liver Disease/American Liver Foundation Grant. We thank Alina Fernandez-Revuelta for the preparation of cell cultures.
Abbreviations
- ALF
acute liver failure
- AA
arachidonic acid
- APO
apocyanin
- BAPTA-AM
1,2-bis-(o-aminophenoxy)-ethane-N,N,-N′,N′-tetraacetic acid tetraacetoxy-methyl ester
- BSA
bovine serum albumin
- cNOS
constitutive nitric oxide synthase
- cPLA2
cytosolic phopholipase A2
- DMEM
Dulbecco’s modified Eagle’s medium
- DTNB
5,5′-dinitrobis-(2-dinitrobenzoic acid
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- FAD
flavin adenine dinucleotide
- HE
hepatic encephalopathy
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- iNOS
inducible nitric oxide synthase
- MAPKs
mitogen-activated protein kinases
- mPT
mitochondrial permeability transition
- NOX
NADPH oxidase
- 7-NI
7-nitroindazole
- NO
nitric oxide
- NOS
nitric oxide synthase
- OMG
O-methyl-[3H]glucose
- ONS
oxidative/nitrosative stress
- QNC
quinacrine
- RONS
reactive oxygen/nitrogen species
References
- Blei AT. Cerebral edema and intracranial hypertension in acute liver failure: distinct aspects of the same problem. Hepatology. 1991;13:376–379. [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capocaccia L, Angelico M. Fulminant hepatic failure. Clinical features, etiology, epidemiology, and current management. Dig Dis Sci. 1991;36:775–779. doi: 10.1007/BF01311236. [DOI] [PubMed] [Google Scholar]
- Chan PH, Longar S, Chen S, Yu AC, Hillered L, Chu L, Imaizumi S, Pereira B, Moore K, Woolworth V. The role of arachidonic acid and oxygen radical metabolites in the pathogenesis of vasogenic brain edema and astrocytic swelling. Ann N Y Acad Sci. 1989;559:237–247. doi: 10.1111/j.1749-6632.1989.tb22612.x. [DOI] [PubMed] [Google Scholar]
- Chan PH, Yurko M, Fishman RA. Phospholipid degradation and cellular edema induced by free radicals in brain cortical slices. J Neurochem. 1982;38:525–531. doi: 10.1111/j.1471-4159.1982.tb08659.x. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Liao SL, Kuo JS. Gliotoxic action of glutamate on cultured astrocytes. J Neurochem. 2000;75:1557–1565. doi: 10.1046/j.1471-4159.2000.0751557.x. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648–653. doi: 10.1002/hep.510290309. [DOI] [PubMed] [Google Scholar]
- Cocco T, Di Paola M, Papa S, Lorusso M. Arachidonic acid interaction with the mitochondrial electron transport chain promotes reactive oxygen species generation. Free Radic Biol Med. 1999;27:51–59. doi: 10.1016/s0891-5849(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Ducis I, Norenberg LO, Norenberg MD. The benzodiazepine receptor in cultured astrocytes from genetically epilepsy-prone rats. Brain Res. 1990;531:318–321. doi: 10.1016/0006-8993(90)90793-b. [DOI] [PubMed] [Google Scholar]
- Ganz R, Swain M, Traber P, DalCanto M, Butterworth RF, Blei AT. Ammonia-induced swelling of rat cerebral cortical slices: implications for the pathogenesis of brain edema in acute hepatic failure. Metab Brain Dis. 1989;4:213–223. doi: 10.1007/BF01000297. [DOI] [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A2. Biochim Biophys Acta. 2000;1488:124–138. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B, 3rd, Reddy PV, Norenberg MD. Na-K-Cl cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J Biol Chem. 2008 doi: 10.1074/jbc.M804016200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Murthy CR, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Hughes RD, Keays RT, Williams R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology. 1992;15:1060–1066. doi: 10.1002/hep.1840150615. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Anisotonic media and glutamate-induced ion transport and volume responses in primary astrocyte cultures. J Physiol (Paris) 1987;82:294–303. [PubMed] [Google Scholar]
- Kimelberg HK, Bonville DJ, Goderie SK. Use of 51Cr cell labelling to distinguish between release of radiolabelled amino acids from primary astrocyte cultures being due to efflux or cell damage. Brain Res. 1993;622:237–242. doi: 10.1016/0006-8993(93)90824-7. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Pariza MW, Becker JE, Potter VR. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975;68:537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Kaminsky Y, Kaminsky A, Valencia M, Lee L, Hermenegildo C, Felipo V. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res. 1997;27:637–644. doi: 10.3109/10715769709097867. [DOI] [PubMed] [Google Scholar]
- Kramer RM, Sharp JD. Structure, function and regulation of Ca2+-sensitive cytosolic phospholipase A2 (cPLA2) FEBS Lett. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- Martinez A. Electron microscopy in human hepatic encephalopathy. Acta Neuropathol (Berl) 1968;11:82–86. doi: 10.1007/BF00692797. [DOI] [PubMed] [Google Scholar]
- Master S, Gottstein J, Blei AT. Cerebral blood flow and the development of ammonia-induced brain edema in rats after portacaval anastomosis. Hepatology. 1999;30:876–880. doi: 10.1002/hep.510300428. [DOI] [PubMed] [Google Scholar]
- Mayer B, John M, Bohme E. Purification of a Ca2+/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor-role of tetrahydrobiopterin. FEBS Lett. 1990;277:215–219. doi: 10.1016/0014-5793(90)80848-d. [DOI] [PubMed] [Google Scholar]
- Murthy CR, Rama Rao KV, Bai G, Norenberg MD. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:282–288. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ohtaki S, Makino R, Tanaka T, Ishimura Y. Superoxide anion is the initial product in the hydrogen peroxide formation catalyzed by NADPH oxidase in porcine thyroid plasma membrane. J Biol Chem. 1989;264:4759–4761. [PubMed] [Google Scholar]
- Norenberg MD. Astrocytic-ammonia interactions in hepatic encephalopathy. Sem Liver Dis. 1996;16:245–253. doi: 10.1055/s-2007-1007237. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Oxidative and nitrosative stress in ammonia neurotoxicity. Hepatology. 2003;37:245–248. doi: 10.1053/jhep.2003.50087. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT. Ammonia-induced astrocyte swelling in primary culture. Neurochem Res. 1991;16:833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis. 2007;22:219–234. doi: 10.1007/s11011-007-9062-5. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rama Rao KV, Jayakumar AR. Mechanisms of ammonia-induced astrocyte swelling. Metab Brain Dis. 2005;20:303–318. doi: 10.1007/s11011-005-7911-7. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Rama Rao KV, Jayakumar AR. The mitochondrial permeability transition in ammonia neurotoxicity. In: Jones E, Meijer AJ, Chamuleau AF, editors. Encephalopathy and Nitrogen Metabolism in Liver Failure. Kluwer; Dordrecht: 2003. pp. 267–286. [Google Scholar]
- Olson JE, Evers JA, Holtzman D. Astrocyte volume regulation and ATP and phosphocreatine concentrations after exposure to salicylate, ammonium, and fatty acids. Metab Brain Dis. 1992;7:183–196. doi: 10.1007/BF01000245. [DOI] [PubMed] [Google Scholar]
- Poulsen KA, Pedersen SF, Kolko M, Lambert IH. Induction of group VIA phospholipase A2 activity during in vitro ischemia in C2C12 myotubes is associated with changes in the level of its splice variants. Am J Physiol Cell Physiol. 2007;293:C1605–1615. doi: 10.1152/ajpcell.00012.2007. [DOI] [PubMed] [Google Scholar]
- Rao VL, Audet RM, Butterworth RF. Increased nitric oxide synthase activities and L-[3H]arginine uptake in brain following portacaval anastomosis. J Neurochem. 1995;65:677–678. doi: 10.1046/j.1471-4159.1995.65020677.x. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Görg B, Becker S, Qvartskhava N, Bidmon HJ, Selbach O, Haas HL, Schliess F, Häussinger D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia. 2007;55:758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J Biol Chem. 2005;280:20937–20944. doi: 10.1074/jbc.M412448200. [DOI] [PubMed] [Google Scholar]
- Schliess F, Görg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, Butterworth RF, Zilles K, Häussinger D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J. 2002;16:739–741. doi: 10.1096/fj.01-0862fje. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Pabst MJ, Johnston RB., Jr Enhancement by Ca2+ or Mg2+ of catalytic activity of the superoxide-producing NADPH oxidase in membrane fractions of human neutrophils and monocytes. J Biol Chem. 1985;260:3635–3639. [PubMed] [Google Scholar]
- Swain M, Butterworth RF, Blei AT. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology. 1992;15:449–453. doi: 10.1002/hep.1840150316. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Koehler RC, Brusilow SW, Traystman RJ. Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am J Physiol. 1991;261:H825–829. doi: 10.1152/ajpheart.1991.261.3.H825. [DOI] [PubMed] [Google Scholar]
- Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67:1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- Traber P, DalCanto M, Ganger D, Blei AT. Effect of body temperature on brain edema and encephalopathy in the rat after hepatic devascularization. Gastroenterology. 1989;96:885–891. [PubMed] [Google Scholar]
- Widmer R, Kaiser B, Engels M, Jung T, Grune T. Hyperammonemia causes protein oxidation and enhanced proteasomal activity in response to mitochondria-mediated oxidative stress in rat primary astrocytes. Arch Biochem Biophys. 2007;464:1–11. doi: 10.1016/j.abb.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Zielinska M, Law RO, Albrecht J. Excitotoxic mechanism of cell swelling in rat cerebral cortical slices treated acutely with ammonia. Neurochem Int. 2003;43:299–303. doi: 10.1016/s0197-0186(03)00015-9. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of Ca2+-dependent enzyme blockers on ammonia-induced RONS production. All inhibitors were added 15 min before exposure to ammonia (5 mM NH4Cl) and RONS production was determined at 3 min and 2h after ammonia exposure. Values are expressed as arbitrary fluorescence intensity units (AFIU) of DCFDA per mg cell protein. *p<0.01 vs. control; **p<0.01 vs. NH4+ (NH4Cl). C, control; 7-NI, 7-nitroindazole, inhibitor of cNOS; APO, apocyanin, inhibitor of NADPH oxidase; QNC, quinacrine, inhibitor of PLA2.
Effect of BAPTA on ammonia-induced astrocyte cell volume. Cultures were pretreated with BAPTA/AM (25 μM) prior to exposure to ammonia (5 mM NH4Cl) and cell volume was determined 24 h after ammonia treatment. *p<0.01 vs. control; **p<0.01 vs. NH4+.
Effect of cNOS, NOX and PLA2 inhibitors on ammonia-induced astrocyte cell volume. All inhibitors were added 15 min before exposure to ammonia (5 mM NH4 Cl) and cell volume was determined 24 h later. *p<0.01 vs. control; **p<0.01 vs. NH4 (5 mM NH4Cl). C, control; 7-NI, 7-nitroindazole, inhibitor of cNOS; APO, apocyanin, inhibitor of NADPH oxidase; QNC (5 μM), quinacrine, inhibitor of PLA2.