Abstract
Hepatic encephalopathy (HE) is major neuropsychiatric disorder occurring in patients with severe liver disease and ammonia is generally considered to represent the major toxin responsible for this condition. Ammonia in brain is chiefly metabolized (“detoxified”) to glutamine in astrocytes due to predominant localization of glutamine synthetase in these cells. While glutamine has long been considered innocuous, a deleterious role more recently has been attributed to this amino acid. This article reviews the mechanisms by which glutamine contributes to the pathogenesis of HE, how glutamine is transported into mitochondria and subsequently hydrolyzed leading to high levels of ammonia, the latter triggering oxidative and nitrative stress, the mitochondrial permeability transition and mitochondrial injury, a sequence of events we have collectively termed as the Trojan horse hypothesis of hepatic encephalopathy.
Keywords: Ammonia, astrocytes, glutamine, glutaminase, hepatic encephalopathy, L-histidine, 6-diazo-5-oxo-norleueine (DON), mitochondrial permeability transition, oxidative stress
Introduction
Hepatic encephalopathy (HE) is the major neurological disorder associated with severe liver disease which presents in chronic and acute forms [1]. Complications of chronic HE (usually in the setting of alcohol-induced cirrhosis) are principally neuropsychiatric abnormalities characterized by personality disorders, altered mood, increased irritability, changes in sleep/wake cycles, decline in intellectual capacity and abnormal muscle tone [2].
The symptoms of acute HE (acute liver failure, ALF), on the other hand, progress more rapidly, wherein patients present with seizures, delirium, alterations in the level of consciousness and coma [3]. The major manifestation of ALF is brain edema (increased brain water content) resulting in increased intracranial pressure (ICP) and brain herniation. While acute HE has been associated with a high mortality (80–90%) [3,4], recent surveys have reported a somewhat lower mortality rate (approximately 60%) [5,6], likely due to better clinical management of this condition. ALF is usually a consequence of acetaminophen toxicity, viral-mediated hepatitis, or exposure to various hepatotoxins [7]. The major etiological factor in both chronic and acute HE is increased blood and brain levels of ammonia.
The pathogenetic manifestations of ALF primarily develop in astrocytes, as these are the cells that are most affected histopathologically [8]. This is likely due to the fact that ammonia is exclusively metabolized in these cells by glutamine synthetase, which converts ammonia and glutamate into glutamine [9]. Accordingly, high brain and CSF levels of glutamine are also characteristic features of HE [10–12].
Ammonia has been shown to result in oxidative/nitrative stress, mitochondrial dysfunction, and alterations in the activity of various metabolic signaling pathways, including activation of mitogen activated protein kinases and the transcriptional factors (NF-κB, p53) as well as cerebral inflammation, and all of these factors have been shown to contribute to the cerebral complications of ammonia toxicity and ALF [13–16]. Thus, increased production of reactive oxygen and nitrogen species, lipid peroxidation, oxidation of mRNA, oxidation/nitration of key astrocytic proteins, and induction of the mitochondrial permeability transition (mPT) have been reported in experimental models of ALF, as well as in cultured astrocytes exposed to a pathophysiological concentration of ammonia [13,14,17,18]. Additionally, strategies geared towards a reduction of the above abnormalities have been shown to exert beneficial effects in ALF [18, 19]. More recently, most of the above noted astrocytic abnormalities have been attributed to glutamine as a consequence of the ammonia “detoxification” process [20,21].
This article reviews the Trojan horse hypothesis in HE whereby glutamine is transported into mitochondria, where it is subsequently hydrolyzed to yield high levels of ammonia, ultimately resulting in the induction of the mPT, mitochondrial dysfunction and the generation of oxidative/nitrative stress (ONS).
Historical perspective on glutamine in the pathogenesis of HE
Early in the evolution of the role of ammonia in the pathogenesis of hepatic encephalopathy [22–24], a seminal finding by Warren and Schenker [25] documented that methionine sulfoximine (MSO), an inhibitor of glutamine synthetase, significantly protected mice from acute ammonia toxicity, including a lowering of the seizure threshold, prevention of coma and improvement of their survival. These investigators thus proposed that glutamine may be a harmful factor in the pathogenesis of HE. Subsequent studies by other groups disclosed that MSO normalized the decreased glucose utilization in a rat model of chronic HE [26], and restored altered vascular CO2 responsiveness [27] in a rat model of chronic HE. MSO was also shown to prevent the cytotoxic edema in experimental models of HE and hyperammonemia [28–30], as well as the cell swelling in cultured astrocytes following exposure to ammonia [31]. These critical studies highlighted the crucial role of glutamine in the pathogenesis of HE.
Mechanisms by which glutamine may contribute to brain edema/astrocyte swelling are not clear. A commonly held view is that the accumulation of glutamine leads to an osmotic shift of water into astrocytes [32]. While glutamine is indeed an osmolyte [33], it remains to be proven whether this amino acid is responsible for the osmotic shift of water into neural cells (principally astrocytes). Noteworthy, there is data showing a lack of temporal correlation of astrocyte glutamine concentration with the extent of cell swelling, as well as reports documenting the reduction of brain edema by various modalities, in the absence of a commensurate reduction in cerebral glutamine concentrations [34–36]. Furthermore, one study showed no temporal correlation between glutamine concentration and the extent of cell swelling in cultured astrocytes following treatment with ammonia [37]. Thus, while glutamine appears to play a crucial role in the mechanism of ALF, it is unlikely that it does so by an osmotic effect.
Recently, another view on the osmotic aspects of glutamine in the production of brain edema has been proposed. According to this hypothesis, glutamine is synthesized in astrocytes during the process of ammonia removal, released into the brain extracellular space via the small neutral amino acid transporter 5 (SNAT5), and then taken up by neurons to generate glutamate. A defect in the inter-cellular trafficking of glutamine between neurons and astrocytes has been postulated to occur which may contribute to the pathogenesis of ALF [38]. This proposal was based on the observation that mRNA levels of SNAT5 were found to be reduced in cerebral cortex of rats with ALF [38], a process postulated to result in the accumulation of glutamine in astrocytes, ultimately leading to cell swelling by an osmotic effect.
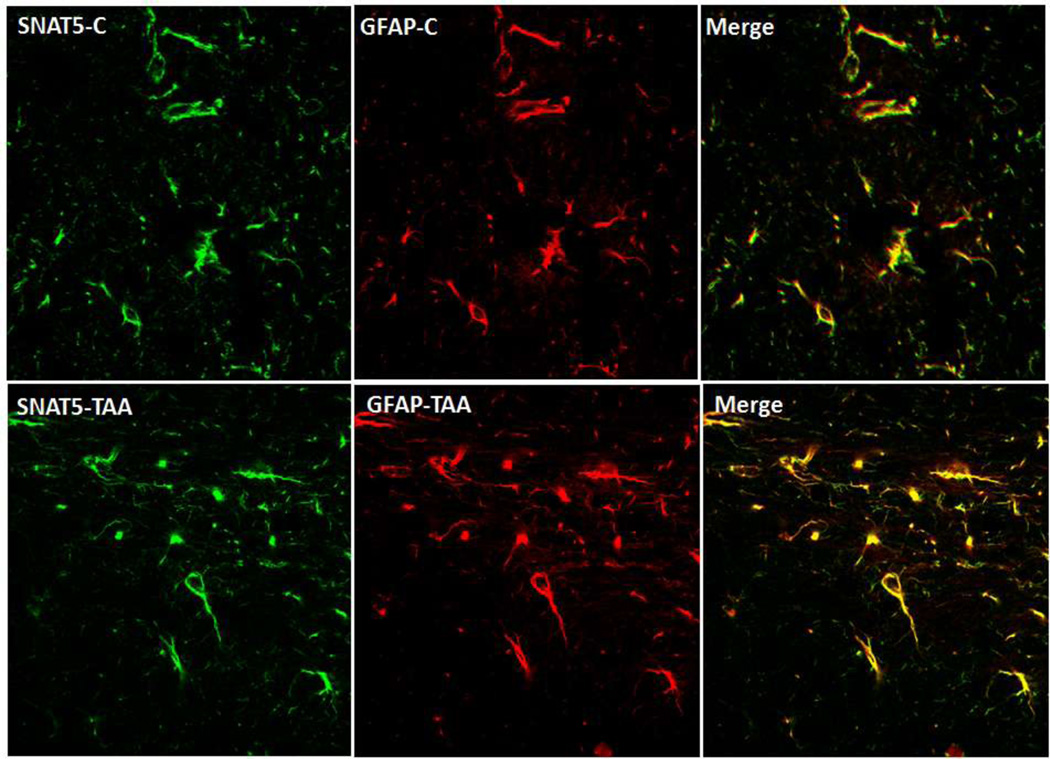
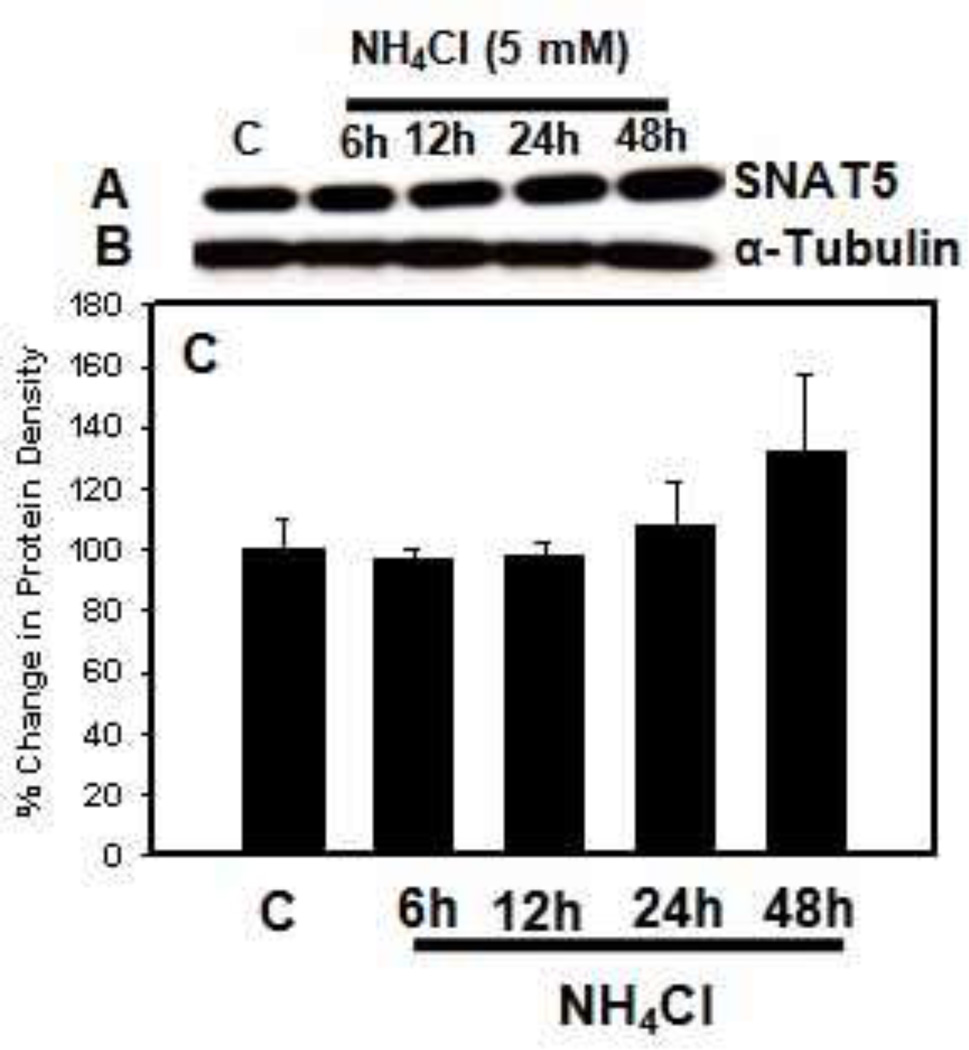
This view, however, is at variance with the well known fact that extracellular glutamine levels in brain are increased by over 5-fold in patients and in experimental models of ALF [11,39,40]. Accordingly, if high levels of glutamine were to be achieved in astrocytes, brain extracellular levels of glutamine would have shown a commensurate reduction (since astrocytes are the major source of extracellular glutamine in brain). This proposal, moreover, relied on a reduction in mRNA levels of SNAT5; yet, a comparable reduction in SNAT5 protein was never documented. In recent studies, we in fact found that protein levels of SNAT5 were unchanged in cerebral cortical sections of mice with ALF induced by hepatotoxin thioacetamide (TAA) (Figure 2). Likewise, cultured astrocytes treated with a pathophysiological concentration of ammonia (5 mM NH4Cl) did not result in any change in SNAT5 protein expression (Figure 3).
Figure 2.
Immunohistochemistry (IHC) of the glutamine transporter SNAT5 (green fluorescence) in brain cortical sections from control and mice treated with hepatotoxin thioacetamide (TAA) to induce ALF . Immunohistochemistry of frozen brain sections were performed as described in Figure 1. The antibodies used included SNAT5 (goat polyclonal, 1:100) and GFAP (red fluorescence, rabbit polyclonal, 1:400). Note that SNAT5 expression did not change in mice with TAA-induced ALF as compared to control animals (SNAT5-C).
Figure 3.
Effect of ammonia (5 mM NH4Cl) treatment to cultured astrocytes on SNAT5 protein expression. A. Representative immunoblot of SNAT5 protein density. B. Immunoblot of tubulin protein density (loading control) corresponding to the immunoblot of SNAT5. C. Quantification of SNAT5 protein densities. Note that ammonia treatment of astrocytes had no significant effect on SNAT5 expression at any time point. Values in each group are mean ± S.E.M of 2 individual culture plates taken from 2 separate seeding batches (n=4).
The Trojan horse hypothesis
In 2006, an alternate mechanism was proposed whereby glutamine may result in harmful effects in brain, the so called Trojan horse hypothesis. Fundamental studies prior to formulating this hypothesis were carried out by Zieminska et al. [41] in isolated mitochondria from rat brain wherein, glutamine caused a marked Ca2+-dependent mitochondria swelling (mPT), which was sensitive to cyclosporine A (CsA), an inhibitor of the mPT [41]. Notably, the direct exposure of ammonia to mitochondria did not elicit the mPT [41]. These observations were subsequently extended with the use of cultured astrocytes. We found that glutamine also resulted in the induction of the mPT in these cultures [42]. Further studies on mechanisms by which glutamine, presumably an innocuous amino acid, induces the mPT, disclosed that glutamine is transported into mitochondria where it undergoes hydrolysis, thereby yielding high levels of ammonia; the latter triggers oxidative stress and mitochondrial dysfunction which ultimately leads to the mPT and astrocyte swelling [42], a process that was blocked by MSO [43].
While the results documenting the mitigation of the mPT and oxidative stress by MSO implied the involvement of glutamine in this process, the precise mechanisms by which such protection occurred was not apparent. In order for glutamine to exert mitochondrial abnormalities, it must first be carried into mitochondria, where it undergoes hydrolysis by phosphate-activated glutaminase (PAG) to generate ammonia and glutamate. Accordingly, studies employing L-histidine, an inhibitor of glutamine transport into mitochondria, showed a significant attenuation in the ammonia-induced mPT and oxidative stress. Likewise, 6-diazo-5-oxo-L-norleucine (DON), an inhibitor of phosphate-activated glutaminase (PAG), blocked the mPT and free radical production by ammonia. This clearly established a Trojan horse role for glutamine in the mechanism of ammonia neurotoxicity, whereby glutamine enters mitochondria, followed by its hydrolysis that yields toxic levels of ammonia in the organelle. For further details on the Trojan horse hypothesis, see references [20,21,44].
Studies showing inhibition by DON and L-histidine in the activation of mitogen-activated protein kinases (MAPKs) also support this hypothesis [21]. Likewise, some of the astrocytic abnormalities caused by ammonia, including activation of the transcriptional factors NF-κB, p53, as well as the decreased uptake of glutamate were rectified by treatment with either DON or L-histidine, all of which support the Trojan horse hypothesis.
This hypothesis is also in keeping with in vivo conditions in ALF induced by hepatotoxin TAA. L-histidine treatment of rats with ALF significantly blocked the induction of oxidative stress, the mPT and the development of brain edema [45]. A recent study also disclosed that reduction of mitochondrial glutathione levels in brains of rats with ALF contribute to the induction of oxidative stress [46], a phenomenon that was attenuated by treatment with L-histidine.
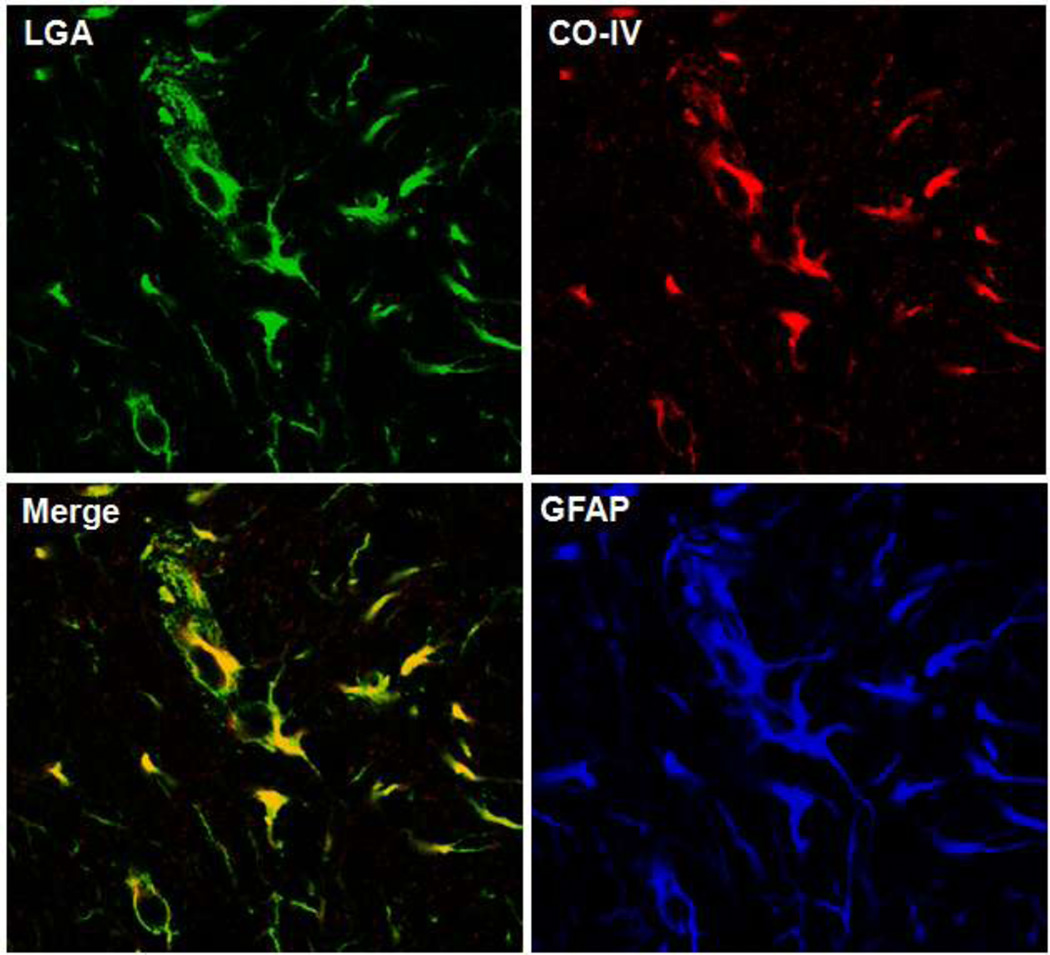
While the Trojan horse hypothesis represents a major mechanism by which glutamine mediates the deleterious effects of ammonia in HE, concerns have been raised as to its validity [47]. The presence of glutaminase in astrocytes, a key factor implicated in the Trojan horse hypothesis, was questioned, since glutaminase was generally considered to be exclusively present in neurons and brain levels of PAG were reported to be relatively low [48]. However, a study by Wurdig and Kugler [49] clearly identified PAG rat brain astrocytes by enzyme histochemistry. Likewise, cultured astrocytes were shown to express abundant quantities of PAG [50–52], which subsequently was shown to be localized in mitochondria isolated from cultured astrocytes [53]. A subsequent study unequivocally confirmed the presence of L-type glutaminase (LGA) in astrocytes in rat brain [54]. Employing cortical sections of mouse brain, we also localized LGA in astrocytic mitochondria as observed by triple immunocytochemistry (Figure 1). Together, these reports clearly document the presence of LGA in astrocytic mitochondria, a finding that strongly is in keeping with the Trojan horse mechanism.
Figure 1.
Immunohistochemistry (IHC) of L-type glutaminase (LGA) (green fluorescence) in normal mouse brain cortical sections. Frozen brain sections were performed as described previously [45]. Briefly, sections were fixed in ice-cold methanol and incubated overnight at 4°C with antibodies to LGA (goat polyclonal, 1:100), cytochrome oxidase subunit IV (red fluorescence, CO-IV, mouse monoclonal, 1:100) and GFAP (blue fluorescence, rabbit polyclonal, 1:400); washed 3-times with phosphate-buffered saline containing 0.1% Triton X 100; incubated with fluorescent secondary antibodies with different excitation wavelengths; and fluorescence was visualized with a confocal microscope. Note the merged image showing marked co-localization of LGA with CO-IV consistent with the mictochondrial localization of LGA.
Concluding remarks
The Trojan horse hypothesis continues to represent a major mechanism by which glutamine contributes to the pathogenesis of HE. It postulates that glutamine is transported into mitochondria, where it undergoes hydrolysis to yield high levels of ammonia, resulting in deleterious effects, including induction of the mitochondrial permeability transition and oxidative/nitrative stress. Accordingly, inhibition of glutamine synthesis, inhibition of glutamine transport into mitochondria, or reducing the activity of phosphate-activated glutaminase were all found to exert beneficial effects in ALF, as shown by a reduction in astrocyte swelling/brain edema, improvement of mitochondrial function, enhanced cerebral energy metabolism, increased astrocytic glutamate uptake, as well as inhibition of the activity of detrimental signaling mechanisms. We propose that targeting astrocytic glutamine transport and/or its hydrolysis in mitochondria remains an attractive strategy for the treatment of HE and other hyperammonemic disorders.
Acknowledgments
This work was supported by NIH Grant DK06331 and by a Department of Veterans Affairs Merit Review Award. The contributions of Drs. Arumugam Jayakumar and Pichili V.B. Reddy to the development of Trojan horse hypothesis are gratefully acknowledged. The helpful discussions with Dr. Javier Marquez, University of Malaga, relative to the localization of PAG in astrocytic mitochondria are gratefully acknowledged.
References
- 1.Williams R. Hepatic encephalopathy. J R Coll Physicians Lond. 1973;8:63–74. [PMC free article] [PubMed] [Google Scholar]
- 2.Jones EA, Weissenborn K. Neurology and the liver. J Neurol Neurosurg Psychiatry. 1997;63:279–293. doi: 10.1136/jnnp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capocaccia L, Angelico M. Fulminant hepatic failure. Clinical features, etiology, epidemiology, and current management. Dig Dis Sci. 1991;36:775–779. doi: 10.1007/BF01311236. [DOI] [PubMed] [Google Scholar]
- 4.Hoofnagle JH, Carithers RL, Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 5.Escorsell A, Mas A, de la Mata M. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl. 2007;13:1389–1395. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]
- 6.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee WM. Acute liver failure. Am J Med. 1994;96:3S–9S. doi: 10.1016/0002-9343(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 8.Norenberg MD. The role of astrocytes in hepatic encephalopathy. Neurochem Pathol. 1987;6:13–33. doi: 10.1007/BF02833599. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 10.Hourani BT, Hamlin EM, Reynolds TB. Cerebrospinal fluid glutamine as a measure of hepatic encephalopathy. Arch Intern Med. 1971;127:1033–1036. [PubMed] [Google Scholar]
- 11.Record CO, Buxton B, Chase RA, Curzon G, Murray-Lyon IM, Williams R. Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976;6:387–394. doi: 10.1111/j.1365-2362.1976.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 12.McConnell JR, Antonson DL, Ong CS, Chu WK, Fox IJ, Heffron TG, Langnas AN, Shaw BW., Jr Proton spectroscopy of brain glutamine in acute liver failure. Hepatology. 1995;22:69–74. [PubMed] [Google Scholar]
- 13.Norenberg MD, Rao KV, Jayakumar AR. Mechanisms of ammonia-induced astrocyte swelling. Metab Brain Dis. 2005;20:303–318. doi: 10.1007/s11011-005-7911-7. [DOI] [PubMed] [Google Scholar]
- 14.Norenberg MD, Jayakumar AR, Rama Rao KV, Panickar KS. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis. 2007;22:219–234. doi: 10.1007/s11011-007-9062-5. [DOI] [PubMed] [Google Scholar]
- 15.O'Grady JG, Williams R. Management of acute liver failure. Schweiz Med Wochenschr. 1986;116:541–544. [PubMed] [Google Scholar]
- 16.Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–739. doi: 10.1053/jhep.2000.17687. [DOI] [PubMed] [Google Scholar]
- 17.Haussinger D, Schliess F. Astrocyte swelling and protein tyrosine nitration in hepatic encephalopathy. Neurochem Int. 2005;47:64–70. doi: 10.1016/j.neuint.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Haussinger D, Gorg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care. 2010;13:87–92. doi: 10.1097/MCO.0b013e328333b829. [DOI] [PubMed] [Google Scholar]
- 19.Norenberg MD, Rama Rao KV, Jayakumar AR. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis. 2009;24:103–117. doi: 10.1007/s11011-008-9113-6. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44:788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- 21.Rama Rao KV, Jayakumar AR, Norenberg MD. Glutamine in the pathogenesis of acute hepatic encephalopathy. Neurochem Int. 2012;61:575–580. doi: 10.1016/j.neuint.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Pearlman DM. Ammonia metabolism and hepatic coma. N Y State J Med. 1957;57:3162–3166. [PubMed] [Google Scholar]
- 23.Walshe JM, De Carli L, Davidson CS. Some factors influencing cerebral ammonia production in relation to hepatic coma. Clin Sci (Lond) 1958;17:27–36. [PubMed] [Google Scholar]
- 24.McDermot W. The role of ammonia intoxication in hepatic coma. Bull N Y Acad Med. 1958;34:357–365. [PMC free article] [PubMed] [Google Scholar]
- 25.Warren KS, Schenker S. Effect of an Inhibitor of Glutamine Synthesis (Methionine Sulfoximine) on Ammonia Toxicity and Metabolism. J Lab Clin Med. 1964;64:442–449. [PubMed] [Google Scholar]
- 26.Hawkins RA, Jessy J. Hyperammonaemia does not impair brain function in the absence of net glutamine synthesis. Biochem J. 1991;277(Pt 3):697–703. doi: 10.1042/bj2770697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Koehler RC, Hirata T, Brusilow SW, Traystman RJ. Restoration of cerebrovascular CO2 responsivity by glutamine synthesis inhibition in hyperammonemic rats. Circ Res. 1992;71:1220–1230. doi: 10.1161/01.res.71.5.1220. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi H, Koehler RC, Brusilow SW, Traystman RJ. Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am J Physiol. 1991;261:H825–H829. doi: 10.1152/ajpheart.1991.261.3.H825. [DOI] [PubMed] [Google Scholar]
- 29.Blei AT, Olafsson S, Therrien G, Butterworth RF. Ammonia-induced brain edema and intracranial hypertension in rats after portacaval anastomosis. Hepatology. 1994;19:1437–1444. [PubMed] [Google Scholar]
- 30.Willard-Mack CL, Koehler RC, Hirata T, Cork LC, Takahashi H, Traystman RJ, Brusilow SW. Inhibition of glutamine synthetase reduces ammonia-induced astrocyte swelling in rat. Neuroscience. 1996;71:589–599. doi: 10.1016/0306-4522(95)00462-9. [DOI] [PubMed] [Google Scholar]
- 31.Norenberg MD, Bender AS. Astrocyte swelling in liver failure: role of glutamine and benzodiazepines. Acta Neurochir Suppl (Wien) 1994;60:24–27. doi: 10.1007/978-3-7091-9334-1_6. [DOI] [PubMed] [Google Scholar]
- 32.Brusilow SW. Hyperammonemic encephalopathy. Medicine (Baltimore) 2002;81:240–249. doi: 10.1097/00005792-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood AH, Yap EW, Rhoades HM, Wong WH. Altered cerebral blood flow and glucose metabolism in patients with liver disease and minimal encephalopathy. J Cereb Blood Flow Metab. 1991;11:331–336. doi: 10.1038/jcbfm.1991.66. [DOI] [PubMed] [Google Scholar]
- 34.Cordoba J, Crespin J, Gottstein J, Blei AT. Mild hypothermia modifies ammonia-induced brain edema in rats after portacaval anastomosis. Gastroenterology. 1999;116:686–693. doi: 10.1016/s0016-5085(99)70191-5. [DOI] [PubMed] [Google Scholar]
- 35.Chatauret N, Zwingmann C, Rose C, Leibfritz D, Butterworth RF. Effects of hypothermia on brain glucose metabolism in acute liver failure: a H/C-nuclear magnetic resonance study. Gastroenterology. 2003;125:815–824. doi: 10.1016/s0016-5085(03)01054-0. [DOI] [PubMed] [Google Scholar]
- 36.Zwingmann C, Chatauret N, Rose C, Leibfritz D, Butterworth RF. Selective alterations of brain osmolytes in acute liver failure: protective effect of mild hypothermia. Brain Res. 2004;999:118–123. doi: 10.1016/j.brainres.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 37.Jayakumar AR, Rao KV, Murthy Ch R, Norenberg MD. Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem Int. 2006;48:623–628. doi: 10.1016/j.neuint.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Desjardins P, Du T, Jiang W, Peng L, Butterworth RF. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: role of glutamine redefined. Neurochem Int. 2012;60:690–696. doi: 10.1016/j.neuint.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jorgensen L, Larsen FS. Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cereb Blood Flow Metab. 2006;26:21–27. doi: 10.1038/sj.jcbfm.9600168. [DOI] [PubMed] [Google Scholar]
- 40.Michalak A, Rose C, Butterworth J, Butterworth RF. Neuroactive amino acids and glutamate (NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology. 1996;24:908–913. doi: 10.1002/hep.510240425. [DOI] [PubMed] [Google Scholar]
- 41.Zieminska E, Dolinska M, Lazarewicz JW, Albrecht J. Induction of permeability transition and swelling of rat brain mitochondria by glutamine. Neurotoxicology. 2000;21:295–300. [PubMed] [Google Scholar]
- 42.Rama Rao KV, Jayakumar AR, Norenberg MD. Induction of the mitochondrial permeability transition in cultured astrocytes by glutamine. Neurochem Int. 2003;43:517–523. doi: 10.1016/s0197-0186(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 43.Murthy CR, Rama Rao KV, Bai G, Norenberg MD. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:282–288. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- 44.Albrecht J, Zielinska M, Norenberg MD. Glutamine as a mediator of ammonia neurotoxicity: A critical appraisal. Biochem Pharmacol. 80:1303–1308. doi: 10.1016/j.bcp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rama Rao KV, Reddy PV, Tong X, Norenberg MD. Brain edema in acute liver failure: inhibition by L-histidine. Am J Pathol. 176:1400–1408. doi: 10.2353/ajpath.2010.090756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruszkiewicz J, Fresko I, Hilgier W, Albrecht J. Decrease of glutathione content in the prefrontal cortical mitochondria of rats with acute hepatic encephalopathy: prevention by histidine. Metab Brain Dis. 2012 doi: 10.1007/s11011-012-9342-6. [DOI] [PubMed] [Google Scholar]
- 47.Brusilow SW, Koehler RC, Traystman RJ, Cooper AJ. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7:452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanamori K, Ross BD. In vivo activity of glutaminase in the brain of hyperammonaemic rats measured by 15N nuclear magnetic resonance. Biochem J. 1995;305(Pt 1):329–336. doi: 10.1042/bj3050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wurdig S, Kugler P. Histochemistry of glutamate metabolizing enzymes in the rat cerebellar cortex. Neurosci Lett. 1991;130:165–168. doi: 10.1016/0304-3940(91)90388-a. [DOI] [PubMed] [Google Scholar]
- 50.Schousboe A, Hertz L, Svenneby G, Kvamme E. Phosphate activated glutaminase activity and glutamine uptake in primary cultures of astrocytes. J Neurochem. 1979;32:943–950. doi: 10.1111/j.1471-4159.1979.tb04579.x. [DOI] [PubMed] [Google Scholar]
- 51.Kvamme E, Svenneby G, Hertz L, Schousboe A. Properties of phosphate activated glutaminase in astrocytes cultured from mouse brain. Neurochem Res. 1982;7:761–770. doi: 10.1007/BF00965528. [DOI] [PubMed] [Google Scholar]
- 52.Hogstad S, Svenneby G, Torgner IA, Kvamme E, Hertz L, Schousboe A. Glutaminase in neurons and astrocytes cultured from mouse brain: kinetic properties and effects of phosphate, glutamate, and ammonia. Neurochem Res. 1988;13:383–388. doi: 10.1007/BF00972489. [DOI] [PubMed] [Google Scholar]
- 53.Kvamme E, Nissen-Meyer LS, Roberg BA, Torgner IA. Novel form of phosphate activated glutaminase in cultured astrocytes and human neuroblastoma cells, PAG in brain pathology and localization in the mitochondria. Neurochem Res. 2008;33:1341–1345. doi: 10.1007/s11064-008-9589-9. [DOI] [PubMed] [Google Scholar]
- 54.Olalla L, Gutierrez A, Jimenez AJ, Lopez-Tellez JF, Khan ZU, Perez J, Alonso FJ, de la Rosa V, Campos-Sandoval JA, Segura JA, Aledo JC, Marquez J. Expression of the scaffolding PDZ protein glutaminase-interacting protein in mammalian brain. J Neurosci Res. 2008;86:281–292. doi: 10.1002/jnr.21505. [DOI] [PubMed] [Google Scholar]