Abstract
Post-transcriptional modifications at the anticodon first (wobble) position of tRNA play critical roles in precise decoding of genetic codes. 5-carboxymethoxyuridine (cmo5U) and its methyl ester derivative 5-methoxycarbonylmethoxyuridine (mcmo5U) are modified nucleosides found at the anticodon wobble position in several tRNAs from Gram-negative bacteria. cmo5U and mcmo5U facilitate non-Watson–Crick base pairing with guanosine and pyrimidines at the third positions of codons, thereby expanding decoding capabilities. By mass spectrometric analyses of individual tRNAs and a shotgun approach of total RNA from Escherichia coli, we identified mcmo5U as a major modification in tRNAAla1, tRNASer1, tRNAPro3 and tRNAThr4; by contrast, cmo5U was present primarily in tRNALeu3 and tRNAVal1. In addition, we discovered 5-methoxycarbonylmethoxy-2′-O-methyluridine (mcmo5Um) as a novel but minor modification in tRNASer1. Terminal methylation frequency of mcmo5U in tRNAPro3 was low (≈30%) in the early log phase of cell growth, gradually increased as growth proceeded and reached nearly 100% in late log and stationary phases. We identified CmoM (previously known as SmtA), an AdoMet-dependent methyltransferase that methylates cmo5U to form mcmo5U. A luciferase reporter assay based on a +1 frameshift construct revealed that terminal methylation of mcmo5U contributes to the decoding ability of tRNAAla1.
INTRODUCTION
RNA molecules are frequently modified post-transcriptionally on their nucleobase and ribose moieties. These modifications carry qualitative information embedded in RNA molecules associated with various biological processes. To date, more than 100 species of modifications have been identified in various RNAs from all domains of life (1), the majority of which are found in tRNAs. tRNA modifications play critical roles in decoding properties and stabilization of tertiary structure (2,3). In particular, a wide variety of modifications occur at the first (wobble) position of the anticodon. These modifications play pivotal roles in modulating codon recognition and ensuring accurate translation of the genetic code (4).
In the classical wobble hypothesis proposed by Crick (5), uridine (U34) at the wobble position pairs with A and G at the third letter of the codon. In the decoding system of Mycoplasma species and mitochondria (6–9), however, U34 can recognize any of the four bases in a family box due to its conformational flexibility, a phenomenon termed ‘four-way wobbling’ (4), although efficiency of this type of wobbling strongly depends on the second and third letters of codons. To restrict decoding capability, tRNAs responsible for two codon sets ending in a purine (NNR) often contain 5-methyl-(2-thio)uridine derivatives [xm5(s2)U] at the wobble position (3,4), including 5-carboxymethylaminomethyl-(2-thio)uridine [cmnm5(s2)U] and 5-methylaminomethyl-(2-thio)uridine [mnm5(s2)U] in bacterial tRNAs, 5-methoxycarbonylmethyl-(2-thio)uridine [mcm5(s2)U] and its derivatives in eukaryotic cytoplasmic tRNAs and 5-taurinomethyl-(2-thio)uridine [τm5(s2)U] in mitochondrial tRNAs. Due to the conformational rigidity of xm5s2U modifications, which are largely fixed in the C3′-endo ribose pucker conformation (10), xm5s2U prefers to base-pair with A and G, thus preventing misreading of near-cognate codons ending in pyrimidine (NNY) (2,11). In addition, C5-substituent of xm5U modification plays a critical role in stabilizing U-G wobble pairing at the A-site of ribosome (12,13).
By contrast, to expand decoding capacity in most bacterial species, 5-hydroxyuridine derivatives (xo5U) are present at the wobble position of tRNAs responsible for recognizing family boxes. 5-carboxymethoxyuridine (cmo5U, also called uridine-5-oxy acetic acid) (Figure 1A) is present in tRNAs from Gram-negative bacteria including Escherichia coli and Salmonella enterica (3,4,14). cmo5U was first reported as a minor nucleoside at the wobble position of E. coli tRNAVal1 (15,16). The chemical structure of cmo5U was determined in 1970 (17). Subsequently, cmo5U was also found in E. coli tRNASer1 (18), tRNAAla1 (19) and tRNALeu3 (20). In addition, cmo5U is present in tRNAPro3 from S. enterica (serovar Typhimurium)(21). The presence of the methyl ester derivative of cmo5U, 5-methoxycarbonylmethoxyuridine (mcmo5U, also called uridine-5-oxy acetic acid methyl ester) (Figure 1A) was predicted by a series of in vitro studies (22–24). In Gram-positive bacteria, 5-methoxyuridine (mo5U) is present in tRNAThr from Bacillus subtilis (25). Collectively, these findings suggested that cmo5U or mcmo5U are present at the anticodon wobble position in E. coli tRNAVal1, tRNAAla1, tRNASer1, tRNAThr4, tRNAPro3 and tRNALeu3 (14,20). However, the exact frequency of these modifications in each tRNA has not been determined.
Figure 1.
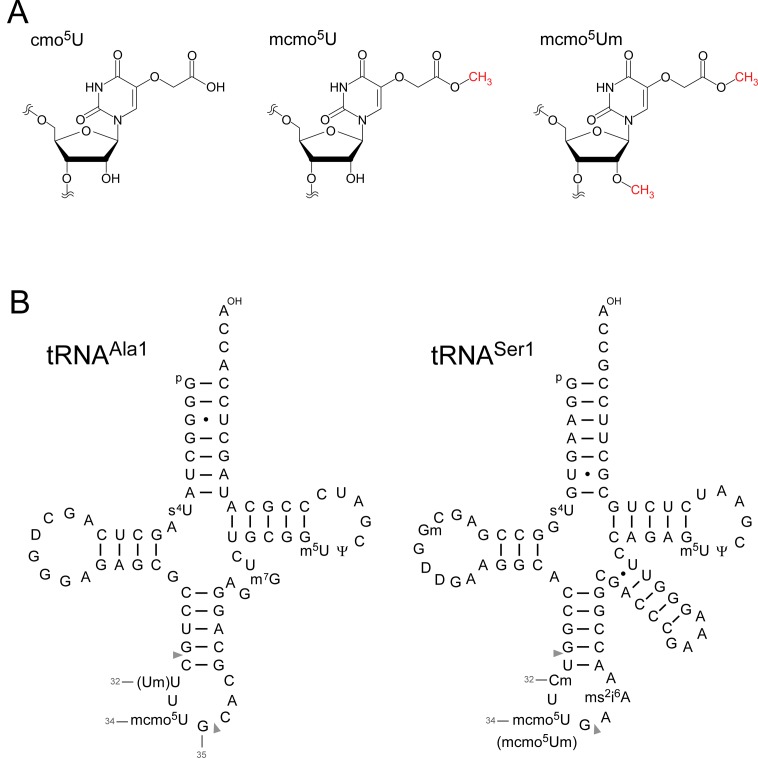
5-carboxymethoxyuridine (cmo5U) and E. coli tRNAs. (A) Chemical structures of 5-carboxymethoxyuridine (cmo5U, left), 5-methoxycarbonylmethoxyuridine (mcmo5U, center) and 5-methoxycarbonylmethoxy-2′-O-methyluridine (mcmo5Um, right). (B) Secondary structures of E. coli tRNAAla1 (left) and tRNASer1 (right) with post-transcriptional modifications: 4-thiouridine (s4U), 2′-O-methylguanosine (Gm), dihydrouridine (D), 2′-O-methylcytidine (Cm), 2′-O-methyluridine (Um), 5-methoxycarbonylmethoxyuridine (mcmo5U), 5-methoxycarbonylmethoxy-2′-O-methyluridine (mcmo5Um), 2-methylthio-N6-isopentenyladenosine (ms2i6A), 7-methylguanosine (m7G), 5-methyluridine (m5U) and pseudouridine (Ψ). The position numbers of the residues (gray letters) are displayed according to the nucleotide numbering system (64). Pairs of gray triangles indicate the positions of cleavage by RNase T1 that generate RNA fragments containing the wobble positions. The sequence of E. coli tRNAAla1 is the same as that of tRNAAla1B (65).
cmo5U and mcmo5U enable non-Watson–Crick base pairing with guanosines and pyrimidines at the third positions of codons, thereby expanding decoding capability. In vitro studies revealed that cmo5U at wobble position allows tRNAs to recognize codons ending in G or U (18,26–29). In addition, tRNAs with cmo5U are able to read codons ending in C in the family boxes for Ala, Pro and Val in vivo (30–32). Solution structure of cmo5U nucleoside analyzed by NMR prefers to adopt the C2′-endo ribose pucker conformation, providing a mechanistic insight into base pairing between cmo5U and pyrimidines (10). Such base pairing at the ribosomal A-site was visualized in a crystal structure of the 30S ribosome in complex with an anticodon stem-loop (ASL) bearing cmo5U and its cognate codon (33). cmo5U forms an intramolecular hydrogen bond that pre-structures the anticodon loop, enabling cmo5U to pair with all four bases at the wobble position in the mRNA. Unexpectedly, cmo5U adopts the C3′-endo form and pairs with G at the third letter of codon by the standard Watson–Crick geometry, rather than classical U-G wobble geometry, indicating that the enol form of the uracil base is involved in this base pairing interaction (33).
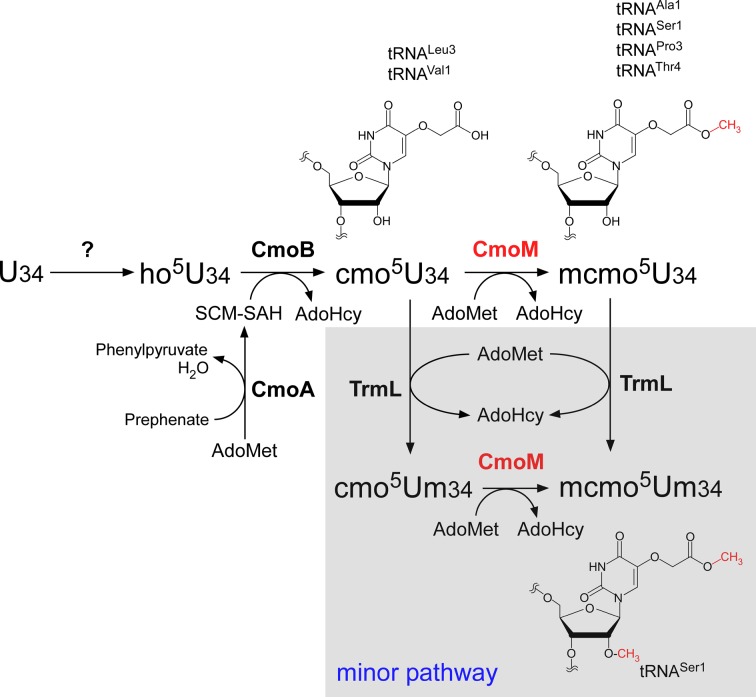
A pathway consisting of multiple enzymatic reactions has been proposed for biogenesis of cmo5U and mcmo5U (see Figure 7). Björk and colleagues made a primary contribution to characterizing this pathway (3,14). cmo5U biogenesis requires chorismate, an end product of the shikimate pathway, which is involved in biosynthesis of aromatic amino acids and vitamins (34,35). First, U34 is hydroxylated to form 5-hydroxyuridine (ho5U) via an unknown pathway. One carbon atom of the 5-carboxymethoxy-group in cmo5U is derived from methionine, indicating the involvement of AdoMet methyltransferase in this step (35). Two genes, cmoA and cmoB, both of which have AdoMet binding motifs, are responsible for formation of cmo5U (31). Subsequent structural analyses revealed that CmoA contains a novel derivative of AdoMet, S-adenosyl-S-carboxymethyl-l-homocysteine (SCM-SAH or Cx-SAM) (36,37). Kim and Almo's group successfully reconstituted cmo5U formation in tRNA in vitro using recombinant CmoA and CmoB in the presence of AdoMet and prephenate (a derivative of chorismate) (37). In this process, CmoA first synthesizes SCM-SAH from AdoMet and prephenate. Next, CmoB employs SCM-SAH as a substrate to transfer its carboxymethyl-group to the 5-hydroxy group of ho5U, thereby generating cmo5U on tRNA (see Figure 8). In some tRNAs, cmo5U is further methylated to form mcmo5U. Biochemical studies in S. enterica cell lysate revealed that cmo5U in tRNASer1 and tRNAAla1 is further methylated by an unidentified AdoMet-dependent methyltransferase, whereas cmo5U in tRNAVal1 is not (22). Initially, the cmo5U methyltransferase was predicted to be encoded by supK (23); however, supK was subsequently identified as prfB which encodes release factor 2 (RF2) (38). CmoA was also speculated to use its second function to methylate cmo5U to generate mcmo5U (31). However, it is now clear that CmoA is responsible for catalyzing SCM-SAH formation (37). Thus, the gene responsible for the cmo5U methyltransferase remains to be identified.
Figure 7.
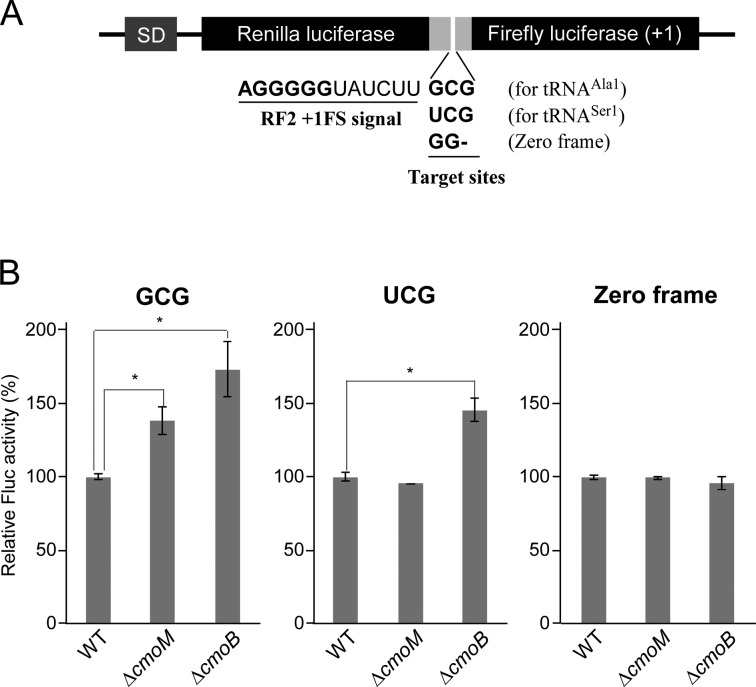
Terminal methylation of mcmo5U contributes to GCG decoding. (A) Schematic depiction of the dual-luciferase reporter constructs based on the RF2 recoding system. SD, Shine-Dalgarno sequence. Renilla and firefly luciferases were fused with a linker containing the +1 frameshift signal of the RF2 recoding site. The frameshift target site was replaced with a GCG codon for tRNAAla1, a UCG codon for tRNASer1or GG for zero frame (used as a control). (B) Relative pausing activity at the frameshift site with GCG (left), UCG (middle) or zero frame (right) was calculated based on relative Fluc activity normalized to Rluc activity in wild-type, ΔcmoM and ΔcmoB strains. Data are presented as means ± SD (n = 4). *, P < 0.01 versus control (Student's t-test).
Figure 8.
Biosynthesis of mcmo5U. In E. coli, U34 of six tRNAs responsible for decoding NCN codons is modified to ho5U34 by an unknown pathway, and subsequently modified to cmo5U in a reaction catalyzed by CmoM. CmoB uses SCM-SAH as a substrate and transfers its carboxymethyl group to ho5U34 on tRNAs. SCM-SAH is synthesized from prephenate and AdoMet catalyzed by CmoA. Four tRNAs (Ala1, Ser1, Pro3 and Thr4) are further modified to mcmo5U by CmoM, using AdoMet as a substrate. mcmo5U frequency is altered by growth phase only in tRNAPro3. In a minor pathway, mcmo5U34 in tRNASer1 is further methylated by TrmL to yield mcmo5Um34. Alternatively, cmo5U34 could be first converted to cmo5Um34, then to mcmo5Um34.
In this study, we isolated individual tRNAs from E. coli and analyzed the modification status of each tRNA by mass spectrometry. We found that mcmo5U was present as a major modification in tRNAAla1, tRNASer1, tRNAPro3 and tRNAThr4, whereas cmo5U is primarily present in tRNALeu3 and tRNAVal1. In addition, we discovered 5-methoxycarbonylmethoxy-2′-O-methyluridine (mcmo5Um) (Figure 1A) as a novel derivative of mcmo5U in tRNASer1. Frequency of terminal methylation of mcmo5U in tRNAPro3 was dependent on growth phase. Moreover, we identified the cmo5U methyltransferase, which we named CmoM, that methylates cmo5U to form mcmo5U in the presence of AdoMet. This terminal methylation of mcmo5U contributes to the decoding ability of tRNAAla1.
MATERIALS AND METHODS
Strains and plasmid construction
The E. coli deletion strains ΔcmoB::Kmr, ΔtrmL::Kmr, ΔcmoM (ΔsmtA)::Kmr, and their parental strain BW25113, were obtained from the National BioResource Project (NBRP), National Institute of Genetics, Japan (39). All strains were cultured in LB media at 37°C.
To generate a vector for expression of recombinant CmoM with an N-terminal His6-tag, cmoM was polymerase chain reaction (PCR)-amplified from the BW25113 genome and inserted into the NdeI/NotI site of pET-28a (Novagen) to yield pET-cmoM-N-His6. To complement ΔcmoM, cmoM with its 5′ flanking region (including the promoter) was PCR-amplified and cloned into the low-copy plasmid pMW118 (Nippon Gene) to yield pMW−cmoM (psmtA). Point mutations were introduced in pMW-cmoM by QuikChangeTM site-directed mutagenesis (Agilent Technologies). For dual-luciferase reporters, the fusion gene of Renilla and firefly luciferases was PCR-amplified from pQE-Luc(+1) (40) using primers containing NcoI and XhoI sites, and then inserted into the corresponding site of pBAD/Myc-His (Invitrogen) to yield pBAD-RFLuc. Subsequently, the RF2 recoding site (32,41), including an SD-like sequence and GCG as a test codon, was introduced by PCR into the linker region between the two luciferases to yield pBAD-RFLucGCG. Two variants in which the test codon was replaced by UCG (pBAD-RFLucUCG) and GG (pBAD-RFLucGG) were generated by QuikChangeTM site-directed mutagenesis. All constructs used in this study were verified by Sanger sequencing. The primers used in this study are listed in Supplementary Table S1.
RNA extraction and tRNA isolation
Total RNA from each E. coli strain was extracted by phenol in acidic condition (42). Individual tRNAs were isolated by reciprocal circulating chromatography, as described previously (42,43). The 5′-terminal ethylcarbamate amino-modified DNA probes used in this method are listed in Supplementary Table S1.
Mass spectrometry of tRNA modifications
For RNA fragment analysis, isolated tRNA (1.25 pmol) was digested at 37°C for 1 h in 12.5 μl of a solution containing 20 mM NH4OAc (pH 5.3) and 125 U RNase T1. The digested RNA was mixed with 12.5 μl of 0.1 M triethylamine-acetate (pH 7.0), and 10 μl of the digest was analyzed by capillary liquid chromatography (LC) coupled to nano electrospray (ESI)/mass spectrometry (MS) on a linear ion trap-Orbitrap hybrid mass spectrometer (LTQ Orbitrap XL; Thermo Fisher Scientific) as described (42,44).
Total nucleosides were analyzed basically as described (44,45). Isolated tRNASer1 (110 pmol) was digested at 37°C for 3 h in 15 μl of a solution containing 20 mM trimethylamine-HCl (TMA-HCl) (pH 7.0), 0.05 U of nuclease P1 and 0.1 U of BAP. The digested RNA (100 pmol) was adjusted as 50 μl of 90% acetonitrile and subjected to HILIC/ESI-MS using Q ExactiveTM Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Fisher Scientific) equipped with a Dionex UltiMateTM 3000 LC System (Thermo Fisher Scientific) using a ZIC-cHILIC column (3 μm, 2.1 × 150 mm, Merck Millipore). For ribonucleome analysis, a reverse genetic approach combined with RNA-MS (44), nucleosides of total RNAs obtained from knockout strains were subjected to RPC-ESI/MS using LCQ Advantage ion-trap mass spectrometer (Thermo Fisher Scientific) equipped with an HP1100 liquid chromatography system (Agilent Technologies) using an Inertsil ODS-3 column (2.1 × 250 mm, GL science).
Shotgun analysis of tRNA fragments by RNA-MS
Total RNA of each strain cultured in 2–20 ml of LB medium was extracted with TRIzolTM (Life Technologies). Then, 50–250 μg of total RNA was dissolved in 800 μl of 3 M NH4OAc (pH 5.3), mixed with 640 μl (0.8 vol) of isopropanol at room temperature and centrifuged at 15 000 rpm for 10 min to precipitate long RNAs including rRNAs. The supernatant was collected and precipitated with ethanol. Depletion of rRNA was verified by denaturing PAGE. The resultant small RNA fraction (50 ng) was digested for 1 h at 37°C with RNase T1 (125 U) in 20 mM NH4OAc (pH 5.3), and the digested RNA (20 ng) was subjected to capillary LC coupled to nanoESI/MS as described above.
Mutation study of CmoM by genetic complementation
The E. coli ΔcmoM strain was transformed with psmtA, its mutant derivatives or an empty vector (pMW118), and the functional importance of the mutations was assessed by monitoring restoration of mcmo5U in the transformants. Transformants were cultured overnight at 37°C in 2 ml of LB medium containing 100 μg/ml ampicillin. Total RNA was subjected to the shotgun analysis as described above to detect the anticodon-containing fragment of tRNAPro3. Modification frequency was determined by calculating the intensity ratio of mass chromatograms between UmUcmo5UGp (m/z 683.567, z = 2) and UmUmcmo5UGp (m/z 690.575, z = 2).
Preparation of recombinant protein
E. coli BL21 (DE3) transformed with pET-cmoM-N-His6 was cultured at 37°C to an OD600 of ≈0.7, supplemented with 0.1 mM IPTG and cultured at 37°C for an additional 4 h. Cells were harvested and disrupted by sonication in lysis buffer consisting of 50 mM HEPES-KOH (pH 7.5), 100 mM KCl, 10 mM MgCl2, 1 mM dithiothreitol and 0.2 mM PMSF. Purification of recombinant protein was performed basically as described (42,46) using a HisTrap column (GE Healthcare) with a linear gradient of imidazole (25–500 mM). The purified protein was dialyzed in lysis buffer, added with glycerol to f.c. 30% and stored at –20°C.
In vitro reconstitution of mcmo5U
tRNASer1, a substrate for CmoM, was isolated from the ΔcmoM strain. In vitro methylation was performed for 1 h at 37°C in a 10 μl reaction mixture containing 50 mM HEPES-KOH (pH 7.5), 100 mM KCl, 10 mM MgCl2, 7 mM 2-mercaptoethanol, 1 mM AdoMet, 10 pmol of tRNASer1 and 1 pmol of recombinant CmoM. After the reaction, tRNA was extracted with acidic phenol and chloroform, followed by ethanol precipitation. The tRNAs were digested with RNase T1 and subjected to LC/MS as described above.
Luciferase reporter assay
The luciferase reporter assay was performed essentially as described (40). E. coli wild-type, ΔcmoM and ΔcmoB strains were transformed with the series of dual-luciferase reporters described above. Each transformant was pre-cultivated at 37°C in 2 ml LB medium containing 100 μg/ml ampicillin overnight. The preculture (1%) was inoculated to 2 ml of LB medium containing 100 μg/ml ampicillin and 100 μM arabinose to induce expression of the reporter. When the OD600 reached 0.3–0.7, 1 ml aliguot was centrifuged, and the pelleted cells were resuspended in 200 μl of lysis buffer [10 mM HEPES-KOH (pH 7.5), 100 mM NaCl, 10 mM MgCl2, 7 mM 2-mercaptoethanol, 400 μg/ml lysozyme]. Cell lysates were prepared by the freeze-thaw method (47) and cleared by centrifugation. The reporter assay was carried out on 5 μl of lysate using GloMaxTM 96 Microplate Luminometer (Promega) with the Dual-LuciferaseTM Reporter Assay System (Promega). The Fluc luminescence signal was normalized against the Rluc luminescence signal.
RESULTS
Modification status of individual tRNAs analyzed by mass spectrometry
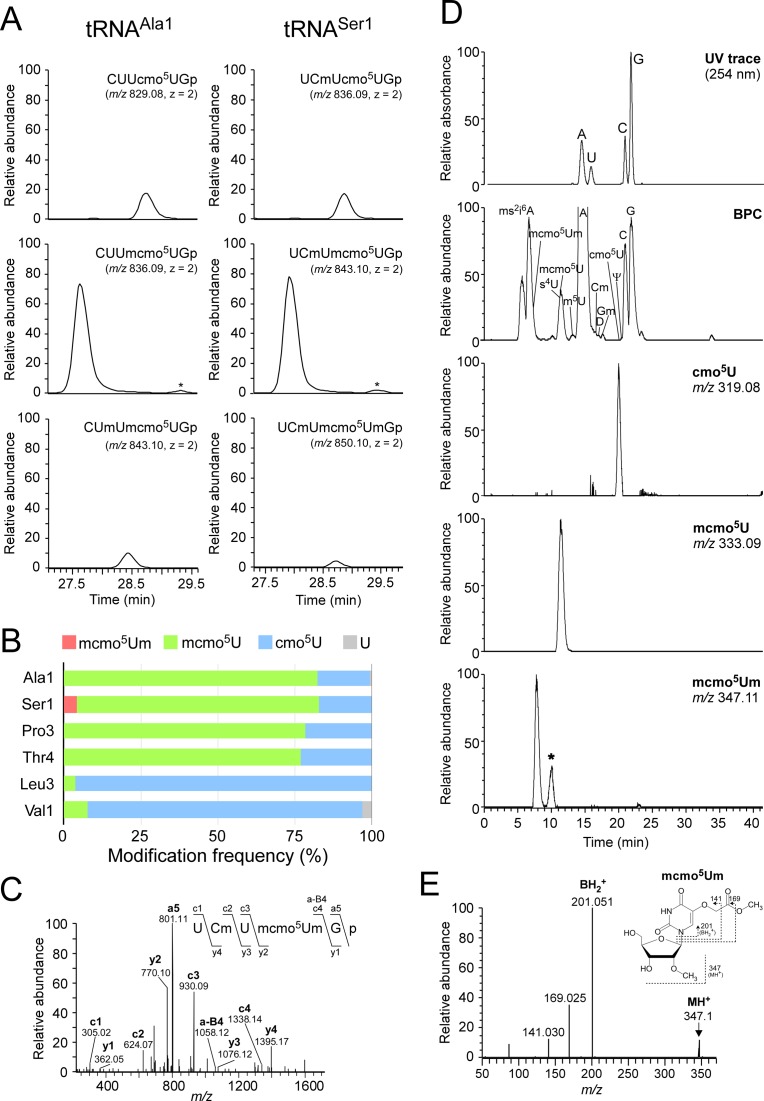
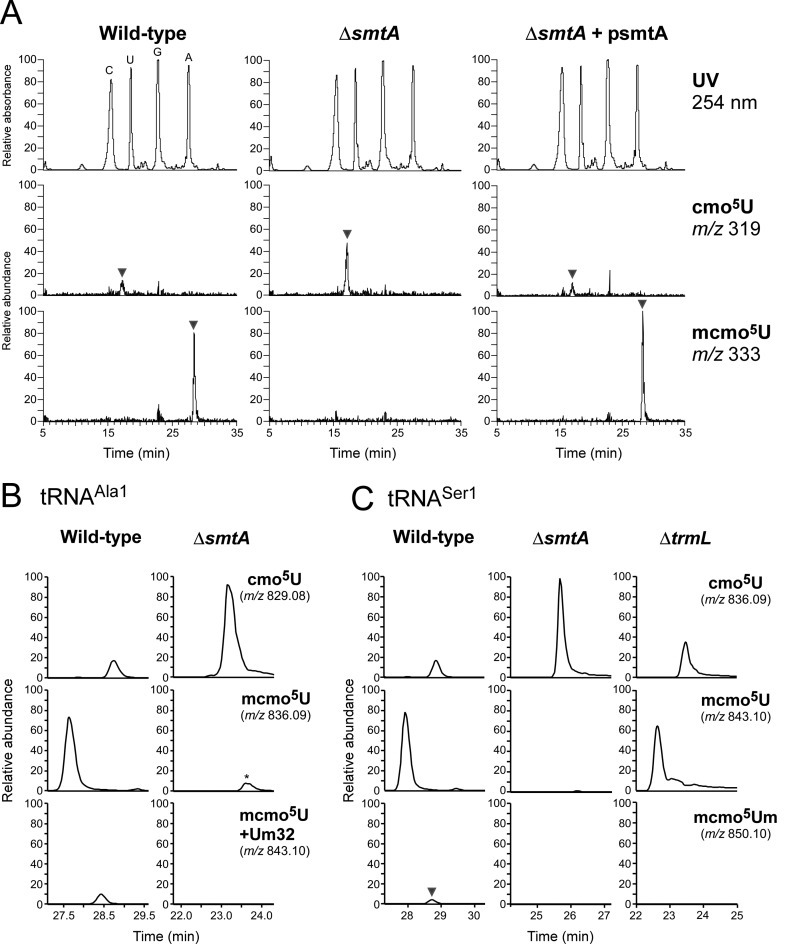
Because the methyl ester of mcmo5U is easily hydrolyzed during preparation and handling of tRNAs (22), little information is available regarding the presence of mcmo5U in individual tRNAs. To determine which tRNA species contain this modification, we employed reciprocal circulating chromatography (RCC) (43) to isolate six tRNA species (tRNAAla1, tRNALeu3, tRNAPro3, tRNASer1, tRNAThr4 and tRNAVal1) (Figure 1B) predicted to contain cmo5U or mcmo5U from E. coli cells harvested at stationary phase. Each individual tRNA was digested with RNase T1 and subjected to capillary liquid chromatography (LC)/nanoelectrospray ionization mass spectrometry (MS) to precisely analyze its post-transcriptional modifications (44). The RNA fragment containing the wobble modification was detected as multiply-charged negative ions (Figure 2A and Supplementary Figure S1) and further probed by collision-induced dissociation (CID) to determine the position of each modification (Supplementary Figure S2). Frequencies of the wobble modifications in these six tRNA species were calculated from the peak areas of mass chromatograms (Figure 2B). The wobble modifications of tRNAAla1, tRNAPro3, tRNASer1 and tRNAThr4 consisted of ≈80% mcmo5U and ≈20% cmo5U. By contrast, over 90% of tRNALeu3 and tRNAVal1 molecules contained cmo5U, whereas the remaining 10% contained mcmo5U or unmodified U. In tRNAAla1, we observed partial modification (≈11%) of 2′-O-methyluridine (Um) at position 32 (Figure 2A), which was confirmed by CID analysis (Supplementary Figure S2A).
Figure 2.
Mass spectrometric analysis of individual tRNAs isolated from stationary-phase E. coli. (A) Mass chromatograms of RNase T1-digested fragments containing cmo5U and its derivatives from tRNAAla1 (left panels) and tRNASer1 (right panels) isolated from stationary-phase E. coli. Top and middle panels: extracted-ion chromatograms (XIC) for doubly-charged negative ions of cmo5U34-containing fragments (CUUcmo5UGp of tRNAAla1, MW 1660.18, m/z 829.08; UCmUcmo5UGp of tRNASer1, MW 1674.19, m/z 836.09) and mcmo5U34-containing fragments (CUUmcmo5UGp of tRNAAla1, MW 1674.19, m/z 836.09; UCmUmcmo5UGp of tRNASer1, MW 1688.21, m/z 843.10), respectively. Bottom left and bottom right panels: XICs for doubly-charged negative ions of Um32/mcmo5U34-containing fragment of tRNAAla1 (CUmUmcmo5UGp, MW 1688.21, m/z 843.10) and mcmo5Um34-containing fragment of tRNASer1 (UCmUmcmo5UmGp, MW 1702.22, m/z 850.10), respectively. The peaks marked with asterisks represent Um32/cmo5U-containing fragment (CUmUcmo5UGp, MW 1674.19, m/z 836.09) in tRNAAla1 and cmo5Um-containing fragment (UCmUcmo5UmGp, MW 1688.21, m/z 843.10) in tRNASer1. The RNA fragments containing unmodified C32 are also present in tRNASer1, but they are not described here due to high frequency of Cm32 in tRNASer1 isolated from stationary-phase E. coli. (B) Modification frequencies of cmo5U and its derivatives at position 34 in six species of tRNAs isolated from stationary-phase E. coli. Relative composition of each modification was calculated from the peak area ratio of mass chromatograms of RNase T1-digested fragments containing mcmo5Um34 (red), mcmo5U34 (green), cmo5U34 (blue) or U (gray). (C) Collision-induced dissociation (CID) spectrum of a fragment of E. coli tRNASer1 containing mcmo5Um34. The doubly-charged negative ion of the RNase T1-digested fragment containing mcmo5Um34 (m/z 850.10) was used as the precursor ion for CID. The product ions were assigned as described previously (66). Sequences of parent ion and assigned product ions are described on the upper left side of this panel. (D) Nucleoside analysis of E. coli tRNASer1. Top panel: UV chromatogram at 254 nm of the four major nucleosides (C, U, G and A). Second panel: mass chromatograms of the protonated nucleosides (MH+) on the base peak chromatogram (BPC). Third to bottom panels: XICs for cmo5U (m/z 319.08), mcmo5U (m/z 333.09) and mcmo5Um (m/z 347.11), respectively. The peak marked with an asterisk represents unspecific peak. (E) CID spectrum of mcmo5Um nucleoside. The protonated mcmo5Um (MH+, m/z 347.11) was used as the precursor ion for CID. The N-glycoside bond cleaved to generate the base-related ion (BH2+) and other product ions are assigned on the chemical structure.
Discovery of mcmo5Um as a novel modification
At the wobble position of tRNASer1, we found a novel minor modification with a molecular mass 14 Da larger than that of mcmo5U (Figure 2A). CID analysis of this fragment (Figure 2C) revealed a specific product ion (a-B4) lacking the 5-methoxycarbonylmethoxyuracil-base (mcmo5U-base), strongly suggesting that the 2′ hydroxyl group of the ribose was methylated. To confirm this result, we analyzed total nucleosides of tRNASer1 and detected a proton-adduct (MH+) of this minor nucleoside (m/z 347) (Figure 2D) which we then probed by CID analysis (Figure 2E). We clearly detected a base-related fragment (BH2+) with m/z 201, which also appears in mcmo5U, confirming that the ribose portion is methylated. We also verified the absence of this modification in a knockout strain of trmL, which encodes a 2′-O-methyltransferase that targets position 34 (see Figure 4C). Taken together, we conclude that the novel modification found in tRNASer1 is 5-methoxycarbonylmethoxy-2′-O-methyluridine (mcmo5Um) (Figure 1A,B). About 4.2% of tRNASer1 molecules contain this modification (Figure 2A,B). Given that 2′-O-methylation stabilizes C3′-endo ribose pucker conformation (48), mcmo5Um is likely to allow tRNASer1 to recognize UCG codon more efficiently than mcmo5U.
Figure 4.
Reverse genetic approach identified a gene responsible for terminal methylation of mcmo5U. (A) Nucleoside analyzes by LC/MS using reverse phase chromatography of total RNA from wild-type (left panels), ΔsmtA (middle panels) and ΔsmtA rescued with psmtA (right panels). Top panels: UV trace at 254 nm. Second and bottom panels: XICs for cmo5U (m/z 319) and mcmo5U (m/z 333), respectively. Intensity of each peak was normalized to that of cyclic t6A (m/z 395). (B) Mass chromatograms of RNase T1-digested fragments containing cmo5U and its derivatives from tRNAAla1 isolated from wild-type (left panels) and ΔsmtA (right panels) strains. Top, middle and bottom panels: XICs for doubly-charged negative ions of the cmo5U34-containing fragments (CUUcmo5UGp, m/z 829.08), the mcmo5U34-containing fragments (CUUmcmo5UGp, m/z 836.09) and the Um32/mcmo5U34-containing fragment (CUmUmcmo5UGp, m/z 843.10), respectively. The peak marked with an asterisk represent the Um32/cmo5U-containing fragment (CUmUcmo5UGp, m/z 836.09). (C) Mass chromatograms of RNase T1-digested fragments containing cmo5U and its derivatives from tRNASer1 isolated from wild-type (left panels), ΔsmtA (middle panels) and ΔtrmL (right panels) strains. Top, middle and bottom panels: XICs for doubly-charged negative ions of the cmo5U34-containing fragments (UCmUcmo5UGp, m/z 836.09), the mcmo5U34-containing fragments (UCmUmcmo5UGp, m/z 843.10) and the mcmo5Um34-containing fragment (UCmUmcmo5UmGp, m/z 850.10), respectively.
Frequency of mcmo5U in cellular tRNAs estimated by shotgun approach
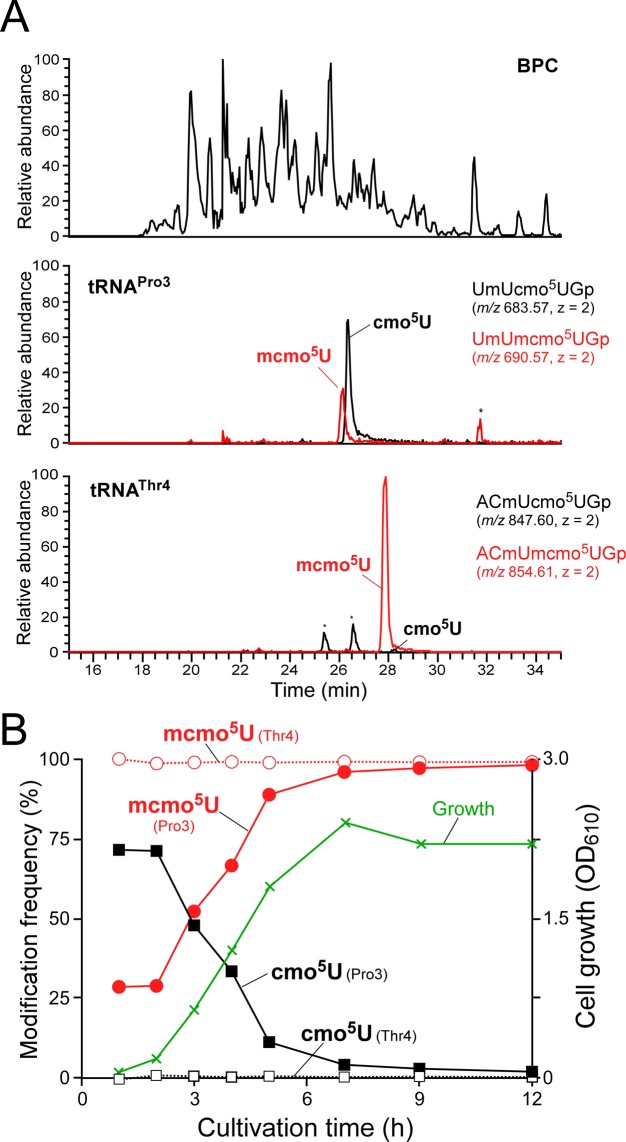
Although mcmo5U was detected in the four tRNAs with a frequency of ≈80% (Figure 2B), it is possible that the methyl ester of mcmo5U was partially hydrolyzed during isolation of individual tRNAs by RCC. To exclude the effects of the inevitable hydrolysis of mcmo5U during tRNA isolation, we analyzed the wobble modifications in total tRNAs using a shotgun approach. In order to profile the huge number of RNA fragments derived from all species of tRNAs, we prepared crude E. coli tRNA fractions from different growth phases, digested them with RNase T1 and subjected the digested material to LC/MS (Figure 3A). Among the four tRNAs bearing mcmo5U, we clearly detected an anticodon-containing fragment of tRNAPro3 and tRNAThr4 containing mcmo5U or cmo5U, each of which has a unique molecular mass and does not overlap with other fragments. Judging from the peak area of mass chromatograms for UmUmcmo5UGp (m/z 690.575) and UmUcmo5UGp (m/z 683.567) in tRNAPro3 isolated from cells in stationary phase, mcmo5U was present in tRNAPro3 with a frequency of 98% (Figure 3B). Similarly, tRNAThr4 isolated from cells in stationary phase contained nearly 100% mcmo5U (Figure 3B). These results suggest that about 20% of the cmo5U detected in tRNAPro3 and tRNAThr4 (Figure 2B) was generated by artificial hydrolysis of mcmo5U during tRNA isolation. Given that mcmo5U was present in the other two isolated tRNAs (Ala1 and Ser1) at a frequency of ≈80%, nearly 100% mcmo5U should be present in all four tRNAs in stationary-phase E. coli.
Figure 3.
Shotgun analysis of tRNA fragments and growth phase-dependent alteration of mcmo5U. (A) Shotgun analysis of total tRNA digested by RNase T1. Top panel: base peak chromatogram (BPC) of RNase T1-digested total tRNA isolated from early log phase (2 h) E. coli. Second panel: XICs for doubly-charged negative ions of the cmo5U34-containing fragment (UmUcmo5UGp, m/z 683.57) and the mcmo5U34-containing fragment (UmUmcmo5UGp, m/z 690.57) from tRNAPro3. Bottom panel: XICs for doubly-charged negative ions of the cmo5U34-containing fragment (ACmUcmo5UGp, m/z 847.60) and the mcmo5U34-containing fragment (ACmUmcmo5UGp, m/z 854.61) from tRNAThr4. (B) mcmo5U level is altered by growth phase. tRNAPro3 (filled circle and rectangle) or tRNAThr4 (open circle and rectangle) were calculated from the peak area ratio of XICs for the fragments containing mcmo5U34 and cmo5U34 at different cultivation times. Cell growth (green line) was monitored by absorbance at 610 nm.
Growth phase-dependent alteration of mcmo5U in tRNAPro3
To investigate the effect of growth phase on mcmo5U, we prepared total tRNA from E. coli cells harvested at various growth phases. The total tRNA was digested with RNase T1, and analyzed by LC/MS to monitor the RNA fragment containing mcmo5U or cmo5U derived from tRNAPro3 (Figure 3A). Based on the ratio of the two fragment peaks, we calculated the frequency of mcmo5U over the course of E. coli cultivation (Figure 3B and Supplementary Figure S3A). In early log phase (1–2 h after inoculation), mcmo5U was present in tRNAPro3 with a frequency of ≈30%, whereas cmo5U was present in the remaining molecules. The mcmo5U frequency gradually increased as growth proceeded, reaching nearly 100% in late log and stationary phase. By contrast, mcmo5U frequency in tRNAThr4 was consistently high (>99%) in all growth phases (Figure 3B and Supplementary Figure S3B).
The shotgun analysis failed to detect specific fragments bearing mcmo5U or cmo5U derived from tRNAAla1 and tRNASer1, because the molecular masses of these fragments overlapped with those of other fragments. To investigate the modification status of these tRNAs in log phase, we isolated four tRNAs containing mcmo5U from E. coli cells harvested in mid-log phase (OD600 = 0.4). Mass spectrometric analysis revealed that both tRNAAla1 and tRNASer1 contained mcmo5U with frequencies of 82% and 84%, respectively (Supplementary Figure S4). Because 20% of mcmo5U is hydrolyzed and converted to cmo5U during isolation of tRNAs by RCC (Figure 2B), we concluded that both tRNAAla1 and tRNASer1 were fully modified with mcmo5U in mid-log phase. Meanwhile, tRNAPro3 and tRNAThr4 contained mcmo5U at frequencies of 34% and 80%, respectively (Supplementary Figure S4). These results are consistent with those observed in the shotgun analysis (Figure 3B). Based on these findings, we conclude that mcmo5U content in tRNAPro3 is dependent on growth phase, whereas the other three tRNAs are fully modified with mcmo5U during all phases of cell growth.
Identification of a gene responsible for terminal methylation of mcmo5U
To identify the gene encoding the AdoMet-dependent methyltransferase that methylates cmo5U to form mcmo5U, we conducted a genome-wide screen to identify genes responsible for RNA modifications. The method we employed, ribonucleome analysis, uses a reverse genetic approach combined with RNA-MS (44). This approach has been used to successfully identify many genes responsible for tRNA/rRNA modifications among uncharacterized genes in E. coli (40,46,49–52) and Saccharomyces cerevisiae (53–56). Screening of E. coli knockout strains revealed that mcmo5U (m/z 333) was completely absent in the ΔsmtA strain; instead, the level of cmo5U (m/z 319) was higher than that in the wild-type (Figure 4A). When smtA was introduced on a plasmid (psmtA) into the ΔsmtA strain, formation of mcmo5U (m/z 333) was restored (Figure 4A). These data indicate that SmtA is a methyltransferase that produces mcmo5U from cmo5U.
Next, we isolated tRNAAla1 and tRNASer1 from the ΔsmtA strain, and analyzed their wobble modifications by LC/MS (Figure 4B,C). As expected, mcmo5U-containing fragments were completely converted to cmo5U-containing fragments in both strains.
As mentioned above, we discovered mcmo5Um (Figure 1A) as a minor modification in tRNASer1. We confirmed the absence of mcmo5Um in both ΔsmtA and ΔtrmL (Figure 4C). trmL encodes a 2′-O-methyltransferase responsible for 2′-O-methylation of cmnm5Um34 of tRNALeu4 and Cm34 of tRNALeu5 (57). Therefore, we conclude that mcmo5Um in tRNASer1 is generated by 2′-O-methylation of mcmo5U by TrmL.
In vitro reconstitution of mcmo5U mediated by CmoM
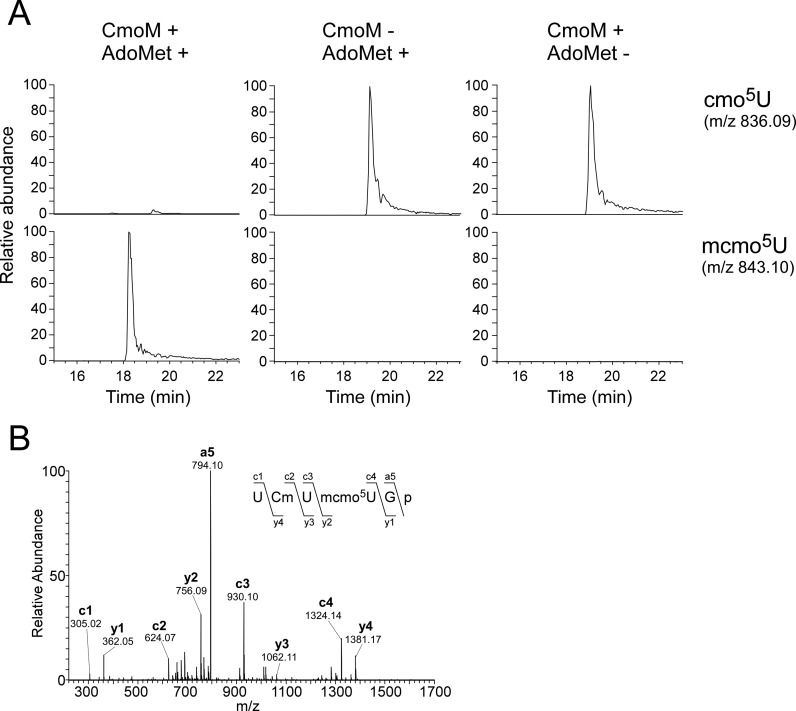
To determine whether SmtA actually has methyltransferase activity, we conducted in vitro reconstitution of mcmo5U formation by recombinant SmtA. In this experiment, tRNASer1 bearing cmo5U was isolated from the ΔsmtA strain and used as a substrate. We clearly detected mcmo5U in the tRNASer1 only in the presence of both recombinant SmtA and AdoMet (Figure 5A). CID analysis of the anticodon-containing fragment confirmed the methylation occurred at the wobble position (Figure 5B). This result demonstrated that SmtA is an AdoMet-dependent methyltransferase that transfers a methyl group to cmo5U34 of tRNAs to form mcmo5U34. Based on the enzymatic activity, we renamed this gene CmoM (cmo5U methyltransferase).
Figure 5.
In vitro reconstitution of cmo5U methylation by recombinant CmoM. (A) E. coli tRNASer1 bearing cmo5U isolated from the ΔcmoM strain was incubated in the presence or absence of recombinant CmoM with or without AdoMet. Top and bottom panels: XICs for doubly-charged negative ions of the cmo5U34-containing fragments (UCmUcmo5UGp, m/z 836.09) and the mcmo5U34-containing fragments (UCmUmcmo5UGp, m/z 843.10), respectively. (B) A CID spectrum of RNase T1-digested fragment of E. coli tRNASer1 incubated in the presence of recombinant CmoM with AdoMet. The doubly-charged negative ion of the mcmo5U34-containing fragment (UCmUmcmo5UGp, m/z 843.10) was used as the precursor ion for CID. The product ions were assigned according to the literature (66). Sequences of parent ion and assigned product ions are described upper left side in this panel.
Characterization of CmoM
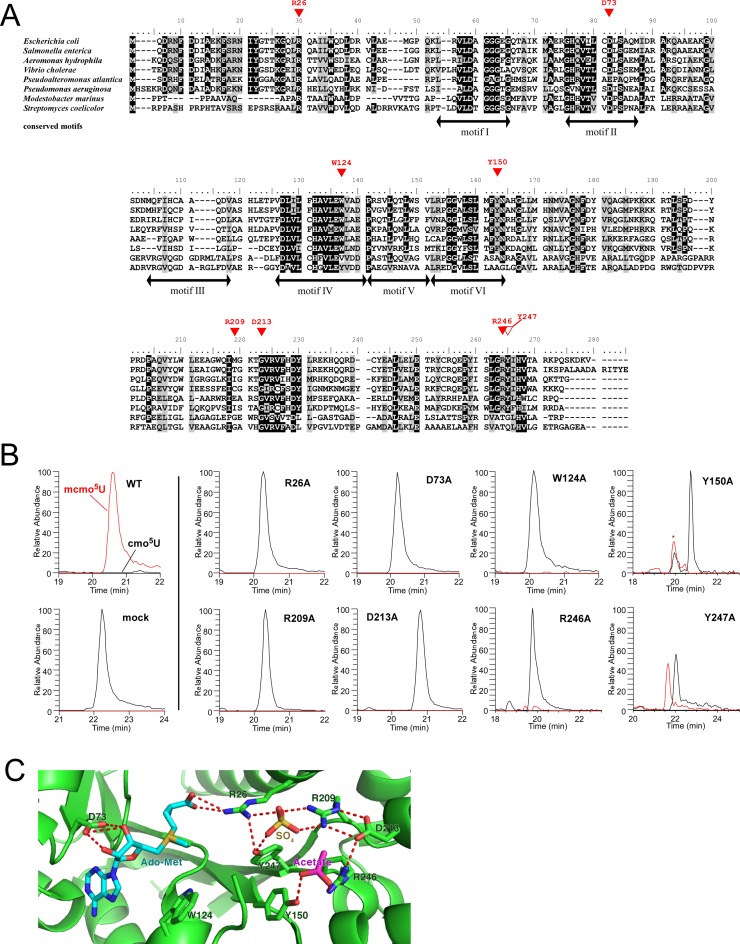
CmoM belongs to the Class I AdoMet-dependent methyltransferase (MTase) family, whose members contain a Rossmann-fold as a characteristic structural motif (Figure 6A) (58–60). The high-resolution crystal structure of E. coli CmoM (SmtA) (PDB ID:4HTF) revealed that CmoM forms a homodimer, and that each subunit contains one molecule each of AdoMet, sulfate, acetate and 2-mercaptoethanol as ligands (Figure 6C and Supplementary Figure S5). Based on this structure, we designed eight mutant cmoM constructs bearing single amino-acid alterations (Figure 6A). The mutated residues, which are conserved in cmoM homologs, are located at the catalytic site where AdoMet is bound (Figure 6C). The ΔcmoM strain was transformed with each of the mutant constructs, and total RNA extracted from each construct was digested with RNase T1 and subjected to LC/MS to detect the RNA fragment of tRNAPro3 bearing mcmo5U or cmo5U (Figure 6B). The positive and negative controls behaved as expected: mcmo5U was fully restored by wild-type cmoM, whereas no mcmo5U was formed in cells transfected with an empty vector. Little or no mcmo5U was observed in the ΔcmoM strain introduced by the mutant constructs R26A, D73A, W124A, Y150A, R209A, D213A and R246A, whereas mcmo5U formation was partially restored by the Y247A mutant. The data indicate that the highly conserved residues in the catalytic center are essential for normal methyltransferase activity.
Figure 6.
Characterization of CmoM. (A) Sequence alignment of CmoM homologs from six γ-proteobacteria, Escherichia coli (NP_415441.1), Salmonella enterica (NP_455477.1), Aeromonas hydrophila (YP_856903.1), Vibrio cholerae (NP_231353.1), Pseudoalteromonas atlantica (ABG40695.1), Pseudomonas aeruginosa (NP_253478.1), and two Actinobacteria, Modestobacter marinus (WP_014741474.1), Streptomyces coelicolor (WP_011028138.1). Identical or similar residues are shaded in black or gray, respectively. Red triangles indicate residues that are essential (filled) or non-essential (open) for generic complementation. Motifs I to VI are conserved in Class I AdoMet-dependent methyltransferases. (B) XICs for doubly-charged negative ions of the cmo5U34-containing fragment (black lines, UmUcmo5UGp, m/z 683.57) and the mcmo5U34-containing fragment (red lines, UmUmcmo5UGp, m/z 690.57) from tRNAPro3 in the ΔcmoM strain rescued by plasmid-encoded wild-type cmoM or its mutant derivatives. The peak marked with an asterisk represents unspecific peak. (C) Close-up view of the AdoMet-binding site in the crystal structure of E. coli CmoM (PDB ID: 4HTF) containing ligands, AdoMet, acetate and sulfate. Predicted hydrogen bonds between ligands and CmoM are indicated by red dotted lines.
Terminal methylation of mcmo5U contributes to the decoding process
To investigate the functional role of the terminal methylation of mcmo5U, we constructed dual-luciferase reporters based on the RF2 recoding system (32,41). Renilla luciferase (Rluc) was fused to firefly luciferase (Fluc) by a linker sequence bearing the +1 frameshift signal of the RF2 recoding site (Figure 7A). The original UGA codon at the frameshift site was replaced with GCG or UCG to examine the decoding abilities of tRNAAla1 and tRNASer1, respectively. The GCG codon is exclusively deciphered by tRNAAla1 (mcmo5UGC), whereas the UCG codon is redundantly recognized by tRNASer1(mcmo5UGA) and tRNASer2(CGA) (Supplementary Figure S6) (14). We also prepared a control reporter construct lacking the +1 frameshift site (zero frame) (Figure 7A). Each of these reporters was introduced into wild-type (WT), ΔcmoM and ΔcmoB strains. The decoding ability of the test codon at the frameshift site is reflected by the +1 frameshift activity. Because the +1 frameshift activity in this system is promoted by the ‘hungry’ A-site, the ability of the cognate tRNA to decode the test codons can be estimated indirectly. The +1 frameshift activity was calculated from the Fluc signal against the Rluc signal (F/R value). No difference in the frameshift activity of the zero frame construct, used as a negative control, was observed in the three strains (Figure 7B). In the ΔcmoB strain, in which all mcmo5U/cmo5U should be converted to ho5U, we observed clear stimulation of +1 frameshift activity at both GCG and UCG codons (Figure 7B), indicating that the carboxymethyl group of cmo5U contributes to decoding of G-ending codons. This result is consistent with the previous reports (32,61). On the other hand, in the ΔcmoM strain, we detected a slight but significant stimulation of frameshift activity at the GCG codon (Figure 7B), but not at UCG. This observation indicates that the terminal methyl group of mcmo5U is partially involved in GCG decoding by tRNAAla1. The absence of any reduction in UCG decoding in ΔcmoM strain can be explained by the fact that this codon is redundantly deciphered by tRNASer1 and tRNASer2; tRNASer2 might have compensated for the reduced decoding ability of hypomodified tRNASer1 in the ΔcmoM strain.
DISCUSSION
It is difficult to estimate the exact frequency of an RNA modification with unstable chemical structure, such as an ester group, in individual tRNAs, because such modifications are easily hydrolyzed during tRNA isolation (42). Although the presence of mcmo5U in cellular tRNAs was reported previously, the exact frequency of this modification in individual tRNAs remained unknown (14,22). Using the shotgun approach, we showed here that mcmo5U is present in nearly 100% of tRNAPro3 and tRNAThr4 molecules isolated from stationary-phase E. coli (Figure 3B). This method can be applied to analysis of other RNA modifications with unstable chemical structures from various sources, including 5-methoxycarbonylmethyluridine (mcm5U), wybutosine (yW), cyclic N6-threonylcarbamoyladenosine (ct6A), glutamylqueuosine (GluQ) and their derivatives. In addition, we used RCC to estimate the fraction of mcmo5U hydrolyzed during tRNA isolation. Judging from the mcmo5U frequency (≈80%) in the isolated tRNAs, we concluded that ≈20% of mcmo5U was converted to cmo5U during tRNA isolation (Figure 2B), indicating that all four tRNAs (tRNAAla1, tRNASer1, tRNAPro3 and tRNAThr4) are fully modified with mcmo5U in stationary-phase E. coli. The four tRNAs containing mcmo5U all have G35 at the second letter of the anticodon, and therefore specify NCN codons, implying that CmoM preferentially recognizes G35. However, because mcmo5U was present (albeit at a low frequency, <10%) in tRNALeu3 and tRNAVal1, which do not have G35 (Figure 2B), this residue is not an essential determinant for CmoM.
By applying the shotgun approach to total tRNA, we observed growth phase-dependent alteration of mcmo5U in tRNAPro3 (Figure 3B). In all phases of cell growth, cmo5U was fully incorporated into this tRNA. In early log phase, cmo5U of tRNAPro3 was partially modified by CmoM to yield mcmo5U with a frequency of 30%. As growth proceeded, the level of mcmo5U gradually increased, and the level of cmo5U concomitantly decreased. At late log and stationary phases, tRNAPro3 was fully modified with mcmo5U. According to the GEO profile database (ID: 35525121 and 35543521) (62), the steady-state level of cmoM mRNA is temporarily elevated in early log phase, and is expressed at a constant in late log and stationary phases. Therefore, we speculate that hypomodification of mcmo5U in tRNAPro3 might be due to slow methylation by CmoM that fails to catch up with fast production of tRNA in early log phase; as growth rate decreases, mcmo5U accumulates gradually. By contrary, tRNAThr4 was fully modified with mcmo5U in all growth phases (Figure 3B). In addition, a high level of mcmo5U was also found in tRNAAla1, tRNASer1 as well as tRNAThr4 isolated from log phase E. coli. These results imply that cmo5U34 is a better substrate for CmoM in these three tRNAs than in tRNAPro3. The growth phase dependency of mcmo5U in tRNAPro3 might be involved in regulatory decoding of CCN codons in growth phase-specific gene expression. Further studies will be necessary to examine this speculation.
Because ΔcmoM exhibited no obvious growth phenotype (data not shown)(63), the functional role of the terminal methyl group of mcmo5U may be limited. To characterize this modification, we employed a reporter assay based on the RF2 recoding system to estimate the ability to decode GCG and UCG codons in the presence or absence of cmoM (Figure 7B). The +1 frameshift activity at a GCG codon increased specifically in the absence of cmoM, indicating that mcmo5U facilitates decoding of GCG by tRNAAla1. However, we observed no change in UCG decoding in the absence of cmoM, because the UCG codon is redundantly recognized by the other isoacceptor. Similarly, it is difficult to assess the decoding ability of other NCN codons in the absence of cmoM, because NCA codons are recognized exclusively by Watson–Crick base pairs, and other NCN codons are redundantly deciphered by two isoacceptors (14). However, in light of our findings, it is reasonable to assume that the terminal methyl group of mcmo5U contributes to NCN decoding in general.
According to the crystal structure of the 30S ribosomal subunit in complex with ASL of tRNAVal bearing the cmo5UAC anticodon and its cognate codons (33), the carboxylate of cmo5U forms a hydrogen bond with the N6 amino group of A35 (the second letter of the anticodon). This interaction is one component of the intramolecular hydrogen bonding network that pre-structures the anticodon loop, so that cmo5U can pair with all four bases at the third letters of codons. We showed here that mcmo5U is primarily present in tRNAs with anticodons containing G35. When cmo5U is present in these tRNAs at the ribosome A-site, the carboxylate of cmo5U cannot form a hydrogen bond with G35. To make the matter worse, cmo5U might be destabilized due to electrostatic repulsion between the carboxylate and the O6 carbonyl oxygen of G35, both of which are negatively charged. The terminal methyl group of mcmo5U neutralizes the negative charge of cmo5U carboxylate, suggesting that mcmo5U is involved in stabilizing the wobble base in the anticodons containing G35.
The crystal structure of CmoM also reveals AdoMet and other ligands bound to the positively-charged surface, which might be involved in tRNA recognition (Supplementary Figure S5). Seven residues essential for mcmo5U formation, R26, D73, W124, Y150, R209, D213 and R246, which we identified in this study, reside near the ligand-binding site on the positively charged surface of CmoM (Figure 6C). R26 and D73 play a critical role in positioning AdoMet by forming hydrogen bonds. D73 is a conserved carboxylate in motif II of AdoMet MTase (Figure 6A) (58,59). W124 may participate in AdoMet binding via a stacking interaction with the adenine base of AdoMet. Given that W124 is in close proximity to the methyl group of AdoMet, it might be involved in the interaction with the cmo5U base of tRNA and thereby facilitate the cmo5U methylation. R26 forms a network of hydrogen bonds with two other essential residues, R209 and Y247, along with a sulfate. R209 extends the network to D213, which interacts with R246. R246 and Y150 to form the binding site for an acetate. The functional roles of the sulfate and the acetate bound to the catalytic site remain unknown, but these ligands may act as mimics for the phosphate group of tRNA bound to CmoM.
Phylogenetic analysis revealed that cmoM is present in γ-proteobacteria, actinobacteria and a few species in other bacterial clades (Figure 6A). Because actinobacteria doesn't have homologs of cmoB, cmo5U is not predicted to be present in this organism, indicating that actinobacterial counterpart is not a functional homolog of cmoM. Consistent with this, two essential residues, W124 and Y150, in E. coli CmoM are not conserved in there organisms (Figure 6A). Thus, CmoM and mcmo5U are mainly distributed in γ-proteobacteria. Presence of cmoM homologs in Spirochaeta cellobiosiphila (Spirochaeta), Zetaproteobacteria bacterium (ζ-proteobacteria) and Paenibacillus sophorae (Firmicutes) indicates horizontal gene transfer of cmoM from γ-proteobacteria to these species.
In the biogenesis of cmo5U and mcmo5U (Figure 8), six tRNA species (tRNAAla1, tRNALeu3, tRNAPro3, tRNASer1, tRNAThr4 and tRNAVal1) first undergo hydroxylation at C5 of the uracil base in U34 to yield ho5U34; the enzyme and substrate involved in this process are unknown. Next, ho5U34 is further modified by CmoB using SCM-SAH as a substrate to yield cmo5U34. SCM-SAH is generated from AdoMet and prephenate in a reaction catalyzed by CmoA. For four tRNA species (tRNAAla1, tRNAPro3, tRNASer1 and tRNAThr4), cmo5U34 is methylated by CmoM in the presence of AdoMet to yield mcmo5U34. Only in tRNASer1, small portion of mcmo5U34 is further methylated by TrmL to yield mcmo5Um34. Alternatively, cmo5U34 could be first converted to cmo5Um34, then to mcmo5Um34. Growth phase-dependent alteration of mcmo5U34 takes place in tRNAPro3, implying a possible mechanism of translational control mediated by the regulatory decoding efficiency of CCN codons.
Supplementary Material
Acknowledgments
We are grateful to the members of the Suzuki laboratory, in particular Yuriko Sakaguchi, for technical support and many insightful discussions.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan [to T.S.]; JSPS Fellowship for Japanese Junior Scientists [to Y.S. and S.K.]. Funding for open access charge: Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan [to T.S.].
Conflict of interest statement. None declared.
REFERENCE
- 1.Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M., et al. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokoyama S., Nishimura S. In: tRNA: Structure, Biosynthesis, and Function. Soll DRUL, editor. Washington, D.C.: American Society for Microbiology; 1995. pp. 207–224. [Google Scholar]
- 3.Bjork G. In: tRNA: Structure, Biosynthesis, and Function. Soll DRUL, editor. Washington, D.C.: American Society for Microbiology; 1995. pp. 165–205. [Google Scholar]
- 4.Suzuki T. In: Fine-Tuning of RNA Functions by Modification and Editing. Grosjean H, editor. Vol. 12. Springer-Verlag Berlin and Heidelberg: GmbH & Co. KG; 2005. pp. 23–69. [Google Scholar]
- 5.Crick F.H. Codon–anticodon pairing: the wobble hypothesis. J. Mol. Biol. 1966;19:548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 6.Barrell B.G., Anderson S., Bankier A.T., de Bruijn M.H., Chen E., Coulson A.R., Drouin J., Eperon I.C., Nierlich D.P., Roe B.A., et al. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inagaki Y., Kojima A., Bessho Y., Hori H., Ohama T., Osawa S. Translation of synonymous codons in family boxes by Mycoplasma capricolum tRNAs with unmodified uridine or adenosine at the first anticodon position. J. Mol. Biol. 1995;251:486–492. doi: 10.1006/jmbi.1995.0450. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T., Nagao A., Suzuki T. Human Mitochondrial tRNAs: Biogenesis, Function, Structural Aspects, and Diseases. Annu. Rev. Genet. 2011;45:299–329. doi: 10.1146/annurev-genet-110410-132531. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T., Suzuki T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2014;42:7346–7357. doi: 10.1093/nar/gku390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. U.S.A. 1985;82:4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agris P.F., Soll D., Seno T. Biological function of 2-thiouridine in Escherichia coli glutamic acid transfer ribonucleic acid. Biochemistry. 1973;12:4331–4337. doi: 10.1021/bi00746a005. [DOI] [PubMed] [Google Scholar]
- 12.Kirino Y., Yasukawa T., Ohta S., Akira S., Ishihara K., Watanabe K., Suzuki T. Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15070–15075. doi: 10.1073/pnas.0405173101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurata S., Weixlbaumer A., Ohtsuki T., Shimazaki T., Wada T., Kirino Y., Takai K., Watanabe K., Ramakrishnan V., Suzuki T. Modified uridines with C5-methylene substituents at the first position of the tRNA anticodon stabilize U.G wobble pairing during decoding. J. Biol. Chem. 2008;283:18801–18811. doi: 10.1074/jbc.M800233200. [DOI] [PubMed] [Google Scholar]
- 14.Bjork G.R., Hagervall T.G. Transfer RNA Modification: Presence, Synthesis and Function. EcoSal Plus. 2014;1 doi: 10.1128/ecosalplus.ESP-0007-2013. doi:10.1128/ecosalplus.ESP-0007-2013. [DOI] [PubMed] [Google Scholar]
- 15.Yaniv M., Barrell B.G. Nucleotide sequence of E. coli B tRNA1-Val. Nature. 1969;222:278–279. doi: 10.1038/222278a0. [DOI] [PubMed] [Google Scholar]
- 16.Harada F., Kimura F., Nishimura S. Nucleotide sequence of valine tRNA 1 from Escherichia coli B. Biochim. Biophys. Aacta. 1969;195:590–592. doi: 10.1016/0005-2787(69)90671-6. [DOI] [PubMed] [Google Scholar]
- 17.Murao K., Saneyoshi M., Harada F., Nishimura S. Uridin-5-oxy acetic acid: a new minor constituent from E. coli valine transfer RNA I. Biochem. Biophys. Res. Commun. 1970;38:657–662. doi: 10.1016/0006-291x(70)90631-5. [DOI] [PubMed] [Google Scholar]
- 18.Ishikura H., Yamada Y., Nishimura S. Structure of serine tRNA from Escherichia coli. I. Purification of serine tRNA's with different codon responses. Biochim. Biophys. Acta. 1971;228:471–481. doi: 10.1016/0005-2787(71)90052-9. [DOI] [PubMed] [Google Scholar]
- 19.Williams R.J., Nagel W., Roe B., Dudock B. Primary structure of E. coli alanine transfer RNA: relation to the yeast phenylalanyl tRNA synthetase recognition site. Biochem. Biophys. Res. Commun. 1974;60:1215–1221. doi: 10.1016/0006-291x(74)90328-3. [DOI] [PubMed] [Google Scholar]
- 20.Sørensen M.A., Elf J., Bouakaz E., Tenson T., Sanyal S., Bjork G.R., Ehrenberg M. Over expression of a tRNALeu isoacceptor changes charging pattern of leucine tRNAs and reveals new codon reading. J. Mol. Biol. 2005;354:16–24. doi: 10.1016/j.jmb.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 21.Kuchino Y., Yabusaki Y., Mori F., Nishimura S. Nucleotide sequences of three proline tRNAs from Salmonella typhimurium. Nucleic Acids Res. 1984;12:1559–1562. doi: 10.1093/nar/12.3.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pope W.T., Brown A., Reeves R.H. The identification of the tRNA substrates for the supK tRNA methylase. Nucleic Acids Res. 1978;5:1041–1058. doi: 10.1093/nar/5.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pope W.T., Reeves R.H. Purification and characterization of a tRNA methylase from Salmonella typhimurium. J. Bacteriol. 1978;136:191–200. doi: 10.1128/jb.136.1.191-200.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesiewicz J., Dudock B. In vitro methylation of E. coli alanine trna with homologous E. coli methylase. Fed. Proc. 1977;36:705. [Google Scholar]
- 25.Murao K., Hasegawa T., Ishikura H. 5-methoxyuridine: a new minor constituent located in the first position of the anticodon of tRNAAla, tRNAThr, and tRNAVal from Bacillus subtilis. Nucleic Acids Res. 1976;3:2851–2860. doi: 10.1093/nar/3.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai K., Takaku H., Yokoyama S. Codon-reading specificity of an unmodified form of Escherichia coli tRNA1Ser in cell-free protein synthesis. Nucleic Acids Res. 1996;24:2894–2899. doi: 10.1093/nar/24.15.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelps S.S., Malkiewicz A., Agris P.F., Joseph S. Modified Nucleotides in tRNALys and tRNAVal are Important for Translocation. J. Mol. Biol. 2004;338:439–444. doi: 10.1016/j.jmb.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 28.Samuelsson T., Elias P., Lustig F., Axberg T., Fölsch G., Akesson B., Lagerkvist U. Aberrations of the classic codon reading scheme during protein synthesis in vitro. J. Biol. Chem. 1980;255:4583–4588. [PubMed] [Google Scholar]
- 29.Takemoto T., Takeishi K., Nishimura S., Ukita T. Transfer of valine into rabbit haemoglobin from various isoaccepting species of valyl-tRNA differing in codon recognition. Eur. J. Biochem. 1973;38:489–496. doi: 10.1111/j.1432-1033.1973.tb03084.x. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel K., Schneider J., McClain W.H. Functional evidence for indirect recognition of G.U in tRNA(Ala) by alanyl-tRNA synthetase. Science. 1996;271:195–197. doi: 10.1126/science.271.5246.195. [DOI] [PubMed] [Google Scholar]
- 31.Nasvall S., Chen P., Bjork G.R. The modified wobble nucleoside uridine-5-oxyacetic acid in tRNA(cmo5UGG)(Pro) promotes reading of all four proline codons in vivo. RNA. 2004;10:1662–1673. doi: 10.1261/rna.7106404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasvall S.J., Chen P., Bjork G.R. The wobble hypothesis revisited: Uridine-5-oxyacetic acid is critical for reading of G-ending codons. RNA. 2007;13:2151–2164. doi: 10.1261/rna.731007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weixlbaumer A., Murphy F.V., Dziergowska A., Malkiewicz A., Vendeix F.A.P., Agris P.F., Ramakrishnan V. Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat. Struct. Mol. Biol. 2007;14:498–502. doi: 10.1038/nsmb1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjork G.R. A novel link between the biosynthesis of aromatic amino acids and transfer RNA modification in Escherichia coli. J. Mol. Biol. 1980;140:391–410. doi: 10.1016/0022-2836(80)90391-5. [DOI] [PubMed] [Google Scholar]
- 35.Hagervall T.G., Jönsson Y.H., Edmonds C.G., McCloskey J.A., Bjork G.R. Chorismic acid, a key metabolite in modification of tRNA. J. Bacteriol. 1990;172:252–259. doi: 10.1128/jb.172.1.252-259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrne R.T., Whelan F., Aller P., Bird L.E. S-Adenosyl-S-carboxymethyl-L-homocysteine: a novel cofactor found in the putative tRNA-modifying enzyme CmoA. Acta Crystallogr. D Biol. Crystallogr. 2013;D69:1090–1098. doi: 10.1107/S0907444913004939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J., Xiao H., Bonanno J.B., Kalyanaraman C., Brown S., Tang X., Al-Obaidi N.F., Patskovsky Y., Babbitt P.C., Jacobson M.P., et al. Structure-guided discovery of the metabolite carboxy-SAM that modulates tRNA function. Nature. 2013;498:123–126. doi: 10.1038/nature12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakami K., Jönsson Y.H., Bjork G.R., Ikeda H., Nakamura Y. Chromosomal location and structure of the operon encoding peptide-chain-release factor 2 of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5620–5624. doi: 10.1073/pnas.85.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2 doi: 10.1038/msb4100050. 20060008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura S., Suzuki T. Fine-tuning of the ribosomal decoding center by conserved methyl-modifications in the Escherichia coli 16S rRNA. Nucleic Acids Res. 2010;38:1341–1352. doi: 10.1093/nar/gkp1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curran J.F. Analysis of effects of tRNA:message stability on frameshift frequency at the Escherichia coli RF2 programmed frameshift site. Nucleic Acids Res. 1993;21:1837–1843. doi: 10.1093/nar/21.8.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyauchi K., Kimura S., Suzuki T. A cyclic form of N6-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat. Chem. Biol. 2013;9:105–111. doi: 10.1038/nchembio.1137. [DOI] [PubMed] [Google Scholar]
- 43.Miyauchi K., Ohara T., Suzuki T. Automated parallel isolation of multiple species of non-coding RNAs by the reciprocal circulating chromatography method. Nucleic Acids Res. 2007;35:e24. doi: 10.1093/nar/gkl1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki T., Ikeuchi Y., Noma A., Suzuki T., Sakaguchi Y. Mass spectrometric identification and characterization of RNA-modifying enzymes. Methods Enzymol. 2007;425:211–229. doi: 10.1016/S0076-6879(07)25009-8. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi Y., Miyauchi K., Kang B.I., Suzuki T. Nucleoside Analysis by Hydrophilic Interaction Liquid Chromatography Coupled with Mass Spectrometry. Methods Enzymol. 2015;560:19–28. doi: 10.1016/bs.mie.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Kimura S., Miyauchi K., Ikeuchi Y., Thiaville P.C., Crecy-Lagard V., Suzuki T. Discovery of the beta-barrel-type RNA methyltransferase responsible for N6-methylation of N6-threonylcarbamoyladenosine in tRNAs. Nucleic Acids Res. 2014;42:9350–9365. doi: 10.1093/nar/gku618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirabayashi N., Sato N.S., Suzuki T. Conserved Loop Sequence of Helix 69 in Escherichia coli 23 S rRNA Is Involved in A-site tRNA Binding and Translational Fidelity. J. Biol. Chem. 2006;281:17203–17211. doi: 10.1074/jbc.M511728200. [DOI] [PubMed] [Google Scholar]
- 48.Kawai G., Yamamoto Y., Kamimura T., Masegi T., Sekine M., Hata T., Iimori T., Watanabe T., Miyazawa T., Yokoyama S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2′-hydroxyl group. Biochemistry. 1992;31:1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- 49.Ikeuchi Y., Shigi N., Kato J., Nishimura A., Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Ikeuchi Y., Kitahara K., Suzuki T. The RNA acetyltransferase driven by ATP hydrolysis synthesizes N4-acetylcytidine of tRNA anticodon. EMBO J. 2008;27:2194–2203. doi: 10.1038/emboj.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soma A., Ikeuchi Y., Kanemasa S., Kobayashi K., Ogasawara N., Ote T., Kato J., Watanabe K., Sekine Y., Suzuki T. An RNA-modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Mol. Cell. 2003;12:689–698. doi: 10.1016/s1097-2765(03)00346-0. [DOI] [PubMed] [Google Scholar]
- 52.Kimura S., Ikeuchi Y., Kitahara K., Sakaguchi Y., Suzuki T. Base methylations in the double-stranded RNA by a fused methyltransferase bearing unwinding activity. Nucleic Acids Res. 2012;40:4071–4085. doi: 10.1093/nar/gkr1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noma A., Ishitani R., Kato M., Nagao A., Nureki O., Suzuki T. Expanding role of the jumonji C domain as an RNA hydroxylase. J. Biol. Chem. 2010;285:34503–34507. doi: 10.1074/jbc.M110.156398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noma A., Kirino Y., Ikeuchi Y., Suzuki T. Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J. 2006;25:2142–2154. doi: 10.1038/sj.emboj.7601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noma A., Sakaguchi Y., Suzuki T. Mechanistic characterization of the sulfur-relay system for eukaryotic 2-thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Res. 2009;37:1335–1352. doi: 10.1093/nar/gkn1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noma A., Yi S., Katoh T., Takai Y., Suzuki T. Actin-binding protein ABP140 is a methyltransferase for 3-methylcytidine at position 32 of tRNAs in Saccharomyces cerevisiae. RNA. 2011;17:1111–1119. doi: 10.1261/rna.2653411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benítez-Páez A., Villarroya M., Douthwaite S., Gabaldón T., Armengod M.-E. YibK is the 2′-O-methyltransferase TrmL that modifies the wobble nucleotide in Escherichia coli tRNA(Leu) isoacceptors. RNA. 2010;16:2131–2143. doi: 10.1261/rna.2245910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin J.L., McMillan F.M. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr. Opin. Struct. Biol. 2002;12:783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 59.Schubert H.L., Blumenthal R.M., Cheng X. Many paths to methyltransfer: a chronicle of convergence. Trends Biochem. Sci. 2003;28:329–335. doi: 10.1016/S0968-0004(03)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kozbial P.Z., Mushegian A.R. Natural history of S-adenosylmethionine-binding proteins. BMC Struct. Biol. 2005;5:19. doi: 10.1186/1472-6807-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takai K., Okumura S., Hosono K., Yokoyama S., Takaku H. A single uridine modification at the wobble position of an artificial tRNA enhances wobbling in an Escherichia coli cell-free translation system. FEBS Lett. 1999;447:1–4. doi: 10.1016/s0014-5793(99)00255-0. [DOI] [PubMed] [Google Scholar]
- 62.Barrett T., Wilhite S.E., Ledoux P., Evangelista C., Kim I.F., Tomashevsky M., Marshall K.A., Phillippy K.H., Sherman P.M., Holko M., et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2012;41:D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamanaka K., Ogura T., Niki H., Hiraga S. Characterization of the smtA gene encoding an S-adenosylmethionine-dependent methyltransferase of Escherichia coli. FEMS Microbiol. Lett. 1995;133:59–63. doi: 10.1111/j.1574-6968.1995.tb07861.x. [DOI] [PubMed] [Google Scholar]
- 64.Sprinzl M., Vassilenko K.S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005;33:D139–D140. doi: 10.1093/nar/gki012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lund E., Dahlberg J.E. Spacer transfer RNAs in ribosomal RNA transcripts of E. coli: processing of 30S ribosomal RNA in vitro. Cell. 1977;11:247–262. doi: 10.1016/0092-8674(77)90042-3. [DOI] [PubMed] [Google Scholar]
- 66.McLuckey S.A., Van Berkel G.J., Glish G.L. Tandem mass spectrometry of small, multiply charged oligonucleotides. J. Am. Soc. Mass Spectrom. 1992;3:60–70. doi: 10.1016/1044-0305(92)85019-G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.