Ectothermic lizards become endothermic in the breeding season, supporting a parental care model for the origins of endothermy.
Keywords: Life sciences, animal science, reptile, lizard, Evolution, endothermy, breeding season, thermogenesis, parental care
Abstract
With some notable exceptions, small ectothermic vertebrates are incapable of endogenously sustaining a body temperature substantially above ambient temperature. This view was challenged by our observations of nighttime body temperatures sustained well above ambient (up to 10°C) during the reproductive season in tegu lizards (~2 kg). This led us to hypothesize that tegus have an enhanced capacity to augment heat production and heat conservation. Increased metabolic rates and decreased thermal conductance are the same mechanisms involved in body temperature regulation in those vertebrates traditionally acknowledged as “true endotherms”: the birds and mammals. The appreciation that a modern ectotherm the size of the earliest mammals can sustain an elevated body temperature through metabolic rates approaching that of endotherms enlightens the debate over endothermy origins, providing support for the parental care model of endothermy, but not for the assimilation capacity model of endothermy. It also indicates that, contrary to prevailing notions, ectotherms can engage in facultative endothermy, providing a physiological analog in the evolutionary transition to true endothermy.
INTRODUCTION
The origin of endothermy remains one of the most debated questions in vertebrate evolutionary physiology (1), particularly because modern-day birds and mammals do not share a common endothermic ancestor, suggesting different possible scenarios for its evolution. The proposed proximate and ultimate origins of the rise in metabolic expenditure required to fuel an endothermic rate of living are numerous, ranging from an enhanced metabolic or aerobic capacity (2, 3), nocturnal behavior enabling selection on enhanced thermogenic capacity (4), to gigantism-associated heat retention (5). Metabolic rate in reptiles is influenced by body size (6), season (6), thyroid hormone (7), and reproductive hormones (8). Other than the special case of muscular thermogenesis in brooding pythons (9), no reptile has been shown to exhibit a sustained metabolic rate approaching that of a similarly sized endotherm because of the absence of a constitutive form of endogenous thermogenesis. Recently, a parental care and reproductive model for the origin of endothermy (10–13) has argued that enhanced metabolic activity related to reproductive synthesis drove the selection for a continuously elevated metabolism (1, 14, 15); endothermy is therefore an exaptation of reproductive metabolism, in that “reproduction is a time of synthesis and heat is a by-product”(13). Reproductive processes are indeed metabolically expensive in endotherms (16, 17). In lizards, the amount of energy allocated to egg production is significant, ranging from 13 to 48% of the total energy expended during the breeding season (17). Although evolutionary biologists typically argue sperm to be inexpensive compared to mate competition and territorial behaviors, evidence from certain reptiles suggests otherwise (18).
The traditional view is that ectotherms have such low rates of metabolism that any heat produced is rapidly dissipated and thus body temperature (Tb) does not normally exceed ambient temperature (Ta) (19). Although normally the case, it has long been recognized that larger ectotherms have the potential to exhibit an elevated Tb due to thermal inertia. However, for most small reptiles, it has been estimated that Tb could only exceed ambient temperature by 0.5° to 1.5°C despite very large changes in metabolism associated with activity or with processes related to digestion (20, 21). Indeed, a reptile such as the tegu lizard is predicted to be able to elevate Tb above Ta (that is, ΔT) by only 0.2°C (22). To maintain a temperature differential that is higher than predicted (23) implies a previously unsuspected use of sustainable thermogenesis combined with greater adjustments in thermal conductance than earlier studies have estimated. For reptiles that hibernate underground, a sustained and elevated body temperature should be of significant advantage when coming out of dormancy, regrowing gonads, undergoing gametogenesis, engaging in mating behaviors, and producing eggs and, ultimately, in nest incubation (11–13).
Our data were obtained from tegu lizards [Salvator merianae (24), formerly Tupinambis merianae], a species that exhibits a marked circannual cycle of activity that is reflected in their thermal biology. During spring and summer (late September through March), tegus are active and warm themselves by basking in the sun, reaching maximum body temperatures of 32° to 35°C (25), and retreating to their burrows at night. During most of the autumn and winter (early April to early September), animals enter dormancy (26, 27) by retreating completely into burrows where they hibernate (that is, exhibit torpor) without feeding and with minimal activity during the whole season, with Tb equal to that of the shelter (Fig. 1). During this period, males undergo gonadal involution and quiescence (28), and toward the end of their 5- to 6-month hibernation period (typically early September), testosterone levels in males rise 20- to 30-fold, they emerge from their burrows and engage in territorial and mate searching behaviors as well as increased aggression (29, 30). In males, the pterygoideus muscle, involved in bite force and jaw stabilization, increases from ~1% to up to 4% of body mass during the reproductive season (31). On the other hand, females remain within their burrows, gather nesting materials, and begin egg laying in the early-mid spring (October to November) (29). Females invest heavily in egg production; a clutch of eggs typically corresponds to ~40% of her body mass (32). Field observations of increased aggression in female tegus remaining with their nests during and up to hatching suggest that incubation maintenance and rudimentary parental care occur (33).
Fig. 1. Life cycle and seasonal changes in the daily profile of body temperature variation in the tegu lizard, with concomitant changes in the outside and burrow temperatures.
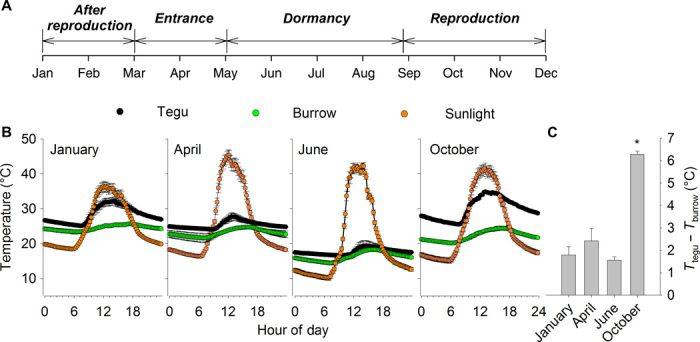
(A to C) The annual cycle (A) of tegu lizards is characterized by a reproductive season of mating and egg laying, a postreproductive season during the wet, warm summer months, a period of prolonged entrance into burrows during inclement weather, before a dormant period during the dry winter months. Averaged (±SEM; n = 4) monthly values (B) of tegu, burrow, and sunlight are shown plotted against hour of the day for the four periods of the year. The temperature difference (ΔT) between tegu and burrow temperature at the coldest time of the day (typically 4:00 a.m. to 6:00 a.m.) when tegus are inside their burrows is shown for the four different months (C). The temperature difference between tegu and burrow was greatest during the reproductive period in the month of October, as denoted by the asterisk (F3,9 = 43.2; P < 0.001).
RESULTS AND DISCUSSION
Our first key observation was that, although tegu Tb equilibrated with that of their burrows during the night throughout most of the year as predicted from heat transfer equations, it did not do so during the reproductive season. To quantify this, we determined the magnitude of the temperature differential (Tb − Ta) of animals kept under seminatural conditions during late night (that is, between 4:00 a.m. and 6:00 a.m.) throughout the year. At this time, any heat attained by basking during the day would be expected to have dissipated. The Tb − Ta differential was substantially elevated during the reproductive season (Figs. 1 and 2).
Fig. 2. Infrared thermal images of tegu lizards following prolonged absence of solar heat gain.
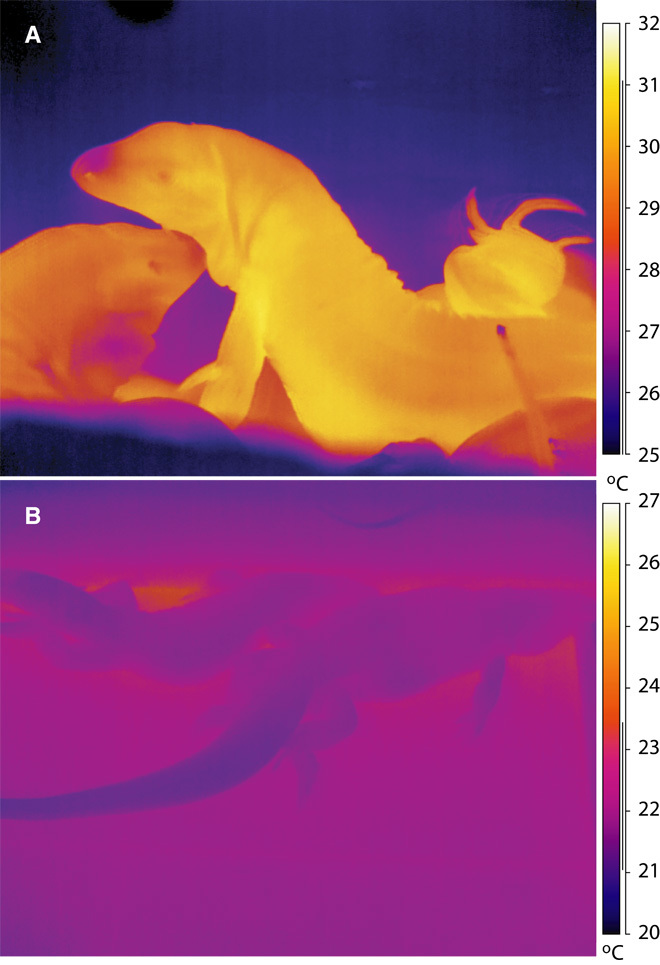
(A and B) A tegu lizard viewed before emerging from its burrow at first light in the morning (6:00 a.m.) during the reproductive season, demonstrating thermogenesis in an outside burrow (A). On a separate occasion, tegu lizards were imaged (10:00 a.m.) after extensive equilibration in a constant temperature environmental chamber, demonstrating cool skin temperature (B) whereas the core temperature, recovered from implanted data loggers, was much warmer.
Could this Tb − Ta difference be explained simply by improved heat conservation during this season, resulting in a very slow rate of dissipation of the heat acquired earlier through basking in the sun (that is, thermal inertia due to the insulative properties of the lizard and the burrow)? To determine this, we placed them into an indoor environment at constant ambient temperature for a prolonged period (8 days). With no access to a heat source for basking or to any material that could act as an insulative shelter, we could test the thermal inertia hypothesis. Our results unequivocally showed that tegus were able to maintain a Tb differential for the entire 8 days without access to heat from basking (figs. S1 and S2). These data were further corroborated by the fact that the individual tegu’s indoor and outdoor temperature differentials were positively correlated (Fig. 3). To sustain this temperature differential, heat had to be endogenously produced, and it could only have originated from thermogenesis.
Fig. 3. Body temperature–ambient temperature differences in outdoor burrows and under indoor conditions in male and female tegus.
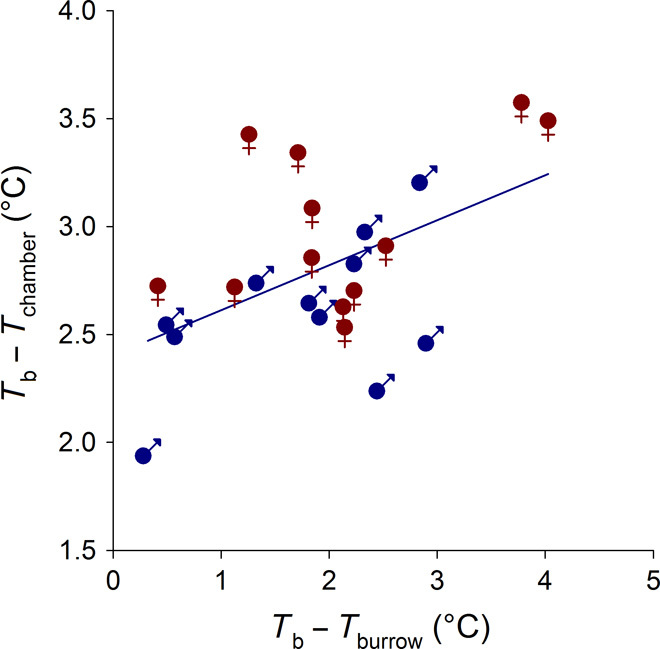
Temperature differentials (mean daily values from the coldest period of the day: 4:00 a.m.) for individual tegus held under seminatural outdoor conditions for 7 days (Tb − Tburrow) and subsequently after transfer to constant temperature conditions indoors for 7 days (Tb − Tchamber). The thermogenic response observed indoors shows strong correlations with the thermogenic response observed outdoors as well as with sex, but not with body mass (r2 = 0.352; outdoor P = 0.016; sex P = 0.037; mass P = 0.85), with males having an indoor ΔT 0.29°C cooler than females.
We questioned whether the heat could have been generated by alternative means, such as activity or the specific dynamic action (SDA) of feeding. Because tegus were fasted for most of the outdoor-indoor comparisons, SDA was not able to explain the increased Tb. When the tegus were held indoors at constant temperature but with a light/dark daily cycle, they were observed to exhibit a distinct circadian rhythm in ΔTb that could not be attributed to changes in ambient temperature (fig. S1). It could not be attributed to activity either. During the early morning and especially when the lights came on indoors, we observed a clear and transient decline in deep-core Tb (fig. S3, see also fig. S4). The greatest temperature differential occurred later in the day and during the night when the animals were quiescent; thus, activity was not responsible for the rise in Tb. Feeding status could also not explain the ΔT results from the year-long study (Fig. 4) because ΔT values rose between day 160 and day 180 of their hibernation fasting period.
Fig. 4. Thermogenesis correlates with HRs in tegus in natural enclosures.
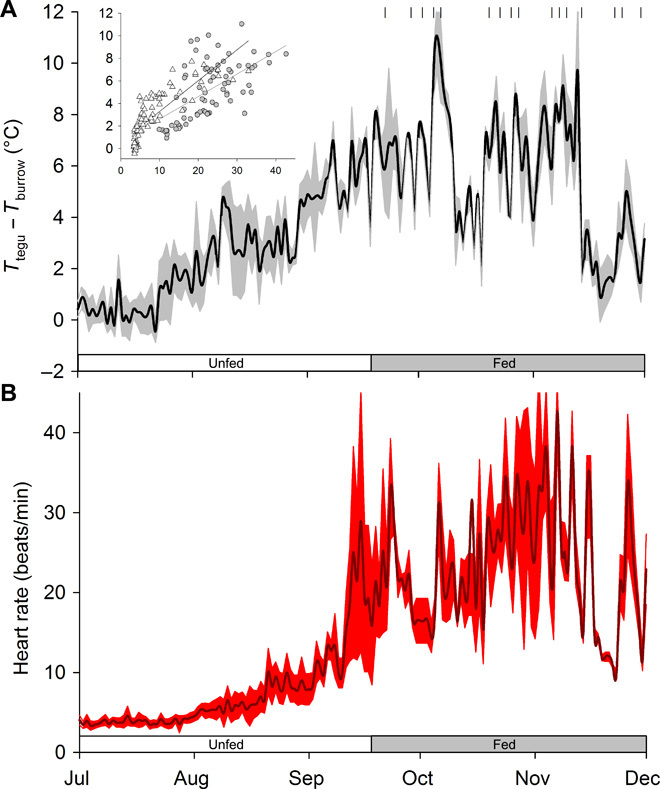
(A) Average (±SEM; shaded area) daily ΔT values (Ttegu − Tburrow) obtained during the daily minimum body temperature period (between 4:00 a.m. and 6:00 a.m.) from tegu lizards (n = 4) free to behave within outdoor enclosures. (B) Continuous records of average HRs (± SEM; shaded area) from the same time period of each day are also shown. Discrete feeding events are marked by small vertical lines in (A). The elevation in ΔT shows a strong correspondence (r2 = 0.56) to the seasonal elevation in HR that cannot be explained strictly on the basis of biochemical Q10 effects alone (that is, HR from July to November increases from 3 to 18 beats/min, whereas body temperature itself only changes from 16° to 26°C; the estimated Q10 for this would be 6, which far exceeds normal biochemical Q10 values of 2 to 3). Inset figure shows ΔT correlation with HR [type II Wald χ2df = 1 = 129; P < 0.0001] for the fed (gray symbol) and unfed (open symbols) periods; neither feeding status [type II Wald χ2df = 1 = 1.93; P = 0.16] nor body mass [type II Wald χ2df = 1 = 1.74; P < 0.18] had effects on ΔT.
We speculate that the cause of the paradoxical drop in body temperature with morning activity is related to cardiovascular changes seen at this time of the year that prepare the animals to warm rapidly by basking. Throughout most of the year, average daily heart rate (HR) is temperature-dependent; it rises with body temperature after the animals leave the burrow to bask. However, following arousal from hibernation and during the reproductive season, we observed a rise in HR that preceded the daily emergence from the burrow [fig. S5; (25)]. Assuming that blood pressure was roughly maintained, this would be associated with a large vasodilation of peripheral vascular beds, one that would normally facilitate the rapid warming of the lizards if they were basking (25). However, under the indoor conditions, the onset of the light phase was not associated with the availability of an external heat source, and we hypothesize that the decline in ΔT seen under these conditions was due to a similar vasodilation leading to increased thermal conductance and, in this instance, a loss of core heat.
Further evidence that the heat is produced by thermogenesis can be indirectly inferred from changes in HR (Fig. 4). It has been shown that through the Fick equation, HR can be used as a relatively accurate proxy for metabolism under many conditions (34, 35). On close examination, we saw that both the nighttime temperature differential and the HR increased during the spring (September to November) reproductive season (Fig. 4). At this time, the lizards are coming out of dormancy and growing gonads, undergoing gametogenesis, engaging in mating behaviors, nest building, laying eggs, and guarding nests (36–38). Significantly, we found that female lizards, who carry a much greater burden of parental investment than males [88% more energy devoted to reproduction across five species of lizards (17)], exhibited a higher ΔT than males under the indoor condition (Fig. 3), although the magnitude of this difference is small.
The evidence that the tegus also decreased thermal conductance at this time is provided by the observed difference between core and skin temperatures in the early morning. This differential was greater in the reproductive season both when tegus were maintained outdoors and indoors (fig. S2A). However, when tegus were held outdoors, they had the ability to both bask during the day and huddle together at night in insulated burrows. Not surprisingly, the Tb − Ta differential in these animals was even larger than that in the animals held indoors. Under natural conditions, the tegus take advantage of behavioral warming along with the insulative features of the burrow to enhance the physiological changes in heat production and thermal conductance. Furthermore, tegu occupation contributes to burrow heat (fig. S2B); empty burrows measured were significantly cooler than occupied burrows, which have consequences for egg incubation temperatures in natural burrows.
To verify the energetic validity of our hypothesis, we modeled tegu thermogenesis using our estimates of seasonal metabolic rates in tegus with the standard model for heat production/transfer in endotherms (Fig. 5). Given the known correlation between HR and metabolic rate (34), we estimated that metabolic rate shows a progressive decline from the postreproductive season to the dormant season and then rises two- to threefold during the reproductive season. Allowing for normal biochemical Q10 effects, and given the thermal conductance of model tegu lizards (see fig. S6), we estimate that such a rise in metabolism could reasonably account for the observed ΔT of 5° to 6°C. This seasonal thermogenesis is further supported by data on average daily HRs obtained from lizards under seminatural conditions (fig. S5). Although average daily HR follows a temperature dependency (that is, ectothermy), during the reproductive season and arousal from dormancy, HR departs from this and demonstrates a seasonal hysteresis, suggesting a rise in metabolism associated with a seasonal reproductive drive (that is, facultative endothermy). Further, the estimates of early-morning metabolic rates during the reproductive season (Fig. 5B) are approximately five times higher than the dormant tegu metabolic rates at the same temperature (27), ~25% lower than the rates of basal metabolism from similarly sized tenrecs [2.7 ml O2/kg per minute; (39)], an insectivorous mammal, and ~2.5-fold higher than predictions for a similarly sized ectotherm at rest [a resting varanid lizard at a body temperature of 25°C: 0.75 O2/kg per minute (40)], although still ~25 to 50% of (maximal oxygen uptake) levels (Q10-corrected) recorded in similarly lizards (41, 42), suggesting that, although they have increased metabolism, they are not maximizing their cardiorespiratory potential.
Fig. 5. Thermogenesis in tegu lizards depends on changes in metabolism and in whole-body thermal conductance.
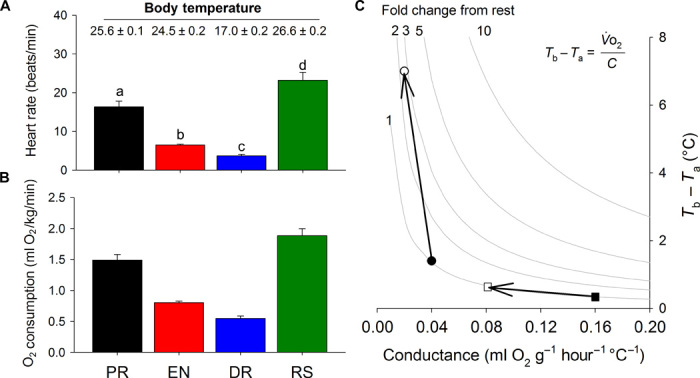
(A and B) Proposed model of seasonal endothermy in tegu lizards obtained from monthly averaged (early morning, predawn; n = 4 animals, two males and two females; 344 days of observations) HR values (A) and associated predicted metabolic rates (B) [from Piercy et al. (34)] from the postreproductive period (PR), entrance into dormancy period (EN), dormancy period (DR), and reproductive season (RS). Between the winter dormant period and the reproductive season, HR increases more than sixfold (despite body temperature changing by less than 10°C; see mean temperature ± SEM). (C) Graphical representation of the calculated relationship between thermal conductance and ΔT at different levels of metabolic rate for lizards. The thermal conductance for tegus (see fig. S6) is plotted against the ΔT values obtained for the nonreproductive tegus (solid circle; April) and reproductive tegus (open circle; October). Isopleths (gray lines) indicate the fold increase from the dormancy metabolic rate (of a tegu at Tb = 25°C) needed to produce the observed change in ΔT. The values calculated are consistent with those measured. Small changes in thermal conductance can have large effects on the ΔT. Squares represent conductance values (for comparison) from 300-g agamid lizards with ectothermic rates of metabolism, showing that a change from high air flow (filled) to low air flow (open) conditions in a burrow would have little effect on ΔT.
Compared to other reptiles, this finding is unique. In other similarly sized reptiles, large increases in metabolic rate (4- to 10-fold) only lead to small (0.5° to 1.5°C) increases in Tb (20, 21). Traditional interpretations (43) posit that endothermic homeothermy must have evolved through mechanisms other than size-induced thermal inertia because from their measurements, Bartholomew and Tucker (43) predicted that an ectotherm would have to reach 100 kg before being capable of a ΔT (of 10°C) of any significance to endothermy. Here, we demonstrate that ~2-kg lizards are capable of remarkable adjustments in thermal conductance combined with sustained changes in metabolism that lead to substantial rises in body temperature under the low air flow conditions of their underground burrows. Is this unreasonable? McNab (22) estimated that reptiles on the order of ~10 kg in size could exhibit thermal conductances similar to mammals, consistent with the data we show here. Tegu lizards can certainly produce high metabolic rates. They have an active predatory lifestyle and a posthepatic septum that enhances ventilatory efficiency and are capable of sustaining metabolic rates similar to maximum rates observed in varanid lizards (42). Other than our estimates, thermal conductance has not been measured in tegus, but previous research has shown many reptiles to exhibit the capacity to alter thermal conductance by two to three times when comparing rates of heating and cooling (43–45). It is reasonable then to suggest that these normally ectothermic lizards have the capacity for endothermy, facultatively dependent on adjustments in thermal conductance. That this degree of endothermy has not been reported in other similarly sized lizards (for example, varanid lizards) may be due to the use of techniques that promote peripheral blood flow and heat dissipation (that is, forced exercise enhances blood and air convection), thereby disrupting any endothermy bestowed by diminished thermal conductance (21, 22).
For comparison, temporal facultative endothermy does occur in some mammalian lineages in a similar context. Both the echidna (a protoendotherm) and the greater hedgehog tenrec (a primitive placental mammal) exhibit increased endothermy and enhanced homeothermy during the reproductive season; egg laying and other reproductive activities are associated with a cessation of daily torpor, a steep rise in body temperature, and lower Tb variability (46, 47). That an ectotherm the size of the earliest mammals can engage in reproductive endothermy is significant. It bolsters the proposal that the burrowing lifestyle of small protoendotherms in combination with the high metabolic demands of reproduction provided appropriate conditions for the origins of mammalian endothermy. Because female tegus remain with their egg clutches throughout the incubation period (33) and lepidosaurs are the sister taxa to birds and other archosaurs, the results also lend support to the parental care model of endothermy in which enhanced parental metabolic capacity could be selected to facilitate increased nest temperature or enhance nest thermal stability, which are known to speed up or alter the developmental rates of embryos in reptiles (13). The reason male tegus also exhibit these changes in temperature could be common seasonal hormonal (for example, estradiol, leptin, or thyroid hormone) surges with pyrogenic potential (48–50), that sperm production and gonadal regrowth are energetically expensive (18), that spermatogenesis requires elevated temperatures (37, 38), that the rapid rise in testosterone following a period of hibernation, leads to intense androgenic effects (28) such as seasonal changes in secondary sexual morphologies (31), or simply that the genetic processes supporting maternal endothermy are shared in offspring of both sexes and not linked to sex chromosomes (13).
MATERIALS AND METHODS
Animals
Adult black and white tegu lizards (S. merianae) were reared from eggs within a captive colony at the State University of Sao Paulo, Rio Claro, Brazil. At the time of study, animals were of an average mass of 2.0 ± 0.55 kg and sexually mature (for example, males had secondary sexual characteristics and females laid eggs following the completion of the study). Two cohorts of animals were used: one group was studied completely in outdoor enclosures (~30 m2) for an entire year and was part of a larger study investigating cardiorespiratory physiology (25, 34), and another group was studied under both outdoor and indoor conditions over the course of three reproductive seasons (typically early to late October). Tegus were fed on a regular basis (once every 4 to 5 days, except during the hibernation period when they voluntarily withdrew to their burrows and were not fed from 23 March until 23 September) a primarily meat-based diet (chicken) supplemented with necessary vitamins and minerals. Tegus were implanted with multichannel biopotential radiotelemeters (group 1; Konigsberg Instruments) or temperature data loggers (group 2; Thermochron iButtons). Surgeries were conducted as described in our previous studies (17, 18). Experiments using biopotential radio-telemeters lasted an entire year, whereas those conducted with temperature data loggers were conducted during the reproductive season (October) over separate years and lasted approximately 16 days. All experimental procedures conformed to and were approved by the University of British Columbia animal care and use committee.
Experimental protocol
Tegus were examined under four different conditions. The initial group (n = 4, two males and two females) was allowed to behave naturally for a period of a year in outdoor enclosures. The outdoor enclosures contained sites for voluntary solar basking and shade cover as well as a burrow (1 m wide × 1 m high × 0.5 m deep) where tegus always retreated at night. This year-long group of tegus revealed strong seasonal differences in the degree of thermogenesis, leading us to design further experiments to ascertain the nature of the thermogenesis during the reproductive season with subsequent groups. Subsequently, we compared the thermogenesis of tegus held under seminatural conditions and following a transfer to fixed thermal conditions in the laboratory. These latter groups of tegus were tested under different states: outdoor for 8 days and transferred to indoor conditions at 18° to 20°C for 8 days (n = 23). A final comparison was made with a subset (n = 11) of the latter group of tegus (transferred from outdoor to constant indoor temperature of 18°C) with either a normal photoperiod [12 hours light/ 12 hours dark (12L/12D)] or constant darkness (0L/24D). In the protocol with photoperiod, lights were gradually increased or decreased in intensity. Three cold fluorescent lamps were on from 6:00 a.m. to 6:00 p.m., whereas additional cold fluorescent lamps were on from 10:00 a.m. to 4:00 p.m. and additional cold fluorescent lamps were turned on from 12:00 p.m. to 2:00 p.m. The net result was that the light intensity was highest from 12:00 p.m. to 2:00 p.m.
Temperature measurements
All loggers measuring ambient temperature (Ta) were placed inside water-filled copper pipes of similar volume and mass to a tegu lizard and thus provided an estimate of operative temperature (51). In the outdoor enclosures, loggers were placed in the sun, shade, and burrows where tegus were free to roam. Indoors, the environmental chamber was set to 18° or 20°C, and loggers were placed on the floor at the same level as the lizards. Tegus were free to move inside the environmental chamber. All temperature loggers and telemeters were calibrated before use and verified following explant. Calibrations were conducted by immersing loggers into a water bath of known temperature for 30 min at three different temperatures spanning from 10° to 40°C. Temperature loggers were set to capture body temperature (Tb) every 10 min, and telemeters captured Tb at 125 Hz, but values were averaged over 15-min intervals. Infrared thermal imaging was conducted using a FLIR (Model SC660) thermal imager; images of tegus were captured in the early morning in their outside burrow or indoors after turning the lights on.
HR and Tb analysis
Daily minimum Tb and HR data were extracted from the continuous annual recording using custom-written scripts in MATLAB (25). Voltage data traces from the telemetered biopotential leads were digitally filtered (high pass, 0.1 Hz) to provide a stable signal to derive QRS complexes for detection of the ventricular depolarization. A complete rendition of the individual R-R intervals was obtained over 15-min time periods, which were visually verified by overlaying the detected peaks on top of the raw voltage trace. HR and Tb from tegus were subsequently binned into 15-min periods over the entire year in the outdoor enclosures, and hourly or daily averages or minimum values were extracted where appropriate. Oxygen consumption () was estimated from HR using empirically derived equations (18) for tegu lizards tested under various metabolic states (sleep, inactivity, rest, and activity). Thermogenesis was modeled using standard approaches to endothermic heat metabolism (14).
Thermal conductance estimates
Estimating thermal conductance in ectotherms can be confounded by the potential contribution of endogenous heat production, which would slow down the rates of cooling or raise the rates of warming. Bartholomew and Tucker (43, 44) attempted to account for this effect by estimating heat production from existing HR/metabolic rate relationships during temperature transitions and removing this influence from their estimate of C. However, their values of C are still overestimates due to the high air flow rates used in their experiments as well as a lack of resting conditions in their restrained subjects (52). Therefore, we estimated C from a recently deceased specimen, which allowed for a matching of body size and removal of metabolic and circulatory contributions to conductance estimates. Whole-lizard thermal conductance was estimated using cooling constants (22). The cooling constant of a dead specimen (2 kg) was obtained following transfer of the individual to a constant temperature environment. The slope of ln(Tb − Ta) versus time allows the estimate of an exponential cooling constant, which was converted into a whole-animal thermal conductance using the following equation [derived by McNab (22)]
where a is the cooling constant (per hour), ctissue is the specific heat capacity of tissue (3.35 J/g per °C), and Qox is the oxycaloric equivalent heat production per milliliter of oxygen consumed [20.1 J/ml O2; (53)].
Statistical analysis
Unless otherwise stated, temperature differentials (ΔT = Ttegu − Ta) and physiological data were taken from the early-morning time period (predawn, 4:00 a.m. to 6:00 a.m.) before emergence in the sun or before the onset of lights indoors. These values ensured minimal locomotory activity and as long a period of time as possible for lizards to equilibrate with the external environmental temperature and exhibit any potential thermogenesis independent of thermal inertia. Temperature differentials were analyzed using one-way analysis of variance (ANOVA) (repeated measures for seasonal or outdoor-indoor comparisons) with Holm-Sidak post hoc tests. If normality was violated, log transformation was used and normality was confirmed on residuals. On occasions where normality was not possible, corresponding nonparametric assessments were performed on ranked data. Temperature differentials were also analyzed using linear mixed-effects models using the lme4 package in R (54) with HR, body mass, and fed status as fixed effects and animal identity and month of year as random effects. Where appropriate, an α of 0.05 was used as the critical level for statistical comparisons. SigmaPlot (version 11) and R (55) were used for statistical analyses.
Supplementary Material
Acknowledgments
We thank São Paulo State University (UNESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and São Paulo Research Foundation (FAPESP) for their financial assistance that supported the animal rearing facility; the staff of the Jacarezario providing care for the tegus throughout this study; and C. Farmer for her helpful comments on the manuscript. Funding: This research was funded by the Natural Sciences and Engineering Research Council of Canada (to W.K.M. and G.J.T.), FAPESP, and CNPq through the INCT in Comparative Physiology (proceeding nos. 2008/57712-4 and 573921/2008-3). D.V.A. was supported by research grants from the FAPESP (proceeding nos. 2010/05473-6 and 2013/04190-9) and CNPq. Author contributions: G.J.T., W.K.M., C.E.S., and C.A.C.L. designed the experiments; C.E.S. conducted the year-long experiments investigating tegus in outdoor enclosures; C.A.C.L. and W.K.M. conducted the outdoor-indoor comparison experiments; V.C. conducted the thermal imaging measurements; C.E.S., W.K.M., G.J.T., C.A.C.L., and D.V.A. conducted the surgeries; G.J.T. wrote the scripts for analyzing HR; and C.E.S. and G.J.T. analyzed the data. G.J.T. conducted the statistical analysis and wrote the manuscript. W.K.M., C.A.C.L., A.S.A., and D.V.A. edited the manuscript. A.S.A. and D.V.A. provided support for logistical aspects of the project, including animal care and permit acquisition. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data are available from the Brock University Respository (http://hdl.handle.net/10464/7737).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/1/e1500951/DC1
Fig. S1. Temperature differentials for tegus (n = 11) held under constant temperature conditions (Ta = 18°C) indoors with and without a photoperiod plotted against days after transfer indoors (±SD, one direction, gray area).
Fig. S2. Mean (±SD) temperature differences (A) between tegu (Tb), skin (Ts), and ambient (Ta) temperature in a seminatural burrow (B), indoor chamber with (C-P) and without (C-NP) a photoperiod.
Fig. S3. Transient thermal changes in tegu lizards associated with photoperiod.
Fig. S4. Trace from a tegu in a natural enclosure during 3 days in January when it did not emerge out of its burrow.
Fig. S5. Average (±SEM) minimum daily HRs (that is, between 4:00 a.m. and 6:00 a.m.) as a function of body temperature in tegu lizards in seminatural outdoor enclosures.
Fig. S6. Thermal equilibrium curves from tegus that had previously warmed in the sun and then transferred themselves to their lower temperature burrows.
REFERENCES AND NOTES.
- 1.Kemp T. S., The origin of mammalian endothermy: a paradigm for the evolution of complex biological structure. Zool. J. Linn. Soc. 147, 473–488 (2006). [Google Scholar]
- 2.Hayes J. P., Garland T., The evolution of endothermy: Testing the aerobic capacity model. Evolution 49, 836–847 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Bennett A. F., Ruben J. A., Endothermy and activity in vertebrates. Science 206, 649–654 (1979). [DOI] [PubMed] [Google Scholar]
- 4.Crompton A. W., Taylor C. R., Jagger J. A., Evolution of homeothermy in mammals. Nature 272, 333–336 (1978). [DOI] [PubMed] [Google Scholar]
- 5.McNab B. K., The evolution of endothermy in the phylogeny of mammals. Am. Nat. 112, 1–21 (1978). [Google Scholar]
- 6.Toledo L. F., Brito S. P., Milsom W. K., Abe A. S., Andrade D. V., Effects of season, temperature, and body mass on the standard metabolic rate of tegu lizards (Tupinambis merianae). Physiol. Biochem. Zool. 81, 158–164 (2008). [DOI] [PubMed] [Google Scholar]
- 7.A. J. Hulbert, in Advances in Physiological Research, H. McLennan, J. R. Ledsome, C. H. S. McIntosh, D. R. Jones, Eds. (Plenum Publishing Corp., New York, 1987), pp. 305–319. [Google Scholar]
- 8.Marler C. A., Walsberg G., White M. L., Moore M., Marler C. A., Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behav. Ecol. Sociobiol. 37, 225–231 (1995). [Google Scholar]
- 9.Harlow P., Grigg G., Shivering thermogenesis in a brooding diamond python, Python spilotes spilotes. Copeia 1984, 959–965 (1984). [Google Scholar]
- 10.Farmer C. G., Hot blood and warm eggs. Integr. Comp. Biol. 38, 93A (1998). [Google Scholar]
- 11.Farmer C. G., Parental care: The key to understanding endothermy and other convergent features in birds and mammals. Am. Nat. 155, 326–334 (2000). [DOI] [PubMed] [Google Scholar]
- 12.C. G. Farmer, in New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom, J. A. Gauthier, L. F. Gall, Eds. (Peabody Museum of Natural History, Yale University, New Haven, CT, 2001), pp. 389–412. [Google Scholar]
- 13.Farmer C. G., Reproduction: The adaptive significance of endothermy. Am. Nat. 162, 826–840 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Oelkrug R., Goetze N., Exner C., Lee Y., Ganjam G. K., Kutschke M., Müller S., Stöhr S., Tschöp M. H., Crichton P. G., Heldmaier G., Jastroch M., Meyer C. W., Brown fat in a protoendothermic mammal fuels eutherian evolution. Nat. Commun. 4, 2140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavijo-Baquet S., Bozinovic F., Testing the fitness consequences of the thermoregulatory and parental care models for the origin of endothermy. PLOS One 7, e37069 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond K. A., Lloyd K. C., Diamond J., Is mammary output capacity limiting to lactational performance in mice? J. Exp. Biol. 199, 337–349 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Kunz T., Orrell K., Energy costs of reproduction. Encycl. Energy 5, 423–442 (2004). [Google Scholar]
- 18.Olsson M., Madsen T., Shine R., Is sperm really so cheap? Costs of reproduction in male adders, Vipera berus. Proc. R. Soc. B. 264, 455–459 (1997). [Google Scholar]
- 19.Cowles R. B., Possible origin of dermal temperature regulation. Evolution 12, 347–357 (1958). [Google Scholar]
- 20.Tattersall G. J., Milsom W. K., Abe A. S., Brito S. P., Andrade D. V., The thermogenesis of digestion in rattlesnakes. J. Exp. Biol. 207, 579–585 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Bennett A. F., Hicks J. W., Cullum A. J., An experimental test of the thermoregulatory hypothesis for the evolution of endothermy. Evolution 54, 1768–1773 (2000). [DOI] [PubMed] [Google Scholar]
- 22.B. K. McNab, The Physiological Ecology of Vertebrates : A View from Energetics (Comstock Publishing Associates, Ithaca, 2002), pp. xxvii. [Google Scholar]
- 23.Garrick D., Body surface temperature and length in relation to the thermal biology of lizards. Biosci. Horiz. 1, 136–142 (2008). [Google Scholar]
- 24.Harvey M. B., Ugueto G. N., Gutberlet R. L., Review of Teiid morphology with a revised taxonomy and phylogeny of the Teiidae (Lepidosauria: Squamata). Zootaxa 3459, 1–156 (2012). [Google Scholar]
- 25.Sanders C. E., Tattersall G. J., Andrade D. V., Abe A. S., Milsom W. K., Daily and annual cycles in thermoregulatory behaviour and cardio-respiratory physiology of black and white tegu lizards. J. Comp. Physiol. B 185, 905–915 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Andrade D. V., Brito S. P., Toledo L. F., Abe A. S., Seasonal changes in blood oxygen transport and acid–base status in the tegu lizard, Tupinambis merianae. Resp. Physiol. Neurobiol. 140, 197–208 (2004). [DOI] [PubMed] [Google Scholar]
- 27.de Andrade D. V., Abe A. S., Gas exchange and ventilation during dormancy in the tegu lizard Tupinambis merianae. J. Exp. Biol. 202, 3677–3685 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Chamut S., Jahn G. A., Arce O. E. A., Manes M. E., Testosterone and reproductive activity in the male tegu lizard, Tupinambis Merianae. Herpetol. Conserv. Biol. 7, 299–305 (2012). [Google Scholar]
- 29.Winck G. R., Cechin S. Z., Hibernation and emergence pattern of Tupinambis merianae (Squamata: Teiidae) in the Taim Ecological Station, southern Brazil. J. Nat. Hist. 42, 239–247 (2008). [Google Scholar]
- 30.Winck G. R., Blanco C. C., Cechin S. Z., Population ecology of Tupinambis merianae (Squamata, Teiidae): Home-range, activity and space use. Anim. Biol. 61, 493–510 (2011). [Google Scholar]
- 31.Naretto S., Cardozo G., Blengini C. S., Chiaraviglio M., Sexual selection and dynamics of jaw muscle in Tupinambis lizards. Evol. Biol. 41, 192–200 (2014). [Google Scholar]
- 32.H. R. Lopes, Biologia reprodutiva e comportamento do teiú Tupinambis merianae (Linnaeus, 1758) em cativeiro (Reptilia, Teüdae) (Universidade Federal de São Carlos, São Carlos, Brazil, 1986). [Google Scholar]
- 33.L. A. Fitzgerald, J. M. Chani, O. E. Donadio, in Neotropical Wildlife: Use and Conservation, J. Robinson, K. Redford, Eds. (University of Chicago Press, Chicago, 1991), pp. 303–316. [Google Scholar]
- 34.Piercy J., Rogers K., Reichert M., Andrade D. V., Abe A. S., Tattersall G. J., Milsom W. K., The relationship between body temperature, heart rate, breathing rate, and rate of oxygen consumption, in the tegu lizard (Tupinambis merianae) at various levels of activity. J. Comp. Physiol. B 185, 891–903 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Butler P. J., Frappell P. B., Wang T., Wikelski M., The relationship between heart rate and rate of oxygen consumption in Galapagos marine iguanas (Amblyrhynchus cristatus) at two different temperatures. J. Exp. Biol. 205, 1917–1924 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Meiri S., Brown J. H., Sibly R. M., The ecology of lizard reproductive output. Glob. Ecol. Biogeogr. 21, 592–602 (2012). [Google Scholar]
- 37.Yazawa T., Nakayama Y., Fujimoto K., Matsuda Y., Abe K., Kitano T., Abé S.-I., Yamamoto T., Abnormal spermatogenesis at low temperatures in the Japanese red-bellied newt, Cynops pyrrhogaster: Possible biological significance of the cessation of spermatocytogenesis. Mol. Reprod. Dev. 66, 60–66 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Joly J., Saint Girons H., Influence of temperature on the rate of spermatogenesis, duration of spermatogenic activity and development of secondary sex characteristics in the wall-lizard, Lacerta muralis L. (Reptilia, Lacertidae). Arch. Anat. Microsc. Morphol. Exp. 64, 317–336 (1975). [PubMed] [Google Scholar]
- 39.McNab B. K., The evolution of energetics in eutherian "insectivorans": An alternate approach. Acta Theriol. 51, 113–128 (2006). [Google Scholar]
- 40.P. C. Withers, Comparative Animal Physiology (Saunders College, Fort Worth, 1992), pp. xxii, 949, [111]. [Google Scholar]
- 41.Frappell P., Schultz T., Christian K., Oxygen transfer during aerobic exercise in a varanid lizard Varanus mertensi is limited by the circulation. J. Exp. Biol. 205, 2725–2736 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Klein W., Andrade D. V., Abe A. S., Perry S. F., Role of the post-hepatic septum on breathing during locomotion in Tupinambis merianae (Reptilia: Teiidae). J. Exp. Biol. 206, 2135–2143 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Bartholomew G. A., Tucker V. A., Size, body temperature, thermal conductance, oxygen consumption, and heart rate in Australian varanid lizards. Physiol. Zool. 37, 341–354 (1964). [Google Scholar]
- 44.Bartholomew G. A., Tucker V. A., Control of changes in body temperature, metabolism, and circulation by the agamaid lizards, Amphibolurus barbatus. Physiol. Zool. 36, 199–218 (1963). [Google Scholar]
- 45.Seebacher F., Grigg G. C., Changes in heart rate are important for thermoregulation in the varanid lizard Varanus varius. J. Comp. Physiol. B 171, 395–400 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Nicol S., Andersen N. A., Body temperature as an indicator of egg-laying in the echidna, Tachyglossus aculeatus. J Therm Biol 31, 483–490 (2006). [Google Scholar]
- 47.Levesque D. L., Lovegrove B. G., Increased homeothermy during reproduction in a basal placental mammal. J. Exp. Biol. 217, 1535–1542 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Brasfield S. M., Talent L. G., Janz D. M., Reproductive and thyroid hormone profiles in captive Western fence lizards (Sceloporus occidentalis) after a period of brumation. Zoo Biol. 27, 36–48 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Niewiarowski P. H., Balk M. L., Londraville R. L., Phenotypic effects of leptin in an ectotherm: A new tool to study the evolution of life histories and endothermy? J. Exp. Biol. 203, 295–300 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Spanovich S., Niewiarowski P. H., Londraville R. L., Seasonal effects on circulating leptin in the lizard Sceloporus undulatus from two populations. Comp. Biochem. Phys. B Biochem. Mol. Biol. 143, 507–513 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Hertz P. E., Huey R. B., Stevenson R. D., Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am. Nat. 142, 796–818 (1993). [DOI] [PubMed] [Google Scholar]
- 52.McNab B. K., Auffenberg W., The effect of large body size on temperature regulation of komodo dragon, Varanus komodoensis. Comp. Biochem. Physiol. A Comp. Physiol. 55, 345–350 (1976). [DOI] [PubMed] [Google Scholar]
- 53.Elliott J. M., Davison W., Energy equivalents of oxygen consumption in animal energetics. Oecologia 19, 195–201 (1975). [DOI] [PubMed] [Google Scholar]
- 54.Bates D., Mächler M., Bolker B. M., Walker S. C., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 55.R Core Team, R (Programming Language) (R Foundation for Statistical Computing, Vienna, Austria, 2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/1/e1500951/DC1
Fig. S1. Temperature differentials for tegus (n = 11) held under constant temperature conditions (Ta = 18°C) indoors with and without a photoperiod plotted against days after transfer indoors (±SD, one direction, gray area).
Fig. S2. Mean (±SD) temperature differences (A) between tegu (Tb), skin (Ts), and ambient (Ta) temperature in a seminatural burrow (B), indoor chamber with (C-P) and without (C-NP) a photoperiod.
Fig. S3. Transient thermal changes in tegu lizards associated with photoperiod.
Fig. S4. Trace from a tegu in a natural enclosure during 3 days in January when it did not emerge out of its burrow.
Fig. S5. Average (±SEM) minimum daily HRs (that is, between 4:00 a.m. and 6:00 a.m.) as a function of body temperature in tegu lizards in seminatural outdoor enclosures.
Fig. S6. Thermal equilibrium curves from tegus that had previously warmed in the sun and then transferred themselves to their lower temperature burrows.


