Abstract
Ubiquinone (coenzyme Q10, Q10) represents an endogenously synthesized lipid‐soluble antioxidant which is crucial for cellular energy production but is diminished with age and under the influence of external stress factors in human skin. Here, it is shown that topical Q10 treatment is beneficial with regard to effective Q10 replenishment, augmentation of cellular energy metabolism, and antioxidant effects. Application of Q10‐containing formulas significantly increased the levels of this quinone on the skin surface. In the deeper layers of the epidermis the ubiquinone level was significantly augmented indicating effective supplementation. Concurrent elevation of ubiquinol levels suggested metabolic transformation of ubiquinone resulting from increased energy metabolism. Incubation of cultured human keratinocytes with Q10 concentrations equivalent to treated skin showed a significant augmentation of energy metabolism. Moreover, the results demonstrated that stressed skin benefits from the topical Q10 treatment by reduction of free radicals and an increase in antioxidant capacity. © 2015 BioFactors, 41(6):383–390, 2015
Keywords: coenzyme Q10, energy metabolism, mitochondrial activity, antioxidant, topical Q10 treatment, skin, skin aging
Abbreviations
- Q10
coenzyme Q10
- ROS
reactive oxygen species
- UV
ultraviolet light
- SSL
skin surface lipids
- DMQ
demethoxyubiquinone
1. Introduction
Skin as the outermost human organ is in direct contact with the environment and is, therefore, exposed to external stress factors. To combat resulting damages, cutaneous cells are constantly involved in tissue regeneration and repair, processes that require a high amount of energy and a well‐regulated cellular metabolism. With increasing age, however, energy production as well as mitochondrial activity decline 1, 2. As a consequence, cell and tissue functions are impaired and visible structural alterations occur. These result in the well‐known signs of skin aging, for example, the appearance of wrinkles and lines 3 as well as loss of elasticity 4.
Reactive oxygen species (ROS) and free radicals represent predominant causes of damages to cellular components. In aging cells, ROS are frequently generated due to changes in cell respiration 5, 6, 7. Especially in skin cells, formation of ROS is also promoted by exposition to external insults such as ultraviolet (UV) light 8.
ROS damage not only lipid membranes and DNA but also at the same time structural and catalytic proteins which play a crucial role in cellular energetic pathways. As a result, the cutaneous energy metabolism is further impaired by actions of ROS, which are not only the cause of the aging process, but also its consequence 9.
Along these lines, two important points of action arise in order to strengthen skin in the fight against age‐associated alterations. First, maintenance of sufficient cellular energy levels to stop the decline of mitochondrial activity and, second, antioxidant protection against ROS originating from any source.
Coenzyme Q10 (Q10), also known as ubiquinone, is an important coenzyme that is present in all human cells. It was originally shown to be a necessary component of the mitochondrial respiratory chain, working as an electron carrier between the complexes I, II, and III 10, 11 and is, thus, crucial for this energy production process of the human body. It has also been well established that Q10 has many other important functions, for example, as a native constituent of the lysosomal electron transport chain where it promotes proton translocation 12.
Moreover, Q10 is widely accepted for its intra‐ and extracellular antioxidant capacity 9, 13, 14, 15, 16. A recent publication showed that global loss of Q10 levels in a mouse model leads to gradual loss of mitochondrial function, the development of aging‐like disease phenotypes, and a shortened lifespan of the mice 17. This phenotype is reversible, when ubiquinone levels are partially restored 17, underlining the importance of Q10 for proper function of the entire organism.
During the process of energy production and in extracellular enzymatic processes ubiquinone is converted into its reduced form (designated as ubiquinol) which serves specific functions as a lipid‐soluble antioxidant. Ubiquinol acts as a radical scavenger and protects mitochondria, lipid membranes, lipoproteins, and also DNA from oxidative damage 9, 18, 19.
In skin, endogenous Q10 levels decline with increasing age 14. Additionally, UV‐irradiation, which leads to oxidative damage, significantly reduces skin's Q10 levels 20. In this context, the objective of this study was to investigate whether human skin may benefit from a topical Q10 treatment with regards to the two aforementioned important points of action: increase in cellular energy metabolism as well as antioxidant effects.
2. Methods
2.1. In Vivo Treatment with Q10‐Containing Formulas
A controlled, randomized study was carried out enrolling 73 healthy, non‐smoking, female volunteers (20–66 years).
The recommendations of the current version of the Declaration of Helsinki and the guideline of the International Conference on Harmonization Good Clinical Practice (ICH GCP) were observed as applicable to a non‐drug study. All volunteers provided written, informed consent.
For the study, two formulas, a cream and a serum, containing Q10 in different concentrations were used (cream 348 µM ubiquinone [formula 1]; serum 870 µM ubiquinone [formula 2]).
During a 5‐day preconditioning period and throughout the study, volunteers were required to desist from using skin care products and to avoid excessive contact with surfactants and sun exposure on both forearms. Visits to saunas, solariums, swimming‐pools as well as very demanding exercise were prohibited for 1 day prior to measurements. Measurements were performed by trained and experienced personnel after acclimatization for at least 30 minutes under standard atmospheric conditions (21.5°C ± 1.0°C and 45% ± 5% relative humidity).
After the preconditioning period, a total of three test areas were established: One inner forearm of each volunteer was used for product treatment (two test areas) and the other forearm was left untreated and utilized as control (one test area). The positioning of treatment locations was left–right randomized and a stencil was used to mark the test areas. Volunteers applied the test formulas twice daily (morning and evening; 2 mg/cm2) for 2 weeks according to written instructions.
2.2. Collection of Skin Samples
After 2 weeks of application, the following skin samples were obtained on the morning after the last treatment: (I) uppermost layers of stratum corneum (skin surface), (II) suction blister fluid, and (III) suction blister epidermis (Fig. 1).
Figure 1.
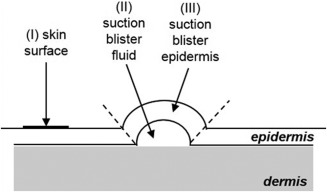
Schematic illustration of collection of skin samples (not shown to scale). Samples from the skin surface (I) were obtained using adhesive sampling discs (D‐Squames®). After raising suction blisters, the blister fluid (II) was collected using a sterile syringe. In the last step, suction blister epidermis (III) was harvested using sterile forceps and scissors (III).
(I) To collect samples from the skin surface, adhesive sampling discs (D‐Squame®, Cuderm Corporation) were used and samples were immediately stored at −85°C. (II) Suction blisters of 8 mm diameter were generated from each test area as previously described 21, 22. Blister fluid was isolated using a sterile syringe, stored on ice and immediately processed as described below. (III) Suction blister epidermis was harvested utilizing a sterile pair of scissors in combination with a forceps and was stored at −85°C.
2.3. Preparation of Extracts from Suction Blister Epidermis and D‐Squames®
For analysis, suction blister epidermis and D‐Squames® were thawed. D‐Squames® were extracted with 1 mL acetone. Suction blister epidermis was lysed in 350 µL isopropanol utilizing a Precellys® 24™ homogenizer (Peqlab). Extracts were used for analysis without further processing.
2.4. HPLC–MS/MS Measurements and Data Analysis
Concentrations of ubiquinone, ubiquinol, and cholesterol were determined using High Performance Liquid Chromatography (1200 series, Agilent Technologies) coupled to a triple quadrupol mass spectrometer (6490 Triple Quadrupol LC/MS system, Agilent Technologies). The separation was achieved utilizing a YMC‐Pack Pro C8 50 × 3 mm (3 µm) column (YMC Europe) and gradient separation using water containing 2 mM ammonium acetate and methanol containing 2 mM ammonium acetate; 0.5 mL/min. Mass spectrometric detection was carried out as follows: ubiquinone (Sigma‐Aldrich): 880.5 amu [M + NH4]+ → 197 amu (Quantifier); ubiquinol (Kaneka Nutrients): 882.7 amu [M + NH4]+ → 197 amu (Quantifier); 882.7 amu [M + NH4]+ → 81 amu (Qualifer); cholesterol (Sigma‐Aldrich): 369.4 amu [M + H‐H2O]+ → 91 amu (Quantifier); 369.4 amu [M + NH4]+ → 81 amu (Qualifer).
For the comparison of young (20–25 years; n = 28) versus aged (60–66 years; n = 28) subjects, only samples obtained from these volunteers were selected and all values were normalized to cholesterol. Cholesterol and quinones are readily soluble in isopropyl alcohol which was used for extraction of suction blister material. Also, cholesterol showed uniform levels throughout the study population. To obtain levels of quinones in each sample, values of ubiquinone and ubiquinol were added and depicted as [ng quinones/µg cholesterol].
For the comparison of untreated and treated subjects, samples obtained from all volunteers (n = 73, age: 20–66 years) were chosen. For analysis, it is most appropriate to consider the formula‐treated area for normalization. Thus, all values were normalized to the size of the surface area of the respective sample (D‐Squames®: 380.13 mm2; suction blister: 50.27 mm2) and were depicted as [ng/mm2].
In order to determine levels of ubiquinone, ubiquinol, and both quinones in the deeper layers of the epidermis, the respective value obtained from the skin surface (D‐Squame®) was subtracted from the value determined in the suction blister epidermis.
2.5. Analysis of Suction Blister Fluids
Freshly isolated suction blister fluid samples were analyzed using the FORM Analyzer (Micro‐Medical Instrumente GmbH). The concentration of hydroperoxides was determined in untreated skin utilizing the Free Oxygen Radicals Test (FORT, Calligari) 23 in accordance with the manufacturer's instructions and the “FORM‐CR3000‐Minilab” device (Micro‐Medical). One FORT unit corresponds to 0.26 mg/L H2O2.
Baseline levels in suction blister fluids were ≤160 FORT units. According to reference values of blood samples given by the manufacturer and obtained from a recent publication 24, increased oxidative stress levels are considered as such starting at a value of 55% over baseline. Thus, the limit value for oxidative stress in our samples starts at 250 FORT units and was displayed by 16 out of 73 volunteers which are depicted in Fig. 6.
Figure 6.
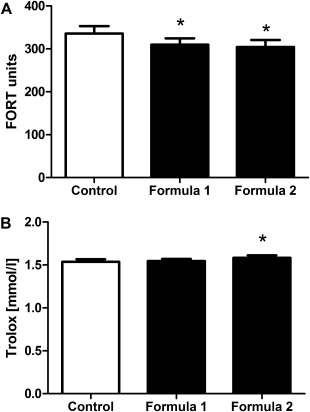
Antioxidant properties of stressed skin are improved after treatment with Q10‐containing formulas. Volunteers displaying elevated oxidative stress (≥250 FORT units) in untreated skin, were analyzed following a 14‐day treatment with formula 1 and formula 2. The level of free oxygen radicals (A) and the free oxygen radical defense (B) were determined in suction blister fluid obtained from treated compared with untreated control samples. Results are depicted as mean ± SEM (n = 16). Significant differences are marked with an asterisk [*P < 0.05 with respect to the untreated control (repeated measures analysis of variance, Dunnett's post hoc test)].
The free oxygen defense was quantified in samples of the 16 volunteers displaying elevated levels of oxidative stress using the Free Oxygen Radicals Defense (FORD) test 23 in accordance with the manufacturer's instructions and the ‘FORM‐CR3000‐Minilab’ device. One FORD unit corresponds to 1.53 mmol/L Trolox.
2.6. Oxygen Consumption Rate of Q10‐Stimulated Human Keratinocytes In Vitro
Human epidermal keratinocytes were obtained from six different adult donors and cultured in KGM‐GoldTM Keratinocyte Growth Medium (Lonza) in an incubator at 37°C and 5% CO2.
The relevant Q10 concentration was calculated based on (i) the Q10 concentration determined within the epidermis after treatment with formula 2 (1.5 ng/mm2) and (ii) an epidermis thickness of 0.1 mm. Q10 (Ubidecarenone, Kyowa Hakko Europe GmbH) was dissolved as described by Failla et al. 25 using PEG‐40 hydrogenated castor oil (BASF Personal Care and Nutrition) instead of PEG‐60 hydrogenated castor oil and was then added to KGM‐GoldTM Keratinocyte Growth Medium at a final concentration of 18 µM.
The experiments were performed separately with cells of the six different adult donors. Cells were seeded into XF96 Cell Culture Microplates (Seahorse Bioscience) in a density of 20,000 cells per well and incubated with Q10‐supplemented medium the following day. After 24 H, keratinocytes were washed twice with assay medium [XF Base Medium (Seahorse Bioscience) containing 2 mM l‐glutamine (Sigma), 500 μM sodium pyruvate (Sigma), and 8.7 mM glucose (Sigma)] and then supplemented with assay medium. Following a 1‐H incubation at 37°C, the OCR as a parameter of energy metabolism was determined using the Extracellular Flux Analyzer XF 96 system (Seahorse Bioscience) according to the manufacturer's instruction. After measurement, the supernatant was replaced by propidium iodide staining solution [20% (v/v) ethanol in Dulbecco's Phosphate‐Buffered Saline containing 0.48% (v/v) 1.0 mg/mL propidium iodide solution in water (Life Technologies)]. Microplates were then sealed with sealing film, protected from light using an aluminum foil cover and stored at 4°C until cell counting was performed utilizing fluorescence microscopy. OCR values were normalized to cell count and are displayed as [fmol/min].
2.7. Statistical Analysis
For the descriptive representation of data, mean and standard error of the mean (SEM) were calculated. Before inductive analysis, data were transformed by Blom transformation. Comparisons between two groups were performed by Student's t‐test (unpaired data) or by paired t‐test (paired data) as appropriate. All other comparisons were carried out by repeated measures analysis of variance using the Dunnett's post hoc test. The significance level was 0.05.
3. Results
3.1. Quinone Levels are Reduced with Age in Human Epidermis
In order to investigate total quinone (ubiquinone plus ubiquinol) levels and connected biological effects in human skin in more detail, suction blister epidermis material was used for the determination of quinone concentrations. A comparison of samples obtained from young and aged volunteers showed that the quinone content in aged epidermis (8.04 ± 0.26 ng/µg cholesterol) was significantly lower than in young epidermis (9.45 ± 0.37 ng/µg cholesterol) indicating a loss of quinones with age (Fig. 2). Out of this reason, we set out to investigate whether the epidermal quinone content can be improved by topical application.
Figure 2.
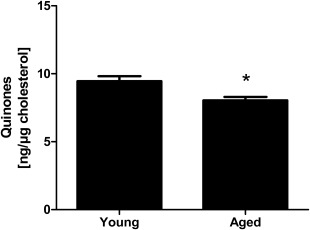
Age‐dependent decline of quinone levels within human epidermis. Quinone concentrations of young (20–25 years; n = 28) and aged (60–66 years; n = 28) volunteers measured in suction blister epidermis obtained from untreated forearm skin. Data are depicted as mean ± SEM. Significant differences are marked with an asterisk [*P < 0.05 for comparison between young and aged subjects (Student's t‐test)].
3.2. Topical Treatment with Q10‐Containing Formulas Increases Epidermal Quinone Content on Multiple Levels
After 14 days of treatment with Q10‐containing formulas, quinone levels were assessed in D‐Squame® samples collected from the skin surface. To take the size of the treatment area into account, values were related to the area of the sample (mm2). The untreated control sample was compared with two individual samples which were obtained from test areas treated with ubiquinone‐containing formula 1 or formula 2 (containing more than twice as much ubiquinone than formula 1). The untreated control sample displayed a level of 0.024 ± 0.003 ng quinones/mm2. Compared with the untreated control, treatment with formula 1 resulted in a significant increase to 0.133 ± 0.02 ng quinones/mm2, whereas application of formula 2 led to an even more pronounced and significant augmentation to 0.717 ± 0.083 ng quinones/mm2 (Fig. 3A).
Figure 3.
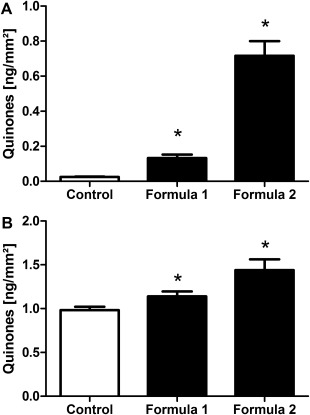
Increase in quinone levels after treatment with Q10‐containing formulas. Following a 14‐day treatment with Q10‐containing formulas 1 and 2, quinone levels were assessed on the skin surface using samples obtained from D‐Squames® (A) and within the epidermis using suction blister material (B). Results are shown as mean ± SEM (20–66 years; n = 73). Significant differences are marked with an asterisk [*P < 0.05 with respect to the untreated control (repeated measures analysis of variance, Dunnett's post hoc test)].
Next, we investigated whether topical treatment can increase quinone levels also in deeper layers of the epidermis. For this purpose, quinone content was analyzed in suction blister epidermis obtained from the treated and untreated areas described above. Quinone values determined in the respective D‐Squame® samples were subtracted to exclude quinone residues present on the skin surface. After treatment with formula 1, quinone levels were significantly increased (1.14 ± 0.06 ng quinones/mm2) compared with the untreated control (0.98 ± 0.04 ng quinones/mm2). Application of formula 2 elevated the quinone content even more (1.44 ± 0.12 ng quinones/mm2) and also showed a significant augmentation compared with the untreated control (Fig. 3B).
According to this data, topical application of the two test formulas increased quinone levels not only on the skin surface but also within the epidermis.
3.3. Topically Applied Ubiquinone is in Part Transformed into Ubiquinol in Human Epidermis
In order to investigate the fate of the topically applied Q10 within the epidermis, ubiquinone and ubiquinol levels were analyzed separately using the calculation described above to exclude any residues of quinones on the skin surface. Ubiquinone levels were significantly increased (Fig. 4A) after treatment with formula 1 (0.57 ± 0.04 ng ubiquinone/mm2) compared with the untreated control (0.46 ± 0.03 ng ubiquinone/mm2). This effect was even more pronounced in the area treated with formula 2 (0.86 ± 0.11 ng ubiquinone/mm2).
Figure 4.
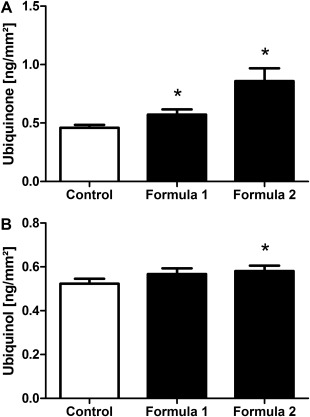
Increase in ubiquinone and ubiquinol content within the epidermis after treatment with Q10‐containing formulas. Following a 14‐day treatment with formula 1 and formula 2 ubiquinone (A) and ubiquinol (B) levels were determined within the epidermis obtained from treated forearm skin compared with untreated control skin. Results are depicted as mean ± SEM (20–66 years; n = 73). Significant differences are marked with an asterisk [*P < 0.05 with respect to the untreated control (repeated measures analysis of variance, Dunnett's post hoc test)].
Regarding the level of ubiquinol (Fig. 4B), which is produced during the process of energy production and is the variant that mainly exerts the antioxidant characteristics of Q10, treatment with formula 1 showed a slight but not significant rise to 0.57 ± 0.03 ng ubiquinol/mm2 compared with the untreated control (0.52 ± 0.02 ng ubiquinol/mm2), whereas treatment with formula 2 induced a stronger and significant increase to 0.58 ± 0.02 ng ubiquinol/mm2. These data indicate that the topically applied ubiquinone is not only able to enter the deeper layers of the epidermis but also may in part be transformed into ubiquinol.
Thus, we asked the question whether this might result in an increase of energy metabolism in skin cells after treatment.
3.4. Energy Metabolism of Cultured Keratinocytes is Increased After Q10 Treatment
The Q10 content found within the epidermis after treatment with formula 2 corresponds to 18 µM Q10. To test the hypothesis that this level may stimulate energy metabolism in epidermal cells, we treated cultured human keratinocytes with 18 µM ubiquinone. The oxygen consumption rate (OCR) as a parameter for energy metabolism was determined using the Seahorse System (Fig. 5). Compared with untreated control cells (2.79 ± 0.62 fmol/min/cell), treatment with Q10 increased the OCR significantly to 3.84 ± 0.80 fmol/min/cell. Thus, treatment with Q10 in quantities comparable to those found within the human epidermis after 2 weeks of topical application of test formula 2 significantly stimulated the energy metabolism of human keratinocytes.
Figure 5.
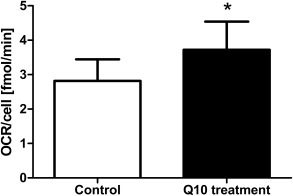
Energy metabolism of cultured human keratinocytes is increased after treatment with Q10. Cultured human keratinocytes were supplemented with 18 µM ubiquinone (representing the amount of Q10 which was determined in the tissue after topical treatment) and the oxygen consumption rate (OCR) was determined. Results are depicted as mean ± SEM (n = 6 donors). Significant differences are marked with an asterisk [*P < 0.05 with respect to the untreated control (paired t‐test)].
3.5. Topical Q10 Treatment Improves Antioxidant Properties of Stressed Skin
To analyze if the augmented post‐treatment ubiquinol levels found within the epidermis (Fig. 4B) translate into a reduction of oxidative stress within the tissue, the Free Oxygen Radicals Test (FORT) was performed on suction blister fluid samples. Volunteers displaying at least 250 FORT units in the untreated area were selected to represent a population of stressed skin. Compared with the untreated control area (335.8 ± 17.3 FORT units), the areas treated with formula 1 and formula 2 showed a significant decrease in free radicals (formula 1: 309.8 ± 14.7 FORT units; formula 2: 304.7 ± 16.3 FORT units; Fig. 6A) indicating that the treatment effectively reduced oxidative stress within the tissue.
To further elucidate the effects of the test formulas, the antioxidant capacity was investigated in the same samples using the Free Oxygen Radicals Defense (FORD) test. As depicted in Fig. 6B, FORD levels increased slightly but not significantly after treatment with formula 1 (1.55 ± 0.03 mmol/L Trolox) compared with untreated controls (1.54 ± 0.03 mmol/L Trolox). Application of formula 2, however, lead to a pronounced and significant effect (1.58 ± 0.03 mmol/L Trolox) compared with the untreated control, indicating that stressed skin benefits from the topical treatment not only by reduction of free radicals but also by increase in antioxidant capacity.
4. Discussion
Skin is constantly exposed not only to intrinsic but also to environmental stressors producing augmented internal ROS concentrations which cause damage throughout the tissue. Therefore, the “Free Radical Theory of Aging” 5 postulating that aging is the result of cellular damage inflicted over time by free radicals is not only one of the most prominent ideas concentrating on the complex phenomenon of aging, but also highly relevant for skin.
Coenzyme Q10 constitutes the only endogenously synthesized lipid‐soluble antioxidant 26. At the same time, it plays a crucial role in cellular energy production. Intracellular synthesis is the major source of human Q10 but it can also be delivered through the diet or dietary supplements 27 which can increase human total Q10 levels in plasma 28.
However, in skin, Q10 is not only found in living cells but also in the skin surface lipids (SSL), which are part of the stratum corneum, forming the outermost barrier of the skin. SSL are composed of a mixture of sebum secreted from sebaceous glands and lipids originating mainly from corneocytes. Due to their location on the skin surface SSL are constantly exposed to UV irradiation, air pollution, chemical oxidants, and microorganisms 29. SSL Q10 levels have been shown to decline with age and also decrease after UV exposure in vitro 28.
We showed that quinone values on the skin surface were significantly increased after treatment with Q10‐containing formulas demonstrating that the powerful antioxidant Q10 can be delivered directly to the uppermost layer of the skin. Our data are of special relevance since an oral supplementation with Q10 did not result in the enhancement of Q10 content in SSL 28. With a topical Q10 treatment, however, short‐term environmental stress‐induced as well as age‐related Q10 deficits may be counteracted directly at the skin surface. Young individuals displaying normal Q10 values may benefit since spontaneously occurring external oxidative stress can be neutralized quickly. In the aged population already decreased Q10 levels may be replenished. Thus, using Q10‐containing formulas on a regular basis to protect the outermost skin layer is favorable for skin at any age.
We detected a significant age‐dependent decline in quinone levels in suction blister epidermis samples which is well in line with other findings documenting decreased Q10 levels not only in several human organs 30 but also in the epidermis of volunteers aged 30–80 years 14. In human plasma, approximately 96% of total Q10 is available as ubiquinol and an age‐dependent shift toward the oxidized form, ubiquinone, is observed 31, 32. In contrast, approximately 46% of total Q10 was found to be present in the reduced form in human epidermis 33, which is in line with our data. With increasing age we did not detect a significant shift in the epidermis toward one of the Q10 variants (data not shown). Thus, with advancing age the decline of both quinones represents the major issue in skin. Besides the chronological aging process, occasional external stress events inside the epidermis may have an impact on the levels of both quinones as demonstrated by Podda et al. 20 in human skin equivalents after UV‐irradiation. In skin, both causes of Q10 decline (age‐dependent and UV‐induced) may be of significant physiological importance given that even small changes in Q10 concentration could result in substantial alterations in the respiratory rate as shown by the saturation kinetics of Q10‐dependent enzymes 34.
According to our data, topical application serves to replenish ubiquinone levels, which may be decreased for any of the aforementioned reasons, in the deeper layers of the epidermis.
The increase in ubiquinol that goes in parallel may result from a transformation of ubiquinone to ubiquinol during energy production. However, ubiquinol can also be generated extramitochondrially by the flavoenzymes lipoamide dehydrogenase, glutathione reductase, and thioredoxin reductase 35. To test if the increased epidermal quinone levels found in skin after treatment are reflected in an augmented cellular energy metabolism that would result in the production of ubiquinol, the OCR as a parameter of energy metabolism was determined in cultured human keratinocytes. Indeed, cells supplemented with Q10 in a concentration representing that found inside human epidermis after 2 weeks of topical treatment with formula 2 exhibited a significant increase in OCR. Similar results have been obtained using the mitochondrial membrane potential as another parameter of energy metabolism: Topical Q10 application showed beneficial effects on mitochondrial membrane potential of UV‐stressed keratinocytes obtained from suction blister epidermis 16. Altogether, these data suggest that topically applied ubiquinone reaches the vital layers of the skin and promotes energy metabolism thereby being transformed into ubiquinol.
Since ubiquinol exerts potent antioxidant properties 9, 18, 19, we tested the importance of its increase for the skin by examining suction blister fluids. This cutaneous interstitial fluid provides useful information about the entire tissue 36. Since states of oxidative stress can be triggered by different short‐term factors, we specifically examined samples of volunteers who displayed elevated oxidative stress levels in the untreated area at the time of sample generation.
After Q10 treatment, decreased FORT units in these subjects showed that the elevated level of free radicals was significantly reduced indicating an antioxidant effect of topical Q10 application. In addition, a distinct increase in FORD was detected. Thus, in case of elevated levels of ROS in the tissue, topical treatment with Q10‐containing formulas may significantly reduce the oxidative stress level.
A very recent study using a mouse model, in which ubiquinone biosynthesis can be activated in the developed organism 17, confirmed the importance of ubiquinone for mitochondrial function and aging phenotypes on the level of the entire organism. Despite the fact that ubiquinone levels were decreased to about 10% in most organs (skin not studied), no signs of oxidative stress were observed. However, the blockage performed in this study still allows the production of a quinone analogue, demethoxyubiquinone (DMQ), which may provide antioxidant activities, explaining the lack of oxidative damage. In another mouse study 37, oxidative damage of specific organs was found. In this model, no analogues of ubiquinone were produced. According to our data obtained from skin, the antioxidative properties of Q10 can be demonstrated and are most relevant in a context of oxidative stress in the respective tissue. The efficacy and benefit of Q10 supplementation may, thus, vary in different types of organisms. Both are definitely dependent on the application form as well as on the bioavailability of Q10 in the respective target organ.
In this context, it is interesting to note that dietary supplementation with ubiquinol is reported to exert beneficial effects in age‐related diseases, such as cardiovascular disease, diabetes, age‐related hearing loss, and Parkinson's disease 38, 39, 40, 41. Moreover, decelerated age‐related accumulation of oxidative damage was shown in ubiquinol‐supplemented mice with accelerated senescence 42.
In summary, the data presented here show that topically applied Q10 can penetrate the skin, is metabolically transformed, exerts antioxidant effects, and can support the maintenance of cellular energy levels. These effects are not only beneficial for the aged population suffering from a Q10 deficit but also to replenish the Q10 level in skin which is lost over time. People of all ages can benefit from regular treatment with Q10‐containing formulas to cope more effectively with short‐term insults inflicted by UV irradiation and stress to foster long‐term anti‐aging effects for their skin.
Acknowledgements
We kindly thank Sören Jaspers for critical discussions during data analysis and manuscript preparation as well as Thomas Hillemann and Urte Koop for support in subject recruitment. In terms of a potential conflict of interest, we would like to indicate that all authors are current or former employees of the Beiersdorf AG.
References
- 1. Conley, K. E. , Marcinek, D. J. , and Villarin, J. (2007) Mitochondrial dysfunction and age. Curr. Opin. Clin. Nutr. Metab. Care 10, 688–692. [DOI] [PubMed] [Google Scholar]
- 2. Trifunovic, A. and Larsson, N. G. (2008) Mitochondrial dysfunction as a cause of ageing. J. Intern. Med. 263, 167–178. [DOI] [PubMed] [Google Scholar]
- 3. Batisse, D. , Bazin, R. , Baldeweck, T. , Querleux, B. , and Lévêque, J. L. (2002) Influence of age on the wrinkling capacities of skin. Skin Res. Technol. 8, 148–154. [DOI] [PubMed] [Google Scholar]
- 4. Escoffier, C. , de Rigal, J. , Rochefort, A. , Vasselet, R. , Lévêque, J. L. , and Agache, P. G. (1989) Age‐related mechanical properties of human skin: an in vivo study. J. Invest. Dermatol. 93, 353–357. [PubMed] [Google Scholar]
- 5. Harman, D. (1956) Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 293–300. [DOI] [PubMed] [Google Scholar]
- 6. Sohal, R. S. (1991) Hydrogen peroxide production by mitochondria may be a biomarker of aging. Mech. Ageing Dev. 60, 189–198. [DOI] [PubMed] [Google Scholar]
- 7. Zwerschke, W. , Mazurek, S. , Stöck, lP. , Hütter, E. , Eigenbrodt, E. , et al. (2003) Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 376, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scharffetter‐Kochanek, K. , Brenneisen, P. , Wenk, J. , Herrmann, G. , Ma, W. , et al. (2000) Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 35, 307–316. [DOI] [PubMed] [Google Scholar]
- 9. Blatt, T. and Littarru, G. P. (2011) Biochemical rationale and experimental data on the antiaging properties of CoQ(10) at skin level. Biofactors 37, 381–385. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell, P. (1975) Protonmotive redox mechanisms of cytochrome b‐c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 56, 1–6. [DOI] [PubMed] [Google Scholar]
- 11. Bentinger, M. , Tekle, M. , and Dallner, G. (2010) Coenzyme Q ‐ biosynthesis and functions. Biochem. Biophys. Res. Commun. 396, 74–79. [DOI] [PubMed] [Google Scholar]
- 12. Gille, L. and Nohl, H. (2000) The existence of a lysosomal redox chain and the role of ubiquinone. Arch. Biochem. Biophys. 375, 347–354. [DOI] [PubMed] [Google Scholar]
- 13. Ernster, L. and Dallner, G. (1995) Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1271, 195–204. [DOI] [PubMed] [Google Scholar]
- 14. Hoppe, U. , Bergemann, J. , Diembeck, W. , Ennen, J. , Gohla, S. , et al. (1999) Coenzyme Q10, a cutaneous antioxidant and energizer. Biofactors 9, 371–378. [DOI] [PubMed] [Google Scholar]
- 15. Blatt, T. , Mundt, C. , Mummert, C. , Maksuik, T. , Wolber, R. , et al. (1999) Modulation des oxidativen Stresses in der humanen Altershaut. Z. Gerontol. Geriat. 32, 83–88. [DOI] [PubMed] [Google Scholar]
- 16. Prahl, S. , Kueper, T. , Biernoth, T. , Wöhrmann, Y. , Münster, A. , et al. (2008) Aging skin is functionally anaerobic: importance of conenzyme Q10 for anti aging skin care. Biofactors 32, 245–255. [DOI] [PubMed] [Google Scholar]
- 17. Wang, Y. , Oxer, D. , and Hekimi, S. (2015) Mitochondrial function and lifespan of mice with controlled ubiquinone biosynthesis. Nat. Commun. 6, 6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Littarru, G. P. and Tiano, L. (2007) Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol. Biotechnol. 37, 31–37. [DOI] [PubMed] [Google Scholar]
- 19. Schmelzer, C. and Döring, F. (2012) Micronutrient special issue: coenzyme Q(10) requirements for DNA damage prevention. Mutat. Res. 733, 61–68. [DOI] [PubMed] [Google Scholar]
- 20. Podda, M. , Traber, M. G. , Weber, C. , Yan, L. J. , and Packer, L. (1998) UV‐irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic. Biol. Med. 24, 55–65. [DOI] [PubMed] [Google Scholar]
- 21. Kiistala, U. (1968) Suction blister device for separation of viable epidermis from dermis. J. Invest. Dermatol. 50, 129–137. [DOI] [PubMed] [Google Scholar]
- 22. Südel, K. M. , Venzke, K. , Knußmann‐Hartig, E. , Moll, I. , Stäb, F. , et al. (2003) Tight control of matrix metalloproteinase‐1 activity in human skin. Photochem. Photobiol. 78, 840–845. [DOI] [PubMed] [Google Scholar]
- 23. Palmieri, B. and Sblendorio, V. (2007) Oxidative stress tests: overview on reliability and use: part II. Eur. Rev. Med. Pharmacol. Sci. 11, 383–399. [PubMed] [Google Scholar]
- 24. Kamhieh‐Milz, J. and Salama, A. (2014) Oxidative stress is predominant in female but not in male patients with autoimmune thrombocytopenia. Oxid. Med. Cell Longev., 2014; 2014:720347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Failla, M. L. , Chitchumroonchokchai, C. , and Aoki, F. (2014) Increased bioavailability of ubiquinol compared to that of ubiquinone is due to more efficient micellarization during digestion and greater GSH‐dependent uptake and basolateral secretion by Caco‐2 cells. J. Agric. Food Chem. 62, 7174–7182. [DOI] [PubMed] [Google Scholar]
- 26. Bentinger, M. , Brismar, K. , and Dallner, G. (2007) The antioxidant role of coenzyme Q. Mitochondrion 7(Suppl), S41–S50. [DOI] [PubMed] [Google Scholar]
- 27. Kwong, L. K. , Kamzalov, S. , Rebrin, I. , Bayne, A. C. , Jana, C. K. , et al. (2002) Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic. Biol. Med. 33, 627–638. [DOI] [PubMed] [Google Scholar]
- 28. Passi, S. , de Pità, S. , Puddu, O. , and Littarru, P. G. P. (2002) Lipophilic antioxidants in human sebum and aging. Free Radic. Res. 36, 471–477. [DOI] [PubMed] [Google Scholar]
- 29. Thiele, J. J. , Schroeter, C. , Hsieh, S. N. , Podda, M. , and Packer, L. (2001) The antioxidant network of the stratum corneum. Curr. Probl. Dermatol. 29, 26–42. [DOI] [PubMed] [Google Scholar]
- 30. Kalén, A. , Appelkvist, E. L. , and Dallner, G. (1989) Age‐related changes in the lipid compositions of rat and human tissues. Lipids 24, 579–584. [DOI] [PubMed] [Google Scholar]
- 31. Miles, M. V. , Horn, P. S. , Tang, P. H. , Morrison, J. A. , Miles, L. , et al. (2004) Age‐related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin. Chim. Acta 347, 139–144. [DOI] [PubMed] [Google Scholar]
- 32. Wada, H. , Goto, H. , Hagiwara, S. , and Yamamoto, Y. (2007) Redox status of coenzyme Q10 is associated with chronological age. J. Am. Geriatr. Soc. 55, 1141–1142. [DOI] [PubMed] [Google Scholar]
- 33. Shindo, Y. , Witt, E. , Han, D. , Epstein, W. , and Packer, L. (1994) Enzymic and non‐enzymic antioxidants in epidermis and dermis of human skin. J. Invest. Dermatol. 102, 122–124. [DOI] [PubMed] [Google Scholar]
- 34. Estornell, E. , Fato, R. , Castelluccio, C. , Cavazzoni, M. , Parenti Castelli, G. , et al. (1992) Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Lett. 311, 107–109. [DOI] [PubMed] [Google Scholar]
- 35. Nordman, T. , Xia, L. , Björkhem‐Bergman, L. , Damdimopoulos, A. , Nalvarte, I. , et al. (2003) Regeneration of the antioxidant ubiquinol by lipoamide dehydrogenase, thioredoxin reductase and glutathione reductase. Biofactors 18, 45–50. [DOI] [PubMed] [Google Scholar]
- 36. Kool, J. , Reubsaet, L. , Wesseldijk, F. , Maravilha, R. T. , Pinkse, M. W. , et al. (2007) Suction blister fluid as potential body fluid for biomarker proteins. Proteomics 7, 3638–3650. [DOI] [PubMed] [Google Scholar]
- 37. Quinzii, C. M. , Garone, C. , Emmanuele, V. , Tadesse, S. , Krishna, S. , et al. (2013) Tissue‐specific oxidative stress and loss of mitochondria in CoQ‐deficient Pdss2 mutant mice. Faseb J. 27, 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winkler‐Stuck, K. , Wiedemann, F. R. , Wallesch, C. W. , and Kunz, W. S. (2004) Effect of coenzyme Q10 on the mitochondrial function of skin fibroblasts from Parkinson patients. J. Neurol. Sci. 220, 41–48. [DOI] [PubMed] [Google Scholar]
- 39. Safarinejad, M. R. , Safarinejad, S. , and Shafiei, N. (2012) Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double‐blind, placebo controlled, randomized study. J. Urol. 188, 526–531. [DOI] [PubMed] [Google Scholar]
- 40. Someya, S. , Xu, J. , Kondo, K. , Ding, D. , Salvi, R. J. , et al. (2009) Age‐related hearing loss in C57BL/6J mice is mediated by Bak‐dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA 106, 19432–19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Someya, S. , Yu, W. , Hallows, W. C. , Xu, J. , Vann, J. M. , et al. (2012) Sirt3 mediates reduction of oxidative damage and prevention of age‐related hearing loss under caloric restriction. Cell 143, 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tian, G. , Sawashita, J. , Kubo, H. , Nishio, S. Y. , Hashimoto, S. , et al. (2014) Ubiquinol‐10 supplementation activates mitochondria functions to decelerate senescence in senescence‐accelerated mice. Antioxid. Redox Signal. 20, 2606–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]


