Abstract
Objective
To determine the prevalence and risk factors associated with Chagas disease in pregnant women in an endemic area of Santander, Colombia.
Methods
Cross‐sectional study included 23 municipalities of Santander, Colombia. Serological IFAT and ELISA tests were undertaken to detect IgG anti‐ Trypanosoma cruzi. A questionnaire was conducted for assessing the risk factors of each participant. Newborns were evaluated at birth and followed up to 1 year of age to determine congenital infection.
Results
An overall prevalence of 3.2% (95% CI 2.4–4.2) among 1518 pregnant women was detected. Prevalences by provinces were as follows: Guanentina: 6.0% (95% CI 4.1–8.5), García Rovira: 2.9% (95% CI: 1.5–4.8) and Comunera: 0.4% (0.4–2.3). The main risk factors identified were age >32 years old (OR: 2.1; 95% CI: 1.1–3.9); currently having a thatched roof (OR: 11.8; CI95% 2.2–63.2) and a thatched roof during childhood (OR: 3.0; 95% CI: 1.4–6.6); having below primary school education level (OR: 4.6; 95% CI: 2.2–9.5); and a history of a close contact with the vector (triatomine bugs) at least once during their lifetime (OR: 6.9; 95% CI: 3.7–12.9). No congenital cases were detected by parasitological or serological techniques.
Conclusions
Prevalence of Chagas disease in pregnant women is a potential source of infection in this Colombian endemic area. The main risk factors associated with seropositivity were related to conditions favouring the contact with the vector. The results show that it is necessary to continue an active surveillance in order to offer diagnosis and treatment to mothers and their newborns in addition to screening to pregnant women from endemic areas.
Keywords: Chagas disease, serological diagnosis, Trypanosoma cruzi, pregnancy, congenital Chagas disease, risk factors
Abstract
Objectif
Déterminer la prévalence et les facteurs de risque associés à la maladie de Chagas chez les femmes enceintes dans une zone endémique de Santander, en Colombie.
Méthodes
Etude transversale couvrant 23 municipalités de Santander, en Colombie. Les tests sérologiques IFAT et ELISA ont été effectués pour détecter les anticorps IgG de T. cruzi. Un questionnaire a été administré pour évaluer les facteurs de risque de chaque participant. Les nouveau‐nés ont été évalués à la naissance et suivis jusqu’à un an pour déterminer l'infection congénitale.
Résultats
Une prévalence globale de 3,2% (IC95%: 2,4 à 4,2) chez 1518 femmes enceintes a été déterminée. Les prévalences par provinces étaient: Guanentina: 6,0% (IC95%: 4,1 à 8,5), García Rovira: 2,9% (IC95%: 1,5 à 4,8) et Comunera: 0,4% (IC95%: 0,4 à 2,3). Les principaux facteurs de risque identifiés étaient l’âge > 32 ans (OR: 2,1; IC95%: 1,1 à 3,9); avoir en cours un toit de chaume (OR: 11,8; IC95%: 2,2 à 63,2) et avoir eu un toit de chaume pendant l'enfance (OR: 3,0; IC95%: 1,4 à 6,6); avoir un niveau d’éducation en dessous de celui de l’école primaire (OR: 4,6; IC95%: 2,2 à 9,5) et une histoire d'un contact étroit avec le vecteur (les triatomes) au moins une fois au cours de sa vie (OR: 6,9; IC95%: 3,7 à 12,9). Aucun cas congénital n'a été détecté par les techniques parasitologiques ou sérologiques.
Conclusions
La prévalence de la maladie de Chagas chez les femmes enceintes est une source potentielle d'infection dans cette zone endémique Colombienne. Les principaux facteurs de risque associés à la séropositivité étaient liés à des conditions favorisant le contact avec le vecteur. Les résultats montrent que la surveillance active dans le but d'offrir un diagnostic et un traitement aux mères et à leurs nouveau‐nés et le dépistage des femmes enceintes dans les zones endémiques doit être poursuivie.
Keywords: maladie de Chagas, diagnostic sérologique, Trypanosoma cruzi, grossesse, maladie de Chagas congénitale, facteurs de risque
Abstract
Objetivo
Determinar la prevalencia y los factores de riesgo asociados con la enfermedad de Chagas entre mujeres embarazadas de un área endémica de Santander, Colombia.
Métodos
Estudio crosseccional que incluyó 23 municipios de Santander, Colombia. Se realizaron las pruebas de IFAT y ELISA en muestras de suero para detectar IgG anti‐T. cruzi. Se pasó un cuestionario para evaluar los factores de riesgo de cada participante. Los recién nacidos se evaluaron en el momento del parto y se les realizó un seguimiento hasta que cumplieron el primer año, con el fin de determinar la presencia de una infección congénita.
Resultados
Se detectó una prevalencia total del 3.2% (IC 95% 2.4 – 4.2) entre 1,518 mujeres embarazadas. Las prevalencias por provincias eran: Guanentina: 6.0% (IC 95% 4.1 – 8.5), García Rovira: 2.9% (IC 95%: 1.5 – 4.8) y Comunera: 0.4% (0.4‐2.3). Los principales factores de riesgo identificados eran tener >32 años (OR: 2.1; IC 95%: 1.1 – 3.9); vivir en un hogar con un techo de paja (OR: 11.8; IC 95% 2.2 – 63.2) y haber vivido en un hogar con techo de paja durante la infancia (OR: 3.0; IC 95%: 1.4 – 6.6); tener un nivel de estudios menor que la primaria (OR: 4.6; IC 95%: 2.2 – 9.5) y una historia de contacto cercano con el vector (insectos triatominos) de al menos una vez durante el trascurso de su vida (OR: 6.9; 95% CI: 3.7 – 12.9). No se detectaron casos congénitos mediante técnicas parasitológicas o serológicas.
Conclusiones
La prevalencia de la enfermedad de Chagas entre mujeres embarazadas es una fuente potencial de infección en esta área endémica de Colombia. Los principales factores de riesgo asociados con la seropositividad estaban relacionados con condiciones que favorecían el contacto con el vector. Estos resultados muestran que se debe continuar con una vigilancia activa que ofrezca diagnóstico y tratamiento a las madres y a sus recién nacidos, así como el cribado de mujeres embarazadas pertenecientes a áreas endémicas.
Introduction
Chagas disease is an important public health problem in Latin American countries. In Colombia, vectorborne is the main transmission route and Rhodnius prolixus is the predominant species of vector 1. Risk factors relate to environmental, cultural and social vulnerabilities such as living conditions, housing and poverty in rural areas; and these have allowed national programmes to target the most endemic areas for vector control 2. However, additional strategies are needed to control other routes of parasite transmission (congenital, oral, transfusion) 3.
Congenital transmission has gained special importance in some zones where vector transmission has been interrupted. Hence, in some endemic countries like Argentina, the rate of congenital transmission can account for up to 10 times the number of acute vectorborne cases 4. Congenital cases have been widely reported even in countries where the infection is non‐endemic due to international migration from endemic countries 5, 6.
Congenital transmission occurs when the parasites cross the placental barrier during pregnancy 7, 8, 9. There are two effective trypanocidal drugs (benznidazole and nifurtimox) for reducing parasitemia, but they are explicitly contraindicated during pregnancy due to their potential teratogenic adverse effects 8, 10. However, there is recent evidence of the potential of trypanocidal drugs for preventing congenital transmission when women are treated before pregnancy 11.
In South American countries, the prevalence of Trypanosoma cruzi seropositivity in pregnancy ranges from 0.3% to 49.5% 12, 13, 14, 15, 16 and the transmission probability from infected mother to child has been estimated at 4.7% (95% CI: 3.9–5.6) in a meta‐analysis 17. Although about 90% of congenital cases are asymptomatic at birth, some cases present varied symptoms such as low weight, hepatosplenomegaly, jaundice and anaemia 18.
Both the prevalence of infection in pregnant women and the transmission pattern of congenital infection in Colombia are unknown 19. Only two previous studies have determined the prevalence in pregnant women in parts of the country: 3.3% in Boyacá 20 and 4% in Casanare 21.
This study determines the prevalence of T. cruzi seropositivity in pregnant women and accordingly the frequency of congenital transmission in an endemic area previously unexplored in this regard.
Methods
Study design
A cross‐sectional study was performed between August 2010 and December 2013 in the Department of Santander (first administrative order), specifically in three provinces (intermediate administrative order): García Rovira, Guanentina and Comunera, and 23 municipalities (second administrative order) (Figure 1).
Figure 1.
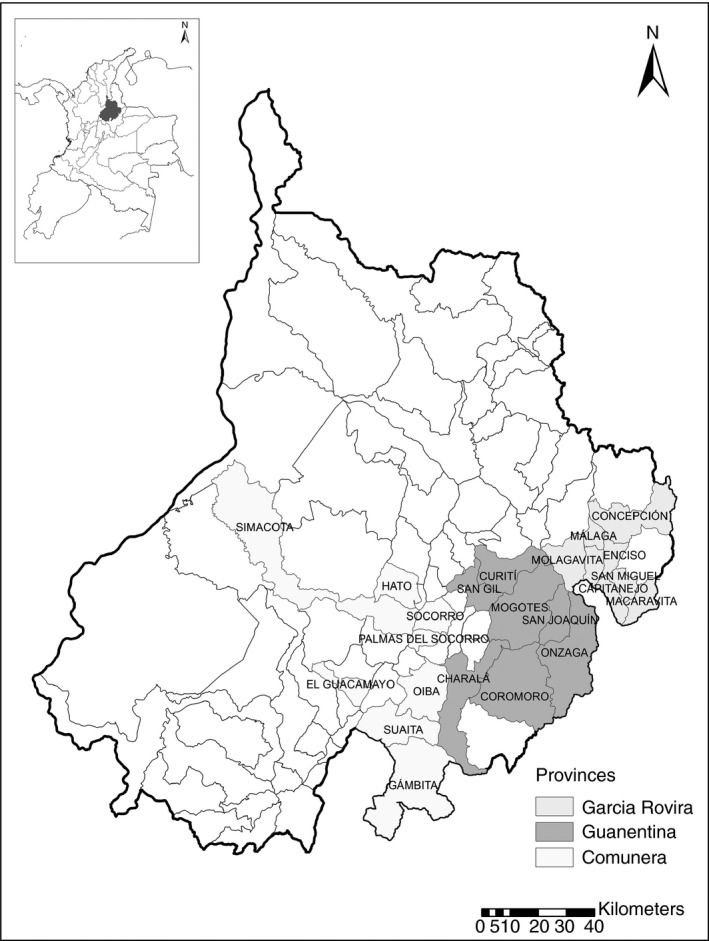
Map of the participant municipalities in the Guanentina, Comunera and García Rovira provinces, Santander, Colombia.
Pregnant women were recruited from August 2011 until September 2011, and their newborns were followed up until the age of 12 months. The sample size was calculated according to an estimate of about 3000 pregnant women per year attending the health facilities in these 23 municipalities. Based on previous work, the expected prevalence was 3.9% 20, 21, therefore with a confidence level of 95%, and a precision of 1%, a required minimum sample size of 1440 pregnant women was estimated to successfully determine the overall prevalence in this area.
Inclusion of participants and follow‐up
The inclusion criteria for municipalities in these three provinces were being a Chagas endemic area for domiciliary transmission and having a primary care facility with a regular pregnancy care programme. The inclusion criteria for individual participants were as follows: (i) acceptance of pregnant women to participate in the diagnosis phase; (ii) acceptance of the mother to participate in the follow‐up of her newborn once she was identified as seropositive; and (iii) written informed consent.
Several meetings at municipality level were conducted to inform health workers from the 23 hospitals about the study. The local teams who facilitated the inclusion and follow‐up were comprised of a physician, a nurse and laboratory technicians. They were trained for helping out with the inclusion procedures, filling‐in questionnaires, collecting samples, contacting patients and directing infected pregnant women towards attending a series of specific consultations with the researchers for the follow‐up phase of the study.
For diagnosis of pregnant women and children, two techniques were used as follows: ELISA and IFAT to detect anti‐T. cruzi IgG. These tests were standardised and evaluated at the National Health Institute 22, 23, 24. Discordant results were then processed by the indirect haemagglutination (IHA) commercial test (Winner®, Rosario, Argentina). Microhaematocrit was processed according to the technique previously described by Freilij 25 and modified by Torrico 26; the IHA was also used for parasitological diagnosis of newborns. The children underwent serological tests and were considered as cases when the tests were reactive after 9 months of age.
Once a pregnant woman was confirmed as positive by two serological tests, she was informed about the need to follow‐up her baby after birth. Clinical examination and samples for microhaematocrit and serology were taken at birth and up to 12 months of age. Aetiological (parasiticidal) treatment with nifurtimox (Lampit®) was offered to all confirmed cases.
Ethical aspects
Pregnant women were invited to participate voluntarily during the prenatal consultation at the local hospital, after being informed about the goal, objectives, procedures and benefits of the study. Written informed consent was obtained from all participants, following national and international regulations. The research protocol was approved by the Committee on Research Ethics of Colombia's National Health Institute.
Survey questionnaires and data quality control
The questionnaires contained information on socio‐demographic variables (residency, age, marital status, education and occupancy), housing characteristics (construction materials of the roof, walls and floor) for both current and childhood homes, recognition and contact with the vector (past and current), history of transfusions, and information on relatives with previous diagnosis of Chagas disease. During the interview, a box with desiccated insects of the four principal triatomine species (R. prolixus, Triatoma maculata, Triatoma dimidiata, Panstrongylus geniculatus) was shown to the pregnant women, who were asked to identify their interaction with the vectors as either ‘simple recognition’ or ‘close contact’. Simple recognition was defined as having seen the vector at least once in their lifetime in their houses or in another place (trees, palms, other houses). This was intended as a proxy indicator of a potential exposure but not remembering any particular contact. Close contact was defined as remembering having been bitten by vectors or seeing them full of blood emerging from the bed where the women slept.
To control for potential information bias, the local teams participating in the study received specific training sessions on the questionnaires and procedures and as a quality control they were regularly reassessed and if necessary received additional training. For data quality control, the fieldwork coordinator revised and evaluated the quality of the questionnaires. In the case of missing data, patients were re‐contacted to obtain the specific information. The questionnaires were entered in an MS Excel® database, a random sample of 2% of the questionnaires was reviewed in detail, and patients were called to corroborate the validity and reliability of the information provided.
Data analysis
Frequencies were calculated and displayed as percentages with their 95% confidence intervals (95% CI). Potential risk factors were evaluated through bivariate analysis as odds ratio (OR) and then through stratified and multivariate analysis by logistic regression. For the latter, a stepwise backward removal method, dropping variables with P values <0.2, was used for controlling confounding variables and choosing the final model, for which only variables with P value <0.05 were included. Akaike information criteria (AIC) were also used to choose the best model fit. All the data analysis was performed using Stata 10.0 (Stata Corp. 2007, release 10; StataCorp LP, College Station, TX, USA).
Results
Univariate analysis
A total of 23 health facilities from 23 municipalities in the three endemic provinces participated in the study. All municipalities and all the mothers invited to participate in the study accepted. A total of 1518 pregnant women aged 13–46 years (average: 24.5 years; standard deviation: 6.9 years) were included. The overall prevalence was 3.2%. The Guanentina province had the largest number of cases (30 infected pregnant women). At municipality level, Mogotes presented the highest prevalence (18.3%). The frequency of infected pregnant women by municipalities and provinces is presented in Table 1.
Table 1.
Prevalence of Trypanosoma cruzi seropositivity in pregnant women in the municipalities of three provinces, Santander, Colombia
| Province/Municipalitya | Negative | Positive | Total | Proportion (%) of sero[+] | 95% CI |
|---|---|---|---|---|---|
| Guanentina Province | |||||
| Curiti | 29 | 1 | 30 | 3.3 | 0.1–17.2 |
| Charalá | 14 | 0 | 14 | 0.0 | 0.0–23.1 |
| Coromoro | 36 | 2 | 38 | 5.3 | 0.6–17.7 |
| Mogotes | 94 | 21 | 115 | 18.3 | 11.6–26.5 |
| Onzaga | 25 | 0 | 25 | 0.0 | 0.0–13.7 |
| San Gil | 222 | 4 | 226 | 1.8 | 0.5–4.5 |
| San Joaquín | 47 | 2 | 49 | 4.1 | 0.5–14.0 |
| Subtotal | 467 | 30 | 497 | 6.0 | 4.1–8.5 |
| Comunera Province | |||||
| Gámbita | 13 | 0 | 13 | 0.0 | 0.0–24.7 |
| Guacamayo | 23 | 0 | 23 | 0.0 | 0.0–14.8 |
| Socorro | 324 | 5 | 329 | 1.5 | 0.5–3.5 |
| Suaita | 40 | 0 | 40 | 0.0 | 0.0–8.8 |
| Oiba | 138 | 1 | 139 | 0.7 | 0.0–3.9 |
| Hato | 4 | 0 | 4 | 0.0 | 0.0–60.2 |
| Palmas del Socorro | 3 | 0 | 3 | 0.0 | 0.0–70.8 |
| Simacota | 15 | 0 | 15 | 0.0 | 0.0–21.8 |
| Subtotal | 560 | 6 | 566 | 1.1 | 0.4–2.3 |
| García Rovira Province | |||||
| Capitanejo | 91 | 4 | 95 | 4.2 | 1.2–10.4 |
| Concepción | 25 | 0 | 25 | 0.0 | 0.0–13.7 |
| Enciso | 6 | 2 | 8 | 25.0 | 3.2–65.1 |
| Macaravita | 35 | 2 | 37 | 5.4 | 0.7–18.2 |
| Molagavita | 51 | 2 | 53 | 3.8 | 0.5–13.0 |
| Málaga | 186 | 1 | 187 | 0.5 | 0.0–2.9 |
| San Jose de Miranda | 40 | 1 | 41 | 2.4 | 0.1–12.9 |
| San Miguel | 8 | 1 | 9 | 11.1 | 0.3–48.2 |
| Subtotal | 442 | 13 | 455 | 2.9 | 1.5–4.8 |
| Total | 1469 | 49 | 1518 | 3.2 | 2.4–4.2 |
Department is the first administrative order. Municipality is second administrative order. Province is an intermediate level between department and municipality.
52.2% were from rural areas and 47.8% from urban areas; 67.7% belonged to the lower socio‐economic strata (1 or 2) and 80.0% were affiliated to subsidised health care. 55.5% had finished high school and 33.2% had no education at all. 77.9% were either married or had a partner at the time of the survey. 78.5% were housewives and 13.8% were high school students; 43.2% recognised the Chagas disease vector (Triatominae), and only 11.1% reported any contact with it sometime in the past (Table 2).
Table 2.
Bivariate analysis for potential risk factors associated with T. cruzi seropositive pregnant women in Santander, Colombia
| Variable | n | sero[+] | % | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Socio‐demographic factors | ||||||
| Residence area | ||||||
| Urban area | 725 | 13 | 1.79 | 1 | 0.003 | |
| Rural area | 793 | 36 | 4.54 | 2.6 | 1.3–5.4 | |
| Age | ||||||
| 13–19 | 382 | 4 | 1.05 | 1 | ||
| 20–24 | 447 | 13 | 2.91 | 2.8 | 0.9–8.4 | <0.001 |
| 25–29 | 301 | 5 | 1.66 | 1.6 | 0.4–5.9 | |
| 30–42 | 388 | 27 | 6.96 | 6.6 | 2.3–18.8 | |
| Socio‐economic strata | ||||||
| High (3,4,5) | 491 | 9 | 1.83 | 1 | 0.034 | |
| Low (0,1,2) | 1027 | 40 | 3.89 | 2.2 | 1.0–4.5 | |
| Healthcare insurance | ||||||
| Contributive | 151 | 3 | 1.99 | 1 | 0.211 | |
| Non‐insured | 152 | 2 | 1.32 | 1.5 | 0.3–9.1 | |
| Subsidised | 1215 | 44 | 3.62 | 2.8 | 0.7–11.7 | |
| Education level | ||||||
| University | 172 | 2 | 1.16 | 1 | <0.001 | |
| High School | 842 | 9 | 1.07 | 0.9 | 0.2–4.3 | |
| Primary School | 493 | 36 | 7.30 | 6.7 | 1.6–28.1 | |
| None | 11 | 2 | 18.18 | 18.9 | 2.4–149.9 | |
| Marital status | ||||||
| Married | 1183 | 43 | 3.63 | 1 | 0.124 | |
| Single | 321 | 5 | 1.56 | 0.4 | 0.2–1.1 | |
| Widow‐separated | 14 | 1 | 7.14 | 2 | 0.3–15.9 | |
| Occupation | ||||||
| Housewife | 1192 | 46 | 3.86 | 1 | 0.068 | |
| Student | 209 | 2 | 0.96 | 0.2 | 0.6–1.0 | |
| Worker in urban area | 96 | 1 | 1.04 | 0.3 | 0.0–1.9 | |
| Worker in rural area (agriculture) | 21 | 0 | 0.00 | – | – | |
| Factors related with triatomine vectors exposure | ||||||
| Recognition of the vector | ||||||
| No | 809 | 10 | 1.24 | 1 | <0.001 | |
| Yes | 656 | 39 | 5.95 | 5.1 | 2.5–10.2 | |
| Close contact with vectors at least once in lifetime | ||||||
| No | 1186 | 21 | 1.77 | 1 | <0.001 | |
| Yes | 169 | 25 | 14.79 | 9.6 | 5.3–17.7 | |
| No response | 163 | 3 | 1.84 | 1 | 0.3–3.5 | |
| Close contact with vectors during the last year | ||||||
| No | 1336 | 44 | 3.29 | 1 | 0.283 | |
| Yes | 26 | 2 | 7.69 | 2.4 | 0.6–10.7 | |
| Close contact with animals | ||||||
| No | 462 | 16 | 3.46 | 1 | 0.732 | |
| Yes | 1056 | 33 | 3.13 | 0.9 | 0.5–1.7 | |
| Housing conditions | ||||||
| Current floor | ||||||
| Cement | 1241 | 32 | 2.58 | 1 | <0.001 | |
| Wood | 36 | 2 | 5.56 | 2.2 | 0.5–9.7 | |
| Dirt | 190 | 15 | 7.89 | 3.2 | 1.7–6.1 | |
| Other | 51 | 0 | 0.00 | – | – | |
| Current roof | ||||||
| Clay tiles | 601 | 20 | 3.33 | 1 | ||
| Fibrocement tiles | 552 | 18 | 3.26 | 1 | 0.5–1.9 | <0.001 |
| Thatched/palm leaves | 12 | 3 | 25.00 | 9.7 | 2.4–38.5 | |
| Other | 353 | 8 | 2.27 | 0.7 | 0.3–1.5 | |
| Current wall | ||||||
| Brick/cement | 1109 | 28 | 2.52 | 1 | 0.02 | |
| Wood | 19 | 2 | 10.53 | 4.5 | 1.0–20.6 | |
| Adobe | 371 | 19 | 5.12 | 2.1 | 1.2–3.8 | |
| Other | 19 | 0 | 0.00 | – | – | |
| Floor of housing during childhood | ||||||
| Cement | 796 | 16 | 2.01 | 1 | 0.003 | |
| Wood | 68 | 0 | 0.00 | – | – | |
| Dirt | 639 | 33 | 5.16 | 2.7 | 1.5–4.9 | |
| Other | 15 | 0 | 0.00 | – | – | |
| Roof during childhood | ||||||
| Clay tiles | 753 | 22 | 2.92 | 1 | <0.0001 | |
| Fibrocement tiles | 408 | 8 | 1.96 | 0.7 | 0.3–1.5 | |
| Thatched/palm leaves | 92 | 12 | 13.04 | 5 | 2.4–10.5 | |
| Other | 265 | 7 | 2.64 | 0.9 | 0.4–2.1 | |
| Walls of housing during childhood | ||||||
| Brick/cement | 737 | 12 | 1.63 | 1 | 0.004 | |
| Wood | 70 | 2 | 2.86 | 1.8 | 0.4–8.1 | |
| Adobe | 676 | 34 | 5.03 | 3.2 | 1.6–6.2 | |
| Other | 35 | 1 | 2.86 | 1.8 | 0.2–14.1 | |
| Other factors | ||||||
| Relatives diagnosed with Chagas disease | ||||||
| No | 1320 | 39 | 2.95 | 1 | 0.022 | |
| Yes | 101 | 9 | 8.91 | 3.2 | 1.5–6.8 | |
| No response | 97 | 1 | 1.03 | 0.3 | 0.1–2.5 | |
| Previous transfusions | ||||||
| No | 1467 | 48 | 3.27 | 1 | 0.578 | |
| Yes | 29 | 0 | 0.00 | – | – | |
Bold numbers mean p values statistically signifciant at <0.05.
Bivariate analysis and multivariate analysis
In the bivariate analysis, low socio‐economic status, low level of education, age above 30 years, knowledge of the vector, and contact with the vector at least once during lifetime were significantly associated with seropositivity. Having lived in childhood or living currently in rural area and in houses with dirt floor, thatched roof and adobe walls were also statistically significant (Table 2).
To identify potential interactions, a stratified analysis by age groups was conducted with the variables identified as statistically significant in the bivariate analysis, evidencing no modification of the effect with age. In the logistic regression model, the final variables associated with seropositivity were as follows: age >32 years, less than primary schooleducation, previous contact with the vector at least once in the lifetime, and having lived in a house with thatched roof in childhood (Table 3).
Table 3.
Logistic regression model for T. cruzi seropositive pregnant women of Santander
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Current thatch roof | 11.8 | 2.2–63.2 | 0.004 |
| Close contact with vector at least once on life | 6.9 | 3.7–12.9 | <0.001 |
| Low education level | 4.6 | 2.2–9.5 | <0.001 |
| Thatch roof during childhood | 3.0 | 1.4–6.6 | 0.005 |
| Age >32 years | 2.1 | 1.1–3.9 | 0.024 |
Follow‐up of newborns and search for congenital infection
The 49 infected women had a successful delivery with no abortions or deaths at birth. After birth, one child died due to respiratory infection at 3 months. One participating mother from the Socorro municipality initially refused to continue in the study after being diagnosed as seropositive, so it was not possible to take samples from the newborn, but it was possible to follow‐up the child after 1 year. Follow‐up of 48 newborns continued until they reached 12 months of age; no congenital cases were identified through parasitological or serological tests. The transmission rate from mother to children was then estimated at 0% (95% CI: 0.0–7.3%) for this population.
Discussion
The overall prevalence found in this endemic area was 3.2% (95% CI: 2.39–4.24), similar to the prevalence reported for two other Colombian endemic areas in recent studies: 4% in Casanare in 2011 21 and 3.34% Boyacá in 2007 20. Santander has been one of the high endemic departments in Colombia 1. In previous studies, the prevalence of Chagas disease in general population had reached 52.5% 27 and 6.9% in children 3 in this department. The only previous study in pregnant women in this department was conducted in the capital city, Bucaramanga, in 1993 when a prevalence of 6.9% in women at delivery in a specialised level hospital was found 28. Thus, the prevalence almost 15 years ago was higher than in the current study, which is the observable trend for other endemic areas 21. This difference over time could be explained by the scaling‐up of vector control measures in this area since the beginning of the 21st century 1. A similar trend was found in Paraguay in the prevalence in pregnant women in two endemic municipalities in 1995 (15% and 12%) and 2009, when the prevalence dropped to 6% in both sites 29.
One of the most striking results was the high prevalence found in Mogotes, which is one of the most endemic municipalities in Colombia. As part of the Andean countries initiative for the control of Chagas disease in the 1990s, an integrated domiciliary vector infestation control programme was created to guide the vigilance and control actions of high‐risk municipalities. This initiative involved the participation of 30 municipalities in Santander but Mogotes was not part of such strategy and no systematic vector control took place there 1. This result supports the hypothesis that vector control is an important factor in explaining temporal changes to prevalence and accounts for the substantial difference in prevalence between Mogotes and other municipalities.
In our study, age was an important risk factor for T. cruzi seropositivity. Specifically, being older than 32 resulted in twice the risk of having positive serology. This confirms similar findings in Argentina 30, Bolivia 31, another endemic area of Colombia 21 and recently in El Salvador 32. This recurrent finding reflects the typical seroprevalence profile due to accumulated exposure with age in areas of domiciliary transmission.
A history of living in poor housing conditions (specifically in houses with thatched roofs) was a risk factor for seropositivity (OR 3.0; 95% CI: 1.4–6.6), which increases significantly if women continue living in such conditions (OR 11.8; 95% CI: 2.2–63.2). Housing conditions are the most important risk factors associated with triatomine infestation in Colombia and Argentina 2, 33. Even though some variables such as socio‐economic conditions and living in rural areas were statistically significant in the bivariate analysis, they did were not relevant in the final model. This may be due to correlation between those variables and the ones that were finally presented in the model such as thatch roof and level of education. Recollection of at least one ‘close contact’ (a bite or seeing a vector in bed) also was a clear risk factor, although ‘simple recognition’ did not represent a risk.
In Colombia, and particularly the department of Santander, several oral outbreaks have been reported in the last years 34, 35, 36, 37. Interestingly, for the majority of foodborne outbreaks for which a triatomine infection source has been identified, these have usually been species that are not usually associated with vectorial transmission, and mostly P. geniculatus 38. Given the epidemiological history of vectorial transmission in the areas involved in this study, and also the association with housing conditions for intradomiciliary transmission, oral transmission seems less likely to be a driver of prevalence of infection in this population.
With respect to performance of the diagnostic tests, ELISA and IFI have been evaluated and presented in detailed in previous studies 24. The reproducibility between these two tests was almost perfect with a kappa coefficient of 0.98 so the HAI test was only necessary to confirm the seropositive status in a few cases.
Because the local doctors and health personal participating in this study were trained for both diagnosis and treatment, the study was a great opportunity to generate awareness among healthcare workers and the community about congenital Chagas disease. Recent studies have highlighted the importance of increasing awareness at local level as a crucial tool to improve diagnosis and disease control 39. For all pregnant women diagnosed in this study, aetiological treatment was offered and given after delivery through the local hospitals. The decision of treating all mothers (after pregnancy) despite the absence of congenital transmission in this particular study was considered appropriate given that in other endemic departments of the country this type of transmission has been reported 20 and we cannot dismiss the possibility of transmission in future pregnancies. The trypanocidal treatment of women with chronic Chagas infection before pregnancy prevents congenital transmission of T. cruzi to their children 11. This group of young and mostly healthy women could potentially benefit from aetiological treatment in terms of reducing the possibility of developing heart complications 40.
A potential limitation of this study is that we only included pregnant women who attended antenatal control clinics in hospitals. Despite the fact that the coverage of antenatal care in Colombia has been increasing in the last decades and for 2010 it was estimated at 97% 41, this still could represent a potential bias in omitting the poorest women who only seek hospital care for delivery or who do not seek care at any time and who are the patients at the highest risk of T. cruzi seropositivity. Another potential limitation of this study, considering a recent meta‐analysis has estimated the transmission rate of congenital Chagas disease at 4.7% (95% CI: 3.9–5.6) 17, is that the sample size of newborns (49 children) was not enough to find congenital cases.
This study updates epidemiological data on Chagas disease prevalence in pregnant women in a Colombian endemic area. The identified risk factors will allow the local health providers to target more precisely the necessary interventions for this specific population. The fact that no congenital cases were found in this series of pregnant women does not mean that this transmission route is not present in this department. There is still a need to guarantee the proper diagnosis at birth and the clinical and laboratory follow‐up to all children born from infected women, as recently recommended 42. Providing care and aetiological treatment to infected women of childbearing age before they become pregnant is also imperative in order to reduce the probability of the disease progression and prevent congenital transmission.
Acknowledgements
We thank Santander's Departmental Secretary of Health and its Public Health Laboratory for their most valuable assistance during all stages of this project. We also gratefully appreciate the special collaboration provided by the 23 local hospitals and the three provincial health teams (grupos provinciales) who helped with all the necessary logistics for this study. We thank the two anonymous peer reviewers for critically reading the manuscript and suggesting substantial improvements, and Thomas Crellen for editing the manuscript. Finally, we thank the pregnant women and children who kindly and enthusiastically agreed to participate in this study during more than 2 years. This research was co‐financed by the Colombian Department of Science and Technology, COLCIENCIAS, Colombia's National Health Institute, the Pontificia Universidad Javeriana and Santander's Departmental Secretary of Health. For the publication fees, we received support from the research programme CHAGAS NETWORK.
References
- 1. Guhl F, Restrepo M, Angulo VM, Antunes CMF, Campbell‐Lendrum D, Davies CR. Lessons from a national survey of Chagas disease transmission risk in Colombia. Trends Parasitol 2005: 21: 259–262. [DOI] [PubMed] [Google Scholar]
- 2. Campbell‐Lendrum DH, Angulo VMV, Esteban L et al House‐level risk factors for triatomine infestation in Colombia. Int J Epidemiol 2007: 36: 866–872. [DOI] [PubMed] [Google Scholar]
- 3. Guhl F. Chagas disease in Andean countries. Mem Inst Oswaldo Cruz 2007: 102(Suppl): 29–38. [DOI] [PubMed] [Google Scholar]
- 4. Gürtler RE, Segura EL, Cohen JE. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Infect Dis 2003: 9: 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fumadó V, Juncosa T, Posada E, Fisa R, Gállego M, Gascón J. Chagas pediátrico en zona no endémica. Enferm Infecc Microbiol Clin 2014: 32: 293–296. [DOI] [PubMed] [Google Scholar]
- 6. Imai K, Maeda T, Sayama Y et al Mother‐to‐child transmission of congenital Chagas disease. Japan Emerg Infect Dis 2014: 20: 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bittencourt AL. Possible risk factors for vertical transmission of chagas′ disease. Rev Inst Med Trop San Pablo 1992: 34: 403–408. [DOI] [PubMed] [Google Scholar]
- 8. Carlier Y, Torrico F. Congenital infection with Trypanosoma cruzi: from mechanisms of transmission to strategies for diagnosis and control. Rev Soc Bras Med Trop 2003: 36:767–771. [DOI] [PubMed] [Google Scholar]
- 9. Kemmerling U, Bosco C, Galanti N. Infection and invasion mechanisms of Trypanosoma cruzi in the congenital transmission of chagas' disease: a proposal. Biol Res 2010: 43: 307–316. [PubMed] [Google Scholar]
- 10. Flores‐Chavez M, Merino F, Garcia‐Bujalance S et al Surveillance of Chagas disease in pregnant women in Madrid (Spain), 2008–2010. Trop Med Int Health 2011: 16: 368. [DOI] [PubMed] [Google Scholar]
- 11. Fabbro DL, Danesi E, Olivera V et al Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis 2014: 8: e3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Contreras S, Fernandez MR, Agüero F, Desse Desse J, Orduna T, Martino O. Enfermedad de Chagas‐Mazza congenita en Salta. Rev Soc Bras Med Trop 1999: 32: 633–636. [DOI] [PubMed] [Google Scholar]
- 13. Rassi A, Amato Neto V, Rassi GG et al [A retrospective search for maternal transmission of Chagas infection from patients in the chronic phase] [Portuguesse]. Rev Soc Bras Med Trop 2004: 37: 485–489. [DOI] [PubMed] [Google Scholar]
- 14. Brutus L. Congenital Chagas disease: diagnostic and clinical aspects in an área without vectorial transmission, Bermejo, Bolivia. Acta Trop 2008: 106: 195–199. [DOI] [PubMed] [Google Scholar]
- 15. Araújo AB, Castagno VD, Gallina T, Aires E. Prevalência da doença de Chagas em gestantes da região sul do Rio Grande do Sul Prevalence of Chagas disease among pregnant women in the southern region of Rio Grande do Sul. Rev Soc Bras Med Trop 2009: 42: 732–733. [DOI] [PubMed] [Google Scholar]
- 16. De Rissio AM, Riarte AR, García MM, Esteva MI, Quaglino M, Ruiz AM. Congenital Trypanosoma cruzi infection. Efficacy of its monitoring in an urban reference health center in a non‐endemic area of Argentina. Am J Trop Med Hyg 2010: 82: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howard EJ, Xiong X, Carlier Y, Sosa‐Estani S, Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta‐analysis. BJOG 2014: 121: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zaidenberg M, Segovia A. Congenital Chagas disease in the city of Salta, Argentina. Rev Inst Med Trop Sao Paulo 1993: 35: 35–43. [PubMed] [Google Scholar]
- 19. Pavia PX, Montilla M, Flórez C et al The first case of congenital Chagas' disease analyzed by AP‐PCR in Colombia. Biomedica 2009: 29: 513–522. [PubMed] [Google Scholar]
- 20. Manrique‐Abril F, Ospina J, Herrera G et al Diagnóstico de enfermedad de Chagas en mujeres embarazadas y recién nacidos de Moniquirá y Miraflores, Boyacá, Colombia. Infectio 2014: 7: 28–34. [Google Scholar]
- 21. Cucunubá ZM, Flórez AC, Cárdenas A et al Prevalence and risk factors for Chagas disease in pregnant women in Casanare, Colombia. Am J Trop Med Hyg 2012: 87: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López M, Duque S, Orozco L. Inmunodiagnóstico de la infección chagásica por ELlSA. Biomedica 1999: 19: 159–163. [Google Scholar]
- 23. Orozco LC, Camargo D, Lopez C et al Inmunodiagnóstico de la infección en humanos por Trypanosoma cruzi mediante ELISA utilizando sangre recolectada en papel filtro. Biomedica 1999: 19: 164–168. [Google Scholar]
- 24. Castellanos YZ, Cucunubá ZM, Flórez AC, Orozco‐Vargas LC. Reproducibility of serological tests for the diagnosis of Trypanosoma cruzi infection in pregnant women in an endemic area of Santander, Colombia. Biomedica 2014: 34: 198–206. [DOI] [PubMed] [Google Scholar]
- 25. Freilij H, Altcheh J. Congenital Chagas' disease: diagnostic and clinical aspects. Clin Infect Dis 1995: 21: 551–555. [DOI] [PubMed] [Google Scholar]
- 26. Torrico F, Alonso‐Vega C, Suarez E et al Endemic level of congenital Trypanosoma cruzi infection in the areas of maternal residence and the development of congenital Chagas disease in Bolivia. Rev Soc Bras Med Trop 2005: 38(Suppl 2): 17–20. [PubMed] [Google Scholar]
- 27. Gutierrez R, Angulo VM, Tarazona Z, Britto C, Fernandes O. Comparison of four serological tests for the diagnosis of Chagas disease in a Colombian endemic area. Parasitology 2004: 129: 439–444. [DOI] [PubMed] [Google Scholar]
- 28. Castañeda G & Angulo‐Silva VM. Estudio de prevalencia de la infección por Trypanosoma cruzi en parturientas en el Hospital González Valencia. Determinación de la incidencia de Chagas congénito en hijos. de madres infectadas. 1995: Bucaramanga, Colombia. [Google Scholar]
- 29. Russomando G. Congenital transmission of Chagas disease in Paraguay. Mem Inst Investig Cienc Salud 2009: 7: 55–64. [Google Scholar]
- 30. Blanco SB, Segura EL, Gürtler RE. Control of congenital transmission of Trypanosoma cruzi in Argentina. Medicina (B Aires) 1999: 59(Suppl 2): 138–142. [PubMed] [Google Scholar]
- 31. Brutus L, Castillo H, Bernal C et al Detectable Trypanosoma cruzi parasitemia during pregnancy and delivery as a risk factor for congenital Chagas disease. Am J Trop Med Hyg 2010: 83: 1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sasagawa E, Aiga H, Corado EY et al Risk factors for Chagas disease among pregnant women in El Salvador. Trop Med Int Health 2015: 20: 268–276. [DOI] [PubMed] [Google Scholar]
- 33. Sanmartino M, Crocco L. Conocimientos sobre la enfermedad de Chagas y factores de riesgo en comunidades epidemiológicamente diferentes de Argentina. Rev Panam Salud Pública 2000: 7: 173–178. [DOI] [PubMed] [Google Scholar]
- 34. Hernández LM, Ramírez AN, Cucunubá Z, Zambrano P. Brote de Chagas agudo en Lebrija, Santander 2008. Rev del Obs Salud Pública Santander 2009: 4: 28–36. [Google Scholar]
- 35. Ramírez JD, Montilla M, Cucunubá ZM, Floréz AC, Zambrano P, Guhl F. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl Trop Dis 2013: 7: e2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nicholls RS, Cucunubá ZM, Knudson A et al Acute Chagas disease in Colombia: a rarely suspected disease. Report of 10 cases presented during the 2002–2005 period. Biomedica 2007: 27(Suppl 1): 8–17. [PubMed] [Google Scholar]
- 37. Zambrano P, Cucunubá ZM, Montilla M, Florez AC, Parra E, Cortes LJ. Brotes de síndrome febril asociado a miocarditis aguda chagásica de posible transmisión oral en el departamento de Santander, diciembre de 2008 a mayo de 2009. IQEN 2010: 15: 17–26. [Google Scholar]
- 38. de Noya BA, Díaz‐Bello Z, Colmenares C et al Update on oral Chagas disease outbreaks in Venezuela: epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz 2015: 110: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soriano‐Arandes A, Basile L, Ouaarab H et al Controlling congenital and paediatric chagas disease through a community health approach with active surveillance and promotion of paediatric awareness. BMC Public Health 2014: 14: 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Viotti R, Alarcón de Noya B, Araujo‐Jorge T et al Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrob Agents Chemother 2014: 58: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Bank . Pregnant women receiving prenatal care (%) in Colombia 2015. (Available from: http://www.tradingeconomics.com/colombia/pregnant-women-receiving-prenatal-care-percent-wb-data.html) [21 February 2015]
- 42. Cucunubá ZM, Valencia‐Hernández CA, Puerta CJ et al Primer consenso colombiano sobre Chagas congénito y orientación clínica a mujeres en edad fértil con diagnóstico de Chagas. Infectio 2014: 18: 50–65. [Google Scholar]


