Abstract
In a phase I/IIa study of in situ gene therapy using an adenovirus vector carrying the human REIC/Dkk‐3 gene (Ad‐REIC), we assessed the inhibitory effects of cancer recurrence after radical prostatectomy (RP), in patients with high risk localized prostate cancer (PCa). After completing the therapeutic interventions with initially planned three escalating doses of 1.0 × 1010, 1.0 × 1011, and 1.0 × 1012 viral particles (VP) in 1.0–1.2 mL (n = 3, 3, and 6), an additional higher dose of 3.0 × 1012 VP in 3.6 mL (n = 6) was further studied. Patients with recurrence probability of 35% or more within 5 years after RP as calculated by Kattan's nomogram, were enrolled. They received two ultrasound‐guided intratumoral injections at 2‐week intervals, followed by RP 6 weeks after the second injection. Based on the findings of MRI and biopsy mapping, as a rule, one track injection to the most prominent cancer area was given to initial 12 patients and 3 track injections to multiple cancer areas in additional 6 patients. As compared to the former group, biochemical recurrence‐free survival of the latter showed a significantly favorable outcome. Neoadjuvant Ad‐REIC, mediating simultaneous induction of cancer selective apoptosis and augmentation of antitumor immunity, is a feasible approach in preventing cancer recurrence after RP. (199)
Keywords: REIC/Dkk‐3, gene therapy, neoadjuvant therapy, localized prostate cancer
Introduction
With the advent of prostate specific antigen (PSA) screening, PCa has been detected commonly when localized. Nevertheless, outcomes in patients with high risk localized prostate cancer (PCa) undergoing RP alone, have not improved significantly with time.1 In order to improve long‐term outcomes of these patients, the establishment of a new, efficient neoadjuvant therapy is much awaited. Recently, a variety of clinical trials including in situ gene therapy have been conducted as a form of neoadjuvant therapy, since this approach provides a paradigm for evaluating the activity and mechanism of action of new agents with histopathological analysis using tumor tissues before and after therapy.2
The expression of reduced expression in immortalized cells (REIC)/Dickkoph‐3 (Dkk‐3) gene is significantly reduced in a wide variety of cancer cells including prostate cancer 3, 4, 5, 6, 7, 8, 9, 10 and its forced expression using Ad‐REIC, induces cancer‐selective apoptosis as a result of unfolded protein response, due to endoplasmic reticulum stress (ER stress).6 ER stress mediates the enhanced IL‐7 expression in co‐infected normal fibroblasts, resulting in the activation of innate immunity involving NK cells.11 In addition, secreted REIC protein with potent immunomodulatory function creates an optimal environment for activation of host immune cells, inducing cytotoxic T lymphocytes.12, 13
We are developing an Ad‐REIC gene therapy agent as a therapeutic cancer vaccine in the treatment of various intractable solid cancers. The First‐In‐Human clinical study, a phase I/IIa study of in situ Ad‐REIC gene therapy for prostate cancer was initiated at Okayama University from January 2011. In this phase I/IIa study, two groups of patients were treated: group A consisting of patients with castration‐resistant PCa (CRPC) with or without metastasis, and group B consisting of patients with high‐risk, localized PCa scheduled to undergo radical prostatectomy (neoadjuvant study). In group A, direct and indirect systemic effects induced by in situ gene therapy were clearly illustrated in a case of chemotherapy resistant advanced CRPC with bulky lymph node metastases.14
In the neoadjuvant study, patients treated with the initially planned three escalating dose levels (DLs) of 1.0 × 1010, 1.0 × 1011, and 1.0 × 1012 viral particles (VP) in 1.0–1.2 mL (n = 3, 3, and 6) showed remarkable safety profiles of Ad‐REIC (primary endpoint) and dose‐dependent immunopathological effects (secondary endpoint) without reaching the maximum tolerated dose. Then, an additional study with a higher dose level (DL‐4) of 3.0 × 1012 VP in 3.6 mL (n = 6) was conducted to assess the safety and the inhibitory effects of cancer recurrence after RP. In this preliminary report, we discuss the potential of neoadjuvant Ad‐REIC gene therapy to proceed the next comparative study. The precise clinical data on the entire phase I/IIa study of in situ Ad‐REIC gene therapy including the data for advanced CRPC will be published elsewhere.
Patients and Methods
Kattan's nomogram score of ≥115 (calculated 5‐year recurrence‐free probability of ≤65%) 15, 16 was used to select high risk localized PCa and eighteen patients were enrolled in the study. Initially, three escalating DLs of Ad‐REIC (1.0×1010, 1.0×1011, and 1.0×1012 VP in 1.0–1.2 mL) were studied in 3, 3 and 6 cases, respectively. Based on MRI findings and biopsy mapping, one track injection to the most prominent cancer area was conducted. Secondarily, DL‐4 of 3.0×1012 VP in 3.6 mL with 3 track injections (3 times injection of 1.0×1012 VP in 1.2 mL) to multiple cancer areas was given to 6 cases. The original Ad‐REIC, a replication‐deficient adenovirus vector, was constructed at Okayama University 3 and its cGMP product was supplied by Momotaro‐Gene Inc., a start‐up biotech company originating from Okayama University. All patients received two ultrasound‐guided intraprostatic injections at 2‐week intervals, followed by RP 6 weeks after the second injection.
Using standard sections of RP specimens with hematoxylin and eosin staining, final pathological diagnosis and antitumor effects mediated by Ad‐REIC were determined. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining for the detection of apoptosis of cancer cells and immunohistochemical staining for the analysis of tumor infiltrating lymphocytes were conducted in selected cases. Peripheral blood lymphocyte subsets were analyzed by flow cytometry after Ad‐REIC treatment in all patients. Serum PSA levels were analyzed before and after Ad‐REIC injections. After RP, PSA was measured at months 1, 2, 3 and every 3 months thereafter or as clinically indicated. Biochemical recurrence was defined as an initial PSA value exceeding 0.2 ng/mL, followed by a subsequent confirmatory PSA value >0.2 ng/mL . If PSA levels did not decrease to less than 0.2 ng/mL after surgery, the date of RP was defined as the date of disease recurrence.
The present clinical protocols were approved by the Okayama University Institutional Review Board and the Japanese Government. Patients reviewed the informed consent document and received individual counseling with a thorough discussion as to alternative treatments, including nonparticipation.
Results
Clinical and pathological characteristics of 18 patients in three groups (DL‐1+2, 3, and 4) are demonstrated in Table 1. Although the number of patients in each dose level is small, there are no significant differences in patient characteristics, including Kattan's nomogram scores among three groups of neoadjuvant Ad‐REIC treatment. Most patients were regarded as a very high risk for recurrence; 83% (15/18) had a Gleason score of ≥8 and 72% percent (13/18) had a Kattan's nomogram score of >130 (5‐year recurrence free probability of <5%). All 4 dose levels including the additional dose level 4, were feasible with no adverse events except for fever. Grade 1 or 2 fever was a common symptomatic toxicity in high dose levels of 3 and 4, but was transient and treatable with antipyretics. Neither intraoperative nor postoperative complications related to neoadjuvant Ad‐REIC were observed.
Table 1.
Clinical and pathological characteristics of 18 patients enrolled in the study
| Dose Level 1+2 (n = 6) | Dose Level 3 (n = 6) | Dose level 4 (N = 6) | p Value | ||
|---|---|---|---|---|---|
| Age | 62.5 (59–74) | 68 (63–71) | 66 (57–74) | 0.533 | |
| Clinical T stage | T2a | 1 | 0 | 2 | 0.485 |
| T2b | 0 | 0 | 0 | ||
| T2c | 2 | 2 | 2 | ||
| T3a | 3 | 4 | 2 | ||
| Biopsy Gleason score | 7 | 0 | 2 | 1 | 0.392 |
| 8 | 2 | 3 | 3 | ||
| 9 | 3 | 1 | 2 | ||
| 10 | 1 | 0 | 0 | ||
| PSA | 13.425 (5.02–26.62) | 13.775 (9.82–16.18) | 19.5 (10.87–33.60) | 0.309 | |
|---|---|---|---|---|---|
| Kattan's nomogram score | 140.5 (124–176) | 140 (118–167) | 137 (122–173) | 0.894 | |
| Pathlogical stage | pT2b | 0 | 0 | 0 | 0.148 |
| pT2c | 1 | 3 | 4 | ||
| pT3a | 0 | 1 | 1 | ||
| pT3b | 5 | 2 | 1 | ||
| Margin+ | 4 | 4 | 4 | 1 | |
| Node+ | 2 | 0 | 0 | 0.085 | |
In terms of antitumor effects, no clear effects were detected in DL‐1 but 2 out of 3 in DL‐2 showed PSA decline and moderate cytopathic effects with tumor infiltrating lymphocytes (TIL). All 6 cases in dose level 3 showed PSA decline and clear cytopathic effects with TIL. As illustrated in Figure 1 A, massive degeneration with cytolysis and pyknosis was detected in the targeted tumor areas of Case B‐8. Using serial sections of the surgical specimen from Case B‐8 (Figures 1 B–E), pyknotic cells undergoing apoptosis were confirmed by TUNEL staining (Figure 1 C) and remarkable, concurrent infiltrations of CD8+ lymphocytes (Figure 1 D) and dendritic cells (Figure 1 E) were clearly demonstrated in the area of apoptotic cancer cells by immunohistochemical staining.
Figure 1.
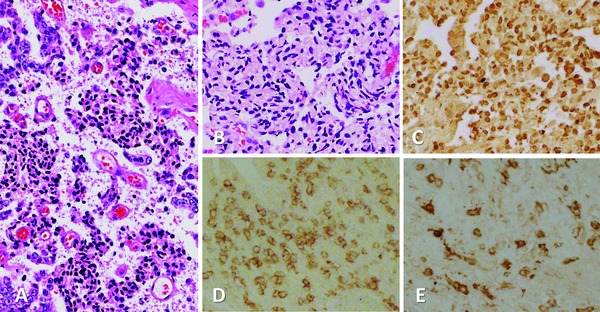
Surgical specimen from Case B‐8 treated with DL‐3 of 1×1012 VP of Ad‐REIC. (A) Massive degeneration with cytolysis and pyknosis detected in the targeted tumor (H&E, x100). Serial sections stained differently (×100). (B) H&E, (C) TUNEL staining, illustrating pyknotic cells undergoing apoptosis. (D) Immunohistochemical staining, illustrating remarkable, concurrent infiltrations of CD8+ lymphocytes and (E) dendritic cells in the area of apoptotic cancer cells.
Biochemical recurrence‐free survival (BRFS) of each dose group was compared using the Kaplan‐Meier survival analysis. Although the follow‐up duration of DL‐4 was short (median 12.0, range 6.1 to 29.7 months), no recurrence was observed in patients treated with dose level 4. BRFS in DL‐4 was significantly more favorable than in DL‐1+2 plus DL‐3 group patients (Figure 2). Peripheral blood lymphocyte subsets were analyzed by flow cytometry after Ad‐REIC treatment. Changes in B cells, T cells, NK cells and CD4+ lymphocytes showed no tendency to increase, while CD8+ lymphocytes increased in response to Ad‐REIC treatment (Figure 3 A). The HLA‐DR marker of activation was used to double label CD3+, CD4+ and CD8+ lymphocytes as a relative measure of activated T cells. HLA‐DR+CD8+ (activated CTL) and HLA‐DR+CD3+ (activated T) lymphocytes showed increases after Ad‐REIC treatment with a tendency of dose‐dependent manner (Figures 3 B and C).
Figure 2.
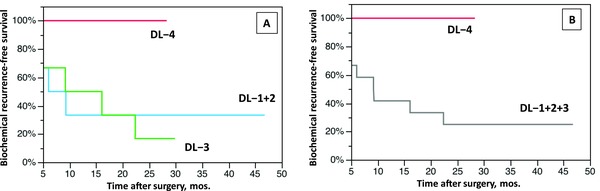
Kaplan–Meier curves representing biochemical recurrence free survival (BRFS) in patients with high risk prostate cancer treated with neoadjuvant Ad‐REIC followed by radical prostatectomy. (A) BRFS curves of 3 dose level groups. The differences were not significant (log‐rank test). (B) BRFS curves of DL‐1,2,3 pooled group and DL‐4 group. The difference was significant (p < 0.05, log‐rank test).
Figure 3.
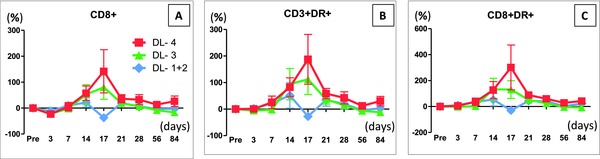
Flow cytometry analysis of circulating peripheral blood lymphocyte at indicated time points after the first injection (Day‐0) of Ad‐REIC. Data for each changing rate are presented as mean ± SE. (A) Changing rates of CD8+ cells in three dose groups. (B) Changing rates of CD3+ DR+ cells in three dose groups. (C) Changing rates of CD8+ DR+ cells in three dose groups. Changing rates of CD8+, CD3+ DR+,CD8+ DR+ cells increased after the second Ad‐REIC injection (Day‐13) in DL‐3 and DL‐4, but the differences among three dose groups were not significant (two‐way ANOVA test).
Discussion
Neoadjuvant therapy is widely accepted in the treatment of patients with localized or locally advanced high‐risk breast cancer and other solid cancers.17, 18, 19 In prostate cancer, however, a number of neoadjuvant trials with androgen deprivation therapy (ADT) and chemotherapy with or without ADT demonstrated that the beneficial effects on pathological outcomes, including pCR rate, did not translate to improved disease‐free survival or overall survival.20, 21, 22, 23, 24, 25 Therefore, neoadjuvant therapy with ADT and chemotherapy is not currently recommended in patients with high‐risk localized PCa undergoing RP. To establish a new standard of neoadjuvant therapy, a variety of clinical trials with various novel agents have been conducted. Different from ordinary systemic therapy, in situ immune gene therapy is expected to provide a new option for neoadjuvant therapy by generating indirect systemic effects.26, 27, 28
REIC/Dkk‐3 gene was isolated and cloned as an immortalization‐related gene at Okayama University in 2000,29 and has rapidly emerged as a key player in most human cancers.30 Recently, we have reviewed previous fundamental studies and summarized the anticancer mechanisms of in situ Ad‐REIC as a therapeutic cancer vaccine.13 These anticancer mechanisms of Ad‐REIC mediating direct and indirect systemic effects by augmented antitumor immunity have been confirmed in treating a patient with metastatic CRPC following chemotherapy.14 In the present neoadjuvant study, the key principal of Ad‐REIC anticancer mechanisms characterized by massive cancer selective apoptosis with concurrent infiltrations of CD8+ lymphocytes and dendritic cells was clearly illustrated in histological sections of surgical specimens treated with Ad‐REIC (see Figure 1). In addition, peripheral blood CD8+ T cells were increased with a tendency of dose‐dependent upregulation of HLA‐DR expression (see Figure 2). Therefore, it is possible that cancer vaccine effects by neoadjuvant Ad‐REIC could translate into improved postoperative outcomes in patients with high‐risk localized PCa. BRFS in dose level 4, with three track injections using an optimum Ad‐REIC dose of 1.0×1012 VP/1.2 mL, was significantly more favorable than other dose levels.
Conclusion
Although historically, PCa was not regarded as an immunogenic cancer, recent clinical results including the efficacy of sipuleucel‐T for metastatic CRPC have led to a renewed interest in immunotherapy for PCa. Consequently, Ad‐REIC gene therapy is regarded as a promising option for immunotherapy in the treatment of patients with high‐risk localized PCa in neoadjuvant setting. Prospective randomized comparative study may warrant exploration. (1,568)
Acknowledgments
We thank Dr. Sabina Mahmood and Prof. Shiro Hinotsu (Okayama University Hospital, Okayama, Japan) for providing valuable suggestions and help with the preparation of this manuscript. This study was supported by scientific research grants (KAKENHI: 22791473, 23390382, and 25462479) and by the Special Coordination Funds for Promoting Science and Technology (Formation of Innovation Center for Fusion of Advanced Technologies) from the Japan Ministry of Education, Culture, Sports, Science, and Technology (MEXT, FY2006‐2009), and Health Labor Sciences Research Grant of Japan (FY2011‐2014).
References
- 1. Kane CJ, Presti JC Jr, Amling CL, Aronson WJ, Terris MK, Freedland SJ. SEARCH Database Study Group. Changing nature of highrisk patients undergoing radical prostatectomy. J Urol. 2007; 177: 113–117. [DOI] [PubMed] [Google Scholar]
- 2. Sonpavde G, Chi KN, Powles T, Sweeney CJ, Hahn N, Hutson TE, Galsky MD, Berry WR, Kadmon D. Neoadjuvant therapy followed by prostatectomy for clinically localized prostate cancer. Cancer. 2007; 110: 2628–2639. [DOI] [PubMed] [Google Scholar]
- 3. Abarzua F, Sakaguchi M, Takaishi M, Nasu Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H, Huh NH. Adenovirus‐mediated overexpression of Dkk‐3 selectively induces apoptosis in human prostate cancer cells through activation of c‐Jun‐HH2‐kinase. Cancer Res. 2005; 65: 9617–9622. [DOI] [PubMed] [Google Scholar]
- 4. Kurose K, Sakaguchi M, Nasu Y, Ebara S, Kaku H, Kariyama R, Arao Y, Miyazaki M, Tsushima T, Namba M, et al. Decreased expression of REIC/Dkk‐3 in human renal clear cell carcinoma. J Urol. 2004; 171: 1314–1318. [DOI] [PubMed] [Google Scholar]
- 5. Tanimoto R, Abarzua F, Sakaguchi M, Takashi M, Nasu Y, Kumon H, Huh NH. REIC/Dkk‐3 as a potential gene therapeutic agent against human testicular cancer. Int J Mol Med. 2007; 19: 363–368. [PubMed] [Google Scholar]
- 6. Kashiwakura Y, Ochiai K, Watanabe M, Abarzua F, Sakaguchi M, Takaoka M, Tanimoto R, Nasu Y, Huh NH, Kumon H. Down‐regulation of inhibition of differentiation‐1 via activation of activating transcription factor3 and Smad regulated REIC/Dickkopf‐3‐induced apoptosis. Cancer Res. 2008; 68: 8333–8341. [DOI] [PubMed] [Google Scholar]
- 7. Kawasaki K, Watanabe M, Sakaguchi M, Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH, Kumon H, et al. REIC/Dkk‐3 overexpression downregulates P‐glycoprotein in multidrug‐resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther. 2009; 16: 65–72. [DOI] [PubMed] [Google Scholar]
- 8. Uchida D, Shiraha H, Kato H, Nagahata T, Iwamuro M, Kataoka J, Horiguchi S, Watanabe M, Takaki A, Nouso K, et al. Potential of adenovirus‐mediated REIC/Dkk‐3 gene therapy for use in the treatment of pancreatic cancer. J Gastroenterol Hepatol. 2014; 29: 973–983. [DOI] [PubMed] [Google Scholar]
- 9. Shimazu Y, Kurozumi K, Ichikawa T, Fujii K, Onishi M, Ishida J, Oka T, Watanabe M, Nasu Y, Kumon H, et al. Integrin antagonist augments the therapeutic effect of adenovirus‐mediated REIC/Dkk‐3 gene therapy for malignant glioma. Gene Ther. 2015; 22: 146–154. [DOI] [PubMed] [Google Scholar]
- 10. Shien K, Tanaka N, Watanabe M, Soh J, Sakaguchi M, Matsuo K, Yamamoto H, Furukawa M, Asano H, Tsukuda K, et al. Anti‐cancer effects of REIC/Dkk‐3‐encoding adenoviral vector for the treatment of non‐small cell lung cancer. PLoS One. 2014; 9: e87900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakaguchi M, Kataoka K, Abarzua F, Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y, Ochiai K, et al. Overexpression of REIC/Dkk‐3 in normal fibroblasts suppresses tumor growth via induction of interleukin‐7. J Biol Chem. 2009; 284: 14236–14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe M, Kashiwakura Y, Huang P, Ochiai K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH, et al. Immunological aspects of REIC/Dkk‐3 in monocyte differentiation and tumor regression. Int J Oncol. 2009; 34: 657–663. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe M, Nasu Y, Kumon H. Adenovirus‐mediated REIC/Dkk‐3 gene therapy: development of an autologous cancer vaccination therapy (Review). Oncol Lett. 2014; 7: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumon H, Sasaki K, Ariyoshi Y, Sadahira T, Ebara S, Hiraki T, Kanazawa S, Yanai H, Watanabe M, Nasu Y. Ad‐REIC gene therapy: promising results in a patient with metastatic CRPC following chemotherapy. Clin Med Insights Oncol. 2015; 9: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998; 90: 766–771. [DOI] [PubMed] [Google Scholar]
- 16. Korets R, Motamedinia P, Yeshchina O, Desai M, McKiernan JM. Accuracy of the Kattan nomogram across prostate cancer risk‐groups. BJU Int. 2011; 108: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, et al. Definitionand impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 18. Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 19. Sonpavde G, Sternberg CN. Neoadjuvant systemic therapy for urological malignancies. BJU Int. 2010; 106: 6–22. [DOI] [PubMed] [Google Scholar]
- 20. Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, Schelin S, Pedersen K. Three‐month neoadjuvant hormonal therapy before radical prostatectomy: a 7‐year follow‐up of a randomized controlled trial. BJU Int. 2002; 90: 561–566. [DOI] [PubMed] [Google Scholar]
- 21. Klotz LH, Goldenberg SL, Jewett MA, Fradet Y, Nam R, Barkin J, Chin J, Chatterjee S; Canadian Uro‐Oncology Group. Long‐term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003; 170: 791–794. [DOI] [PubMed] [Google Scholar]
- 22. Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4‐Year follow‐up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2‐3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer.Eur Urol. 2000; 38: 706–713. [DOI] [PubMed] [Google Scholar]
- 23. Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008; 180: 565–570. [DOI] [PubMed] [Google Scholar]
- 24. Prayer‐Galetti T, Sacco E, Pagano F, Gardiman M, Cisternino A, Betto G, Sperandio P. Long‐term followup of a neoadjuvant chemohormonal taxane‐based phase II trial before radical prostatectomy in patients with non‐metastatic high‐risk prostate cancer. BJU Int. 2007; 100: 274–280. [DOI] [PubMed] [Google Scholar]
- 25. Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S, Barnes AS, Tempany C, Catalona W, Kantoff PW, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high‐risk localized prostate cancer. Clin Cancer Res. 2005; 11: 5233–5240. [DOI] [PubMed] [Google Scholar]
- 26. Sonpavde G1, Thompson TC, Jain RK, Ayala GE, Kurosaka S, Edamura K, Tabata K, Ren C, Goltsov AA, Mims MP, et al. GLIPR1 tumor suppressor gene expressed by adenoviral vector as neoadjuvant intraprostatic injection expressed by adenoviral vector as neoadjuvantintraprostatic injection for localized intermediate or high‐risk prostate cancer preceding radical prostatectomy. Clin Cancer Res. 2011; 15(17): 7174–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rojas‐Martínez A, Manzanera AG, Sukin SW, Esteban‐María J, González‐Guerrero JF, Gomez‐Guerra L, Garza‐Guajardo R, Flores‐Gutiérrez JP, Elizondo Riojas G, Delgado‐Enciso I, et al. Intraprostatic distribution and long term follow‐up after AdV‐tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther. 2013; 20: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Linden RRM, Haagmans BL, Mongiat‐Artus P, van Doornum GJ, Kraaij R, Kadmon D, Aguilar‐Cordova E, Osterhaus AD, van der Kwast TH, Bangma CH. Virus specific immune responses after human neoadjuvant adenovirus‐mediated suicide gene therapy for prostate cancer. Eur Urol. 2005; 48: 153–161. [DOI] [PubMed] [Google Scholar]
- 29. Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows down‐regulation in human immortalized cells and human tumor‐derived cell lines. Biochem Biophys Res Commun. 2000; 268: 20–24. [DOI] [PubMed] [Google Scholar]
- 30. Veeck J, Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf‐3. Biochim Biophys Acta. 2012; 1825: 18–28. [DOI] [PubMed] [Google Scholar]


