Abstract
Aims
To compare the immunogenicity profiles and the potential effects on clinical outcomes of LY2963016 insulin glargine (LY IGlar) and Lantus® insulin glargine (IGlar), products with identical primary amino acid sequences, in patients with type 1 or type 2 diabetes mellitus (T1DM or T2DM).
Methods
To assess immunogenicity, anti‐insulin glargine antibodies (measured as percent binding) were compared between treatments in 52‐week (open‐label) and 24‐week (double‐blind) randomized studies in total study populations of patients with T1DM (N = 535) and T2DM (N = 756), respectively, and two subgroups of patients with T2DM: insulin‐naïve patients and those reporting prestudy IGlar treatment (prior IGlar). Relationships between insulin antibody levels and clinical outcomes were assessed using analysis of covariance and partial correlations. Insulin antibody levels were assessed using Wilcoxon rank sum. Treatment comparisons for treatment‐emergent antibody response (TEAR) and incidence of detectable antibodies were analysed using Fisher's exact test.
Results
No significant treatment differences were observed for insulin antibody levels, incidence of detectable anti‐insulin glargine antibodies, or incidence of TEAR [overall and endpoint, by last‐observation‐carried‐forward (LOCF)] in patients with T1DM or patients with T2DM, including the insulin‐naïve subgroup. A statistically significant difference was noted in the overall incidence of detectable antibodies but not at endpoint (LOCF) nor in TEAR for the prior IGlar subgroup of patients with T2DM. Insulin antibody levels were low (<5%) in both treatment groups. Insulin antibody levels or developing TEAR was not associated with clinical outcomes.
Conclusions
LY IGlar and IGlar have similar immunogenicity profiles; anti‐insulin glargine antibody levels were low for both treatments, with no observed effect on efficacy and safety outcomes.
Keywords: biosimilar insulin, insulin antibody, insulin glargine, LY2963016 insulin glargine
Introduction
Insulin glargine, a long‐acting basal insulin, is a protein product that is a human insulin analogue manufactured using recombinant DNA technology 1. In September 2014, LY2963016 (LY IGlar; Eli Lilly and Co. and Boehringer‐Ingelheim), an insulin glargine product with an identical primary amino acid sequence to Lantus® (recombinant DNA origin; Sanofi‐Aventis, Paris, France) insulin glargine (IGlar) 1, became the first biosimilar insulin to be granted marketing authorization in the European Union 2. LY IGlar has been shown to have similar efficacy and safety to IGlar 3, 4.
The US Food and Drug Administration (FDA) and European Medicines Agency require a comprehensive approach to demonstrating that the proposed biosimilar is highly similar to the reference product, including clinical trial data to assess their immunogenic potential 5, 6, 7. Because subtle differences may exist among protein products manufactured in living cells that can result in different immune responses and clinical effects in patients, evaluating safety and efficacy of LY IGlar compared with IGlar included two phase III, prospective, global, parallel, randomized, clinical trials in patients with type 1 (T1DM) or type 2 diabetes mellitus (T2DM). ELEMENT‐1 was an open‐label study in patients with T1DM that included a 24‐week treatment period for the primary efficacy outcome, and then a 28‐week extension period designed to generate primary data for evaluating immunogenicity after 52 weeks of therapy in patients with T1DM 3. ELEMENT‐2 was a 24‐week double‐blind study in patients with T2DM 4 that provides important supportive evidence of comparative immunogenicity, especially in the subpopulation of insulin‐naïve patients, in whom treatment‐related immune responses may be evaluated without interference from previous exposure to exogenous insulin.
Although similar immunogenicity profiles, including proportions of patients with detectable antibodies, have been reported with LY IGlar and IGlar treatments 3, 4, the present paper presents other immunogenicity‐related findings, such as treatment‐emergent antibody response (TEAR) in patients with T1DM and T2DM and the relationship of antibody levels and TEAR status to clinical outcomes. Findings in the subgroups of patients with T2DM who are insulin‐naïve and those who reported prestudy treatment with IGlar are also provided.
Materials and Methods
Both studies followed the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki 8. The study design and methods for both studies have been previously reported 3, 4. The trials were registered at ClinicalTrials.gov: NCT01421147 and NCT01421459.
Samples for antibody determination were collected before randomization (baseline) and prespecified visits during treatment. Insulin antibody testing was conducted by Millipore (St. Charles, MO, USA). LY IGlar antibodies were quantified as percent binding using a radioimmunoassay where percent binding is the percent of the total amount of radiolabelled tracer (LY IGlar) that coprecipitates with the antibodies. Specificity was determined using excess unlabelled LY IGlar. Cross‐reactivity to human insulin was determined using excess unlabelled insulin. Because of shared epitopes between LY IGlar, IGlar, human insulin and insulin analogues, this anti‐LY IGlar antibody assay also detects antibodies to IGlar, insulin and other insulin analogues. Non‐specific binding ranged from 0 to 0.26% bound/total (or percent binding) using a population of healthy volunteers. The assay's sensitivity was 25 ng/ml using polyclonal affinity‐purified anti‐insulin antibody, satisfying the FDA's recommendation that screening assays be sensitive enough to detect clinically relevant antibody concentrations of 250–500 ng/ml 9. A concentration of 250 ng/ml equated to ∼5% binding in the anti‐LY IGlar antibody assay; therefore, this assay is capable of detecting anti‐LY IGlar and anti‐IGlar antibody levels well below clinically relevant levels.
The proportion of patients with detectable antibodies over time was determined as well as antibody levels as measured by percent binding over time. Analyses of insulin antibody levels (percent binding) included any patient in the full analysis set (FAS), defined as all randomized patients who took ≥ one dose of study medication, with valid antibody testing at baseline and ≥ one postbaseline visit. Further analyses of cross‐reactive insulin antibodies (i.e. anti‐IGlar and anti‐insulin) were conducted to confirm that the immune response to LY IGlar and IGlar were similar with respect to antibodies formed against human insulin. The threshold for the cross‐reactive insulin antibody assay was a 1.06% binding value.
TEAR is a measure of incidence that converts percent binding, a continuous measure, to a dichotomous measure of antibody response at any given timepoint relative to baseline antibody status (non‐detected/detected) and percent binding level. In these studies, TEAR was defined as: (i) for patients who were positive for detectable antibodies at baseline, an absolute increase of at least 1% in percent insulin antibody binding and at least a 30% relative increase in insulin antibody binding from baseline; and (ii) for patients who were negative for insulin antibodies at baseline, a change to a detected insulin antibody binding level of at least 1.26% postbaseline (i.e. 1.26% = 1% + the assay threshold of 0.26%).
The definition noted above of an absolute increase of ≥1% ensures that an absolute meaningful increase must occur near the assay threshold, whereas a ≥30% relative increase from baseline ensures that relative increases at the higher ends of the range are meaningful. Additionally, a 30% relative increase assures identification of a potentially relevant treatment‐emergent change. For patients with non‐detectable antibodies at baseline (i.e. <0.26%), an absolute increase of 1% in insulin antibody level equates to an approximately fourfold increase from baseline, which is a scientifically reasonable margin to account for assay variability 10.
The proportion of patients with detectable antibodies and the proportion of patients with TEAR were compared between treatment groups using Fisher's exact test. Insulin antibody levels were compared between treatments using the Wilcoxon rank sum test.
The potential impact of antibody formation on clinical response was analysed as follows. Relationships between insulin antibody levels and selected efficacy and safety measures [e.g. glycated haemoglobin (HbA1c, %), basal insulin dose (U/kg/day) and total hypoglycaemia rate (events/patients/30 days; blood glucose ≤3.9 mmol/l or ≤70 mg/dl)] were evaluated using scatterplots and analysed using analysis of covariance for the FAS at endpoint [last‐observation‐carried‐forward (LOCF)] and by partial correlations [after adjustment for baseline HbA1c, country, time of basal insulin injection (AM, PM), and sulphonylurea use (ELEMENT‐2 only)]. A significant treatment‐by‐insulin antibody interaction (p < 0.05) indicates a potential differential treatment effect. Relationships between TEAR and selected efficacy and safety measures (e.g. HbA1c, total hypoglycaemia rate, insulin dose and immune‐related adverse events) were also analysed in a similar manner.
Results
Patients
Of the 535 patients in the FAS population (ELEMENT‐1), 532 had a valid antibody testing at baseline and postbaseline, of which 212 (39.8%) had detectable antibodies at any point during the 52‐week treatment period 3. There were 452 patients (84.5%) who reported prestudy treatment with IGlar and 83 (15.5%) who were taking other basal insulin (neutral protamine Hagedorn or determir) at baseline, with fewer patients in the LY IGlar group (81.3%) reporting prestudy treatment with IGlar than in the IGlar group (87.6%). Subgroup analyses based on basal insulin at study entry showed no significant differential treatment effects on safety outcomes (incidence of detectable antibodies, TEAR) for prior IGlar and other prestudy basal insulin subgroups (data not shown); therefore, data on the FAS only for ELEMENT‐1 are presented here.
Of the 756 patients in the FAS population (ELEMENT‐2), 730 had valid antibody testing at baseline and postbaseline, of whom 96 (13.2%) had detectable antibodies at any point during the 24‐week treatment period 4. At study entry, 299 patients (39.6%) comprised the prior IGlar subgroup whereas 457 patients (60.4%) comprised the insulin‐naïve group. In addition to assessing immune responses in the FAS, subgroup analyses for patients with T2DM who were insulin‐naïve [LY IGlar: 221 patients (58.8%); IGlar: 236 patients (62.1%); p = 0.372] and for patients with T2DM who reported prestudy treatment with IGlar [prior IGlar subgroup; LY IGlar: 155 patients (41.2%); IGlar: 144 patients (37.9%); p = 0.372] are presented.
Immunogenicity Outcomes
Total Study Population in ELEMENT‐1 (Type 1 Diabetes)
The proportion of patients with detectable insulin antibodies was similar between the treatment groups. No statistically significant treatment differences were observed at any visit or at endpoint (LOCF) (Figure 1A). Overall incidence, which included all patients with detectable antibodies at any time during the treatment period, was also similar [24 weeks: LY IGlar: 80 patients (30.2%); IGlar: 90 patients (33.7%); p = 0.404; 52 weeks: LY IGlar: 107 patients (40.4%); IGlar: 105 patients (39.3%), p = 0.859]. Median insulin antibody levels were low for both treatment groups, and no significant treatment differences were observed at any visit or at the 52‐week endpoint (LOCF; LY IGlar: 0.92; IGlar: 0.89; p = 0.987; Figure 1B). There were no statistically significant differences between treatment groups in incidence of TEAR at the 52‐week endpoint (LOCF) or overall throughout the 24‐ or 52‐week treatment periods (Figure 1C).
Figure 1.

(A) Proportion of patients with detectable anti‐insulin glargine antibodies in the full analysis set (FAS) population with type 1 diabetes (T1DM). (B) Level of insulin antibodies (percent binding) in patients with detectable antibodies in the T1DM FAS. aData are presented as median + interquartile range. bFive percent binding level in the screening assay approximately equates to 250 ng/ml. Insulin antibody values depicted in the graph are from determinations following screening. (C) Incidence of treatment‐emergent antibody response (TEAR) in the T1DM FAS. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
Subsequent analysis of patients with cross‐reactive antibodies indicated no statistically significant treatment differences in the proportion of patients with cross‐reactive antibodies at any visit (Figure S1), endpoint (LOCF), or overall (52 weeks; Table S1). The median cross‐reactive insulin antibody levels were similar between treatments at all visits (Figure S1) and at the 52‐week endpoint (LOCF; Table S1).
Total Study Population in ELEMENT‐2 (Type 2 Diabetes)
The overall (24 weeks) proportion of patients with T2DM with detectable antibodies was similar between treatment groups [LY IGlar: 56 patients (15.3%); IGlar: 40 patients (11.0%); p = 0.100]. No statistically significant treatment differences were observed at any visit or at endpoint (LOCF), except at week 4 [LY IGlar: 26 patients (7.2%); IGlar: 13 patients (3.6%); p = 0.047; Figure 2A]. No statistically significant treatment differences were observed for median insulin antibody levels at any visit or at the 24‐week endpoint (LOCF; Figure 2B); low median insulin antibody levels were observed up to the 24‐week endpoint (LOCF; LY IGlar: 1.07; IGlar: 0.65; p = 0.331). No statistically significant differences in the incidence of TEAR were observed between the treatment groups at the 24‐week endpoint (LOCF) or overall (Figure 2C).
Figure 2.
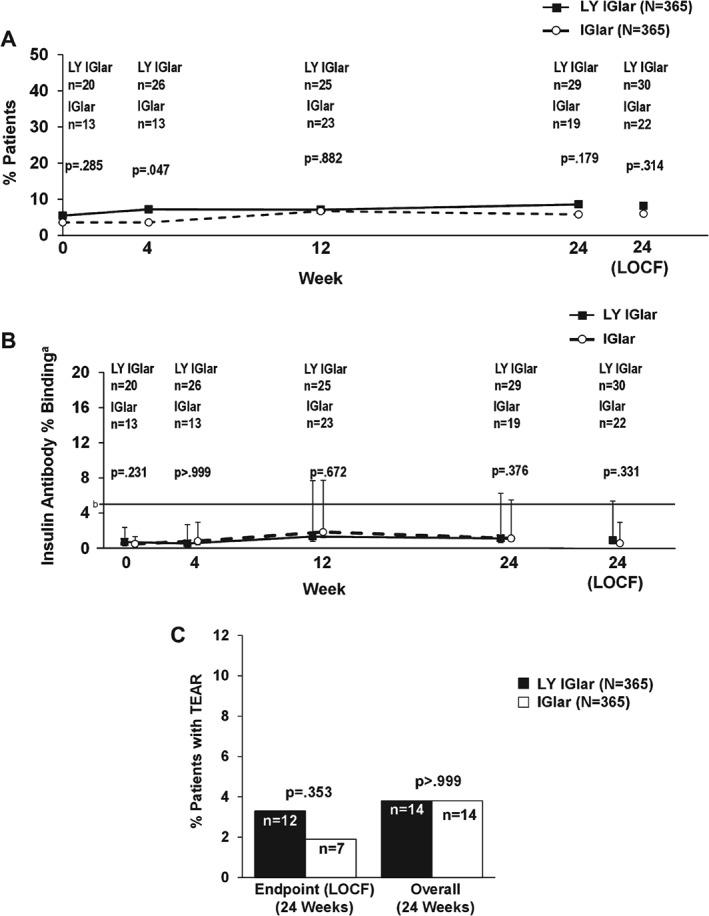
(A) Proportion of patients with detectable anti‐insulin glargine antibodies in the type 2 diabetes (T2DM) total study population. (B) Level of insulin antibodies (percent binding) in patients with detectable antibodies in the T2DM full analysis set (FAS) population. aData are presented as median + interquartile range. bFive percent binding level in the screening assay approximately equates to 250 ng/ml. Insulin antibody values depicted in the graph are from determinations following screening. (C) Incidence of treatment‐emergent antibody response (TEAR) in the T2DM FAS population. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
No statistically significant differences were observed in the proportion of patients with cross‐reactive antibodies at any visit (Figure S2) or at the 24‐week endpoint (LOCF; Table S2). Median cross‐reactive insulin antibody levels were similar between treatments at all visits (Figure S2) and at the 24‐week endpoint (LOCF; Table S2).
Subgroup Analyses Based on Basal Insulin at Study Entry in ELEMENT‐2 (Type 2 Diabetes)
In the insulin‐naïve subgroup of patients with T2DM, the proportion of patients with detectable insulin antibodies was similar in the treatment groups during the 24‐week treatment period (Figure 3A) and overall [LY IGlar: 27 patients (12.6%); IGlar: 29 patients (12.8%); p > 0.999; Figure 3A]. No statistically significant differences were observed in median insulin antibody levels at any visit or at the 24‐week endpoint (LOCF; Figure 3B). No statistically significant treatment differences in the incidence of TEAR were observed at the 24‐week endpoint (LOCF) or overall (Figure 3C).
Figure 3.
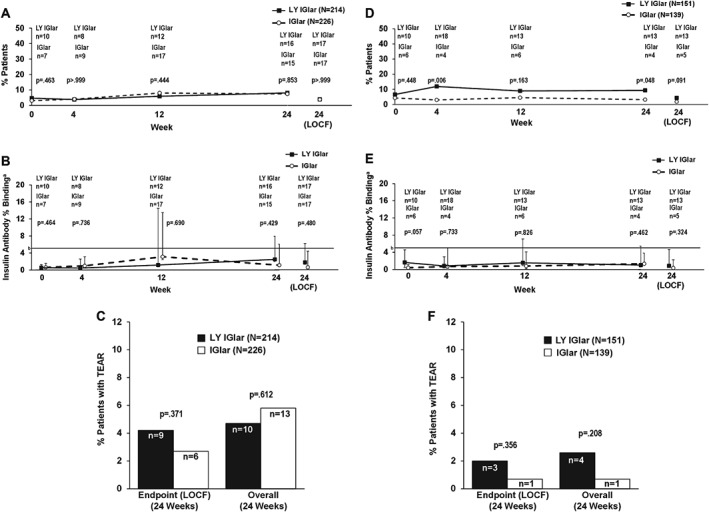
(A) Proportion of patients with detectable antibodies in the type 2 diabetes (T2DM) insulin‐naïve subgroup. (B) Level of insulin antibodies (percent binding) in patients with detectable antibodies in the T2DM insulin‐naïve subgroup. aData are presented as median + interquartile range. bFive percent binding level in the screening assay approximately equates to 250 ng/ml. Insulin antibody values depicted in the graph are from determinations following screening. (C) Incidence of treatment‐emergent antibody response (TEAR) in the T2DM insulin‐naïve subgroup. (D) Proportion of patients with detectable antibodies in the T2DM prior IGlar subgroup. (E) Level of insulin antibodies (percent binding) in patients with detectable antibodies in the T2DM prior IGlar subgroup. aData are presented as median + interquartile range. bFive percent binding level in the screening assay approximately equates to 250 ng/ml. Insulin antibody values depicted in the graph are from determinations following screening. (F) TEAR in the T2DM prior IGlar subgroup. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
In the prior IGlar subgroup, numerically more patients with detectable antibodies at baseline were randomly assigned to LY IGlar [LY IGlar: 10 patients (6.6%); IGlar: six patients (4.3%); p = 0.448], with statistically significant differences noted at weeks 4 and 24 and overall [LY IGlar: 29 patients (19.2%); IGlar: 11 patients (7.9%); p = 0.006], but not at the 24‐week endpoint [LOCF; LY IGlar: 13 patients (8.6%); IGlar: five patients (3.6%); p = 0.091; Figure 3D]. Although median insulin antibody levels were higher for LY IGlar vs IGlar at baseline (1.64 vs 0.35; p = 0.057), these were similar by week 4 and at subsequent visits through the 24‐week endpoint (LOCF; Figure 3E). Few patients developed TEAR, and there were no statistically significant differences in the incidence of TEAR between the groups at the 24‐week endpoint (LOCF) or overall (Figure 3F).
The proportion of patients with cross‐reactive antibodies at any visit (Figure S3) or at endpoint (LOCF) was similar between treatment groups, regardless of basal insulin at study entry (Table S2). Likewise, median levels of cross‐reactive insulin antibodies were similar between the groups at all visits (Figure S3) and at endpoint (LOCF; Table S2).
Relationships between Insulin Antibodies and Clinical Outcomes
In addition to the analyses of immunogenicity measures discussed previously, further analyses were carried out to understand whether TEAR status or insulin antibody levels had any significant effects on clinical outcomes. Figure 4A shows the least‐squares mean (standard error) change from baseline to endpoint (LOCF) by treatment for each clinical outcome [HbA1c (%), basal insulin dose (U/kg/day), and total hypoglycaemia rate (events/patient/30 days)] for patients with T1DM who did or did not develop TEAR during the 52‐week study. Treatment‐by‐TEAR interactions for these clinical outcomes were not statistically significant, indicating no differential treatment effects in patients who did or did not develop TEAR during the 52‐week study. Similarly, no statistically significant treatment‐by‐TEAR interactions were observed for each clinical outcome for patients with T2DM who did or did not develop TEAR during the study (Figure 4B). In both studies, the change in each clinical outcome from baseline to endpoint (LOCF) was similar among treatment groups and across TEAR status at endpoint.
Figure 4.
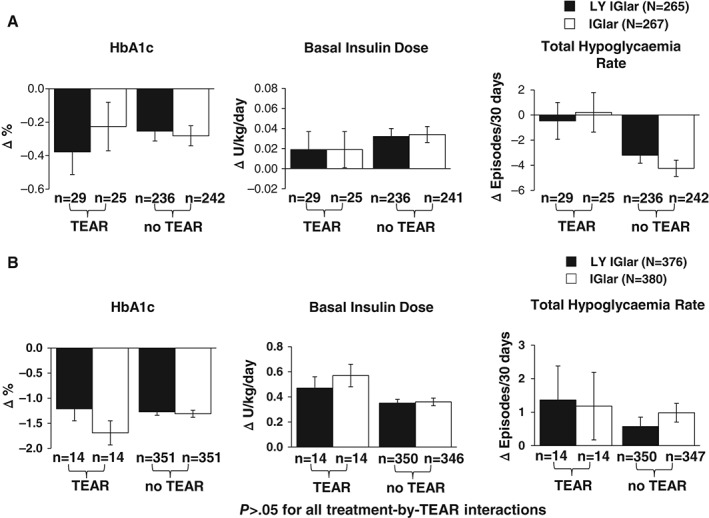
(A) Effect of treatment‐emergent antibody response (TEAR) status on change in clinical outcomes [glycated haemoglobin (HbA1c), basal insulin dose, total hypoglycaemia rate] in the type 1 diabetes (T1DM) full analysis set (FAS) population. (B) Effect of TEAR status on change in clinical outcomes (HbA1c, basal insulin dose, total hypoglycaemia rate) in the type 2 diabetes (T2DM) FAS population. Data are presented as least squares mean ± standard error change from baseline to LOCF endpoint. p > 0.05 for all treatment‐by‐TEAR interactions. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
No significant correlation was seen between endpoint insulin antibody levels and clinical outcomes [HbA1c (%), basal insulin dose (U/kg/day), hypoglycaemia rate (events/patient/30 days)] in either the total study population of patients with T1DM (Figure 5A) or patients with T2DM (Figure 5B).
Figure 5.
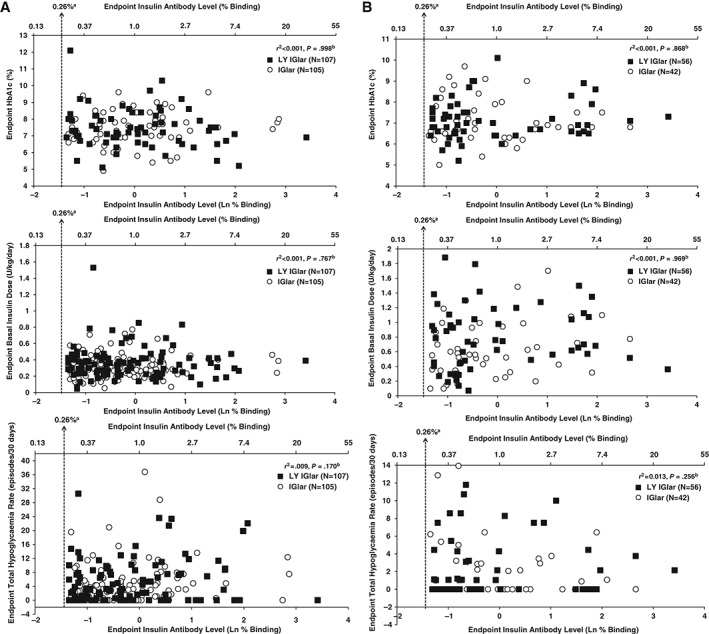
(A) Relationship between antibody level (percent binding) and clinical outcomes [glycated haemoglobin (HbA1c), basal insulin dose, total hypoglycaemia rate] in the type 1 diabetes (T1DM) full analysis set (FAS) population. aQuantitative detection limit of the assay. bPartial correlation measures the relationship between endpoint measure (HbA1c, basal insulin dose, or total hypoglycaemia rate) and endpoint antibody level after adjustment for baseline HbA1c, country, and time of basal injection stratification factors. Only patients with non‐missing endpoint antibody levels and non‐missing baseline value and at least one non‐missing post‐baseline value of the response variable were included in the analysis. (B) Relationship between antibody level (percent binding) and clinical outcomes (HbA1c, basal insulin dose, total hypoglycaemia rate) in the T2DM FAS population. aQuantitative detection limit of the assay. bPartial correlation measures the relationship between endpoint measure (HbA1c, basal insulin dose, or total hypoglycaemia rate) and endpoint antibody level after adjustment for baseline HbA1c, country, time of basal injection (AM, PM), and sulphonylurea use stratification factors. Only patients with non‐missing endpoint antibody levels and non‐missing baseline value and at least one non‐missing post‐baseline value of the response variable were included in the analysis. IGlar, insulin glargine; LY IGlar, LY2963016 insulin glargine.
In both studies, there were no statistically significant treatment‐by‐TEAR interactions for the occurrence of treatment‐emergent adverse events (TEAEs) related to study drug, allergic events, injection site reactions, or serious adverse events (SAEs; Table S3). A significant treatment‐by‐TEAR interaction was observed for patients who experienced ≥1 TEAE in the open‐label ELEMENT‐1 study but was not observed for the double‐blind ELEMENT‐2 study. Closer examination of system organ classes and pertinent individual preferred terms in ELEMENT‐1 showed a statistically significant difference in the incidence of nasopharyngitis, albeit low in both groups [LY IGlar: 6 patients (20.7%); IGlar: 0 patients (0.0%); p = 0.025], but occurrence of TEAEs was otherwise similar between treatment groups in patients with TEAR (Table S4). The incidence of nasopharyngitis in LY IGlar‐treated patients who developed TEAR [six patients (20.7%)] was similar to that in those who did not develop TEAR [LY IGlar: 37 patients (15.5%); IGlar: 45 patients (18.6%)]. Moreover, no IGlar‐treated patients who developed TEAR experienced nasopharyngitis. Evaluation of the timing of these events in relation to TEAR showed no association and no patient who developed TEAR in either treatment group discontinued the study as a result of an adverse event (data not shown). Furthermore, few cases of nasopharyngitis among patients with TEAR were observed in each treatment group in the double‐blind ELEMENT‐2 study (Table S4).
Discussion
Because treatment of patients with therapeutic protein products such as insulin could result in a variety of immune responses ranging from antibody responses without clinical effects to life‐threatening reactions, evaluating the immune response to insulin analogues involves measuring insulin antibody levels and investigating their association with clinical sequelae. Previous insulin studies used assays ranging from ∼5% to at least 20% binding as a clinically relevant level of antibody response 11, 12, 13. We used a sensitive screening assay capable of measuring levels well below the FDA‐recommended antibody concentration threshold for clinically relevant levels of antibody response 9. In both studies, LY IGlar and IGlar both exhibited low levels of insulin antibodies (i.e. <5% binding).
In addition to measuring antibody levels, determining whether significant increases have occurred over time compared with baseline levels is important. TEAR, a measure of immune response indicating a patient's antibody status has changed during the study (postbaseline), was intended to identify a relatively increased level of binding higher than that expected from analytical and biological variability alone (i.e. reflects a real treatment‐related change). The TEAR threshold in patients without detectable antibodies at baseline (≥1.26% binding) was stringent enough to capture potential treatment‐emergent changes in percent binding levels that may be associated with clinical events (<5% binding). In patients with T1DM or T2DM (including insulin‐naïve and prior IGlar subgroups), the proportion of patients with TEAR was similar between both treatment groups.
Because the development of antibody responses can differ in patients with T1DM versus those with T2DM, immunogenicity was studied separately and results are presented separately. As expected, a greater proportion of patients with T1DM (ELEMENT‐1) had detectable antibodies to insulin glargine at baseline and throughout the study relative to patients with T2DM (ELEMENT‐2). This finding is consistent with reports that patients with T1DM, a disease characterized by an autoimmune condition 14, are more likely to have pre‐existing anti‐insulin antibodies 15 and exhibit different immune responses from those with T2DM 16. Within each study, antibody levels (percent binding) were similar between the treatment groups with no statistically significant differences at any visit or at the 52‐week (ELEMENT‐1) or 24‐week (ELEMENT‐2) endpoints in the total study population. The majority of patients [ELEMENT‐1: 320 patients (60.2%); ELEMENT‐2: 634 patients (86.8%)] had no detectable antibodies throughout the studies or changes in total insulin antibody level that ranged from antibody‐negative at baseline to a maximum percent binding value within 0.26–1.26% during the study.
For patients with T1DM, the difference in the proportion of patients with overall detectable antibodies through 52 weeks, but not through 24 weeks, included patients with first on‐treatment detectable antibodies at 52 weeks. Although more LY IGlar‐treated patients had first on‐treatment detectable antibodies than IGlar‐treated patients at 52 weeks, overall percent binding and TEAR for both treatment groups were similar for 0–24 and 24–52 weeks. Moreover, both treatment groups had low (i.e. <5%) and similar median percent insulin binding levels; therefore, the apparent increase in the incidence of detectable antibodies in LY IGlar‐treated patients was not considered to be clinically significant.
Results of subgroup analyses of patients with T2DM based on basal insulin at study entry provide additional details about the similarity of LY IGlar to IGlar. Patients in the insulin‐naïve subgroup, where stronger immune responses to newly administered insulin were expected and not confounded by previous insulin exposure, showed no significant treatment differences in the proportion of patients with detectable antibodies, levels of antibodies, or proportion of patients with TEAR. Findings in the prior IGlar subgroup of patients with T2DM were not as straightforward to interpret as the insulin‐naïve subgroup because of an imbalance in the incidence of detectable antibodies at baseline where more patients allocated to LY IGlar had detectable antibodies at baseline. Although statistically significant treatment differences in the proportion of patients with detectable antibodies were observed at weeks 4 and 24 and overall, actual antibody levels (Figure 3E) at the respective timepoints and the incidence of TEAR were similar between the treatment groups, suggesting the significant differences were probably attributable to chance. Moreover, this finding was not corroborated in patients with T1DM (ELEMENT‐1), a more sensitive population where more patients had prior treatment with IGlar or other insulins and a higher incidence of detectable antibodies.
Because LY IGlar and IGlar have the same primary amino acid sequence, the adaptive immune response should be to the same epitope. Although factors such as glycosylation and impurities in the final preparation may affect the magnitude of the response, antibodies directed against the same epitope on either synthetic insulin will recognize the same epitope on endogenous insulin 15. Analyses of cross‐reactive antibodies in both studies further confirmed the immune response to LY IGlar and IGlar was similar with respect to antibodies formed against human insulin. Clinical evidence from both studies showed similar immune responses in patients with T1DM or T2DM treated with either LY IGlar or IGlar, despite distinct manufacturing processes for these insulin glargine products.
No significant differential treatment effect or relationship was seen between TEAR status or antibody levels and HbA1c, insulin dose, or hypoglycaemia rate in either study, indicating no clinically relevant effect of the observed immune responses on these clinical outcomes. Few and similar incidences of injection site reactions and allergic reactions were noted with LY IGlar and IGlar in both studies, consistent with what has been observed with more recently purified insulin formulations 15. Although our studies did not assess insulin‐neutralizing antibodies, the lack of an association between antibody levels (or incidence of TEAR) and clinical outcomes does not suggest neutralizing effects of anti‐insulin glargine antibodies. The incidence of SAEs and deaths were also similar between LY IGlar and IGlar in both studies 3, 4. The frequency of TEAEs among LY IGlar‐ or IGlar‐treated patients with TEAR in both studies was similar, except for a single preferred term (nasopharyngitis) in patients with T1DM; however, this involved few events, and no temporal association existed between TEAR and the adverse event. Because incidence of nasopharyngitis in the LY IGlar subgroup with TEAR (ELEMENT‐1) was similar to that of LY IGlar‐ or IGlar‐treated patients without TEAR, reported events for IGlar appear to be unusually low in the small subset of TEAR patients. Moreover, the incidence of nasopharyngitis was similar between treatment groups in the double‐blind ELEMENT‐2 study, which suggests the possibility of reporting bias contributing to this isolated finding in the open‐label ELEMENT‐1 study.
In conclusion, the incidence of detectable anti‐insulin glargine antibodies, antibody levels and incidence of TEAR were similar in patients with T1DM or T2DM who received LY IGlar or IGlar. Moreover, levels of antibodies and the incidence of TEAR were low in both treatment groups, and no observed association was seen between clinical outcomes and insulin antibody levels or the incidence of TEAR. Our results add to the evidence supporting similar safety and immunogenicity profiles of LY IGlar and IGlar in patients with T1DM or T2DM and add to the totality of preclinical and clinical data demonstrating similarity of LY IGlar to IGlar.
Conflict of Interest
T. C. B. has served on speaker bureaus for Eli Lilly and Company, Merck, Novo Nordisk, Sanofi‐Aventis, Astra Zeneca, Boehringer‐Ingelheim, Janssen Pharmaceuticals and Medtronic, has received research support from Eli Lilly and Company, Novo Nordisk, Sanofi‐Aventis, Astra Zeneca, Halozyme, Merck, Janssen Pharmaceuticals and Medtronic, and has served on a clinical advisory board for Eli Lilly and Company and Sanofi‐Aventis. P. H. has served on scientific advisory panels for Johnson & Johnson, Merck, Novo Nordisk and Roche Pharmaceuticals. S. V. E. has served as a medical advisor for Eli Lilly and Company, Novo Nordisk and Sanofi. L. L. I., T. C., R. J. K., R. A. O., M. A. D., W. J. H, J. S. Z., R. K. P. and M. J. P. are employees of and hold stock in Eli Lilly and Company.
T. C. B., S. V. E., and P. H. contributed to the interpretation and discussion of the research, participated in conducting the study, and reviewed and edited the manuscript. W. J. H. and T. C. participated in the study design, designed and conducted the statistical analyses, participated in the interpretation and discussion of the research, and in writing the manuscript. L. L. I, R. J. K., R. A. O., M. A. D., J. S. Z., R. K. P. and M. J. P. participated in the study design, in conducting the study, in the data analysis, in the interpretation and discussion of the research, and in writing the manuscript. L. L. I. is the guarantor of this work and, as such takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. E. G. (non‐author) prepared the draft manuscript and provided editorial support.
Supporting information
Table S1. Summary of cross‐reactive antibodies in patients with type 1 diabetes (ELEMENT‐1).
Table S2. Summary of cross‐reactive antibodies in patients with type 2 diabetes (ELEMENT‐2).
Table S3. Relationship between overall treatment‐emergent antibody response status and adverse events.
Table S4. Summary of treatment‐emergent adverse events for patients with type 1 or type 2 diabetes who developed treatment‐emergent antibody response.
Figure S1 . (A) Proportion of patients with cross‐reactive antibodies in the full analysis set (FAS) of patients with type 1 diabetes (T1DM). (B) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the T1DM FAS. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
Figure S2. (A) Proportion of patients with cross‐reactive antibodies in the full analysis set (FAS) of patients with type 2 diabetes (T2DM). (B) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the T2DM FAS. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
Figure S3. (A) Proportion of patients with cross‐reactive antibodies in the insulin‐naive subgroup of patients with type 2 diabetes (T2DM). (B) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the insulin‐naive subgroup of patients with T2DM. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. (C) Proportion of patients with cross‐reactive antibodies in the prior IGlar subgroup of patients with T2DM. (D) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the prior IGlar subgroup of patients with T2DM. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
Acknowledgements
This study was funded by Eli Lilly and Company and Boehringer‐Ingelheim. The authors acknowledge the investigators and patients who participated in the ELEMENT‐1 and ELEMENT‐2 studies and Eileen Girten, MS, of inVentiv Health Clinical, for assistance with the preparation of this manuscript. This work was previously published as an abstract presented at the American Diabetes Association's 74th Scientific Sessions, San Francisco, CA, June 2014 (70‐OR) and the European Association for the Study of Diabetes 50th Annual Meeting, Vienna, Austria, September 2014 (P‐969).
References
- 1. European Medicines Agency . Abasaglar. 2014. Available from URL: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002835/human_med_001790.jsp&mid=WC0b01ac058001d124. Accessed 28 April 2015.
- 2. Eli Lilly and Company Press Release . European Commission grants Lilly and Boehringer Ingelheim's insulin glargine product marketing authorisation in Europe. 2014. Available from URL: http://lilly.mediaroom.com/index.php?s=9042&item=137348. Accessed 18 May 2015.
- 3. Blevins TC, Dahl D, Rosenstock J et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomised controlled trial (The ELEMENT 1 Study). Diabetes Obes Metab 2015; 17: 726–733. [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock J, Hollander P, Bhargava A et al. Similar efficacy and safety with LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin‐naïve or previously treated with insulin glargine: a randomized, double blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab 2015; 17: 734–741. [DOI] [PubMed] [Google Scholar]
- 5. European Medicines Agency . Guideline on non‐clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues. 2015. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184161.pdf. Accessed 29 April 2015.
- 6. Food and Drug Administration . Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. 2015. Available from URL: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed 16 April 2015.
- 7. European Medicines Agency. Committee for Medicinal Products for Human Use . Annex to guideline on similar biological medicinal products containing biotechnology‐derived proteins as active substance: non‐clinical and clinical issues. Guidance on similar medicinal products containing recombinant human soluble insulin. EMEA/CHMP/BMWP/32775/2005. London, 22 February 2006. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003957.pdf. Accessed 28 April 2015.
- 8. World Medical Association Declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277: 925–926. [PubMed] [Google Scholar]
- 9. Food and Drug Administration . Guidance for industry assay development for immunogenicity testing of therapeutic proteins. Draft guidance. 2009. Available from URL: http://www.fda.gov/downloads/Drugs/…/Guidances/UCM192750.pdf. Accessed 16 December 2014.
- 10. Shankar G, Arkin S, Cocea L et al. Assessment and reporting of clinical immunogenicity of therapeutic proteins and peptides—harmonized terminology and tactical recommendations. AAPS J 2014; 16: 658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care 2000; 23: 639–643. [DOI] [PubMed] [Google Scholar]
- 12. Lindholm A, Jensen LB, Home PD, Raskin P, Boehm BO, Råstam J. Immune responses to insulin aspart and biphasic insulin aspart in people with type 1 and type 2 diabetes. Diabetes Care 2002; 25: 876–882. [DOI] [PubMed] [Google Scholar]
- 13. Zinman B, Philis‐Tsimikas A, Cariou B et al. Insulin degludec versus insulin glargine in insulin‐naive patients with type 2 diabetes: a 1‐year, randomized, treat‐to‐target trial (BEGIN Once Long). Diabetes Care 2012; 35: 2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . National diabetes fact sheet. 2011. Available from URL: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed 29 April 2015.
- 15. Fineberg SE, Kawabata TT, Finco‐Kent D, Fountaine RJ, Finch GL, Krasner AS. Immunological responses to exogenous insulin. Endocr Rev 2007; 28: 625–652. [DOI] [PubMed] [Google Scholar]
- 16. Eibl N, Spatz M, Fischer GF et al. Impaired primary immune response in type‐1 diabetes: results from a controlled vaccination study. Clin Immunol 2002; 103: 249–259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of cross‐reactive antibodies in patients with type 1 diabetes (ELEMENT‐1).
Table S2. Summary of cross‐reactive antibodies in patients with type 2 diabetes (ELEMENT‐2).
Table S3. Relationship between overall treatment‐emergent antibody response status and adverse events.
Table S4. Summary of treatment‐emergent adverse events for patients with type 1 or type 2 diabetes who developed treatment‐emergent antibody response.
Figure S1 . (A) Proportion of patients with cross‐reactive antibodies in the full analysis set (FAS) of patients with type 1 diabetes (T1DM). (B) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the T1DM FAS. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
Figure S2. (A) Proportion of patients with cross‐reactive antibodies in the full analysis set (FAS) of patients with type 2 diabetes (T2DM). (B) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the T2DM FAS. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.
Figure S3. (A) Proportion of patients with cross‐reactive antibodies in the insulin‐naive subgroup of patients with type 2 diabetes (T2DM). (B) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the insulin‐naive subgroup of patients with T2DM. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. (C) Proportion of patients with cross‐reactive antibodies in the prior IGlar subgroup of patients with T2DM. (D) Level of cross‐reactive insulin antibodies (percent binding) in patients with detectable antibodies in the prior IGlar subgroup of patients with T2DM. aData are presented as median + interquartile range. b5% binding level in the screening assay approximately equates to 250 ng/mL. Insulin antibody values depicted in the graph are from determinations following screening. IGlar, insulin glargine; LOCF, last‐observation‐carried‐forward (endpoint); LY IGlar, LY2963016 insulin glargine.


