Abstract
Medial septum (MS) plays a critical role in controlling the electrical activity of the hippocampus (HIPP). In particular, theta-rhythmic burst firing of MS neurons is thought to drive lasting HIPP theta oscillations in rats during waking motor activity and REM sleep. Less is known about MS-HIPP interactions in nontheta states such as non-REM sleep, in which HIPP theta oscillations are absent but theta-rhythmic burst firing in subsets of MS neurons is preserved. The present study used Granger causality (GC) to examine the interaction patterns between MS and HIPP in slow-wave sleep (SWS, a nontheta state) and during its short interruptions called microarousals (a transient theta state). We found that during SWS, while GC revealed a unidirectional MS→HIPP influence over a wide frequency band (2–12 Hz, maximum: ∼8 Hz), there was no theta peak in the hippocampal power spectra, indicating a lack of theta activity in HIPP. In contrast, during microarousals, theta peaks were seen in both MS and HIPP power spectra and were accompanied by bidirectional GC with MS→HIPP and HIPP→MS theta drives being of equal magnitude. Thus GC in a nontheta state (SWS) vs. a theta state (microarousal) primarily differed in the level of HIPP→MS. The present findings suggest a modification of our understanding of the role of MS as the theta generator in two regards. First, a MS→HIPP theta drive does not necessarily induce theta field oscillations in the hippocampus, as found in SWS. Second, HIPP theta oscillations entail bidirectional theta-rhythmic interactions between MS and HIPP.
Keywords: hippocampo-septal projection, neural networks, oscillation, synchrony
the overwhelming majority of prior investigations of the relationship between medial septum (MS) and hippocampus (HIPP) focused on the role of MS input in controlling hippocampal activity. However, the MS and the HIPP are reciprocally connected (Raisman 1966); ascending cholinergic (Frotscher and Leranth 1985) and GABAergic (Freund and Antal 1988) projections from MS to HIPP are reciprocated by descending GABAergic projections from HIPP to MS (Toth and Freund 1992). Although the characteristics of the neurons participating in these two-way projections have been studied extensively (Dragoi et al. 1999; Ford et al. 1989; King et al. 1998; Petsche et al. 1962; Sweeney et al. 1992), the mechanisms of their functional interactions are not well understood (Bland 1986; Vertes and Kocsis 1997).
Functional MS-HIPP interactions are state dependent. During theta states, including waking motor activity, REM sleep, and arousals, when HIPP activity is dominated by rhythmic field oscillations in the 4–10 Hz range (Buzsaki 2002), MS neurons fire rhythmic bursts in synchrony with local field potential (LFP) in HIPP (Petsche et al. 1962). Lesion and pharmacological studies further suggest that theta-rhythmic cells in MS are pacemakers of HIPP theta oscillations (Lawson and Bland 1993). To what extent this theta pacemaker hypothesis extends to non-REM sleep remains unclear. Although MS-HIPP interactions in nontheta states have received less attention, it was noted that theta burst firing is preserved in slow-wave sleep (SWS) in a subset of MS neurons (Sweeney et al. 1992) when HIPP theta is absent. Two hypotheses may be put forth to account for this observation. First, necessary theta drive from MS does not reach HIPP in nontheta states. Second, theta drive from MS reaches HIPP, but low HIPP responsiveness to MS input prevents a theta-rhythmic response in HIPP. Accordingly, switch to theta may be induced either by increased synchronization of MS neuron firing, thereby increasing the ascending MS drive over the threshold to force theta in HIPP, or by an increase of HIPP network responsiveness to MS input, thereby generating intrinsic theta and activating a descending theta drive to synchronize the MS theta pacemaker, as suggested by in vitro and modeling studies (Manseau et al. 2008; Wang 2002).
In this study we examined functional MS-HIPP interactions by recording MS unit activity together with HIPP LFP during SWS and its brief interruptions (<10 s) called microarousals (Halasz et al. 1979; Schieber et al. 1971). SWS is a nontheta state that cycles through several stages characterized by different patterns (varying frequency, amplitude, spindles, etc.) of slow wave activity in HIPP. Microarousals, which represent disruptions of slow wave activity and are characterized by transient muscle activation and HIPP theta rhythm, are theta states recurring during these SWS cycles. To assess the directional influences between MS and HIPP, a novel method called nonparametric Granger causality (GC) was applied to the mixed spike field data to decompose the MS-HIPP relationship into its directional components MS→HIPP and HIPP→MS. Power, coherence, and GC were then compared between the theta and nontheta states to assess the differences in the pattern of MS-HIPP interactions.
METHODS
Experimental procedures.
Experiments were performed on male Sprague-Dawley rats (Charles River Laboratories) treated in accordance with National Institutes of Health guidelines. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. Four rats (300–350 g body wt at the time of surgery) were anesthetized with a mixture of ketamine and xylazine (70–80 and 10 mg/kg, respectively, injected intraperitoneally) for implantation of stainless steel wires for recording hippocampal LFP, stainless steel screws for reference, ground, and cortical EEG recording, and multithreaded wires for recording neck muscle activity (EMG). The rat's head was fixed in a stereotaxic frame, and stereotaxic coordinates, antero-posterior (AP), lateral (Lat), and dorsoventral (DV) relative to bregma, were measured according to the atlas of Paxinos and Watson (1986). Surface EEG was recorded over the frontal (AP: +1.0 mm, Lat: 2 mm) and parietal (AP: −6.5 mm, Lat: −2.5 mm) cortex. The hippocampal electrode was placed in the CA1 region (AP: −3.7 mm, Lat: 2.2 mm, DV: −2.5 mm) to record from the upper theta dipole (Buzsaki 2002), oscillating in phase with theta waves in the parietal cortical EEG. For MS unit recording, three tetrodes made of 13-μm nichrome microwires were mounted on individually movable (0.3-mm axial movement per complete turn) microdrives and led into a guide tube placed above the MS (AP +0.5 mm, Lat 0.0 mm, DV −5.0 mm). The tetrodes were moved another 2 mm out of the cannula at the end of the implantation surgery. All wires were led to a 16-pin Omnetics microconnector. The screws, wires, and electrode-microdrive assembly were fixed to the skull with dental acrylic.
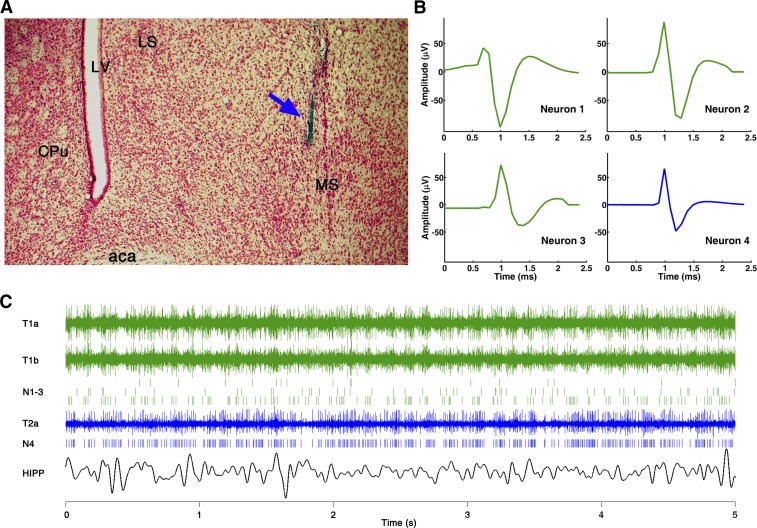
Electrophysiological recordings started after a 7- to 10-day recovery period. Daily recording sessions lasted 2–6 h during the daylight period, in a 26 × 17 × 17-cm recording box. After stable LFP and EMG recordings were attained, the tetrodes were moved slowly into the MS until discriminable unit activities were found. The tetrodes moved through the MS in small steps over several consecutive days. The MS electrode location was marked at the end of the experiment by direct current to generate lesions at several dorsoventral locations, which together with the damage due to the guide cannula served for verification of electrode placement in the MS (Fig. 1A), while the dorsoventral location of individual neurons was estimated using the number of turns of the microdrive. Theta-rhythmic cells were encountered in the MS along the midline in three of four rats; in one rat in which electrode tracks were found more lateral no theta cells were found, and thus this animal was excluded from the analysis.
Fig. 1.
Single-unit recording in the medial septum (MS). A: histological reconstruction of the electrode track (blue arrow) in the MS in 1 experiment. aca, Anterior commissure; CPu, caudal putamen; LS, lateral septum; LV, lateral ventricle. B: waveform of 4 simultaneously recorded units in the MS; 3 units (neurons 1–3, green) were separated from 2 wires of tetrode 1 and the 4th (neuron 4, blue) from a single electrode of tetrode 2 (y-axes use the same scale for all units). C: 3 traces of original recording from tetrode 1 (T1a and T1b) and from tetrode 2 (T2a) in the MS, spike trains of 4 separated units (N1-3 and N4), and hippocampus (HIPP) local field potential (LFP) during a 5-s recording.
The electrical signals were amplified, filtered (LFP: 0.1–100 Hz, EMG: 0.1–3 kHz, units: 600 Hz–3 kHz) and digitally sampled (16-bit, 10 kHz; Neuralynx). MS single neurons were identified and extracted off-line based on their amplitude and waveshape with principal component analysis and k-means clustering algorithms (Spike2, Cambridge Electronic Devices). Units showing a refractory period of 2 ms or higher were considered as single units. The number of simultaneously recorded units varied between 1 and 10 (median 5) (see example in Fig. 1B). SWS periods and microarousal episodes were identified according to standard polysomnographic evaluation criteria based on visual sleep scoring aided by auxiliary signals representing running averages of EMG total power, as well as EEG power in the delta range (1–4 Hz) over the frontal cortex and in the theta range (5–10 Hz) over the parietal cortex and in the hippocampus and the theta-to-delta ratio. Specifically, SWS was characterized by concurrent low EMG activity and high delta and low theta EEG power. Microarousals were short episodes during SWS characterized by abrupt disappearance of large cortical delta waves and concurrent switch of HIPP LFP to theta rhythm and appearance of motor activity on the background of low muscle tone.
Recordings that included SWS and microarousals and had at least one theta-rhythmic single unit in any of the tetrodes were considered for further analysis. All neurons encountered in these recording sites, a total of 79 cells in SWS and 71 cells in microarousal with the 71 cells being a subset of the 79 cells, were then included in the analysis independent of their firing properties. The spike trains of identified MS units along with HIPP LFP signals and markers of sleep stages were transferred to MATLAB for analysis.
Data analysis.
HIPP LFP and MS spike trains were subjected to spectral analysis. Power spectra and MS-HIPP coherence spectra were estimated according to established procedures. MS-HIPP interaction was further decomposed into its directional components, MS→HIPP and HIPP→MS, with a recently proposed nonparametric GC algorithm designed for point processes as well as continuous-valued recordings (Dhamala et al. 2008a, 2008b; Nedungadi et al. 2009). GC is a statistical measure based on the concept of time series forecasting. Specifically, if the current state of a time series is better predicted by incorporating the past knowledge of a second one, the second series is said to have a causal influence on the first. Past work has demonstrated that Granger causal influence can be interpreted in terms of synaptic transmission between neurons and neuronal ensembles (Bollimunta et al. 2008; Ding et al. 2006; Nedungadi et al. 2009). Traditionally, GC is estimated parametrically via autoregressive models of time series data. For spike trains, autoregressive modeling is not directly applicable. A nonparametric framework for estimating GC based on Fourier transforms has been proposed to overcome this problem (Dhamala et al. 2008a, 2008b; Nedungadi et al. 2009). For the present experiment, it consisted of the following steps. First, the continuous recordings were divided into 2-s nonoverlapping epochs that were treated as realizations of an underlying stochastic process. A Kwiatkowski-Phillips-Schmidt-Shin (KSPP) test demonstrated that >99% of the LFP epochs met the stationarity requirement (Kwiatkowski et al. 1992). Second, each epoch was further divided into 1-ms bins where the bin size was chosen such that no more than 1 spike can be found in any bin. Third, HIPP LFP and MS spike train were subjected to separate Fourier transforms; through proper averaging across all the recording epochs within a behavioral state the spectral density matrix was obtained. Fourth, the spectral density matrix was factorized and combined with Geweke's spectral GC formalism to yield MS→HIPP and HIPP→MS in the spectral domain (Ding et al. 2006; Geweke 1982). For statistical analysis, a random permutation procedure was used to generate the significance thresholds for coherence and GC. Specifically, for each neuron, the epoch labels for LFP and the epoch labels for spike train were permuted randomly 1,000 times. Coherence and GC were computed for each of the 1,000 permuted data sets. Null hypothesis distributions were constructed based on these coherence and GC values. Thresholds corresponding to P = 0.01 were determined, and neurons whose coherence or GC was above their respective thresholds were considered statistically significant.
RESULTS
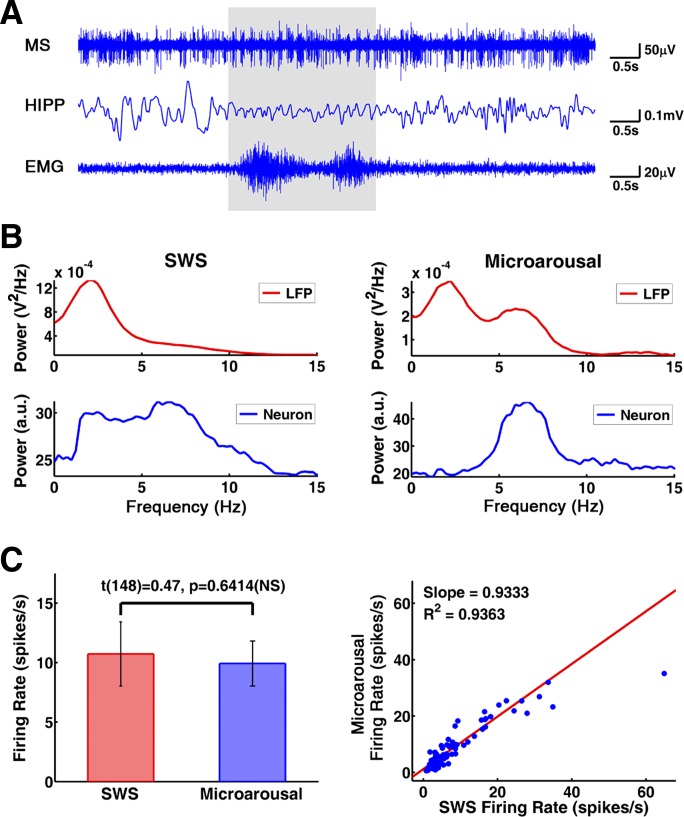
Microarousals were identified and extracted from continuous SWS recordings. These microarousal episodes, characterized by the appearance of HIPP theta and abrupt motor activity appearing on the background of low muscle tone, were brief, usually lasting less than ∼10 s (Fig. 2A); there were no discernible transition periods between SWS and microarousal. A total of 271 microarousal episodes with average length of 7.60 ± 0.58 s extracted from 15 SWS recordings in three rats (data from 1 rat were excluded; see methods) were used for analysis. Power spectra of both MS unit activity and HIPP LFP showed prominent theta peaks in microarousals, whereas delta activity dominated HIPP LFP during SWS (Bland 1986). In MS, spectral power was distributed fairly broadly during SWS, with relatively weak local peaks present in most neurons in the 4–12 Hz range (Fig. 2B). The firing rate of the MS units was in the range reported in earlier studies (Dragoi et al. 1999; Ford et al. 1989; King et al. 1998; Sweeney et al. 1992) and was not significantly different (t[148] = 0.47, P = 0.6414) between SWS (10.73 ± 2.70 spikes/s) and microarousal (9.93 ± 1.89 spikes/s) (Fig. 2C).
Fig. 2.
MS and hippocampal activity during slow-wave sleep (SWS) and microarousals. A: sample recording of MS spikes (top), HIPP LFP (middle), and neck muscle EMG (bottom). Note sudden switch during a short (2.4 s) microarousal (shaded area) from irregular spike train to theta burst in MS, from large amplitude irregular activity to theta waves in HIPP LFP, and from stable, low tone to phasic motor activity in the EMG. B: power spectra of HIPP LFP (top) and MS activity (bottom) during SWS (left) and microarousals (right); power spectra were autoscaled to focus on the pattern. a.u., Arbitrary unit. C: firing rate of MS units in SWS and microarousal. Left: group averages. Right: scatterplot of individual neurons. NS, not significant.
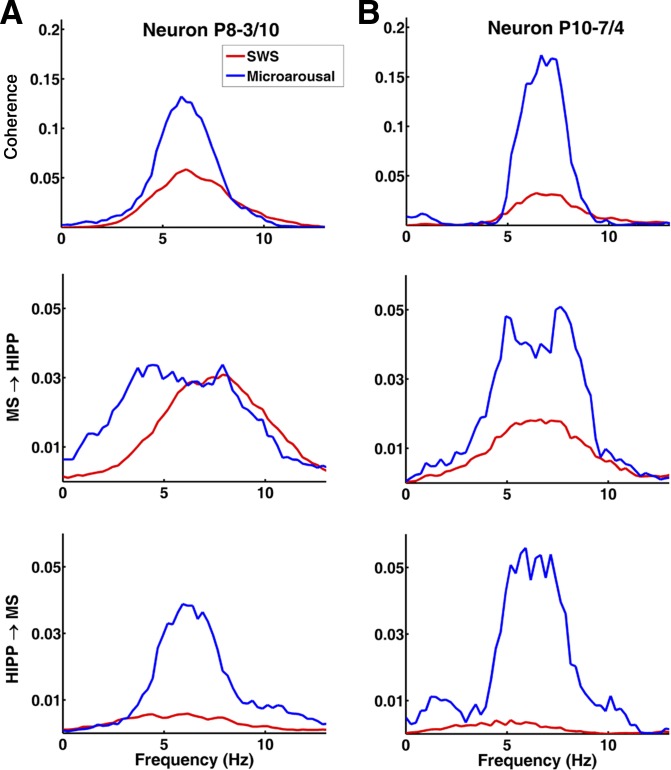
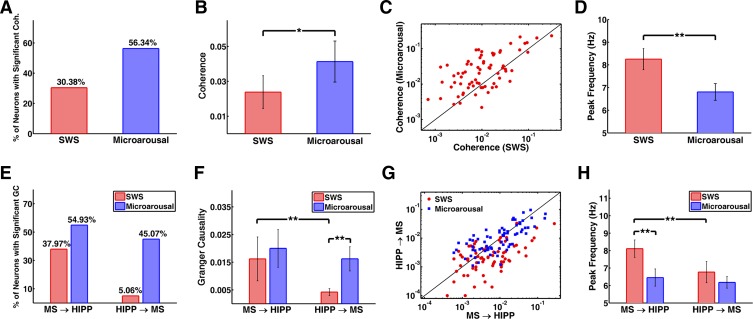
Functional MS-HIPP interactions were investigated by spectral coherence and GC; the latter offers the advantage of characterizing the strength of causal influences in different frequency components. Results for two neurons are demonstrated in Fig. 3; group averages are summarized in Fig. 4. During SWS, MS-HIPP unit-LFP coherence was weak but significant in 24 of 79 (30.38%) neurons (group average coherence: 0.0238 ± 0.0094; Fig. 4, A–C); the peak frequency varied in the range of 3–12 Hz (average 8.25 ± 0.46 Hz; Fig. 4D). In microarousals, MS-HIPP coherence was significant in 56.34% of the neurons, the coherence was significantly higher (0.0413 ± 0.0118, t[148] = −2.30, P = 0.0228), and the peak frequency was concentrated in a narrow frequency band (6–7 Hz; average 6.81 ± 0.37 Hz; Fig. 4D).
Fig. 3.
MS-HIPP relationship in SWS and microarousal in 2 representative neurons (A and B; B is the same neuron presented in Fig. 2A): MS-HIPP coherence (top), MS→HIPP Granger causality (GC) (middle), and HIPP→MS GC (bottom) during SWS and microarousal. Note dramatically increased HIPP→MS theta GC in both neurons during microarousals, reaching the level of MS→HIPP GC; in contrast, MS→HIPP GC is associated with no change (A) or moderate change (B). Note also the similarity between the frequency distribution (shape of the spectra) of coherence and HIPP→MS GC in microarousal, in contrast to MS→HIPP during SWS, which extends over a wider band.
Fig. 4.
Coherence and GC of MS unit and HIPP LFP activity. A–D: comparison of MS-HIPP coherence in SWS and microarousals. A: % of neurons with significant coherence. B: group averages of coherence. C: coherence of individual neurons. D: group averages of peak frequency of coherence in different states. E–H: comparison of MS→HIPP and HIPP→MS GC in SWS and microarousal. E: % of neurons with significant GC. F: group averages of GC. G: GC of individual neurons. H: group averages of peak frequency of GC in different states. Significance: *P < 0.05, **P < 0.005.
GC during SWS indicated a nearly unidirectional MS→HIPP drive in which MS neuronal activity affected HIPP LFP (group average: 0.0162 ± 0.0079) at frequencies within the theta range (see examples in Fig. 3), but the firing activity of these neurons was much less affected by HIPP activity (HIPP→MS GC = 0.0042 ± 0.0013; comparison of GC in the 2 directions shows that MS→HIPP is significantly greater than HIPP→MS: t[156] = −2.93, P = 0.0039; Fig. 4, E–G). During microarousals, the interaction became bidirectional, with the advent of a strong descending HIPP→MS GC component (0.0162 ± 0.0044; the increase was significant: t[148] = −5.40, P < 0.0001). In contrast, MS→HIPP GC in microarousal (0.0200 ± 0.0068) did not change significantly compared with that in SWS (t[148] = −0.70, P = 0.4820); moreover, in microarousal, HIPP→MS and MS→HIPP GC no longer differed (t[140] = −0.92, P = 0.3614). Furthermore, in microarousal the peak frequency of MS→HIPP GC spectra shifted to lower frequencies around the theta peak, with maxima either at ∼4 Hz or at ∼8 Hz (group average: 6.46 ± 0.50 Hz; Fig. 4H), whereas the shape of HIPP→MS GC was similar to theta components in the power spectra and in coherence function (Fig. 3) with peak at the same frequency (6.18 ± 0.33 Hz; Fig. 4H).
In individual neurons, if MS→HIPP GC was significant in SWS it remained significant in microarousal as well, although the GC value could either increase or decrease compared with SWS (Fig. 5A). In microarousal, there was further recruitment of MS neurons from the pool showing insignificant MS→HIPP in SWS (Fig. 5A, red dots). Recruitment was substantially stronger for MS neurons receiving the descending HIPP→MS drive; almost half of the MS population (45.07%) showed significant HIPP→MS GC in microarousal, whereas very few neurons (5.06%) showed HIPP→MS GC in SWS (Fig. 4E).
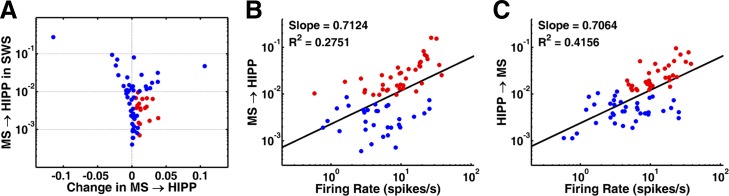
Fig. 5.
Further characterization of GC. A: change in MS→HIPP GC after transitioning from SWS to microarousal, relative to GC in SWS. Each dot represents 1 neuron; red dots represent neurons in which MS→HIPP GC was significant in microarousal but not in SWS. B and C: relationship between firing rate and GC during microarousal. B: MS→HIPP and firing rate. C: HIPP→MS and firing rate. Red dots represent neurons with significant GC.
Furthermore, both MS→HIPP (Fig. 5B) and HIPP→MS (Fig. 5C) GC positively correlated with the neurons' firing rate, indicating that fast-firing neurons were more likely engaged in MS-HIPP theta synchrony. Although identification of the type of neurons is impossible when using extracellular recordings, it was suggested earlier, based on spike shape and firing rate analysis (King et al. 1998; Matthews and Lee 1991), that rhythmic theta bursting activity is the property of fast-firing GABAergic neurons in the MS.
DISCUSSION
The present study examined the causal interactions between MS and HIPP within the theta frequency band in SWS and its short, waking interruptions, called microarousals. A novel nonparametric GC method was applied to decompose the MS-HIPP synchrony into its directional components (Dhamala et al. 2008a, 2008b; Nedungadi et al. 2009). Because our data consisted of mixed spike field recordings, time-domain GC methods developed specifically for spike trains are difficult to apply (Kim et al. 2011), whereas our GC method, formulated in the frequency domain, overcomes this limitation.
The main finding is that there is a significant unidirectional MS→HIPP influence over a wide band (2–10 Hz) in SWS that switches to bidirectional theta drive during microarousals, with MS→HIPP and HIPP→MS GC being of equal magnitude. Unidirectional MS→HIPP influence in SWS was accompanied by significant MS-HIPP coherence (max at 8.25 ± 0.46 Hz) but no theta peak in HIPP power spectra. In microarousal, a rise in HIPP→MS close to the level of the MS→HIPP drive appeared together with elevated, sharp theta coherence and strong theta power in both structures. These findings are in agreement with predictions of a computational model (Wang 2002), indicating that even though MS neurons possess the membrane machinery to generate rhythmic firing in the theta range (Serafin et al. 1996), robust theta synchronization only emerges with the addition of a second GABAergic population, which in the present case is the HIPP GABAergic network projecting back to the MS.
Theta burst firing in single MS units co-occurring with nontheta HIPP LFP was observed in the very first reports on MS theta activity (Petsche et al. 1962). A detailed, quantitative account of such MS cells by Sweeney (1992) documented strong burst firing, equivalent to that in theta states, in 8% of MS neurons during SWS in head-restrained rats and in 20% during nontheta state under urethane anesthesia. Comparable, systematic investigations of this problem in freely moving rats have been lacking, as most studies have focused on theta states (Dragoi et al. 1999; Ford et al. 1989; King et al. 1998) or on spike wave ripple associations in SWS (Dragoi et al. 1999; Jinno et al. 2007; Vandecasteele et al. 2014). Weaker or transient theta-rhythmic MS unit autocorrelograms have been frequently presented, however (Alonso et al. 1987; Apartis et al. 1998; Dragoi et al. 1999; Dutar et al. 1995; Macadar et al. 1970; Ranck 1976).
These findings from nontheta states have been interpreted in the framework of the MS theta pacemaker hypothesis by contending that the lack of effect of MS theta rhythm on HIPP LFP was due to either a lack of synchrony or a weak ascending compound theta signal resulting from a reduced number of burst-firing MS neurons. The present investigation quantified, for the first time, the effect of MS input on HIPP LFP and found detectable, i.e., significant (see methods), MS→HIPP GC in 38% of MS neurons during SWS. Critically, on group average, the MS→HIPP GC value during SWS was not significantly below the level of MS→HIPP GC during microarousals, with the latter being accompanied by strong theta LFP in the hippocampus. Thus weak “subthreshold” MS-to-HIPP input is not sufficient to explain the lack of theta LFP during SWS. On the other hand, distribution of GC over a wide frequency range in individual neurons and substantial variability across neurons indicate that a lack of a synchronized ascending theta signal generated by MS network may underlie the absence of HIPP theta.
Striking differences between SWS and microarousals were observed, however, at the level of theta influence in the opposite direction carried by the descending HIPP→MS GABAergic pathway. Activation of descending HIPP→MS theta drive during microarousal did not change the magnitude of MS→HIPP. Rather, it led to more regular rhythmic MS bursts, sharpened the MS→HIPP GC spectra, and shifted peak frequency in the MS→HIPP GC spectra to ∼6 Hz, to synchronize with the peak frequency in HIPP→MS GC spectra. These results are consistent with the role of GABAergic input in enhancing MS neuronal synchrony (Wang 2002). They also suggest the possibility of an arousal-dependent mechanism controlling MS-HIPP theta coupling. Under a low-arousal state such as SWS, MS theta drive to HIPP is gated out, as evidenced by the significant MS→HIPP drive and a lack of HIPP theta activity during SWS. Under a higher-arousal state such as microarousal, the HIPP theta oscillator activates and becomes responsive to MS theta drive, resulting in HIPP theta activity and the subsequent HIPP→MS GABAergic drive that in turn affects MS synchrony and firmly establishes the MS-HIPP theta network.
The specific mechanism of the HIPP gating operation is not known. Arousal involves activation of several subcortical, aminergic and cholinergic, neurotransmitter systems that are not directly involved in rhythm generation but exert an important modulatory effect on oscillating neural networks in HIPP. Wake-inducing histaminergic and norepinephrinergic neurons densely innervate all sectors of the hippocampus and were shown to modify theta by acting directly on their respective receptors in HIPP (Hajos et al. 2003; Masuoka and Kamei 2007). Systemic increase of histamine and norepinephrine release was also shown to enhance theta activity (Hajos et al. 2008; Kocsis et al. 2007) either by directly modifying the HIPP network or by indirectly through activation of MS cholinergic neurons (Gorelova and Reiner 1996) leading to increased acetylcholine release in HIPP (Bacciottini et al. 2002). While cholinergic transmission from MS to HIPP is too slow to directly drive theta, cholinergic tone enhances theta activity. Selective lesion of MS cholinergic neurons, sparing MS GABAergic neurons, does not affect theta frequency but dramatically reduces theta amplitude (Apartis et al. 1998; Lee et al. 1994). As shown recently with optogenetic stimulation (Dannenberg et al. 2015; Vandecasteele et al. 2014), the primary effect of the activation of MS cholinergic neurons on HIPP LFP is a strong suppression at delta and 10–25 Hz frequencies surrounding the theta band that leads, indirectly, to increased theta coherence and theta-to-delta ratio. The effect was larger in SWS than in waking and even larger under urethane anesthesia.
In contrast, selective optogenetic stimulation of parvalbumin-expressing GABAergic neurons in the MS had its largest effect on HIPP LFP at ∼10 Hz (Dannenberg et al. 2015). When recruited by cholinergic activation, this group of MS cells fire in synchrony with HIPP theta at lower frequencies. Although identification of the type of neurons is impossible when using extracellular recordings, the rate and characteristics of firing (King et al. 1998; Matthews and Lee 1991) suggest that the neurons engaged in theta-rhythmic firing in our study were GABAergic. Thus the shift in the MS→HIPP GC peak frequency from high theta in SWS to lower theta band when HIPP theta is present (in this study) might be analogous to the 15 Hz to 4 Hz shift in their effect on HIPP LFP (Dannenberg et al. 2015) when activated under urethane either selectively or recruited by cholinergic neurons acting on both MS and HIPP networks.
Several other lines of evidence further suggest that brief activation of these neurotransmitters may be responsible for microarousals. Activation of cortically projecting cholinergic neurons in the nucleus basalis via local microinfusion of histamine (Luo and Leung 2009) or norepinephrine (Pillay et al. 2011) leads to brief episodes of cortical electroencephalographic (delta suppression) and behavioral (spontaneous head and limb movements, sporadic crawling) emergence from light anesthesia that resembled microarousals observed in natural sleep (Halasz et al. 1979; Schieber et al. 1971). Proarousal features of a subset of slow-firing, putative cholinergic, MS neurons were also demonstrated in freely behaving rats (Zhang et al. 2011). These cells showed rapid response (15–30 ms) to auditory stimuli and were activated during transient (1.5–5 s) arousal epochs in SWS associated with small-amplitude irregular HIPP activity (Jackson et al. 2008; Jarosiewicz et al. 2002). Interestingly, during theta states the firing of these neurons followed, rather than led, HIPP theta oscillations, leading to the hypothesis that they were driven by the descending hippocamposeptal input (Toth and Freund 1992). The effect of descending theta drive on single-unit burst firing was also shown under urethane anesthesia in response to brief (10 s) sensory-induced (tail pinch) arousal in subsets of neurons in the MS (Hangya et al. 2009) and posterior hypothalamus (Kocsis et al. 1999; Kocsis and Kaminski 2006).
An important limitation of this study is related to the issue of the topography of projections to the HIPP arising from the MS and the fact that the phase and coherence of theta oscillations systematically change across the septotemporal axis (Long et al. 2015; Penley et al. 2012). This raises the possibility that cells recorded at different locations within the MS that project differentially across the septotemporal axis may not have their strongest relationship to theta recorded at the single septal HIPP recording site, but instead maintain a strong causal relationship with theta oscillations generated at more temporal levels. Further uncertainty may be related to the HIPP LFP recorded in one single location. LFPs are usually considered to reflect synaptic currents in the immediate vicinity of the electrode, although more distant sources may also have significant contributions through volume conduction (Kajikawa and Schroeder 2015). Low-frequency components have relatively larger spatial reach (Leski et al. 2013), and thus the variability in the contribution of different hippocampal regions could certainly have contributed to the results.
GRANTS
This work was supported by National Institute of Mental Health Grant R01 MH-100820.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.K., M.D., I.T., L.S., and B.K. analyzed data; D.K., M.D., and B.K. interpreted results of experiments; D.K. and B.K. prepared figures; D.K., M.D., and B.K. edited and revised manuscript; D.K., M.D., I.T., L.S., and B.K. approved final version of manuscript; M.D., I.T., and B.K. conception and design of research; M.D. and B.K. drafted manuscript; I.T., L.S., and B.K. performed experiments.
REFERENCES
- Alonso A, Gaztelu JM, Buno W Jr, Garcia-Austt E. Cross-correlation analysis of septohippocampal neurons during theta-rhythm. Brain Res 413: 135–146, 1987. [DOI] [PubMed] [Google Scholar]
- Apartis E, Poindessous-Jazat FR, Lamour YA, Bassant MH. Loss of rhythmically bursting neurons in rat medial septum following selective lesion of septohippocampal cholinergic system. J Neurophysiol 79: 1633–1642, 1998. [DOI] [PubMed] [Google Scholar]
- Bacciottini L, Passani MB, Giovannelli L, Cangioli I, Mannaioni PF, Schunack W, Blandina P. Endogenous histamine in the medial septum-diagonal band complex increases the release of acetylcholine from the hippocampus: a dual-probe microdialysis study in the freely moving rat. Eur J Neurosci 15: 1669–1680, 2002. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26: 1–54, 1986. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. J Neurosci 28: 9976–9988, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron 33: 325–340, 2002. [DOI] [PubMed] [Google Scholar]
- Dannenberg H, Pabst M, Braganza O, Schoch S, Niediek J, Bayraktar M, Mormann F, Beck H. Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J Neurosci 35: 8394–8410, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamala M, Rangarajan G, Ding M. Analyzing information flow in brain networks with nonparametric Granger causality. Neuroimage 41: 354–362, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamala M, Rangarajan G, Ding M. Estimating Granger causality from Fourier and wavelet transforms of time series data. Phys Rev Lett 100: 018701, 2008b. [DOI] [PubMed] [Google Scholar]
- Ding M, Chen Y, Bressler SL. Granger causality: basic theory and applications to neuroscience. In: Handbook of Time Series Analysis, edited by Schelter B, Winterhalder M, Timmer J. Weinheim, Germany: Wiley-VCH, 2006, p. 437–460. [Google Scholar]
- Dragoi G, Carpi D, Recce M, Csicsvari J, Buzsaki G. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J Neurosci 19: 6191–6199, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev 75: 393–427, 1995. [DOI] [PubMed] [Google Scholar]
- Ford RD, Colom LV, Bland BH. The classification of medial septum-diagonal band cells as theta-on or theta-off in relation to hippocampal EEG states. Brain Res 493: 269–282, 1989. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336: 170–173, 1988. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol 239: 237–246, 1985. [DOI] [PubMed] [Google Scholar]
- Geweke J. Measurement of linear dependence and feedback between multiple time series. J Am Stat Assoc 77: 303–313, 1982. [Google Scholar]
- Gorelova N, Reiner PB. Histamine depolarizes cholinergic septal neurons. J Neurophysiol 75: 707–714, 1996. [DOI] [PubMed] [Google Scholar]
- Hajos M, Hoffmann WE, Robinson DD, Yu JH, Hajos-Korcsok E. Norepinephrine but not serotonin reuptake inhibitors enhance theta and gamma activity of the septo-hippocampal system. Neuropsychopharmacology 28: 857–864, 2003. [DOI] [PubMed] [Google Scholar]
- Hajos M, Siok CJ, Hoffmann WE, Li S, Kocsis B. Modulation of hippocampal theta oscillation by histamine H3 receptors. J Pharmacol Exp Ther 324: 391–398, 2008. [DOI] [PubMed] [Google Scholar]
- Halasz P, Kundra O, Rajna P, Pal I, Vargha M. Micro-arousals during nocturnal sleep. Acta Physiol Acad Sci Hung 54: 1–12, 1979. [PubMed] [Google Scholar]
- Hangya B, Borhegyi Z, Szilagyi N, Freund TF, Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. J Neurosci 29: 8094–8102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Dickson CT, Bland BH. Median raphe stimulation disrupts hippocampal theta via rapid inhibition and state-dependent phase reset of theta-related neural circuitry. J Neurophysiol 99: 3009–3026, 2008. [DOI] [PubMed] [Google Scholar]
- Jarosiewicz B, McNaughton BL, Skaggs WE. Hippocampal population activity during the small-amplitude irregular activity state in the rat. J Neurosci 22: 1373–1384, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsaki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci 27: 8790–8804, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa Y, Schroeder CE. Generation of field potentials and modulation of their dynamics through volume integration of cortical activity. J Neurophysiol 113: 339–351, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Putrino D, Ghosh S, Brown EN. A Granger causality measure for point process models of ensemble neural spiking activity. PLoS Comput Biol 7: e1001110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Recce M, O'Keefe J. The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. Eur J Neurosci 10: 464–477, 1998. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Bragin A, Buzsaki G. Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis. J Neurosci 19: 6200–6212, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Kaminski M. Dynamic changes in the direction of the theta rhythmic drive between supramammillary nucleus and the septohippocampal system. Hippocampus 16: 531–540, 2006. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Li S, Hajos M. Behavior-dependent modulation of hippocampal EEG activity by the selective norepinephrine reuptake inhibitor reboxetine in rats. Hippocampus 17: 627–633, 2007. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D, Phillips PC, Schmidt P, Shin Y. Testing the null hypothesis of stationarity against the alternative of a unit root. J Econ 54: 159–178, 1992. [Google Scholar]
- Lawson VH, Bland BH. The role of the septohippocampal pathway in the regulation of hippocampal field activity and behavior: analysis by the intraseptal microinfusion of carbachol, atropine, and procaine. Exp Neurol 120: 132–144, 1993. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience 62: 1033–1047, 1994. [DOI] [PubMed] [Google Scholar]
- Leski S, Linden H, Tetzlaff T, Pettersen KH, Einevoll GT. Frequency dependence of signal power and spatial reach of the local field potential. PLoS Comput Biol 9: e1003137, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LL, Bunce JG, Chrobak JJ. Theta variation and spatiotemporal scaling along the septotemporal axis of the hippocampus. Front Syst Neurosci 9: 37, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology 111: 725–733, 2009. [DOI] [PubMed] [Google Scholar]
- Macadar O, Roig JA, Monti JM, Budelli R. The functional relationship between septal and hippocampal unit activity and hippocampal theta rhythm. Physiol Behav 5: 1443–1449, 1970. [DOI] [PubMed] [Google Scholar]
- Manseau F, Goutagny R, Danik M, Williams S. The hippocamposeptal pathway generates rhythmic firing of GABAergic neurons in the medial septum and diagonal bands: an investigation using a complete septohippocampal preparation in vitro. J Neurosci 28: 4096–4107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka T, Kamei C. Role of hippocampal H1 receptors in radial maze performance and hippocampal theta activity in rats. Brain Res Bull 73: 231–237, 2007. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Lee WL. A comparison of extracellular and intracellular recordings from medial septum/diagonal band neurons in vitro. Neuroscience 42: 451–462, 1991. [DOI] [PubMed] [Google Scholar]
- Nedungadi AG, Rangarajan G, Jain N, Ding M. Analyzing multiple spike trains with nonparametric Granger causality. J Comput Neurosci 27: 55–64, 2009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney, Australia: Academic, 1986. [Google Scholar]
- Penley SC, Hinman JR, Sabolek HR, Escabi MA, Markus EJ, Chrobak JJ. Theta and gamma coherence across the septotemporal axis during distinct behavioral states. Hippocampus 22: 1164–1175, 2012. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf C, Gogolak G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells.] Electroencephalogr Clin Neurophysiol 14: 202–211, 1962. [DOI] [PubMed] [Google Scholar]
- Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology 115: 733–742, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G. The connexions of the septum. Brain 89: 317–348, 1966. [DOI] [PubMed] [Google Scholar]
- Ranck JB. Behavioural correlates and firing repertoires of neurons in septal nuclei in unrestrained rats. In: The Septal Nuclei, edited by DeFrance JF. New York: Plenum, 1976, p. 423–462. [Google Scholar]
- Schieber JP, Muzet A, Ferriere PJ. [Phases of spontaneous transitory activation during normal sleep in humans.] Arch Sci Physiol (Paris) 25: 443–465, 1971. [PubMed] [Google Scholar]
- Serafin M, Williams S, Khateb A, Fort P, Muhlethaler M. Rhythmic firing of medial septum non-cholinergic neurons. Neuroscience 75: 671–675, 1996. [DOI] [PubMed] [Google Scholar]
- Sweeney JE, Lamour Y, Bassant MH. Arousal-dependent properties of medial septal neurons in the unanesthetized rat. Neuroscience 48: 353–362, 1992. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience 49: 793–805, 1992. [DOI] [PubMed] [Google Scholar]
- Vandecasteele M, Varga V, Berenyi A, Papp E, Bartho P, Venance L, Freund TF, Buzsaki G. Optogenetic activation of septal cholinergic neurons suppresses sharp wave ripples and enhances theta oscillations in the hippocampus. Proc Natl Acad Sci USA 111: 13535–13540, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81: 893–926, 1997. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Pacemaker neurons for the theta rhythm and their synchronization in the septohippocampal reciprocal loop. J Neurophysiol 87: 889–900, 2002. [DOI] [PubMed] [Google Scholar]
- Zhang H, Lin SC, Nicolelis MA. A distinctive subpopulation of medial septal slow-firing neurons promote hippocampal activation and theta oscillations. J Neurophysiol 106: 2749–2763, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]