Abstract
Normal-hearing human listeners and a variety of studied animal species localize sound sources accurately in reverberant environments by responding to the directional cues carried by the first-arriving sound rather than spurious cues carried by later-arriving reflections, which are not perceived discretely. This phenomenon is known as the precedence effect (PE) in sound localization. Despite decades of study, the biological basis of the PE remains unclear. Though the PE was once widely attributed to central processes such as synaptic inhibition in the auditory midbrain, a more recent hypothesis holds that the PE may arise essentially as a by-product of normal cochlear function. Here we evaluated the PE in a unique human patient population with demonstrated sensitivity to binaural information but without functional cochleae. Users of bilateral cochlear implants (CIs) were tested in a psychophysical task that assessed the number and location(s) of auditory images perceived for simulated source-echo (lead-lag) stimuli. A parallel experiment was conducted in a group of normal-hearing (NH) listeners. Key findings were as follows: 1) Subjects in both groups exhibited lead-lag fusion. 2) Fusion was marginally weaker in CI users than in NH listeners but could be augmented by systematically attenuating the amplitude of the lag stimulus to coarsely simulate adaptation observed in acoustically stimulated auditory nerve fibers. 3) Dominance of the lead in localization varied substantially among both NH and CI subjects but was evident in both groups. Taken together, data suggest that aspects of the PE can be elicited in CI users, who lack functional cochleae, thus suggesting that neural mechanisms are sufficient to produce the PE.
Keywords: sound localization, precedence effect, cochlear implants
sound source localization subserves predator avoidance, prey capture, situational awareness, and, in humans and many other species, communication (for recent reviews see Grothe et al. 2010; Stecker and Gallun 2012). Although acoustic cues to sound location are degraded in many environments by reflections and reverberation, localization accuracy is generally robust across environments. This finding is often attributed to the “precedence effect” (PE), a well-known auditory phenomenon in which observers respond to the cues carried by the first-arriving sound, traveling directly from the source to the ears, rather than spurious cues carried by later-arriving signal reflections (Wallach et al. 1949; for review see Brown et al. 2015; Litovsky et al. 1999). The PE, conventionally studied with simulated echoes in a laboratory setting, consists of two primary phenomena termed fusion and localization dominance. Fusion refers to the perception of early-arriving (leading) and late-arriving (lagging) signals as a single sound source, occurring when the lead-lag delay (LLD) is less than the echo threshold, roughly 5–50 ms depending on a variety of stimulus and paradigm factors. Localization dominance refers to the dominance of the perceived source location by the spatial cues, especially the interaural time difference (ITD), carried by the leading sound (see Brown et al. 2015). Thus, provided the delay between two or more successive similar or identical sounds is sufficiently brief, a single sound is perceived near the location cued by the leading sound (Wallach et al. 1949). In addition to providing for accurate sound source localization, the PE is thought to aid sound identification in reverberant environments, including (in humans) speech intelligibility (e.g., Brandewie and Zahorik 2010; Watkins 2005). The PE is a thus a key facility of normal hearing.
While the basic features of the psychophysical PE have been described for more than half a century, the biological mechanisms of the PE remain obscure. Electrophysiological recordings from central auditory structures in a variety of mammalian and avian species over the past two decades have demonstrated reduced neural responses to simulated echoes (e.g., Keller and Takahashi 1996; Litovsky and Yin 1998; Tollin et al. 2004; Yin 1994), but it has often been unclear to what extent such reductions reflected central processes such as synaptic inhibition (e.g., Burger and Pollak 2001; Pecka et al. 2007; Xia et al. 2010) vs. more peripheral processes.
A recent hypothesis holds that persistent resonance of the auditory epithelium itself (in mammals, the basilar membrane of the cochlea) after signal onset leads to degraded transduction of the lagging signal in each ear, precluding brain stem encoding or salient perception of lagging ITD information (Bianchi et al. 2013; cf. Hartung and Trahiotis 2001; Tollin 1998). While this “cochlear mechanical” hypothesis is appealing for its parsimony given the conservation of inner ear function across species, focal lesions (Litovsky et al. 2002) and pharmacological manipulations (Burger and Pollak 2001; Pecka et al. 2007) of auditory midbrain structures appear to alter the PE specifically. However, a majority of neurophysiological PE studies have used barbiturate anesthetics, which augment synaptic inhibition, clouding the interpretation of reported data (Song et al. 2011; see Brown et al. 2015). Thus data that rigidly dissociate central from peripheral contributions to the PE are lacking.
In the present study we aimed to dissociate cochlear-mechanical from central/neuronal contributions to the PE by measuring the effect in a unique human patient population with demonstrated binaural sensitivity but without functional cochleae. Subjects were adult users of bilateral cochlear implants (CIs), auditory prosthetic devices that bypass the inner ear to stimulate auditory nerve fibers directly with electrical impulses (for review see Wilson and Dorman 2008). One report (Kerber and Seeber 2013) suggested that bilateral CI users with relatively good ITD sensitivity were able to localize sound sources in a simulated reverberant environment nearly as well as in an anechoic environment, suggesting that the PE might be functional in at least some CI users (see also Agrawal 2008; van Hoesel 2007). We report that, given appropriate stimulation, bilateral CI subjects with ITD sensitivity exhibited aspects of the PE similar to normal-hearing (NH) listeners, suggesting that neural mechanisms are sufficient to produce the PE and moreover that provision of ITD information may restore perceptual benefits of the PE among some individuals in this growing clinical population.
MATERIALS AND METHODS
All study procedures complied with ethical guidelines set forth by the National Institutes of Health and were consistent with a protocol submitted to and approved by the institutional review boards of the University of Wisconsin-Madison and the University of Maryland-College Park.
Subjects
Subjects were 1) adult users of bilateral CIs (n = 7: 5 women, 2 men; mean age = 63.9 ± 5.6 yr) or 2) adults with bilaterally normal hearing (n = 8: 4 women, 4 men; mean age = 23.9 ± 4.2 yr), defined by audiometric thresholds <25 dB HL over the range 250-8,000 Hz. Demographic information for all bilateral CI subjects is provided in Table 1. Notably, all CI participants included in the present study 1) were deafened and received their first and second implants during adulthood (cf. Litovsky et al. 2010) and 2) demonstrated sensitivity to ITD in previous studies and/or during enrollment procedures (see below). All CI subjects and a majority of NH subjects were naive to the purposes of the study. Four of the CI subjects (CI1, CI2, CI3, CI4) were tested at the University of Maryland, while the remainder were tested at the University of Wisconsin. Equipment and PE testing conditions (described below) were identical at the two sites. All NH subjects had previous experience participating in binaural psychophysical tasks. One NH participant was an author (H. G. Jones was subject NH3). All NH testing was completed at the University of Wisconsin.
Table 1.
Demographic information for the 7 bilateral CI subjects included in the present study
| Subject |
Loss of Hearing |
CI Internal Device |
CI External Device |
CI Activated |
Electrodes Tested |
Other Measures |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Age, yr | Left | Right | Etiology | Left | Right | Left | Right | Left | Right | Apical | Basal | Best ITD JND*, μs | Speech in quiet/noise† |
| CI1 | 73 | ∼1995 | ∼1995 | Unknown | CI24R(CS) | CI24RE(CA) | Freedom | CP810 | 2/2003 | 3/2008 | L18-R18 | L4-R6 | 58 | 95%/57% |
| CI2 | 62 | 2002 | 2002 | Hereditary | CI24RE(CA) | CI24RE(CA) | Freedom | Freedom | 5/2007 | 11/2005 | L18-R19 | L10-R13 | 23 | N/A |
| CI3 | 55 | ∼1980 | ∼1980 | Unknown | CI24RE(CA) | CI24RE(CA) | Freedom | Freedom | 9/2008 | 6/2009 | L18-R18 | L4-R6 | 141 | 88%/60% |
| CI4 | 67 | 2010 | 2010 | Radiation | Freedom | CI512 | CP810 | CP810 | 4/2012 | 4/2011 | L20-R21 | L8-R10 | 136 | N/A |
| CI5 | 64 | ∼1960 | ∼1960 | Hereditary | CI24RE(CA) | CI24R(CA) | CP810 | CP810 | 9/2005 | 11/2002‡ | L18-R19 | L4-R4 | 193 | 92%/63% |
| CI6 | 65 | ∼1961 | ∼1961 | Unknown | CI512 | CI512 | CP810 | CP810 | 12/2009 | 12/2009 | L20-R16 | L4-R6 | 160 | 80%/47% |
| CI7 | 61 | ∼1970 | ∼1970 | Nerve damage | CI512 | CI24RE(CA) | CP810 | CP810 | 6/2010 | 8/2011§ | L20-R20 | L4-R5 | 121 | 98%/77% |
CI, cochlear implant.
The given interaural time difference (ITD) JNDs were determined previously with 100 pulse/s direct stimulation (see text) of pitch-matched and loudness-balanced electrode pairs (not necessarily the same electrode pairs tested in the present study).
The given speech recognition scores, collected in the free field with subjects wearing their clinical processors, represent % correct word recognition for closed sets of 50 single words presented at 50 dB SPL from a loudspeaker at 0° azimuth, either in quiet or with simultaneous 50 dB SPL broadband noise presented from loudspeakers to the left and right of the subject (±90°). Data were unavailable (N/A) for subjects CI2 and CI4.
Subject CI5 was initially implanted in the right ear in 11/2002 but was reimplanted with the listed devices in 2/2005.
Subject CI7 was initially implanted in the right ear in 8/2011 but was reimplanted with the listed devices in 3/2012.
CI Stimuli and Procedures
Mapping for direct stimulation.
Prior to commencement of testing, CI subjects underwent a custom device programming (“mapping”) procedure designed to identify bilateral pairs of electrodes that provided pitch-matched, loudness-balanced, and binaurally centered auditory percepts for single biphasic pulses (25 μs each phase, 8-μs interphase gap; see Fig. 1). Details of this procedure have been described in prior publications (e.g., Kan et al. 2013). Briefly, left and right clinical processors were replaced with left and right custom research processors [synchronized L34 processors in conjunction with Nucleus Implant Communicator (NIC), Cochlear, Centennial, CO], which were directly controlled by a personal computer. This direct stimulation approach enables, among other advantages, precisely controlled presentation of ITD information, which may not be reliably provided by clinical processors that operate independently at each ear (e.g., Litovsky et al. 2010).
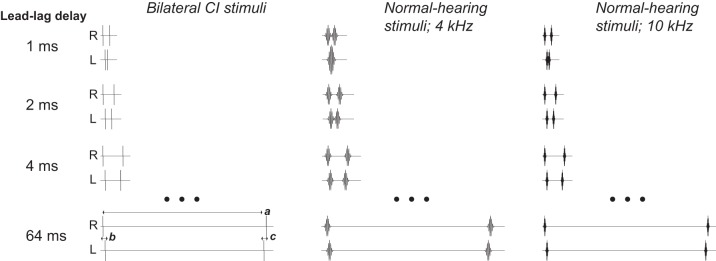
Fig. 1.
Precedence effect stimuli. Left: bilateral cochlear implant (CI) subjects were presented with biphasic current pulses (25 μs/phase, 8-μs interphase gap) at a comfortable current level (“C” level from mapping procedure, see text). Pulses were presented bilaterally in “lead-lag” pairs. The delay between the onset of the leading pulse and onset of the lagging pulse (a) defined the lead-lag delay, the variable of primary interest (rows). Stimuli were lateralized by virtue of a ±500-μs interaural time difference (ITD) in the lead and an opposing ITD in the lag (b, c). Stimuli were delivered to either apical or basal pairs of electrodes (Table 1; see text). Center: normal-hearing (NH) subjects were presented with 4-kHz Gabor clicks (4-kHz cosine multiplied by a Gaussian temporal window), designed to simulate “apical” CI stimulation. Stimuli were otherwise constructed the same as CI stimuli. At a lead-lag delay of 1 ms, the second click of the lead and the first click of the lag overlapped, giving rise to a spurious lag favoring interaural level difference (see text). Right: 10-kHz stimuli were used to simulate “basal” CI simulation.
For each subject, two electrode pairs were identified: a basal pair, intended to stimulate auditory nerve fibers near the base of the cochlea, and an apical pair, intended to stimulate auditory nerve fibers nearer the apex. For each selected electrode pair, “threshold,” “comfortable,” and “maximum comfortable” (hereafter T, C, and M) current levels were determined via subject self-report. Each left-right pair of electrodes was then stimulated simultaneously with left and right C levels. If necessary, C levels were adjusted in each ear until the subject reported perceiving a single compact image near the center of his or her head. In instances where the subject reported the perception of multiple images, e.g., due to a bilateral pitch disparity or other failure to establish binaural fusion, a different electrode pair was selected and tested with the same procedure. Binaurally fused basal and apical pairs were identified in all subjects and are given along with demographic information in Table 1.
ITD lateralization procedure.
To establish that CI subjects were sensitive to ITDs carried by single biphasic pulses, they were tested in a paradigm that assessed the extent of intracranial lateralization produced by pulses carrying a broad range of ITDs, generally −1,000 μs (by convention, left-favoring) to +1,000 μs (right-favoring) at least, in 100- to 200-μs increments. Two of the seven CI participants (CI5 and CI6) did not complete ITD lateralization using single pulses but had previously completed an identical task using pulse trains. All stimuli were presented at the diotically determined C levels for basal and apical pairs with the NIC processors as described above. On each trial (i.e., for each ITD stimulus), subjects indicated the perceived intracranial location within a schematic head displayed on a touch-sensitive monitor. Mean lateralization changed systematically from left to right as the ITD shifted from left-favoring (negative values) to right-favoring (positive values). Despite variability in the dynamic range and slope of lateralization across subjects, all subjects were clearly sensitive to ±500-μs ITD, as would be produced in the free field by sound sources ∼60° to the left or right (e.g., Feddersen et al. 1957). Therefore, these values (±500 μs) were selected for use in subsequent PE testing.
NH Stimuli and Procedures
Simulated CI stimuli.
NH subjects were tested with tones of 4 and 10 kHz multiplied by brief Gaussian temporal windows of 2.8-ms (4 kHz) or 1.2-ms (10 kHz) nominal duration (see Fig. 1). Such “Gabor clicks” have previously been used to simulate the brief yet effectively narrowband nature of CI pulsatile stimuli (e.g., Goupell et al. 2013). By varying stimulus duration with carrier frequency, a constant “cochlear bandwidth” of ∼3 mm (±1.5 mm), simulating the estimated average spread of excitation along the cochlea for a single electrode, can be maintained (cf. Goupell et al. 2013; Kan et al. 2013). While Gabor clicks provide a reasonable acoustic simulation of the CI pulsatile stimuli in terms of bandwidth, their comparability to CI stimuli is limited by their duration: At 4-kHz center frequency, the duration of a single pulse is nearly two orders of magnitude greater than the duration of a CI electrical pulse. Longer pulse duration, in turn, results in significant overlap of lead and lag stimulus waveforms at brief LLDs. Lead-lag overlap is absent from CI PE stimuli of the present study and from the brief/impulsive broadband acoustic stimuli used in a majority of previous NH PE studies. In the 4-kHz condition at 1-ms LLD (see Fig. 1), the temporal overlap was especially severe, leading to summation of overlapping waveforms in the lag channel and a consequent “lag-favoring” interaural level difference (ILD) (cf. Tollin and Henning 1999). Although we tested a version of the experiment in which this issue was averted by shortening pulses in time (and, consequently, increasing their bandwidth) at LLDs ≤2 ms, broader-bandwidth pulses provide a less precise CI simulation, resulting in “cochlear bandwidths” well beyond 3 mm. Subjects also reported changes in sound timbre between trials to be distracting. Thus we elected to use longer-duration pulses at all LLDs, recognizing that acoustic overlap would likely influence NH lateralization at the shortest LLDs (see results). Finally, all stimuli were programmed in MATLAB (MathWorks, Natick, MA), synthesized at a nominal sampling rate of 50 kHz with Tucker-Davis Technologies System 3 hardware (RP2.1, PA5, HB7), and presented via ER-2 insert earphones (Etymotic Research, Elk Grove Village, IL). Pulses were presented at a level of ∼80 dB SPL peak (4 kHz) or ∼90 dB SPL peak (10 kHz) in a background of binaurally uncorrelated low-pass noise (∼50 dB SPL; first-order Butterworth filter with a low-pass cutoff of 100 Hz) in order to mask low-frequency (fine structure) distortion products that may convey ITD information unavailable to CI subjects.
PE testing.
We assessed the PE with a variant of a well-established psychophysical paradigm used in dozens of previous studies, recently employed by Brown and Stecker (2013). CI subjects were seated in front of a touch-sensitive monitor inside a quiet room and listened to stimuli presented via L34 processors, as described above. NH subjects were seated in front of an identical touch-sensitive monitor inside a sound-attenuating booth (IAC, Bronx, NY) and listened to stimuli presented via insert earphones. PE stimuli consisted of lead-lag pairs of single electrical pulses (CI subjects) presented at the previously determined C level or acoustic clicks (NH subjects) presented at ∼80 dB SPL peak (4 kHz) or ∼90 dB SPL peak (10 kHz), as detailed in the preceding sections (see Fig. 1). In either case, the lead carried a 500-μs right- or left-favoring ITD and the lag carried an opposing ITD (500-μs left- or right-favoring, respectively). The variable of primary interest was the LLD, i.e., the temporal disparity between the onset of lead and lag pulse pairs, which was varied randomly across trials from 1 to 64 ms. On each trial, subjects responded to the stimulus by touching a location within one of two panels displayed on the touch-sensitive monitor to indicate 1) whether “one location” (upper panel) or “two locations” (lower panel) had been perceived and 2) the location perceived or, in the event two locations were perceived, the location of the left-most sound perceived (for additional details regarding this method, see Brown and Stecker 2013; cf. Hafter and Jeffress 1968; Seeber and Hafter 2011). The panels were depicted inside a schematic head, centered on the monitor; panels were vertically adjacent, with each centered at the bridge of the nose and extending laterally to either ear. After the subject responded, an asterisk appeared in the selected panel at the location touched by the subject. Trials were presented in counterbalanced blocks for 4 kHz and 10 kHz (NH) or apical and basal (CI) conditions. Each LLD was presented a minimum of 40 times for each condition.
Data Analysis
Lead-lag fusion.
Fusion was assessed according to the proportion of trials on which subjects touched the “two locations” vs. “one location” panel on the touch screen for LLDs from 1 to 64 ms. The resulting psychometric function generally traversed values from 0% “two locations” responses at 1 ms to 100% “two locations” responses at 64 ms. Raw values were then fit with a four-parameter logistic function of the form
| (1) |
where A is the minimum fit number of two-locations responses over the tested range of LLDs, B is the slope (change in proportion of “two” responses per ms LLD), C is the LLD at the inflection point of the function, and D is the maximum number of two-locations responses over the tested range of LLDs. A four-parameter function was chosen over a standard two-parameter logistic function (minimum asymptote at 0, maximum at 1) because it was noted that the minimum proportion of “two locations” responses was >0 for some subjects. Although the y-value of the fit inflection point [f(C)] differed slightly across subjects, we enforced a standard definition of echo threshold, i.e., the delay at which the function reached 50% “two locations” responses. Finally, echo thresholds were compared across CI and NH groups with standard inferential statistics, including ANOVA and t-tests.
Localization dominance.
Localization dominance was assessed according to lateralization responses for trials on which subjects reported perceiving a fused image, i.e., “one location” (see Brown and Stecker 2013). Because absolute values of lateralization responses for a given LLD varied substantially across subjects (ranging from −10, extreme left, to +10, extreme right), responses were analyzed primarily in terms of change in lateralization (i.e., slope of lateralization) across LLD: Whereas localization dominance entails localization (or lateralization) consistently in the direction of the lead, fusion can also occur in the absence of localization dominance. That is, the perceived location of a single, fused image can be strongly influenced by the spatial cues of both the lead and the lag. Reduced localization dominance is most commonly observed near the fusion echo threshold but has also been reported to occur below the fusion echo threshold, suggestive of a divergence of fusion and spatial aspects of the PE under some conditions (e.g., Brown and Stecker 2013; Yang and Grantham 1997; cf. Djelani and Blauert 2001). In the present study, reduced localization dominance with increased LLD would manifest as a negative-going trend in lateralization values across LLD. Thus, for each subject, weighted linear fits (weighted by the number of “one location” responses at each LLD) were computed for lateralization responses at 2-, 4-, and 8-ms LLD. The 1-ms LLD was excluded from the fitting procedure because of the aforementioned acoustic lead-lag interactions at 4 kHz in the NH data; 16- to 64-ms LLDs were excluded because of the paucity of “one location” responses (for some subjects, none) at these LLDs. Finally, the slopes of these lines were compared across NH and CI groups with standard inferential statistics, including ANOVA and independent-samples t-tests to determine whether localization dominance, apart from fusion, differed between groups.
RESULTS
Lead-Lag Fusion Was Similar in NH and CI Subjects
Figure 2, A and B, plot individual subject and mean psychometric functions for lead-lag fusion among NH (Fig. 2A) and CI (Fig. 2B) subjects. As expected, the proportion of “two locations” responses increased with increasing LLD, beginning at or near 0 at 1-ms LLD, sharply increasing for most subjects in the range of 4- to 16-ms LLD, and reaching an asymptote at or near 1 by 32- to 64-ms LLD. Some functions appeared to be shifted to the left (i.e., toward shorter LLDs) for more basal stimulation conditions (CI basal condition, NH 10 kHz condition). Performance appeared to be similar between NH and CI groups. One NH subject (NH6) almost never reported a fused image in the 10 kHz condition. It is possible that this subject's echo threshold at 10 kHz was <1 ms, but this could not be confirmed since LLDs below 1 ms were not tested. Raw data for subject NH6 are thus included in Fig. 2, but his data were excluded from fitting procedures and from group-level statistical analyses.
Fig. 2.
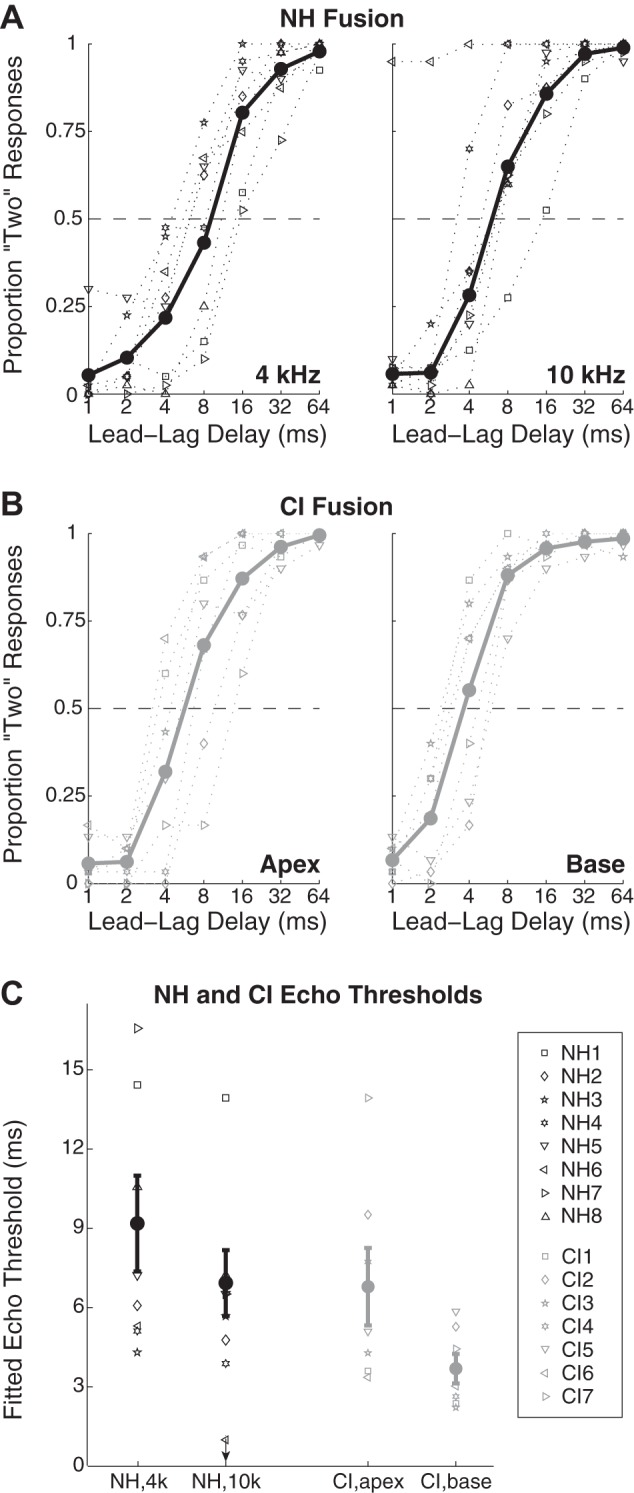
Bilateral CI and NH subjects experience comparable lead-lag fusion. A: proportion of “two locations” responses among NH subjects for 4 kHz (left) and 10 kHz (right) as a function of lead-lag delay. Symbols and dotted lines give data for individual subjects (see key in C); bold points and lines give mean data across subjects, excluding subject NH6, whose performance diverged from the group in the 10 kHz condition (see text). B: data, as in A, for CI subjects in “apical” (left) and “basal” (right) conditions. C: echo thresholds across conditions and groups, computed as the lead-lag delay giving 50% “two locations” responses via a logistic fitting procedure (see materials and methods).
Echo thresholds, computed as described in materials and methods, are given in Fig. 2C. Echo thresholds appeared to be greater for the more apical stimulation conditions (CI apical condition, 4-kHz NH condition) than for more basal conditions in both NH and CI groups and somewhat greater on average for NH than CI subjects. To assess these differences statistically, echo thresholds were submitted to a 2 × 2 ANOVA with group as a between-groups factor and place of stimulation as a repeated measure. The main effect of place of stimulation was significant (F1,12 = 7.72, P = 0.017), while the main effect of group membership (F1,12 = 2.87, P = 0.115) and place × group interaction (F1,12 = 0.19, P = 0.670) were not. Follow-up pairwise tests were conducted on place of stimulation and between CI vs. NH groups with paired and independent-samples t-tests, respectively. NH 4-kHz thresholds (mean = 9.17, SD = 4.80) were higher than NH 10-kHz thresholds (mean = 6.91, SD = 3.30), but not significantly (t6 = 1.61, P = 0.159). Similarly, CI apical thresholds (mean = 6.79, SD = 3.88) were higher than CI basal thresholds (mean = 3.69, SD = 1.48), but not significantly (t6 = 2.35, P = 0.057). Between groups, NH 10-kHz thresholds were marginally higher than CI basal thresholds (t12 = 2.36, P = 0.036), while NH 4-kHz thresholds were not different from CI apical thresholds (t12 = 1.02, P = 0.327). In sum, fusion data were largely similar between NH and CI groups, with marginally weaker fusion (i.e., lower echo thresholds) in the NH 10-kHz condition and particularly the basal CI condition.
Localization Dominance Was Similar in NH and CI Subjects
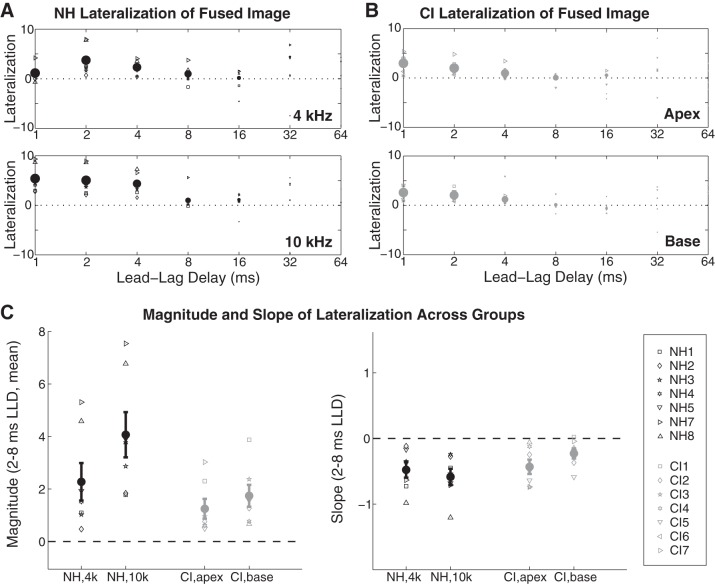
Figure 3, A and B, plot lateralization data for NH and CI individual subject and group mean performance; the sizes of symbols are scaled according to the number of “one location” responses they represent (and are thus largest at 1 ms, smallest or absent at 64 ms). Values on the y-axis in Fig. 3, A and B, represent the average magnitude of lateralization across left-leading and right-leading trials. Positive values indicate lateralization in agreement with the sidedness of the lead, demonstrating localization dominance, whereas values trending close to or below zero indicate a failure of localization dominance (e.g., Brown and Stecker 2013; Dizon and Colburn 2006). Downward-trending values across LLD indicate declining localization dominance despite fusion; for some subjects, at 16 or 32 ms, when “one location” was perceived, stimuli were lateralized at the midline or even slightly toward the lag. As an aside, lateralization responses among NH subjects in the 4-kHz condition were often near 0 at 1-ms LLD, with some subjects lateralizing the fused stimulus to the side opposite the lead (given by negative values along the y-axis in Fig. 3, A and B). We attribute this anomaly to the acoustic lead-lag overlap present at 1-ms LLD in the 4-kHz NH stimuli, which led to a spurious ILD favoring the lagging channel (see Fig. 1 and materials and methods).
Fig. 3.
Bilateral CI and NH subjects experience comparable localization dominance. A: lateralization data are given as magnitudes of lateralization for trials on which subjects reported “one location”; data are combined across left-leading and right-leading trials. Positive values indicate lateralization in agreement with the sidedness of the lead; negative values indicate lateralization in agreement with the sidedness of the lag. The size of individual subject (symbols) and mean (bold circles) data points is proportional to the number of responses recorded at each lead-lag delay (see text). Reduced lateralization at 1 ms lead-lag delay in the NH 4-kHz condition despite strong fusion is ostensibly due to the spurious lag-favoring interaural level difference (see text). B: lateralization data for CI subjects; symbols as in A. C: magnitude (left) and slope (right) of lateralization for CI and NH subjects were computed for values of 2-, 4-, and 8-ms lead-lag delay (LLD) (see text). While the overall magnitude of lateralization was somewhat greater among NH subjects (suggestive of greater ITD sensitivity), the pattern of lateralization across LLD was similar between NH and CI groups. Error bars show ±1 SE.
Weighted means for the magnitude of “one location” lateralization at 2-, 4-, and 8-ms LLD were computed for each subject (weighted according to the number of “one location” responses at each LLD). These values were then submitted to a weighted linear fitting procedure, as described in materials and methods. Computed magnitude and slope values are given in Fig. 3C. Lateralization magnitude appeared to be greater among NH than CI subjects, while the slope of lateralization across LLD was similar across groups—slightly negative in all cases, consistent with weakening localization dominance with increasing LLD.
To assess CI and NH lateralization performance statistically, magnitude and slope data were submitted to two separate 2 × 2 ANOVAs with the same factors as the ANOVA for fusion (previous section). Concerning lateralization magnitude, the main effect of place of stimulation was significant (F1,12 = 27.00, P < 0.001), as was the frequency × group interaction (F1,12 = 8.74, P = 0.012), while the main effect of group membership approached significance (F1,12 = 3.82, P = 0.075). The marginal effect of group is likely attributable to a greater salience of the imposed ±500-μs ITD for NH than CI subjects. This is unsurprising, given the generally better ITD sensitivity in NH than CI subjects reported throughout the literature (see van Hoesel 2012). Nonetheless, CI subjects were clearly sensitive to the imposed ITDs; at 1-ms LLD, the briefest LLD tested, CI lateralization for both left-leading and right-leading trials for both basal and apical stimulation was significant and in the expected direction [1-sample t-test against 0 for raw CI lateralization responses: apex, left lead (P = 0.006, t6 = −4.206); apex, right lead (P = 0.011, t6 = +3.664); base, left lead (P = 0.004, t6 = −4.517); base, right lead (P = 0.003, t6 = +4.794)].
The ANOVA significant main effect of frequency, carried by greater lateralization magnitude for “high” than “low” conditions, was unexpected but may be at least partially attributable to greater acoustic lead-lag overlap in the 4-kHz than 10-kHz stimuli. In support of this interpretation, the high-low disparity was larger among NH subjects than CI users, which gave rise to the significant stimulation place × group interaction. Concerning the more PE-relevant question of lateralization slope in NH vs. CI groups, which indicates the persistence of localization dominance across LLD, no main effects or interactions were significant: place of stimulation (F1,12 = 0.33, P = 0.575), group (F1,12 = 2.39, P = 0.148), or place × group interaction (F1,12 = 3.62, P = 0.082). Thus, despite differences in the overall magnitude of lateralization across groups and some considerable individual differences (e.g., subjects NH7 and CI7 with relatively strong and persistent localization dominance vs. subjects NH2 and CI3 with relatively weak localization dominance), the pattern of localization toward the lead at brief LLDs was similar across CI and NH subjects.
Interim Discussion
We assessed the PE in bilateral CI users with a task that measured both ITD-based lead-lag fusion and lateralization (cf. Brown and Stecker 2013). Although NH PE data from many earlier studies inform the interpretation of our data (see Brown et al. 2015; Litovsky et al. 1999), we also assessed the PE in a group of NH subjects with Gabor click stimuli. These stimuli, which are both brief and relatively narrowband, provide a more appropriate simulation of single-electrode CI stimulation than broadband impulses or similar stimuli used in a majority of NH studies. Key findings of the present study were that CI users experienced both fusion and localization dominance, demonstrating that these aspects of the PE can occur in the absence of functional cochleae.
It is important to note that both CI and NH effects were demonstrated with stimuli lacking low-frequency ITD information. In the case of CI subjects, although the cochlear place of stimulation for “apical” electrode pairs (given in Table 1) is unknown, a typical cochlear array insertion depth of 20 mm would place the apical-most electrode at a roughly ∼1,000-Hz cochlear location (Stakhovskaya et al. 2007; see also Landsberger et al. 2015). Basal electrodes stimulate significantly higher-frequency locations. In the case of NH subjects, stimuli were 4- or 10-kHz center frequency and presented in binaurally uncorrelated low-pass masking noise. Thus even effects demonstrated in NH subjects likely occurred without contributions from low-frequency cochlear-mechanical resonances previously invoked to partially or fully account for the PE (Bianchi et al. 2013; cf. Hartung and Trahiotis 2001; Tollin and Henning 1998). Effects demonstrated in CI users certainly occurred without any cochlear-mechanical contributions.
Nonetheless, we stress that the present data do not rule out important contributions of cochlear-mechanical or other peripheral effects for stimuli that do contain low-frequency ITD information. Indeed, the PE is more robust for low-frequency or broadband than high-frequency stimuli (Shinn-Cunningham et al. 1995; cf. Brown et al. 2015; Wolf et al. 2010). A low-frequency bias in the PE (greater fusion and localization dominance at low frequencies) would be consistent with additive effects of a cochlear mechanism that operates at low frequencies and a second mechanism that operates at all frequencies. Interestingly, we also noted a low-frequency bias in the present data, in that mean echo thresholds in the more apical stimulation conditions were greater than those in more basal stimulation conditions, even among CI subjects. A portion of low-frequency bias reported in the literature thus might also be attributable to noncochlear mechanisms. We also note that the echo thresholds and patterns in lateralization observed in the present study were very similar to those obtained in a previous study that used the same paradigm but employed broadband clicks (which did contain low-frequency ITD information; Brown and Stecker 2013).
While differences between CI and NH groups in the present study generally did not reach statistical significance, lead-lag fusion was marginally weaker in CI than NH subjects. In particular, the “basal vs. 10 kHz” echo threshold contrast bordered on significance (after Bonferroni correction for multiple comparisons). The pattern of localization dominance across LLD did not differ between groups per our assessment, although the overall magnitude of localization (i.e., mean value of lateralization responses) was marginally greater in the NH than the CI group. To the extent that the strength of the PE measured in CI vs. NH subjects did differ, we considered mechanistic differences apart from the cochlea that might give rise to psychophysical differences. Specifically, we considered possible differences in the response of the auditory nerve to lead and lag acoustic (NH) vs. electric (CI) stimulation that might give rise to altered lead-lag responses in more central structures (and perceptually). The next section thus details a simple physiologically inspired stimulus manipulation and our measurement of its psychophysical consequences.
Effects of Lag Attenuation on CI Echo Thresholds
Adaptation of auditory nerve responses to the lag in acoustic hearing.
The response of the auditory nerve to acoustic lead-lag PE stimuli has been studied previously (in unanesthetized cat, Parham et al. 1996). At very brief LLDs (1 and 2 ms), the population response to the lag (or the response of a single auditory nerve fiber to many presentations of the lag) is significantly reduced relative to its response to the lead. As the LLD is increased, the response to the lag gradually recovers. Interestingly, whereas recovery functions (% lag recovery as a function of LLD) across fibers appear to be independent of neuronal characteristic frequency (and thus cochlear origin), low-spontaneous-rate fibers generally recover more rapidly than high-spontaneous-rate fibers (Parham et al. 1996). “Lag adaptation” is also readily visible in the output of auditory nerve models, for example, the model of Zilany et al. (2009; see also Zilany et al. 2014). Figure 4A shows poststimulus time histograms generated with the auditory nerve model of Zilany et al. (2009). The Zilany et al. (2009) model simulates all peripheral stages of hearing, including external and middle ear filtering, half-wave rectification of the filtered signal by inner hair cells, and stochastic firing of auditory nerve fibers. To generate the histograms displayed in Fig. 4A, a model population of 100 medium-spontaneous-rate auditory nerve fibers (3-kHz center frequency) was presented with single 120-μs clicks with paired (“lead-lag”) clicks at LLDs of 1–32 ms at 78 dB SPL. As illustrated in Fig. 4B, model responses replicated the empirical data reported by Parham et al. (1996). Figure 4B gives the peak lag response relative to the peak lead response across LLD for model auditory nerve responses and for the neuron depicted in Fig. 4 of Parham et al. (1996). Even at 8-ms LLD, the lag response was recovered to only ∼80% of the lead response. We observed similar responses for model nerve fiber outputs across a broad range of CFs and for “high”- and “low”-spontaneous-rate fibers, although recovery occurred at relatively shorter LLDs for low-spontaneous-rate fibers (data not shown), consistent with the data of Parham et al. (1996). Thus, although much greater reductions in lag responses have been reported at more central sites (especially the inferior colliculus; see Brown et al. 2015), auditory nerve data collectively suggest adaptation effects in acoustically stimulated auditory nerve fibers that reduce the representation of the lag in the auditory nerve and thereby constrain responses to the lag at more central nuclei (e.g., the cochlear nucleus; Parham et al. 1998).
Fig. 4.
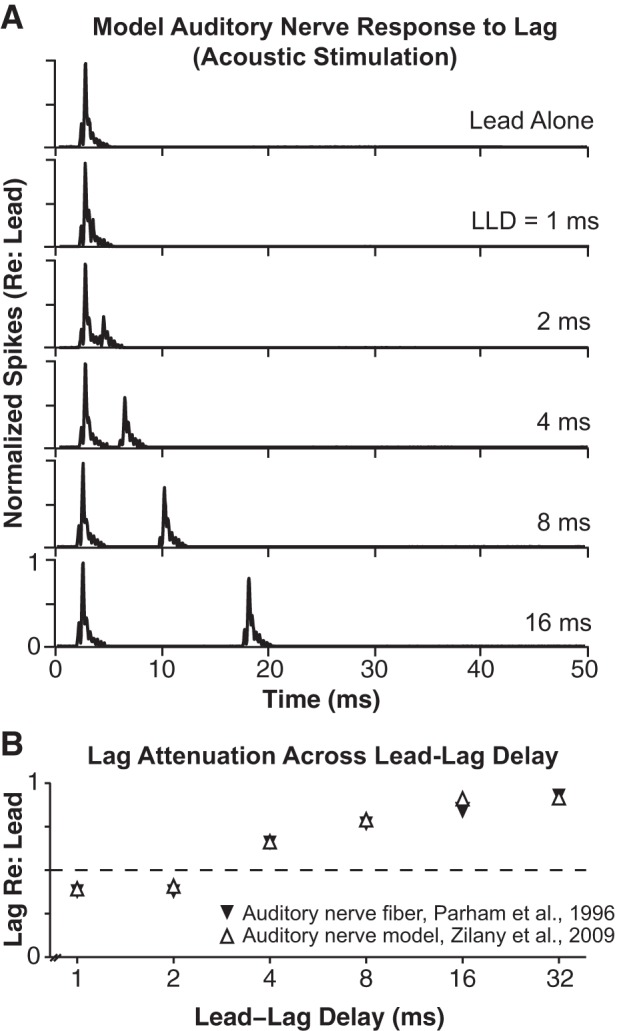
In acoustic hearing, auditory nerve responses to the lag are significantly reduced (re: lead responses). A: poststimulus time histograms for a model population of 100 3-kHz center-frequency, high-spontaneous rate auditory nerve fibers, generated with the auditory nerve model of Zilany et al. (2009). The model population was stimulated with single 120-μs clicks and with paired (“lead-lag”) clicks at LLDs of 1–32 ms. B: peak lag response (relative to peak lead response) across LLD for Zilany model outputs given in A and for a 3-kHz high-spontaneous rate neuron reported by Parham et al. (1996) (see text).
Reduced adaptation of auditory nerve responses to the lag in electric hearing.
Responses of auditory nerve fibers to paired-pulse (sometimes termed “masker-probe”) stimuli have also been studied for electric stimulation (e.g., Cartee et al. 2000; Hartmann et al. 1984; Miller et al. 2001). These studies have generally reported very brief absolute nerve fiber refractory periods, typically <1 ms (dependent on the current level of presented pulses). For example, Cartee et al. (2000) reported a mean refractory time constant of ∼0.7 ms in cat auditory nerve fibers stimulated with paired electrical pulses. While some fibers took longer (≥5 ms) to recover, most fibers exhibited no refractory effects (equal spiking probability for lead and lag at a given current level) by ∼3-ms interpulse interval (equivalent to LLD). Similarly, Miller et al. (2001) demonstrated that cat auditory nerve fiber spiking probability (“firing efficiency”) for lagging (“probe”) pulses was roughly equal to that for a single pulse at LLDs (“masker-probe intervals”) >1–2 ms. Thus electrically stimulated auditory nerve fibers appear to recover sensitivity to a lag impulse more rapidly than acoustically stimulated auditory nerve fibers (cf. Parham et al. 1996). Greater than normal responses to the lag in the auditory nerve at brief LLDs should, in turn, lead to greater than normal responses to the lag at more central sites. Such effects could ultimately lead to greater than normal psychophysical sensitivity to the lag, manifested, for example, by lower than normal fusion echo thresholds.
Lag attenuation experiment.
Toward a coarse simulation of adaptation effects observed in acoustically stimulated auditory nerve fibers (e.g., Fig. 4), we conducted a brief experiment in the same seven CI subjects enrolled in the initial experiment. In this experiment, the amplitude (current level) of the lag stimulus itself was systematically attenuated. In the limit, of course, i.e., with complete lag attenuation, subjects would inevitably perceive only the leading stimulus. Our question was whether LLD-dependent levels of attenuation, scaled according to the LLD-dependent lag recovery functions observed in acoustically stimulated auditory nerve fibers, might precipitate psychophysical performance in CI users yet closer to that observed in NH subjects. This approach makes the simplifying and ultimately incorrect assumption that there is no adaptation in electrically stimulated auditory nerve fibers (cf. Cartee et al. 2000; Miller et al. 2001), i.e., that the electrically evoked population response to the lag is equal to the lead response even at 1-ms LLD. Nonetheless, additional modifications would have required additional assumptions, which we aimed to avoid in this test-of-concept experiment. Simply, we scaled the original current level of the lagging stimulus [the “comfortable” (C) level for each subject] by the values generated by the Zilany et al. (2009) auditory nerve model, as given in Fig. 4B (ranging from 37% of lead level at 1-ms LLD to 100% of lead level at 64-ms LLD). Paired lead-lag PE stimuli with opposing ±500-μs ITD were then presented to CI subjects, with the only difference from the original experiment being the LLD-dependent attenuation of the lag. While it is highly improbable that the implemented attenuation values accurately simulated adaptation for acoustic stimulation across CI subjects and electrode pairs, if the implemented values led to a less-than-NH peripheral response to the lag, CI fusion might be expected to exceed NH fusion.
Figure 5A plots fusion echo thresholds for CI subjects in both conditions (standard lag, attenuated lag) and for NH subjects. Unsurprisingly, lag attenuation augmented lead-lag fusion in CI users. A 2 × 2 repeated-measures ANOVA with factors of electrode place (basal, apical) and lag level (original, attenuated) indicated a significant main effect of lag level (F1,6 = 7.02, P = 0.038) and no other significant effects or interactions. Follow-up pairwise tests indicated increases in echo threshold with lag attenuation for both apical (t6 = 3.27, P = 0.017) and basal conditions, although the increase in the basal condition did not reach statistical significance (t6 = 2.32, P = 0.059). Notably, CI echo thresholds did not exceed NH echo thresholds after lag attenuation was implemented. Indeed, independent-samples t-tests demonstrated that CI lag-attenuated and NH echo thresholds were very similar in both apical/4 kHz [CI (mean = 7.92, SD = 3.87) vs. NH (mean = 9.17, SD = 4.80), t12 = 0.54, P = 0.602] and basal/10 kHz [CI (mean = 5.84, SD = 1.78) vs. NH (mean = 6.91, SD = 3.29), t12 = 0.76, P = 0.465] conditions. Finally, Fig. 5B plots lateralization magnitude and slope data (see Fig. 3) for CI users in standard lag and attenuated lag conditions and for NH subjects. Like fusion, lateralization magnitude and slope were also slightly increased in CI users, suggestive of a reduced influence of the lag on lateralization, i.e., increased localization dominance, following lag attenuation.
Fig. 5.
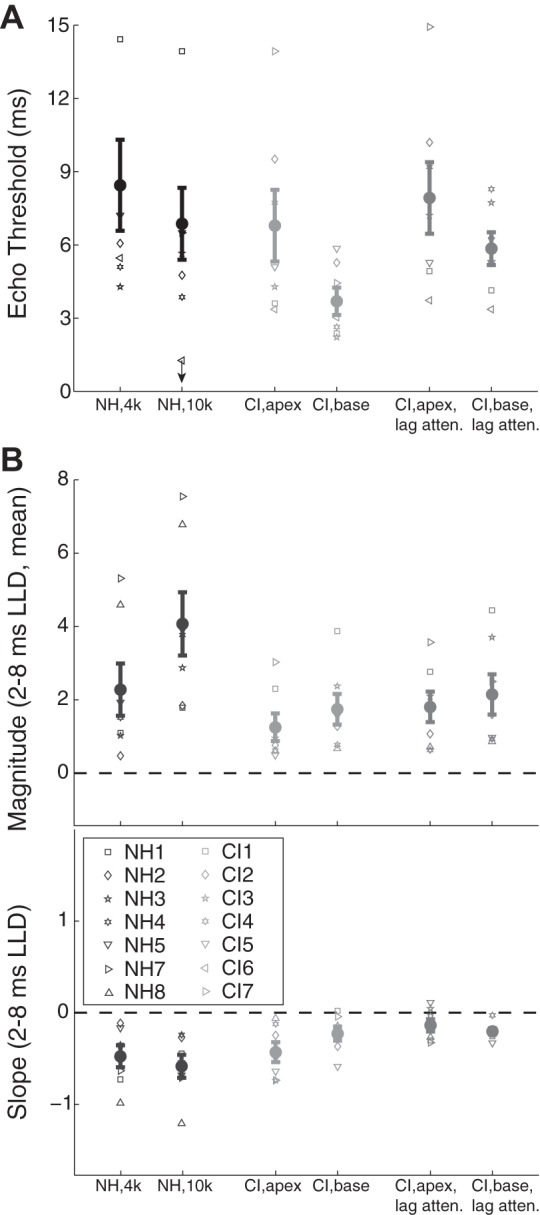
A: adaptation-like lag attenuation augments echo thresholds in bilateral CI users. Echo thresholds are given for NH (black) and bilateral CI subjects in “standard” (light gray) and “lag attenuated” (dark gray) conditions (symbols as in Fig. 2). B: lag attenuation also leads to slight increases in the magnitude and slope of lateralization across brief LLDs. Error bars give ±1 SE.
GENERAL DISCUSSION
The Precedence Effect Without Functional Cochleae
The present data demonstrate that aspects of the PE, including lead-lag fusion and localization dominance, can be elicited in individuals without functional cochleae. This observation, in turn, conflicts with the view that the PE is primarily attributable to cochlear-mechanical effects (Bianchi et al. 2013; cf. Tollin 1998). The model of Hartung and Trahiotis (2001) incorporates a hair cell model proposed by Meddis (1986), which includes contributions of adaptation effects at the hair cell-auditory nerve fiber synapse. Since adaptation effects are still present in electric hearing, it remains possible that the PE as demonstrated in CI users depends on peripheral (though not cochlear-mechanical) mechanisms. Indeed, our simple “attenuation” experiment (Fig. 4 and Fig. 5) was designed to manipulate the peripheral representation of the lag stimulus toward better approximation of the NH condition. Taken together, our data strongly suggest that the PE, as observed for both 4- and 10-kHz acoustic stimuli in NH subjects and for “apical” and “basal” stimulation in CI subjects, depends on central or at least neural processes rather than cochlear processes. Extensive reviews of neurophysiological PE literature (Brown et al. 2015; Fitzpatrick et al. 1999; Litovsky et al. 1999) have considered the temporal extent of PE-like neural responses at multiple levels of the auditory system—from auditory nerve to cortex—in both anesthetized and unanesthetized animal preparations. These reviews collectively implicate the inferior colliculus (auditory midbrain) as the site at which strong suppression of the lag is first observed on a time course consistent with psychophysical observations (localization dominance in particular; Brown et al. 2015). While cochlear-mechanical effects may still contribute to the PE at low frequencies, our data suggest that the cochlea is not necessary for the PE, i.e., that neural sources alone are sufficient to produce the PE, at least over the range of higher frequencies we assessed.
Comparisons to Previous Studies
Two prior studies assessed the PE in CI users with a “lag discrimination” paradigm, in which subjects were required to discriminate the ITD carried by a lagging stimulus across a range of LLDs (see Brown et al. 2015). In one study, van Hoesel (2007) reported that CI subjects, under direct stimulation, appeared to experience the “discrimination suppression” aspect of the PE, as evidenced by elevated lag ITD discrimination thresholds at brief LLDs. In a related study, Agrawal (2008) compared performance of the same subjects under direct stimulation (with binaurally synchronized pulses) and in the free field (listening to signals through clinical processors). The PE was observed only with the binaurally synchronized stimulation, leading Agrawal (2008) to speculate that CI users require high-fidelity ITD information to experience the PE (cf. Brown and Stecker 2013). Recently, Kerber and Seeber (2013) demonstrated particularly poor localization accuracy among CI users wearing their clinical processors in a simulated reverberant environment (relative to anechoic conditions), suggestive of a weak PE, although the PE per se was not directly assessed. Interestingly, the deleterious effect of reverberation on localization appeared to be related to ITD sensitivity: Subjects with better ITD sensitivity tended to be less affected by reverberation, which would be consistent with a relatively more intact PE in those subjects. In conference abstracts, Seeber and Hafter (2007, 2008) reported that the PE was highly variable across subjects using their clinical processors; some subjects experienced fusion without localization dominance, some experienced fusion and localization dominance, and some experienced neither. Finally, in a CI simulation study, Seeber and Hafter (2011) demonstrated a partial failure of the PE using noise-band vocoders of varied interaural correlation, intended to simulate the lack of synchronization in bilateral CI stimulation by present-day clinical speech processors.
Limitations of Present Study
While we assessed the PE in CI and NH subjects with the same paradigm and analogous (brief, narrowband) stimuli, any comparison of CI and NH performance is inherently limited by the dissimilarity of electric and acoustic stimulation. Most especially, it would be desirable to study differences between CI and NH performance at low frequencies (e.g., 500 Hz), where NH cochlear effects should be most prevalent, but 1) CI electrodes generally do not stimulate apically enough to provide for this comparison and 2) the low-frequency, narrowband acoustic stimuli that would be needed to simulate very apical CI stimulation are long in duration and cannot be presented in rapid succession (i.e., at brief LLDs) without extensive acoustic overlap. A future experiment could evaluate the PE in CI subjects by using multielectrode stimulation (spanning multiple frequency channels) to provide for better comparison to traditional NH broadband transient stimuli, though the absence of truly low-frequency information might remain a key dissimilarity. An additional caveat is that our CI subjects were significantly older than our NH subjects. Effects of age on PE measures have been noted (e.g., Cranford et al. 1993), although the recurrent finding has been that old and young subjects alike experience both fusion and localization dominance (see also Lister and Roberts 2005; Roberts and Lister 2004).
Collectively, the present report and existing data indicate that CI users can experience all aspects of the PE (fusion, localization dominance, and discrimination suppression), provided that high-fidelity ITD information is available (see Agrawal 2008). These observations, in turn, suggest that a key facility of audition, the PE, may be restored in some bilateral CI users via binaural synchronization of clinical processors. It remains unclear whether CI users who are insensitive to ITD (particularly congenitally or early-deaf individuals; Litovsky et al. 2010) are able to experience a PE, e.g., via ILD sensitivity (see Brown et al. 2015; Litovsky et al. 1999). Work by Kerber and Seeber (2013) demonstrated that better ITD sensitivity was associated with better localization in reverberation for CI users wearing their clinical speech processors. To this end, future work evaluating the PE in users of bilateral CIs with a more realistic stimulation protocol (e.g., multielectrode) and more extensive stimulus battery, perhaps including speech, will further elucidate the extent to which CI users may obtain clinical benefit from the PE. Nonetheless, the basic observation that the PE can be elicited in the absence of functional cochleae invites reconsideration of exclusively peripheral PE models, and further study of central mechanisms that may give rise to the PE and related effects.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01-DC003083-15 (R. Y. Litovsky), R00-DC010206 (M. J. Goupell), F31-DC050432, F32-DC013927 (A. D. Brown), and R01-DC011548 (G. C. Stecker).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.D.B., H.G.J., A.K., T.T., G.C.S., M.J.G., and R.Y.L. conception and design of research; A.D.B., H.G.J., A.K., T.T., M.J.G., and R.Y.L. performed experiments; A.D.B. analyzed data; A.D.B., H.G.J., A.K., T.T., G.C.S., M.J.G., and R.Y.L. interpreted results of experiments; A.D.B. prepared figures; A.D.B. and R.Y.L. drafted manuscript; A.D.B., H.G.J., A.K., T.T., G.C.S., M.J.G., and R.Y.L. edited and revised manuscript; A.D.B., H.G.J., A.K., T.T., G.C.S., M.J.G., and R.Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Daniel J. Tollin for helpful comments on an earlier version of this manuscript.
Present address of A. D. Brown: Dept. of Physiology and Biophysics, University of Colorado School of Medicine, Aurora, CO 80045.
REFERENCES
- Agrawal SS. Spatial Hearing Abilities in Adults with Bilateral Cochlear Implants (PhD dissertation). Madison, WI: Univ. of Wisconsin-Madison, 2008. [Google Scholar]
- Bianchi F, Verhulst S, Dau T. Experimental evidence for a cochlear source of the precedence effect. J Assoc Res Otolaryngol 14: 767–779, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandewie E, Zahorik P. Prior listening in rooms improves speech intelligibility. J Acoust Soc Am 128: 291–299, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AD, Stecker GC. The precedence effect in sound localization: fusion and lateralization measures for pairs and trains of clicks lateralized by interaural time and level differences. J Acoust Soc Am 133: 2883–2898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AD, Stecker GC, Tollin DJ. The precedence effect in sound localization. J Assoc Res Otolaryngol 16: 1–28, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger RM, Pollak GD. Reversible inactivation reveals the role of the dorsal nucleus of the lateral lemniscus for processing multiple sound sources. J Neurosci 21: 4830–4843, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee LA, van den Honert C, Finley CC, Miller RL. Evaluation of a model of the cochlear neural membrane. I. Physiological measurement of membrane characteristics in response to intrameatal electrical stimulation. Hear Res 146: 143–152, 2000. [DOI] [PubMed] [Google Scholar]
- Cranford JL, Andres MA, Piatz KK, Reissig KL. Influences of age and hearing loss on the precedence effect in sound localization. J Speech Hear Res 36: 437–441, 1993. [DOI] [PubMed] [Google Scholar]
- Dizon RM, Colburn HS. The influence of spectral, temporal, and interaural stimulus variations on the precedence effect. J Acoust Soc Am 119: 2947–2964, 2006. [DOI] [PubMed] [Google Scholar]
- Djelani T, Blauert J. Investigations into the build-up and breakdown of the precedence effect. Acta Acust 87: 253–261, 2001. [Google Scholar]
- Feddersen WE, Sandel TT, Teas DC, Jeffress LA. Localization of high-frequency tones. J Acoust Soc Am 29: 988–991, 1957. [Google Scholar]
- Fitzpatrick DC, Kuwada S, Kim DO, Parham K, Batra R. Responses of neurons to click-pairs as simulated echoes: auditory nerve to auditory cortex. J Acoust Soc Am 106: 3460–3472, 1999. [DOI] [PubMed] [Google Scholar]
- Goupell MJ, Stoelb C, Kan A, Litovsky RY. Effect of mismatched place-of-stimulation on the salience of binaural cues in conditions that simulate bilateral cochlear-implant listening. J Acoust Soc Am 133: 2272–2287, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev 90: 983–1012, 2010. [DOI] [PubMed] [Google Scholar]
- Hafter ER, Jeffress LA. Two-image lateralization of tones and clicks. J Acoust Soc Am 44: 563–569, 1968. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear Res 13: 47–62, 1984. [DOI] [PubMed] [Google Scholar]
- Hartung K, Trahiotis C. Peripheral auditory processing and investigations of the “precedence effect” which utilize successive transient stimuli. J Acoust Soc Am 110: 1505–1513, 2001. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Sensitivity to binaural timing in bilateral cochlear implant users. J Acoust Soc Am 121: 2192–2206, 2007. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Bilateral cochlear implants. In: Auditory Prostheses. Springer Handbook of Auditory Research, vol. 39, edited by Zeng F, Popper AN, Fay RR. New York: Springer, 2012, p. 13–57. [Google Scholar]
- Kan A, Stoelb C, Litovsky RY, Goupell MJ. Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. J Acoust Soc Am 134: 2923–2936, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CH, Takahashi TT. Responses to simulated echoes by neurons in the barn owl's auditory space map. J Comp Physiol A 178: 499–512, 1996. [DOI] [PubMed] [Google Scholar]
- Kerber S, Seeber BU. Localization in reverberation with cochlear implants. J Assoc Res Otolaryngol 14: 379–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger DM, Svrakic M, Roland JT Jr, Svirsky M. The relationship between insertion angles, default frequency allocations, and spiral ganglion place pitch in cochlear implants. Ear Hear 36: e207–e213, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Roberts RA. Effects of age and hearing loss on gap detection and the precedence effect: narrow-band stimuli. J Speech Lang Hear Res 48: 482–493, 2005. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Colburn HS, Yost WA, Guzman SJ. The precedence effect. J Acoust Soc Am 106: 1633–1654, 1999. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Fligor BJ, Tramo MJ. Functional role of the human inferior colliculus in binaural hearing. Hear Res 165: 177–188, 2002. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Jones GL, Agrawal S, van Hoesel RJ. Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans. J Acoust Soc Am 127: 400–414, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Yin TC. Physiological studies of the precedence effect in the inferior colliculus of the cat. II. Neural mechanisms. J Neurophysiol 80: 1302–1316, 1998. [DOI] [PubMed] [Google Scholar]
- Majdak P, Laback B. Effects of center frequency and rate on the sensitivity to interaural delay in high-frequency click trains. J Acoust Soc Am 125: 3903–3913, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddis R. Simulation of mechanical to neural transduction in the auditory receptor. J Acoust Soc Am 79: 702–711, 1986. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Barbara BK. Response properties of the refractory auditory nerve fiber. J Assoc Res Otolaryngol 2: 216–232, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham K, Zhao HB, Kim DO. Responses of auditory nerve fibers of the unanesthetized decerebrate cat to click pairs as simulated echoes. J Neurophysiol 76: 17–29, 1996. [DOI] [PubMed] [Google Scholar]
- Parham K, Zhao HB, Ye Y, Kim DO. Responses of anteroventral cochlear nucleus neurons of the unanesthetized decerebrate cat to click pairs as simulated echoes. Hear Res 125: 131–146, 1998. [DOI] [PubMed] [Google Scholar]
- Pecka M, Zahn TP, Saunier-Rebori B, Siveke I, Wiegrebe L, Klug A, Pollak GD, Grothe B. Inhibiting the inhibition: a neuronal network for sound localization in reverberant environments. J Neurosci 267: 1782–1790, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RA, Lister JJ. Effects of age and hearing loss on gap detection and the precedence effect: broadband stimuli. J Speech Lang Hear Res 47: 965–978, 2004. [DOI] [PubMed] [Google Scholar]
- Saberi K, Antonio JV. Precedence-effect thresholds for a population of untrained listeners as a function of stimulus intensity and interclick interval. J Acoust Soc Am 114: 420–429, 2003. [DOI] [PubMed] [Google Scholar]
- Seeber BU, Hafter ER. Breakdown of precedence with cochlear implants—simulations show importance of spectral cues (Abstract). In: Conference on Implantable Auditory Prostheses Program Book. D27, 2007. [Google Scholar]
- Seeber BU, Hafter ER. Parameters affecting the precedence-effect with cochlear implants (Abstract). J Acoust Soc Am 123: 3055, 2008. [Google Scholar]
- Seeber BU, Hafter ER. Failure of the precedence effect with a noise band vocoder. J Acoust Soc Am 129: 1509–1521, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn-Cunningham BG, Zurek PM, Durlach NI. Adjustment and discrimination measurements of the precedence effect. J Acoust Soc Am 98: 164–171, 1993. [DOI] [PubMed] [Google Scholar]
- Shinn-Cunningham GC, Zurek PM, Durlach NI, Clifton RK. Cross frequency interactions in the precedence effect. J Acoust Soc Am 98: 164–171, 1995. [DOI] [PubMed] [Google Scholar]
- Song P, Wang N, Wang H, Xie Y, Li H. Pentobarbital anesthesia alters neural responses in the precedence effect. Neurosci Lett 498: 72–77, 2011. [DOI] [PubMed] [Google Scholar]
- Stakhovskaya O, Sridhar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol 8: 220–233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecker GC, Gallun FJ. Binaural hearing, sound localization, and spatial hearing. In: Translational Issues in Hearing Science, edited by Tremblay K, Burkhardt R. San Diego, CA: Plural, 2012. [Google Scholar]
- Tollin DJ. Computational model of the lateralization of clicks and their echoes. In: Proceedings of the NATO Advanced Study Institute on Computational Hearing, edited by Greenberg S, Slaney M. Berkeley, CA: ICSI, 1998, p. 77–82. [Google Scholar]
- Tollin DJ, Henning GB. Some aspects of the lateralization of echoed sound in man. II. The role of the stimulus spectrum. J Acoust Soc Am 105: 838–849, 1999. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Yin TC. Neural correlates of the precedence effect in the inferior colliculus of behaving cats. J Neurophysiol 92: 3286–3297, 2004. [DOI] [PubMed] [Google Scholar]
- Wallach H, Newman EB, Rosenzweig R. The precedence effect in sound localization. Am J Psychol 62: 315–336, 1949. [PubMed] [Google Scholar]
- Watkins AJ. Perceptual compensation for effects of echo and of reverberation on speech identification. Acta Acust 91: 892–901, 2005. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res 242: 3–21, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Schuchmann M, Wiegrebe L. Localization dominance and the effect of frequency in the Mongolian gerbil, Meriones unguiculatus. J Comp Physiol A 196: 463–470, 2010. [DOI] [PubMed] [Google Scholar]
- Xia J, Brughera A, Colburn HS, Shinn-Cunningham BG. Physiological and psychophysical modeling of the precedence effect. J Assoc Res Otolaryngol 11: 495–513, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Grantham DW. Echo suppression and discrimination suppression aspects of the precedence effect. Percept Psychophys 59: 1108–1117, 1997. [DOI] [PubMed] [Google Scholar]
- Yin TC. Physiological correlates of the precedence effect and summing localization in the inferior colliculus of the cat. J Neurosci 14: 5170–5186, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany MS, Bruce IC, Carney LH. Updated parameters and expanded simulation options for a model of the auditory periphery. J Acoust Soc Am 135: 283–286, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany MS, Bruce IC, Nelson PC, Carney LH. A phenomenological model of the synapse between the inner hair cell and auditory nerve: long-term adaptation with power-law dynamics. J Acoust Soc Am 126: 2390–2412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]