Abstract
Perception of the visual vertical is strongly based on our ability to match visual inflow with vestibular, proprioceptive, tactile, and even visceral information that contributes to maintaining an internal representation of the vertical. An important cortical region implicated in multisensory integration is the right temporoparietal junction (rTPJ), which also is involved in higher order forms of body- and space-related cognition. To test whether this region integrates body-related multisensory information necessary for establishing the subjective visual vertical, we combined a psychophysical task (the rod-and-frame test) with transient inhibition of the rTPJ via continuous theta burst stimulation (cTBS). A Gabor patch visual detection task was used as a control visual task. cTBS of early visual cortex (V1–V3) was used to test whether early visual cortices played any role in verticality estimation. We show that inhibition of rTPJ activity selectively impairs the ability to evaluate the rod's verticality when no contextual visual information, such as a frame surrounding the rod, is provided. Conversely, transient inhibition of V1–V3 selectively disrupts the ability to visually detect Gabor patch orientation. This anatomofunctional dissociation supports the idea that the rTPJ plays a causal role in integrating egocentric sensory information encoded in different reference systems (i.e., vestibular and somatic) to maintain an internal representation of verticality.
Keywords: rod and frame, multisensory integration, TPJ, TMS
one of the most surprising abilities of the human brain is the ease with which different sources of information are combined within unique, robust, and reliable percepts (Ernst and Bülthoff 2004). Representing the orientation of our head and body in space is one of the strongest examples of multisensory integration (Blanke 2012). The perception of verticality requires a complex integrative mechanism of visual, vestibular, and somatic (proprioceptive/visceral/tactile) information relative to gravity (Mittelstaedt 1996). Accordingly, the ability to represent verticality depends on both accurate processing of unimodal sensory inputs from these modalities and the integrity of brain stem and cortical systems engaged in their integration (Yelnik et al. 2002). The subjective visual vertical is the most widely used index for testing verticality perception, which is typically studied using the rod-and-frame task (RFT; Asch and Witkin 1948) or its variants (Baccini et al. 2014; Docherty and Bagust 2010; Kheradmand et al. 2015; Lopez et al. 2006). Human and animal studies indicate that the posterior insular cortex, inferior parietal lobule, and superior temporal gyrus are key areas involved in combining vestibular inputs with other sensory information (Angelaki and Cullen 2008; Lopez and Blanke 2011). Crucially, somatic information is known to be essential for subjective visual vertical estimation (Anastasopoulos et al. 1997; Barra et al. 2012; Kaptein and Van Gisbergen 2004), possibly because of its important role in head and body position processing. Moreover, the cortical regions involved in spatial body representation (Blanke et al. 2005) partially overlap with areas involved in processing and integrating vestibular inputs in the right temporoparietal junction (rTPJ), a higher order brain region that is also known to be essential for higher order, body-related and spatial, cognitive functions (e.g., perspective taking, attention reorienting, self/other distinction, moral reasoning, in/out-group processing; Chang et al. 2013; Koster-Hale and Saxe 2013; Ruby and Decety 2003; Santiesteban et al. 2012).
Thus far, only one study using noninvasive brain stimulation in healthy participants has investigated the causal role of specific cerebral regions in estimating the visual vertical (Kheradmand et al. 2015). In the present work we expand on this study by adopting a continuous theta burst stimulation (cTBS) paradigm to explore the causal contribution of the rTPJ in solving a multisensory (i.e., somatic, vestibular, and visual) verticality task (RFT) where the presence and the orientation of visual contextual information is manipulated. To confirm the specificity of the involvement of rTPJ in vertical representation, we used a control task in which participants were requested to report the orientation of visual Gabor patches based on low-level visual features (e.g., contrast). Moreover, we used a control site consisting of stimulation of primary visual areas (early visual cortex, likely including stimulation of V1–V3 regions; Rahnev et al. 2013). We found a clear anatomofunctional dissociation, with early visual cortex playing a causal role in the Gabor patches visual detection task but no role in the verticality task, and the rTPJ playing a crucial role in verticality judgments when the integration of vestibular, somatic, and visual cues was needed (internal representation of verticality) but being less involved when this estimation was based on visual information alone (external representation of verticality).
MATERIALS AND METHODS
Participants
Twenty participants (13 women) took part in this study (mean age 27.5 yr, range 20–41 yr). All participants except one were right handed (Briggs and Nebes 1975) and had normal or corrected-to-normal vision. Participants gave their informed consent before beginning the study. The experimental procedures were approved by the Ethical Committee of the Fondazione Santa Lucia (Rome, Italy), and the study was performed in accordance with the 1964 Declaration of Helsinki. Participants were paid 7.50 €/h, were naive as to the aim of the experiment, and were informed of the purpose of the study only after all the experimental procedures were completed. None of the participants had psychiatric, neurological, or medical problems or any contraindications to repetitive transcranial magnetic stimulation (rTMS) (Rossi et al. 2011; Wassermann 1998). No discomfort or adverse effects during TMS were reported or noticed in any participants.
Procedure
The entire experiment lasted about 4 h, during which subjects underwent continuous theta burst stimulations (cTBS). Three stimulation conditions (rTPJ; sham, with the coil positioned approximately on the vertex; and V1–V3) were each followed by two behavioral tasks (the RFT and a visual task in a counterbalanced order across sites and participants). The order of stimulation over rTPJ and V1–V3 was counterbalanced across the subjects, whereas the sham stimulation was performed between the two real stimulations in all participants. There was at least a 1-h time interval between each cTBS. This procedure allowed us to avoid any carry-over effects of prior cTBS for each of the stimulation conditions (Huang et al. 2005).
Experimental Tasks
Rod-and-frame task.
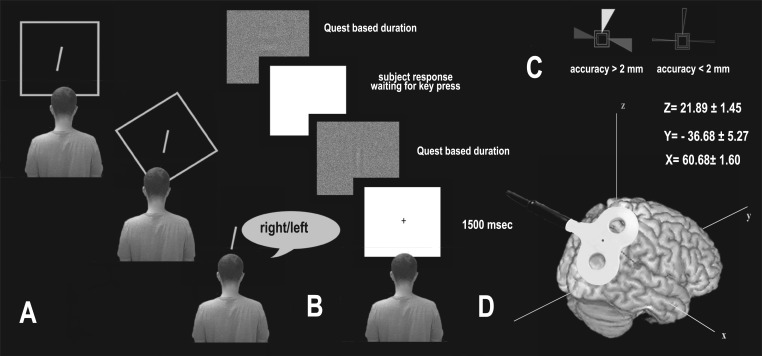
A standard rod-and-frame (RF) device was used for the task (Zoccolotti et al. 1997) (see Fig. 1A for a schematic representation of the apparatus). Each side of the squared frame measured 96 cm, and a single 15-cm-long rod was anchored at the center of the frame. Both the frame and the rod were outlined with 1.2-cm-wide fluorescent tape and were the only visible elements in a completely darkened room during task performance. The frame subtended a visual angle of 34° and the rod a visual angle of 5°. Participants (who had their eyes closed) were led into a pitch-black room and seated on a chair in front of the apparatus. The distance between their eyes and the rod was 160 cm. The starting angle of the rod (and that of the frame) was controlled for by having on the back of the apparatus (where the experimenter was located) a goniometer that allowed the experimenter to position the rod (and the frame) in the desired position. Although the movements were manual, the lever on the back of the apparatus used to rotate the rod and the frame did not make any audible noise. The experimenter asked participants to open their eyes and give verbal instructions about how to adjust the rod to the earth-vertical. No time limit or limited number of chances per trial were given to participants, who were asked to stop the experimenter when they believed the rod was in the vertical position. However, before starting the data collection, the experimenter explained to the participants that it was important to respond as quickly as possible. Once participants estimated the rod's vertical position, the experimenter asked them to close their eyes again and arranged the setting for the following trials. In different experimental conditions, the frame could be tilted [33° clockwise (CW) or counterclockwise (CCW)], upright (0°), or absent, for a total of three frame conditions (TILTED/UP/NO frame). Within each of these three conditions, the rod was tilted 22° CW or CCW for a total of 8 trials per condition (4 with the rod tilted CW and 4 CCW). Please note that the values are analogous to those used by Zoccolotti et al. (1997), David et al. (2014), and Fiori et al. (2014). The conditions were presented randomly.
Fig. 1.
A: example trials of the rod-and-frame test (RFT). From top to bottom are shown the upright (UP), tilted (TILTED), and no (NO)-frame conditions. B: example of trials of the visual Gabor orientation detection task. The Gabor was displayed for a period based on a QUEST algorithm for establishing an individual's perceptual threshold. C: the coil position was kept constant throughout the 20 s of stimulation by a stereotaxic navigation system that provided a 3-dimensional online feedback through which an accuracy threshold of <2 mm concerning the stimulation site was obtained (correct position shown at right). D: stimulation site of the right temporoparietal junction (rTPJ) in Talairach coordinates.
Based on a priori hypotheses and preliminary data analyses (see below), CW and CCW rod conditions were averaged together within each frame condition. Similarly, CW and CCW TILTED frame conditions were averaged together. Participants were asked to adjust the rod to the earth gravitational vertical by verbally guiding the experimenter (i.e., saying “right-left”) in rotating the rod (see Fig. 1A).
Visual detection task.
The Gabor patches task consisted of the identification of the minimum time threshold needed by participants to correctly detect the orientation of a vertical or horizontal Gabor patch. The stimuli were linearly masked by a Gaussian distributed noise of binary values of white and black, with a density of 90%. Gabors had a frequency of 0.26 c/degree, and the Gaussian envelope standard deviation was 50 pixels for both the horizontal and vertical orientations with an amplitude of 0.5. Detecting the orientation of the Gabor patches is not influenced by the integration of vestibular, proprioceptive, and visceral information and is basically resolved at the level of the primary visual cortex. This task was thus used to measure the role of V1–V3 and rTPJ in resolving a purely visual task and to exclude the possibility that any effect found in the RFT after inhibition of the rTPJ could be attributed to low-level visual functions. The experiment was run on MATLAB 7.0 using the Psychtoolbox extension for Windows (Brainard 1997; Pelli 1997). The QUEST algorithm (Watson and Pelli 1983) was used to adjust the duration of Gabor presentation on a trial-by-trial basis (so as to maintain individuals' performance at an accuracy of 80%), which was used as the dependent variable in the analysis. Gabor patches were presented via a LaCie Electron blue II 19-in. CRT monitor with a resolution of 1,024 × 768 pixels. Observers were seated in an erect position 50 cm away from the monitor, and the Gabor patches subtended a visual angle of 5° (see Fig. 1B).
Brain Stimulation
Resting motor threshold.
Stimulation intensity during cTBS was set according to individuals' resting motor threshold (rMT). Participants wore a tight-fitting swim cap on which stimulation points were marked. Motor evoked potentials (MEPs) were recorded from the first dorsal interosseous (FDI) muscle of the right hand. Surface Ag-AgCl electrodes were placed in a belly-tendon montage with the active electrode placed over the muscle belly and the reference over the interphalangeal joint. Electromyographic (EMG) signal was amplified at a gain of 1,000× by a Digitimer D360 amplifier (Digitimer), bandpass filtered (20 Hz–2.5 kHz), and digitized (sampling rate 10 kHz) by means of a CED Power 1401 controlled with Spike2 software (Cambridge Electronic Design). TMS was delivered by means of a Magstim model 200 Monopulse machine (Magstim, Whitland, UK) via a 70-mm figure-of-eight coil. The rMT, defined as the lowest intensity able to evoke 5 of 10 MEPs with an amplitude of at least 50 μV, was determined by holding the stimulation coil over the optimal scalp position (OSP). The OSP for inducing MEPs in the right FDI muscle was found by moving the coil in steps of 1 cm, tangentially to the skull, over the left primary motor cortex with the handle pointing backward and laterally at a 45° angle to the sagittal plane, until the largest MEPs were found. Participants' rMT ranged from 31% to 55% (mean 41.8%, SD 6.1%) of the maximum stimulator output.
cTBS Protocol
The protocol used for cTBS (Huang et al. 2005) consisted of trains of 3 pulses at 50 Hz, each delivered every 200 ms (i.e., at 5 Hz) for 20 s (300 pulses in total) at 80% of rMT. We chose to use the “short” cTBS procedure (20 s of stimulation, 300 pulses) as described in Huang et al. because the inhibitory effect lasts about 20 min after the end of stimulation (Huang et al. 2005), a time window during which participants could complete the experimental tasks. cTBS intensity ranged between 28% and 44% (mean 34.72%, SD 6.5%) of the maximum stimulator output. Coil position was identified on each participant's scalp with the SofTaxic Navigator System (E.M.S., Bologna, Italy). Skull landmarks (nasion, inion, and 2 preauricular points) and 65 points were used to create a uniform representation of the scalp and were digitized by means of Polaris Vicra optical tracking system (NDI, Waterloo, ON, Canada). Talairach coordinates were automatically estimated by the SofTaxic Navigator from an MRI-constructed stereotaxic template. The scalp location that corresponded best to rTPJ was established by means of a selected group of imaging (Bense et al. 2001; Dieterich et al. 2003; Emri et al. 2003; Miller et al. 2008; Miyamoto et al. 2007; Schlindwein et al. 2008), TMS (Blanke et al. 2005; Bosco et al. 2008; Tsakiris et al. 2008), neurophysiological (Kahane et al. 2003; Matsuhashi et al. 2004), and meta-analysis studies (Jakobs et al. 2012) tapping vestibular, proprioceptive, and multisensory integrative functions. Mean (SD) chosen Talairach coordinates corresponded to x = 58.61 (SD 4.48), y = −39.12 (SD 13.03), z = 22.15 (SD 3.70), and the group averages of the stimulated area were x = 60.68 (SD 1.60), y = −36.68 (SD 5.27), z = 21.89 (SD 1.45) (see Fig. 1C). For V1–V3, the stimulation point was localized 2.8 cm above the inion (Salminen-Vaparanta et al. 2012). It should be noted that although this location is thought to be over V1, it may also fall over areas close to V1, such as V2 (Silvanto et al. 2005). TMS was performed by means of 70-mm figure-of-eight coil connected to a Magstim Rapid machine (Magstim). The coil plane was oriented tangentially to the scalp, with the handle pointing upward and 45° backward. Participants wore earplugs to attenuate the sound of the TMS pulses. The position of the stimulation projection on the brain surface was continuously checked during cTBS of both stimulation sites (rTPJ and V1–V3) via SofTaxic Navigator System (E.M.S.).
Data Handling
All statistical analyses were performed with SPSS for Windows (version 22.0; SPSS, Chicago, IL).
Rod-and-frame.
Individuals' absolute deviation (i.e., errors) in evaluating the verticality of the rod with respect to the gravitational vertical relative to earth was measured in angle degrees (°) and used as the dependent variable. Individuals' errors that fell below or above three SD from each frame conditions were removed as outliers (0.9% of the total). Data were not normally distributed, and therefore nonparametric tests were used (Friedman's ANOVA and Wilcoxon signed-rank test). A Wilcoxon signed-rank test was used to determine whether there was a difference between CW and CCW rod positions in each frame condition (TILTED/UP/NO frame) and between CW and CCW frame orientations in the TILTED frame condition. None of these comparisons reached significance, and CW and CCW rod trials were collapsed together, as well as CW and CCW frame trials in the frame TILTED condition.
Visual detection task.
The log-transformed time needed by participants to detect the correct Gabor patch orientation (horizontal vs. vertical) with an accuracy of 80% was used as a dependent variable. Data were not normally distributed, and therefore nonparametric tests were used. One subject was excluded from the analysis due to a technical issue.
RESULTS
Rod-and-Frame Test
Friedman's ANOVA showed a significant difference in the ranks of errors (°) for the frame conditions (TILTED/UP/NO frame) as a function of the three stimulation sites [sham/V1–V3/rTPJ, χ2(8) = 61.246, P < 0.001]. Wilcoxon signed-rank tests were used to follow up this interaction, and Bonferroni correction was applied to disclose significant differences (corrected P = 0.0014).
For the sake of clarity, descriptive statistics are reported in Table 1. In the text below, all the comparisons are described either 1) within the same frame condition across different sites, to show the impact of different sites of stimulation when the task was controlled for, and 2) within each site of stimulation across different frame conditions, to show the impact of different tasks when the stimulation site was controlled for (Fig. 2). All other comparisons are reported in Tables 2 and 3.
Table 1.
Summary of descriptive statistics for RFT dependent variables
| TILTEDsham | UPsham | NOsham | TILTEDV1–V3 | UPV1–V3 | NOV1–V3 | TILTEDrTPJ | UPrTPJ | NOrTPJ | |
|---|---|---|---|---|---|---|---|---|---|
| Median | 1.500 | 0.875 | 1.250 | 1.625 | 1.062 | 1.062 | 1.875 | 1.375 | 2.134 |
| Min | 0.750 | 0.286 | 0.375 | 1.000 | 0.625 | 0.375 | 0.375 | 0.625 | 0.500 |
| Max | 2.625 | 1.375 | 2.500 | 2.750 | 2.500 | 2.500 | 3.125 | 2.714 | 3.000 |
| IQR | 1.156 | 0.706 | 0.594 | 0.844 | 0.812 | 1.094 | 0.969 | 0.875 | 1.094 |
Values are the median, minimum (Min) and maximum (Max), and interquartile range (IQR) of individuals' absolute deviation (errors, in angle degrees) in evaluating verticality for all rod-and-frame task (RFT) dependent variables: tilted (TILTED), upright (UP), or no (NO) frame with sham, early visual cortex (V1–V3), or right temporoparietal junction (rTPJ) stimulation.
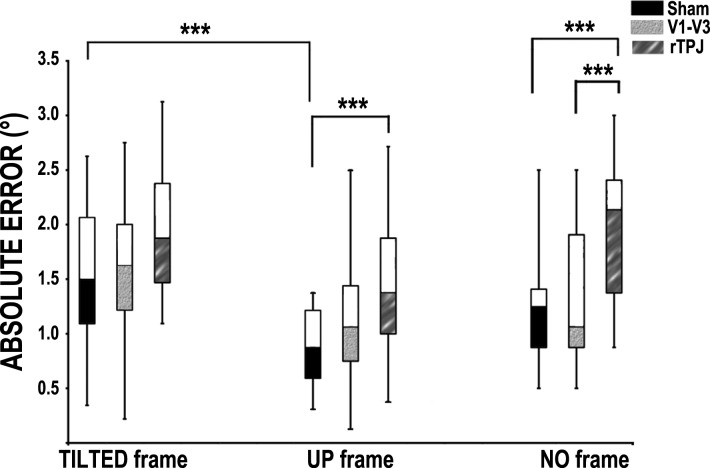
Fig. 2.
RFT results. Displayed is the median value (central line of box) of the errors. On the x-axis, stimulation sites (sham, V1–V3, rTPJ) are grouped by frame condition. Bars represent the error range; the filled part of the box represents errors from the 25th percentile to the median value, whereas the open part of the box represents errors from the median value to the 75th percentile. Asterisks in the plots indicate significance levels (***P < 0.001).
Table 2.
P values of all comparisons between different frame conditions for RFT
| P Value | |
|---|---|
| TILTED frame | |
| TILTEDsham vs. TILTEDV1–V3 | 0.285 |
| TILTEDsham vs. TILTEDrTPJ | 0.034 |
| TILTEDrTPJ vs. TILTEDV1–V3 | 0.131 |
| UP Frame | |
| UPsham vs. UPV1–V3 | 0.009 |
| UPsham vs. UPrTPJ | 0.0008 |
| UPrTPJ vs. UPV1–V3 | 0.214 |
| NO Frame | |
| NOsham vs. NOV1–V3 | 0.284 |
| NOsham vs. NOrTPJ | 0.0005 |
| NOrTPJ vs. NOV1–V3 | 0.0006 |
Bonferroni corrected significant P < 0.0014.
Table 3.
P values of all comparisons between different stimulation site conditions for RFT
| P Value | |
|---|---|
| Sham | |
| TILTEDsham vs. UPsham | 0.0001 |
| TILTEDsham vs. NOsham | 0.005 |
| UPsham vs. NOsham | 0.009 |
| V1–V3 | |
| TILTEDV1–V3vs. UPV1–V3 | 0.015 |
| TILTEDV1–V3vs. NOV1–V3 | 0.058 |
| UPV1–V3vs. NOV1/V2 | 0.532 |
| rTPJ | |
| TILTEDrTPJvs. UPrTPJ | 0.107 |
| TILTEDrTPJ vs. NOrTPJ | 0.697 |
| UPrTPJ vs. NOrTPJ | 0.024 |
Bonferroni corrected significant P < 0.0014.
Within-frame comparisons.
Larger errors were found in the NOrTPJ condition compared with the NOsham (2-tailed Wilcoxon signed-rank test, n = 20, Z = −3.488, P = 0.0005) and the NOV1–V3 condition (2-tailed Wilcoxon signed-rank test, n = 20, Z = −3.421, P = 0.0006). No difference was found between NOsham and NOV1–V3 (P = 0.284); thus the stimulation of rTPJ impairs the ability to establish the vertical when no external visual information is available (with respect to both sham and V1–V3 stimulation).
Errors between TILTEDsham, TILTEDV1–V3, and TILTEDrTPJ did not differ statistically (TILTEDV1–V3 vs. TILTEDsham, P = 0.285; TILTEDrTPJ vs. TILTEDV1–V3, P = 0.131; TILTEDsham vs. TILTEDrTPJ, P = 0.034, respectively). Thus the stimulation site did not change individuals' ability to establish the vertical when tilted contextual visual information was available.
Performance in UPsham was significantly better than that in UPrTPJ (2-tailed Wilcoxon signed-rank test, n = 20, Z = −3.341, P = 0.0008); the difference between UPsham and UPV1–V3 did not survive multiple comparisons (P = 0.009). The difference between UPV1–V3 and UPrTPJ did not reach significance (P = 0.214). Thus the stimulation of rTPJ impairs the ability to establish the vertical when vertical visual information is available, but only with respect to a sham stimulation.
Within-site comparisons.
Participants performed better after the UPsham condition compared with the TILTEDsham condition (2-tailed Wilcoxon signed-rank test, n = 20, Z = −3.874, P = 0.0001), whereas the comparisons between NOsham and UPsham and between NOsham and TILTEDsham did not survive multiple comparison corrections (P = 0.005 and P = 0.009, respectively). Thus, when no brain area is inhibited, errors in the TILTED condition are larger than in the UP condition, as found in previous studies (Fiori et al. 2014; Lester and Dassonville 2014; Lopez et al. 2006; Zoccolotti et al. 1997).
There was no statistically significant difference after V1–V3 stimulation (UPV1–V3 vs. TILTEDV1–V3, P = 0.015; NOV1–V3 vs. UPV1–V3, P = 0.532; NOV1–V3 vs. TILTEDV1–V3, P = 0.058). Thus V1–V3 inhibition seems to abolish the difference between TILTED and UP frame conditions.
No significant differences were found between all tested frame conditions after rTPJ stimulation (UPrTPJ vs. TILTEDrTPJ, P = 0.107; NOrTPJ vs. UPrTPJ, P = 0.24; NOrTPJ vs. TILTEDrTPJ, P = 0.697). Thus rTPJ inhibition seems to abolish the difference between TILTED and UP frame conditions.
Overall, these results indicate worse performance in the NO frame condition after rTPJ stimulation with respect to sham and V1–V3 stimulation. Similarly, rTPJ stimulation impairs verticality judgements when the task is performed in the UP frame condition (i.e., when vertical contextual visual information is available) with respect to sham stimulation. No such effect is found when the frame is tilted. It thus appears that the rTPJ plays a crucial role in verticality judgements when no contextual visual information is available, and individuals need to strongly rely on the integration of vestibular, proprioceptive, and visual cues and match them against an internal frame of reference. At the same time, when vertical contextual visual information is provided and might be used to solve the task, rTPJ activity seems to be necessary to some extent. Furthermore, stimulation of both V1–V3 and rTPJ seems to abolish the known biasing effect of tilted contextual information.
Visual Detection Task.
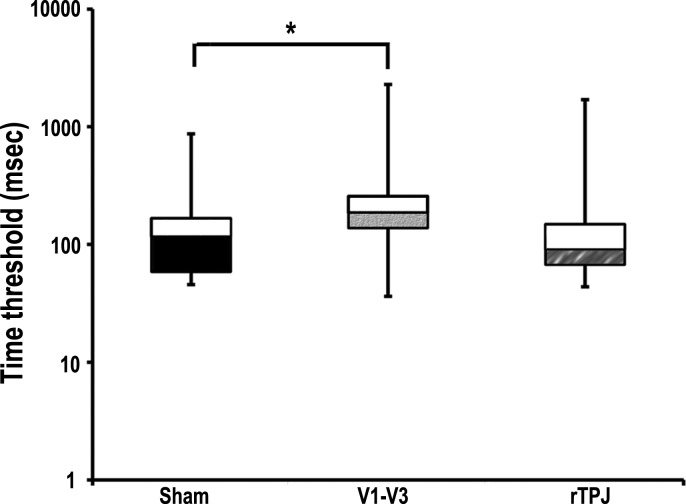
Friedman's ANOVA showed a significant difference in the time of presentation (milliseconds) needed by participants to detect the correct orientation of Gabor patches after cTBS of the three sites [sham/V1–V3/rTPJ, χ2(2) = 10.211, P = 0.006; Fig. 3]. Descriptive statistics are summarized in Table 4. Wilcoxon signed-rank tests were used to follow up this finding, and Bonferroni correction was applied (corrected P = 0.017). Participants needed more time to detect the correct Gabor patch orientation after V1–V3 stimulation compared with sham stimulation (2-tailed Wilcoxon signed-rank test, n = 19, Z = −2.575, P = 0.010). No significant difference was found between the time required after sham and rTPJ cTBS (P = 0.748). The difference between performance after V1–V3 and TPJ cTBS did not survive multiple comparisons (P = 0.033). All results are summarized in Table 5.
Fig. 3.
Visual task results. Displayed is the median value (central line of box) of the time needed by participants to correctly identify the Gabor orientation after each stimulation condition. Bars represent the time range; the filled part of the box represents values from the 25th percentile to the median (fills indicate stimulation sites as denoted in Fig. 2), whereas the open part of the box represents values from the median to the 75th percentile. For better readability of the graph, milliseconds on the y-axis are distributed on a logarithmic scale. Asterisks in the plots indicate significance levels (*P < 0.05).
Table 4.
Summary of descriptive statistics for all visual task dependent variables
| Visual Tasksham | Visual TaskV1/V2 | Visual TaskrTPJ | |
|---|---|---|---|
| Median | 117.490 | 186.209 | 91.201 |
| Min | 45.709 | 36.308 | 43.652 |
| Max | 870.964 | 2290.868 | 1698.244 |
| IQR, ms | 110.940 | 110.220 | 159.578 |
Values are the median, Min and Max, and IQR of time to detect correct Gabor patch orientation for all visual task dependent variables.
Table 5.
P values of all comparisons between different stimulation site conditions for visual task dependent variables
| P Value | |
|---|---|
| Visual taskV1–V3 vs. Visual tasksham | 0.010 |
| Visual tasksham vs. Visual taskrTPJ | 0.748 |
| Visual taskV1–V3 vs. Visual taskrTPJ | 0.033 |
Bonferroni corrected significant P < 0.017.
DISCUSSION
We used repetitive interferential TMS to investigate the causal role of rTPJ and primary visual cortices in a multisensory verticality estimation task and in a in a visual Gabor patches detection task. The ability of individuals to vertically orient a rod using visual, vestibular, and somatic information was explored with the RFT, whereas the ability to visually perceive horizontal or vertical visual gratings was explored by asking participants to perform a Gabor patch orientation task. Interference with rTPJ activity reduced participants' ability to orient the rod vertically, especially when no contextual visual information was provided or when visual contextual information was vertical. Conversely, transient interference with V1–V3 activity increased the time needed to visually detect the orientation of degraded Gabors. Thus interfering with the activity of rTPJ but not V1–V3 influenced the perception of the subjective vertical. This anatomofunctional dissociation supports the idea that the rTPJ plays an essential role in establishing the subjective vertical when information from visual, vestibular, and somatic channels needs to be integrated (i.e., RFT). Conversely, activity of V1–V3 seems necessary for detecting the orientation of visual stimuli in the absence of any relevant contribution of vestibular and somatic modalities.
Internal Vertical Representation in rTPJ
Verticality judgment is mainly viewed as a bottom-up process based on the integration of inputs coming from vestibular (Borel et al. 2008; Lopez et al. 2007), visual (Dichgans et al. 1972; Guerraz et al. 1998), and somatosensory systems (Barbieri et al. 2007; Barra et al. 2010). The vestibular system is thought to contribute to representing an invariant frame of reference based on gravitational vectors. Visual cues are necessary to build an allocentric frame of reference, whereas the somatic system provides information about the position of the head-body in space, an ability that seems fundamental for mapping external stimuli according to an egocentric frame of reference (Li et al. 2014). Many conditions that modulate the activity of the vestibular system (e.g., head torsions or body sway) also activate other sensors such as body (neck) proprioceptors and/or tactile receptors (Barra et al. 2012; Chen et al. 2013). It is worth noting that proprioceptive-vestibular inputs are continuously integrated in the brain stem with signals from muscles, joints, skin, and eyes (Angelaki et al. 2009). The close link between these different signals makes it extremely difficult to disentangle the respective contributions of proprioceptive and vestibular systems. Thus the ability to orient a rod in a vertical position is necessarily based on the integration of visual information with multisensory internal, somatic, and vestibular representations of verticality. An important aspect of our study is that we used a large frame device to lead participants to interpret its axes as a surrogate of the main axes of the environment (Dilorenzo and Rock 1982). Tellingly, visual displays subtending more than 20° are thought to stimulate visuovestibular interactions, whereas visual mechanisms seem to be prominent in guiding evaluation of the axes orientation (for a review, see Spinelli et al. 1991). Furthermore, we added a no-frame condition to the classical upright- and tilted-frame conditions, similar to the one used by Lopez et al. (2006). This procedure allowed us to remove the presence of external information and to make it more likely that the task was accomplished on the basis of an internal representation of verticality. We speculated that the subjective visual vertical estimation in this condition would mainly involve the TPJ, given its role in the integration of vestibular and somatic signals coming from the head and the body with visual information concerning the rod. Our hypothesis was that, given the absence of contextual visual information in the no-frame condition, participants might solve this condition especially on the basis of their ability to use an egocentric frame of reference. Crucially, within the no-frame condition, errors after rTPJ stimulation significantly differed from those after V1–V3 stimulation and those following a sham stimulation. These two conditions did not differ from one another. Thus we suggest that rTPJ plays a causal role in matching the visual inflow with an egocentric, somatic-vestibular representation of the vertical. Our results are in keeping with a recent study in which cTBS was used to test the causal role of the rTPJ in perceiving the upright position (Kheradmand et al. 2015). These authors demonstrate that temporary inhibition of the rTPJ induces the perception that the subjective visual vertical is tilted in the opposite direction of individuals' head tilt. It is worth noting that, at variance with our study, Kheradmand et al. (2015) tested participants in a tilted head condition with the aim of amplifying the magnitude of errors. We tested the role of rTPJ in a condition where no baseline vestibular challenge was imposed (upright head posture). Moreover, to qualify the integrative/multisensory involvement of the rTPJ in vertical representation, we used a Gabor patch visual detection control task and a control area (V1–V3). The hypothesis behind this decision was to highlight the integrative role of the rTPJ in building an internal representation of verticality by showing that rTPJ inhibition impairs the ability to vertically orient a rod when only information related to one's own posture is available. In other words, when proprioceptive-vestibular-somatic inflow is coherent with the vertical, we would expect that any error would specifically concern the visuo/proprioceptive-vestibular-somatic integration. Thus we demonstrated the strong impact of rTPJ inhibition in sensory integration in the service of verticality estimation. Our study expands previous findings by showing that cTBS over the rTPJ impairs verticality judgments even when individuals keep their head in a vertical position, indicating that activity in this region is strongly involved in the maintenance of an internal vertical frame of reference (see Barra et al. 2010). Furthermore, by controlling for the contribution of V1–V3 in the RFT, we were able to rule out the possibility that the multisensory integration required by the RFT was influenced by the transient impairment of low-level visual representations.
It is worth noting that rTPJ inhibition also impaired the accuracy in the upright frame condition, which is generally thought to be the easiest condition because vertical visual information is available. This result supports the notion that the rTPJ is strongly involved in vertical estimation when visual and vestibular information needs to be integrated. The fact that the difference between rTPJ and V1–V3 stimulation did not survive statistical testing in the upright frame condition suggests that the visual cortex is also playing a role in this condition, though less prominently than the rTPJ. Larger errors after sham stimulation in the tilted frame condition compared with the upright frame condition can be explained by the classical disorienting power of the tilted frame. In this condition, impairments in perceiving the vertical have been explained by the conflict between the spatial coordinates given by the tilted frame and the natural direction of gravity (Zoccolotti et al. 1992). Importantly, this difference was abolished by stimulating either V1–V3 or rTPJ. Thus both regions may play a role in the upright frame condition. None of the stimulation sites had an effect on the tilted frame condition. This may be in keeping with a previous rTMS study showing that application of inhibitory 1-Hz stimulation over the right (but not left) superior parietal lobule reduced participants' sensitivity to the illusion compared with a control site (Lester and Dassonville 2014). Unfortunately, however, no TPJ stimulation was performed in that study. Moreover, because the task used in Lester's study seems to be more related to contextual visual processing than to visuo/vestibular/proprioceptive integration, it is highly plausible that inhibition of the superior parietal lobule influenced the participants' performance, disrupting the global visual processing (Urgesi et al. 2007).
Gabor Patch Visual Detection Task
To disentangle the role of different cortical regions in processing internal and external signals in verticality assessment, we included a purely visual detection control task and a primary visual site stimulation condition. The V1–V3 was selected because of its role in the detection of visual orientations and contrast (Hubel and Wiesel 1968). We found an increase in the time needed to correctly identify the orientation of Gabor patches after V1–V3 inhibition compared with the time needed after sham stimulation. The difference between ineffective (sham) and rTPJ stimulation did not reach significance, whereas the impairment of performance after inhibition of V1–V3 with respect to the rTPJ did not survive multiple comparisons. Finding a decrease in the ability to detect the horizontal and vertical orientation of Gabor patches after V1–V3 stimulation is in line with the established function of this region in processing the orientation of visual stimuli. This result helped us ascertain that the role of rTPJ is marginal when visual information alone needs to be processed to determine the orientation of a visual stimulus. It is worth noting that studies applying cTBS to the visual system and measuring visual perception reported both improvement and reduction of performance depending on a number of factors such as the task at hand, the stimulation intensity, and ongoing neural activity (Miniussi et al. 2013; Perini et al. 2012). Repetitive brain stimulation of the primary visual cortex has been reported to affect neural selectivity for orientations in the cat's visual system (Kim et al. 2015). Moreover, single pulses delivered to the primary visual cortex impair orientation detection when applied before and soon after the appearance of a stimulus (de Graaf et al. 2011, 2014). At least two nonmutually exclusive interpretations of the functions tested by the Gabor patches task can be offered. The first is that interference with the activity of V1–V3 areas reduced the reactivity of orientation-sensitive neurons in the primary visual system, thus impairing (i.e., inducing longer detection time) the ability of the participants to detect the orientation of the Gabor patches. The second is that the participants detected the Gabor patches' orientation (vertical/horizontal) as soon as they gained enough contrast information. In this case the time that participants took to answer correctly would be a consequence of contrast detection, and not a matter of orientation. However, in both cases the task tapped functions such as edge orientation and contrast detection that are attributed to low-level visual cortices (Blakemore and Campbell 1969; Hubel and Wiesel 1968). Thus our study expands current knowledge on the causal role of V1–V3 complex in detecting contrast or gratings orientation by showing that offline cTBS over these regions can lower participants' performance in the Gabor patch task. Conversely, activity of rTPJ turned out to be crucial when the integration of visual, vestibular, and proprioceptive information is needed to solve an orientation task.
Conclusions
Taken together, the present results support the hypothesis that verticality estimation is largely based on the functioning of the rTPJ and confirm its role in integrating sensory information encoded in different frames of reference (i.e., vestibular, somatic, and visual).
GRANTS
This work was funded by European Union Information and Communication Technologies VERE Project Grant FP7-ICT-2009-5 (Prot. Num. 257695) and Italian Ministry of Health Grant RF-2010-2312912. N. David was supported by European Union eSCMs Project Grant FP7-ICT-2009-6 (#270212).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.F., M.C., N.D., and S.M.A. conception and design of research; F.F., M.C., and A.A. performed experiments; F.F. and M.C. analyzed data; F.F., M.C., and S.M.A. interpreted results of experiments; F.F. prepared figures; F.F. and M.C. drafted manuscript; F.F., M.C., A.A., N.D., and S.M.A. edited and revised manuscript; F.F., M.C., A.A., N.D., and S.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Martelli, P. Zoccolotti, and G. Antonucci for allowing the use of their RFT setup.
REFERENCES
- Anastasopoulos D, Haslwanter T, Bronstein A, Fetter M, Dichgans J. Dissociation between the perception of body verticality and the visual vertical in acute peripheral vestibular disorder in humans. Neurosci Lett 233: 151–153, 1997. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31: 125–150, 2008. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Klier EM, Snyder LH. A vestibular sensation: probabilistic approaches to spatial perception. Neuron 64: 448–461, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch SE, Witkin HA. Studies in space orientations: I. Perception of the upright with displaced visual fields. J Exp Psychol 38: 325–337, 1948. [DOI] [PubMed] [Google Scholar]
- Baccini M, Paci M, Del Colletto M, Ravenni M, Baldassi S. The assessment of subjective visual vertical: comparison of two psychophysical paradigms and age-related performance. Atten Percept Psychophys 76: 112–122, 2014. [DOI] [PubMed] [Google Scholar]
- Barbieri G, Gissot AS, Fouque F, Casillas JM, Pozzo T, Pérennou D. Does proprioception contribute to the sense of verticality? Exp Brain Res 185: 545–552, 2007. [DOI] [PubMed] [Google Scholar]
- Barra J, Marquer A, Joassin R, Reymond C, Metge L, Chauvineau V, Pérennou D. Humans use internal models to construct and update a sense of verticality. Brain 133: 3552–3563, 2010. [DOI] [PubMed] [Google Scholar]
- Barra J, Pérennou D, Thilo KV, Gresty MA, Bronstein AM. The awareness of body orientation modulates the perception of visual vertical. Neuropsychologia 50: 2492–2498, 2012. [DOI] [PubMed] [Google Scholar]
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol 85: 886–899, 2001. [DOI] [PubMed] [Google Scholar]
- Blakemore CT, Campbell FW. On the existence of neurons in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol 203: 237–260, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J Neurosci 25: 550–557, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci 13: 556–571, 2012. [DOI] [PubMed] [Google Scholar]
- Borel L, Lopez C, Péruch P, Lacour M. Vestibular syndrome: a change in internal spatial representation. Neurophysiol Clin 38: 375–389, 2008. [DOI] [PubMed] [Google Scholar]
- Bosco G, Carrozzo M, Lacquaniti F. Contributions of the human temporoparietal junction and MT/V5+ to the timing of interception revealed by transcranial magnetic stimulation. J Neurosci 28: 12071–12084, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex 11: 230–238, 1975. [DOI] [PubMed] [Google Scholar]
- Chang CF, Hsu TY, Tseng P, Liang WK, Tzeng OJ, Hung DL, Juan CH. Right temporoparietal junction and attentional reorienting. Hum Brain Mapp 34: 869–877, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, DeAngelis GC, Angelaki DE. Eye-centered representation of optic flow tuning in the ventral intraparietal area. J Neurosci 33: 18574–18582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N, Fiori F, Aglioti SM. Susceptibility to the rubber hand illusion does not tell the whole body-awareness story. Cogn Affect Behav Neurosci 14: 297–306, 2014. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, Cornelsen S, Jacobs C, Sack AT. TMS effects on subjective and objective measures of vision: Stimulation intensity and pre-versus post-stimulus masking. Conscious Cogn 20: 1244–1255, 2011. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, Koivisto M, Jacobs C, Sack AT. The chronometry of visual perception: review of occipital TMS masking studies. Neurosci Biobehav Rev 45: 295–304, 2014. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Held R, Young LR, Brandt T. Moving visual scenes influence the apparent direction of gravity. Science 178: 1217–1219, 1972. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex 13: 994–1007, 2003. [DOI] [PubMed] [Google Scholar]
- Dilorenzo JR, Rock I. The rod-and-frame effect as a function of the righting of the frame. J Exp Psychol 8: 536–546, 1982. [DOI] [PubMed] [Google Scholar]
- Docherty S, Bagust J. From line to dots: an improved computerised rod and frame system for testing subjective visual vertical and horizontal. BMC Res Notes 3: 9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emri M, Kisely M, Lengyel Z, Balkay L, Márián T, Mikó L, Berényi E, Sziklai I, Trón L, Tóth A. Cortical projection of peripheral vestibular signaling. J Neurophysiol 89: 2639–2646, 2003. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends Cogn Sci 8: 162–169, 2004. [DOI] [PubMed] [Google Scholar]
- Fiori F, David N, Aglioti SM. Processing of proprioceptive and vestibular body signals and self-transcendence in Ashtanga yoga practitioners. Front Hum Neurosci 8: 734, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerraz M, Poquin D, Ohlmann T. The role of head-centric spatial reference. Percept Psychophys 60: 287–295, 1998. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 45: 201–206, 2005. [DOI] [PubMed] [Google Scholar]
- Hubel D, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol 195: 215–243, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. Neuroimage 60: 2389–2398, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane P, Hoffmann D, Minotti L, Berthoz A. Reappraisal of the human vestibular cortex by cortical electrical stimulation study. Ann Neurol 54: 615–624, 2003. [DOI] [PubMed] [Google Scholar]
- Kaptein RG, Van Gisbergen JA. Interpretation of a discontinuity in the sense of verticality at large body tilt. J Neurophysiol 91: 2205–2214, 2004. [DOI] [PubMed] [Google Scholar]
- Kheradmand A, Lasker A, Zee DS. Transcranial magnetic stimulation (TMS) of the supramarginal gyrus: a window to perception of upright. Cereb Cortex 25: 765–771, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Allen EA, Pasley BN, Freeman RD. Transcranial magnetic stimulation changes response selectivity of neurons in the visual cortex. Brain Stimul 8: 613–623, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J, Saxe R. Theory of mind: a neural prediction problem. Neuron 79: 836–848, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BD, Dassonville P. The role of the right superior parietal lobule in processing visual context for the establishment of the egocentric reference frame. J Cogn Neurosci 26: 2201–2209, 2014. [DOI] [PubMed] [Google Scholar]
- Li D, Karnath O, Rorden C. Egocentric representations of space co-exist with allocentric representations: evidence from spatial neglect. Cortex 58: 161–169, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C, Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev 67: 119–146, 2011. [DOI] [PubMed] [Google Scholar]
- Lopez C, Lacour M, Ahmadi AE, Magnan J, Borel L. Changes of visual vertical perception: a long-term sign of unilateral and bilateral vestibular loss. Neuropsychologia 45: 2025–2037, 2007. [DOI] [PubMed] [Google Scholar]
- Lopez C, Lacour M, Magnan J, Borel L. Visual field dependence-independence before and after unilateral vestibular loss. Neuroreport 17: 797–803, 2006. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M, Ikeda A, Ohara S, Matsumoto R, Yamamoto J, Takayama M, Satow T, Begum T, Usui T, Nagamine T, Mikuni N, Takahashi J, Miyamoto S, Fukuyama H, Shibasaki H. Multisensory convergence at human temporo-parietal junction–epicortical recording of evoked responses. Clin Neurophysiol 115: 1145–1160, 2004. [DOI] [PubMed] [Google Scholar]
- Miller WL, Maffei V, Bosco G, Iosa M, Zago M, Macaluso E, Lacquaniti F. Vestibular nuclei and cerebellum put visual gravitational motion in context. J Neurophysiol 99: 1969–1982, 2008. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Harris JA, Ruzzoli M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci Biobehav Rev 37: 1702–1712, 2013. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. Somatic graviception. Biol Psychol 42: 53–74, 1996. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Fukushima K, Takada T, de Waele C, Vidal PP. Saccular stimulation of the human cortex: a functional magnetic resonance imaging study. Neurosci Lett 423: 68–72, 2007. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Perini F, Cattaneo L, Carrasco M, Schwarzbach JV. Occipital transcranial magnetic stimulation has an activity-dependent suppressive effect. J Neurosci 32: 12361–12365, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnev D, Kok P, Munneke M, Bahdo L, de Lange FP, Lau H. Continuous theta burst transcranial magnetic stimulation reduces resting state connectivity between visual areas. J Neurophysiol 110: 1811–1821, 2013. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallet M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol 122: 1686, 2011. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur J Neurosci 17: 2475–2480, 2003. [DOI] [PubMed] [Google Scholar]
- Salminen-Vaparanta N, Noreika V, Revonsuo A, Koivisto M, Vanni S. Is selective primary visual cortex stimulation achievable with TMS? Hum Brain Mapp 33: 652–665, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiesteban I, Banissy MJ, Catmur C, Bird G. Enhancing social ability by stimulating right temporoparietal junction. Curr Biol 22: 2274–2277, 2012. [DOI] [PubMed] [Google Scholar]
- Schlindwein P, Mueller M, Bauermann T, Brandt T, Stoeter P, Dieterich M. Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. Neuroimage 39: 19–31, 2008. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cereb Cortex 15: 1736–1741, 2005. [DOI] [PubMed] [Google Scholar]
- Spinelli D, Antonucci G, Goodenough DR, Pizzamiglio L, Zoccolotti P. Psychophysiological mechanisms underlying the rod and frame illusion. In: Field Dependence–Independence: Cognitive Style Across the Life Span, edited by Wapner S, Demick J. Hillsdale, NJ: Lawrence Erlbaum, 1991, p. 37–70. [Google Scholar]
- Tsakiris M, Costantini M, Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one's body. Neuropsychologia 46: 3014–3018, 2008. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Calvo-Merino B, Haggard P, Aglioti SM. Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. J Neurosci 27: 8023–8030, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108: 1–16, 1998. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys 33: 113–120, 1983. [DOI] [PubMed] [Google Scholar]
- Yelnik AP, Lebreton FO, Bonan IV, Colle FM, Meurin FA, Guichard JP, Vicaut E. Perception of verticality after recent cerebral hemispheric stroke. Stroke 33: 2247–2253, 2002. [DOI] [PubMed] [Google Scholar]
- Zoccolotti P, Antonucci G, Daini R, Martelli M, Spinelli D. Frame-of-reference and hierarchical - organization effects in the rod-and-frame illusion. Perception 26: 1485–1494, 1997. [DOI] [PubMed] [Google Scholar]
- Zoccolotti P, Antonucci G, Goodenough DR, Pizzamiglio L, Spinelli D. The role of frame size on vertical and horizontal observers in the rod-and-frame illusion. Acta Psychol (Amst) 79: 171–187, 1992. [DOI] [PubMed] [Google Scholar]