Abstract
Consolation behavior toward distressed others is common in humans and great apes, yet our ability to explore the biological mechanisms underlying this behavior is limited by its apparent absence in laboratory animals. Here, we provide empirical evidence that a rodent species, the highly social and monogamous prairie vole (Microtus ochrogaster), greatly increases partner-directed grooming toward familiar conspecifics (but not strangers) that have experienced an unobserved stressor, providing social buffering. Prairie voles also match the fear response, anxiety-related behaviors, and corticosterone increase of the stressed cagemate, suggesting an empathy mechanism. Exposure to the stressed cagemate increases activity in the anterior cingulate cortex, and oxytocin receptor antagonist infused into this region abolishes the partner-directed response, showing conserved neural mechanisms between prairie vole and human.
Consolation, which entails comforting contact directed at a distressed party, is a common empathetic response in humans that emerges in the second year of life (1). Until now, consolation behavior has only been documented in a few nonhuman species and only in the context of naturally occurring aggressive conflicts, as first described in great apes (2, 3) and subsequently in canids (4, 5), corvids (6, 7), and elephants (8). These observations have, so far, been taken to mean that consolation behavior may require advanced cognitive capacities (9). Nonetheless, rodents also manifest some of the empathy-related capacities (10–16) thought to underlie consolation in humans and chimpanzees (1, 17). If consolation behavior were to be observed outside of species with advanced cognition, this would suggest that it rests on much older, more widespread, and less cognitive capacities and may be variably expressed because of species-specific evolutionary context. Moreover, observing consolation behavior in a laboratory rodent under reproducible conditions would allow for empirical research on causal biological mechanisms relevant to human mental health.
Rodents in the genus Microtus display diverse mating strategies and social structures. The prairie vole (Microtus ochrogaster) is a socially monogamous, biparental rodent species in which both males and females may participate in philopatric cooperative breeding in the parental nest (18). These social traits frequently coevolve with other cooperative or altruistic behaviors that increase direct or indirect fitness, including social buffering among colony members (19). In contrast, closely related meadow voles (M. pennsylvanicus) are promiscuous breeders with no formal social structure that show comparatively abbreviated, uniparental care of pups (20). We hypothesized that the prairie vole, but not the meadow vole, would show consolation behavior under reproducible laboratory conditions. Additionally, we hypothesized that as suggested for humans and great apes, consolation behavior in the prairie vole would be based on an empathy mechanism. Last, we hypothesized that consolation behavior would be mediated by conserved neurobiological and neurochemical mechanisms consistent with those implicated in empathy in humans.
Consolation behavior has been defined as an increase in affiliative contact in response to and directed toward a distressed individual, such as a victim of aggression, by an uninvolved bystander, which produces a calming effect (2). This definition emphasizes victims of aggression due to observational constraints in naturalistic studies. In humans, the definition includes individuals experiencing stress from other sources (1), a strategy used in elephants (8) and suggested for primates (9). On the basis of this research, we first developed a set of laboratory conditions under which unstressed male and female prairie voles (“observers”) would respond spontaneously and selectively to stressed conspecifics (“demonstrators”) with a prosocial, other-directed behavior (the “consolation test”) (Fig. 1A). In this protocol, an observer and a demonstrator housed together are separated from each other, and the demonstrator either sits alone in a home cage compartment or is exposed to a stressor consisting of five tones paired with light foot-shocks (0.8 mA, 0.5 s) distributed over the course of 24 min (Pavlovian fear conditioning). The demonstrator is then reunited with the naïve observer, and the natural response is recorded and measured. Under these experimental conditions, licking and grooming directed by observers toward demonstrators (or “allogrooming”) was significantly longer in duration (time-treatment interaction, F1,11 = 6.7, P < 0.025) and shorter in latency (t11 = 3.9, P < 0.003) after a separation during which the demonstrator was stressed (Fig. 1B and fig. S1). Prairie vole observers did not increase allogrooming toward demonstrators after a control separation, demonstrating the selectivity of the response. Both male and female observers showed this behavioral response, differing only in baseline allogrooming (sex-time interaction, F1,73 = 6.4, P < 0.015) (fig. S2). Meta-analysis across 13 experiments shows that observers initiate allogrooming within the first minute and continue for at least the first 10 min of reunion time (Fig. 1C, figs. S3 and S4, and table S1). Additionally, stressed demonstrators that rested alone in the home cage after the stressor subsequently showed increased anxiety-like behavior relative to unstressed controls, whereas those that interacted with the observer for the same period of time showed completely normalized anxiety behavior (interaction effect, F2,63 = 3.2, P < 0.05) (Fig. 1D). This suggests that the observer provided social buffering to the demonstrator, which is consistent with other studies showing stress reduction in rodents (21, 22) and primates (3, 23). In contrast, meadow vole observers showed no differences in allogrooming based on the stress state of the demonstrator (fig. S5). The combination of a selective increase in directed affiliation with a social buffering effect supports the designation of the prairie vole’s natural response as a consolation behavior.
Fig. 1. The consolation test.
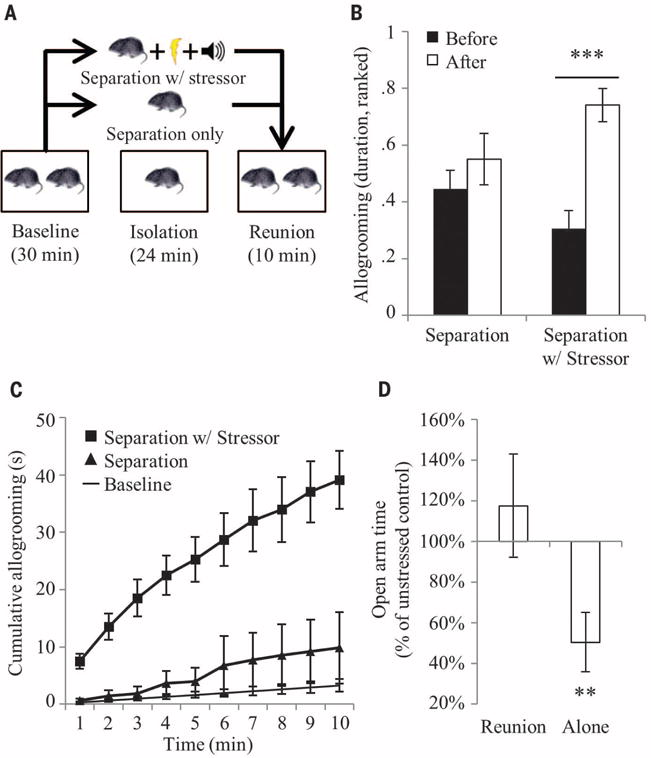
(A) The consolation test protocol. (B) Observer-demonstrator pairs (n = 12 pairs) underwent both control separations without a stressor, and separations in which the demonstrator was stressed. Duration of allogrooming was nonparametric in these experiments and was transformed to ranks, and the ranks normalized to a 0–1 scale. Bars represent the mean ± SEM of the ranked duration of allogrooming directed by the observer toward the demonstrator. (C) A meta-analysis of results from 13 experiments shows the precise expected duration of observer-demonstrator allogrooming over the course of 10 min. Points represent cumulative seconds with 95% confidence intervals. (D) After resting alone in the home cage for 5 min, stressed demonstrators (n = 10 voles) showed a significant decrease in open-arm time on the elevated plus maze test relative to unstressed controls (n = 11 voles). Stressed (n = 11 voles) and unstressed (n = 11 voles) demonstrators reunited with the observer for 5 min showed no differences in open-arm time. Bars represent the mean ± SEM of the percent change in open-arm time between stressed and unstressed demonstrators. **P < 0.005, ***P < 0.0005.
The observation that prairie voles detect the stress state of conspecifics and form a directed prosocial response raises the question of whether the behavior is empathy-based. The empathy hypothesis was tested by assaying for some of its purported characteristics in human and other mammalian species, including emotional contagion, state matching, familiarity bias, and self-other differentiation (24–26). In accordance, prairie vole observers showed behavioral responses consistent with emotional contagion by mimicking the anxiety- and fear-related behaviors of stressed demonstrators (Fig. 2). Observers interacting with a stressed demonstrator after separation matched the increase in self-grooming shown by the demonstrator (main effect of time, F1,23 = 12.7, P < 0.002) (Fig. 2A). Additionally, when observing a fear-conditioned demonstrator freezing during presentations of the conditioned stimulus (tones), the unconditioned observers showed an increase in freezing (main effect of time, F1,22 = 22.2, P < 0.0002) (Fig. 2B) concurrently with the demonstrator’s freezing (Fig. 2C). Observers separated from stressed demonstrators across a clear, perforated barrier had significantly elevated plasma corticosterone afterward (main effect of barrier, F2,27 = 4.8, P < 0.017) (Fig. 3A), which strongly correlated with that of the demonstrator (stressor, R2 = 0.82, P < 0.001; separation, R2 < 0.01, P > 0.98; difference between correlations, Fisher’s transformation, Z = 2.8, P < 0.006) (Fig. 3B), representing a clear example of physiological state–matching similar to that attributed to empathy in humans (27). Observers in full contact with demonstrators without a barrier showed no increase, suggesting that active performance of consolation behavior may ameliorate the observer’s physiological stress response. Consolation behavior was significantly biased toward familiar individuals: Although baseline allogrooming did not differ between groups containing mates, siblings, cagemates, and strangers, observers directed consolation behavior only toward familiar stressed demonstrators and not toward stressed strangers (time-relation interaction, F2,73 = 13.6, P < 0.0001; main effect of relation, F2,73 = 26.6, P < 0.0001; cage-mates, t(8) = −6.1, P < 0.0003) (Fig. 3C and figs. S6 and S7). Last, although observers and stressed demonstrators both showed signs of anxiety and stress during reunion, observers increased allogrooming toward demonstrators, whereas demonstrators themselves did not alter their allogrooming (time-subject interaction, F1,70 = 35.6, P < 0.0001) (Fig. 3D). This differential response dependent on the source of the individual’s stress (vicarious or personal) is an example of self-other differentiation, which shows that the allogrooming response is not a general stress-coping behavior.
Fig. 2. Emotional contagion.
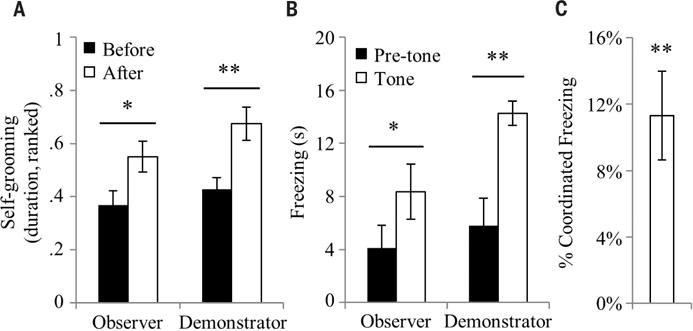
Prairie vole observers exposed to a stressed demonstrator show anxiety-and fear-related responses that match the demonstrator’s responses. (A) Anxiety-related behavior was measured in observers and demonstrators (n = 24 pairs) interacting after reunion. Bars represent the mean ± SEM of the ranked duration of self-grooming performed by the observer and demonstrator. (B) Freezing was measured while fear-conditioned demonstrators and unconditioned observers (n = 12 pairs) were exposed together to a 30-s conditioned stimulus (CS). Bars represent the mean ± SEM of freezing before and after the CS. (C) Coordinated freezing during the CS between observer and demonstrator pairs (n = 12 pairs), calculated as the within-pair difference between the observed percent of simultaneous freezing and the simultaneous freezing expected by chance. *P < 0.05, **P < 0.005.
Fig. 3. State matching, familiarity bias, and self-other differentiation.
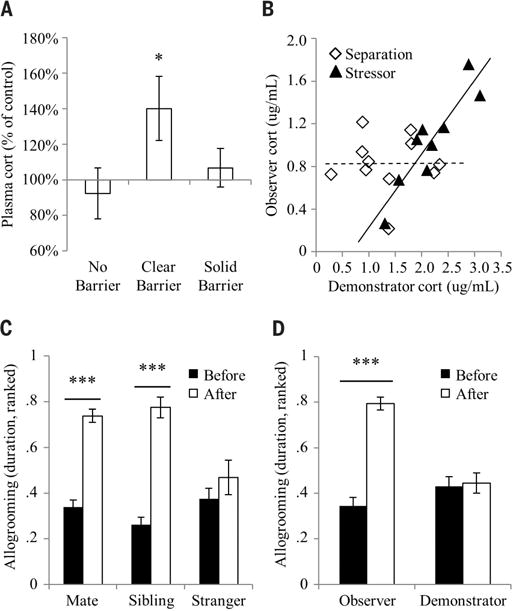
(A) Observer-demonstrator pairs underwent either control separations or separations with stressor and subsequently were either reunited in the home cage with no barrier (separated, n = 11 pairs; stressed, n = 12 pairs), reunited across a clear perforated barrier (separated, n = 11 pairs; stressed, n = 11 pairs), or in independent sections of the home cage separated by a solid opaque barrier (separated, n = 7 pairs; stressed, n = 9 pairs). Bars represent the mean ± SEM percent change in plasma corticosterone concentration in observers between the control separations and separations with stressor in each cage configuration. (B) Correlations between the plasma corticosterone concentrations of observers and demonstrators that interacted across a clear perforated barrier. The dashed and solid lines represent regression lines for the separation (n = 11 pairs) and stressor (n = 9 pairs) conditions, respectively. (C) Prairie vole mated pairs (n = 37 pairs), same-sex sibling pairs (n = 22 pairs), and same-sex stranger pairs (n = 20 pairs) underwent separations in which one cagemate was stressed. Bars represent the mean ± SEM of the ranked duration of allogrooming directed by the observer toward the demonstrator. (D) Observer-demonstrator pairs (n = 37 pairs) underwent separations during which the demonstrator was stressed. Bars represent the mean ± SEM of the ranked duration of allogrooming by either the observer or the demonstrator. *P < 0.05, **P < 0.005, ***P < 0.0005.
The combination of behavioral and physiological state matching in the observer shows that the observer is not neutral to the stress state of the demonstrator, as might be predicted if the allogrooming response were purely information-gathering behavior. Empathy-related responses and behaviors are biased toward familiar individuals in many species, including humans (10, 11, 17, 28); the allogrooming response in prairie voles is also selective for familiar conspecifics (including unrelated long-term cage-mates), representing a true social behavior rather than reproductive or kinship-related. Additionally, the lack of response toward strangers shows that observers are not simply reacting to aversive cues. Whereas some empathy-related studies used training or conditioning (15, 16, 29, 30), the consolation test in the present experiments was administered only once to each set of subjects and therefore captured unconditioned responses. The focus on unconditioned responses means that the consolation test does not assume or necessarily require any particular cognitive capacities, including conscious knowledge or perspective taking—which, in a multilayered view of empathy, may be included but are not required (24–26). Several empathy-related paradigms require priming the observer with direct exposure to the stressor (12–15); in contrast, observers in the present paradigm neither experienced nor witnessed the stressor, and therefore self-referential anticipation of a threat can be ruled out as an explanation. Last, a novel experience alone was not sufficient to elicit a consolation response in absence of a stressor (time-treatment interaction, F1,16 = 7.1, P = 0.017) (fig. S8). This confluence of evidence and exclusion of alternative explanations supports the interpretation that an empathy mechanism underlies the increase in affiliative behavior in prairie voles in response to a stressed conspecific.
In humans, the oxytocin receptor (OTR) has been linked to empathy, emotion recognition, and socioemotional engagement (31–33). Observers that received an injection of an oxytocin antagonist (OTA) into the cerebral ventricle before the consolation test did not change their baseline allogrooming but showed no consolation response (time-treatment interaction, F1,27 = 5.0, P < 0.04) (Fig. 4A), demonstrating that OTR activation in the brain is necessary for consolation behavior. The prairie vole anterior cingulate cortex (ACC), adjacent prelimbic cortex (PLC), and nucleus accumbens shell (NACS) all express high densities of OTR (Fig. 4B); in humans, the ACC and homologous medial prefrontal cortex have been linked to empathy (34), and the NACS is typically linked to social and nonsocial reward (35). Using immunohistochemistry targeting the immediate early gene protein FOS, we determined that the ACC, but not PLC or NACS, is differentially active in observers interacting with stressed demonstrators as compared with unstressed demonstrators (treatment-region interaction, F2,34 = 6.7, P < 0.004; post-hoc t test, P < 0.02 uncorrected) (Fig. 4, C and D, and fig. S9). This result was validated in observers exposed to stressed demonstrators across a clear perforated barrier (t test, P < 0.04) (fig. S10), suggesting that the difference in activity was due to exposure to the stressed demonstrator rather than caused by the observer’s behavior. Following these results, we hypothesized that oxytocin may act region-specifically on OTR in the ACC to enable consolation behavior. An injection of OTA directly into ACC abolished the consolation response in observers (time-treatment interaction, F1,13 = 7.4, P < 0.02) (Fig. 4E and fig. S11A), whereas injections into adjacent PLC had no effect (Fig. 4F and fig. S11B); this shows that OTR signaling within the ACC modulates consolation, possibly by disrupting physiological, emotional, and/or behavioral responses. This evidence demonstrates that the ACC is one node where activity increases during interaction with a stressed conspecific, and where OTR activation is necessary for the expression of consolation behavior. These neural substrates suggest conserved biological mechanisms for consolation behavior between prairie vole and human.
Fig. 4. Neural mechanisms of consolation behavior.
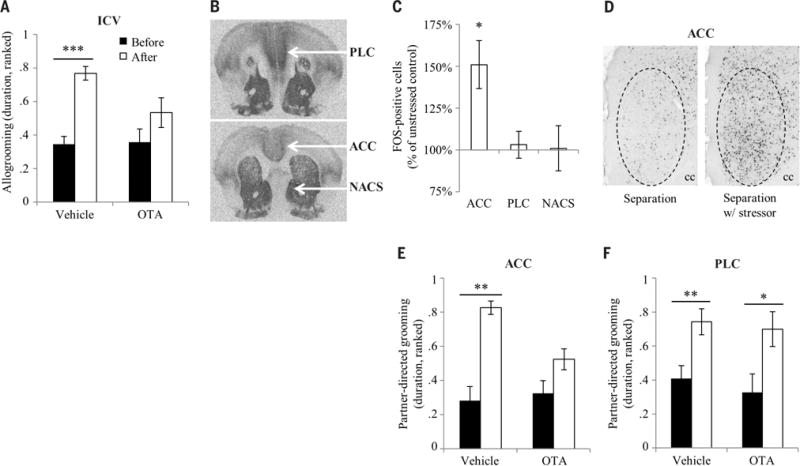
(A) Observers received an intracerebroventricular (ICV) injection of OTA (n = 16 voles) or vehicle (n = 12 voles) before the consolation test. Bars represent mean ± SEM. (B) Receptor autoradiographs show the presence of OTR in prairie vole PLC, ACC, and NACS. (C) Observers were administered a consolation test with control separations (n = 10 voles) or separations with stressor (n = 9 voles). Bars represent mean ± SEM. (D) Images show FOS immunoreactivity in the right ACC of observers representing the mean from each treatment group. Dashed circles show the quantified area. cc, corpus callosum. (E) Observers received a bilateral injection of OTA (n = 8 voles) or vehicle (n = 7 voles) directly into the ACC before the consolation test. Bars represent mean ± SEM. (F) Observers received a bilateral injection of OTA (n = 8 voles) or vehicle (n = 9 voles) directly into the PLC before the consolation test. Bars represent mean ± SEM. *P < 0.05, **P < 0.005, ***P < 0.0005.
The presence of consolation behavior in prairie voles demonstrates that this behavior does not require advanced cognitive capacities, and the conserved neurobiology of consolation between prairie voles and humans suggests a deep homology of the underlying neural substrates. The ancestral biological mechanisms supporting maternal care in mammals have likely served as the basis from which many complex social behaviors evolved, including empathy (24, 36) and pair bonding (37), both of which involve the reorienting of parental behaviors toward adult conspecifics. Nonetheless, the confirmed absence of consolation in the closely related meadow vole and in most macaques (9, 38) shows that consolation behavior emerges only under particular social and evolutionary conditions. Understanding the neurobiology of oxytocin-dependent consolation behavior in prairie voles may help us to understand the diverse deficits in detecting and responding to the emotions of others that are present in many psychiatric conditions, including autism, schizophrenia, and psychopathy.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health (1P50MH100023 and R01MH096983) to L.J.Y. and by predoctoral fellowships from the NIH (NIGMS T32GM08605-10, F31 MH102911-01) and Emory University (Emory Neuroscience Initiative Scholars Program in Interdisciplinary Neuroscience Research) to J.P.B. Additional support was provided by a grant from the NIH (OD P51OD011132) to Yerkes National Primate Research Center. We gratefully acknowledge colony management by L. Mathews, cage design and assembly by G. Feldpausch, and assistance from F. Haddad, L. Hearn, L. S. Jones, K. Kittelberger, R. C. Pearcy, M. Reyes, and M. Carr-Reynolds.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencemag.org/content/351/6271/375/suppl/DC1
Materials and Methods
References (39–47)
REFERENCES AND NOTES
- 1.Zahn-Waxler C, Radke-Yarrow M, Wagner E, Chapman M. Dev Psychol. 1992;28:126–136. [Google Scholar]
- 2.de Waal FBM, van Roosmalen A. Behav Ecol Sociobiol. 1979;5:55–66. [Google Scholar]
- 3.Clay Z, de Waal FBM. PLOS ONE. 2013;8:e55206. doi: 10.1371/journal.pone.0055206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palagi E, Cordoni G. Anim Behav. 2009;78:979–986. [Google Scholar]
- 5.Cools AKA, van Hout AJM, Nelissen MHJ. Ethology. 2008;114:53–63. [Google Scholar]
- 6.Fraser ON, Bugnyar T. PLOS ONE. 2010;5:e10605. doi: 10.1371/journal.pone.0010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seed AM, Clayton NS, Emery NJ. Curr Biol. 2007;17:152–158. doi: 10.1016/j.cub.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Plotnik JM, de Waal FBM. PeerJ. 2014;2:e278. doi: 10.7717/peerj.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal FBM, Aureli F. In: Reaching into Thought: The Minds of the Great Apes. Russon AE, Bard KA, Parker ST, editors. Cambridge Univ. Press; 1996. [Google Scholar]
- 10.Langford DJ, et al. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 11.Jeon D, et al. Nat Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EJ, Kim ES, Covey E, Kim JJ. PLOS ONE. 2010;5:e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Panksepp JB, Lahvis GP. PLOS ONE. 2009;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders J, Mayford M, Jeste D. PLOS ONE. 2013;8:e74609. doi: 10.1371/journal.pone.0074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Church RM. J Comp Physiol Psychol. 1959;52:132–134. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 16.Rice GE, Gainer P. J Comp Physiol Psychol. 1962;55:123–125. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 17.Romero T, Castellanos MA, de Waal FB. Proc Natl Acad Sci USA. 2010;107:12110–12115. doi: 10.1073/pnas.1006991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Getz LL, Mcguire B, Pizzuto T, Hofmann JE, Frase B. J Mammal. 1993;74:44–58. [Google Scholar]
- 19.Nunes S. In: Rodent Societies: An Ecological & Evolutionary Perspective. Wolff JO, Sherman PW, editors. Univ. of Chicago Press; 2007. chap. 13. [Google Scholar]
- 20.Getz LL. J Mammal. 1972;53:310–317. [Google Scholar]
- 21.Kikusui T, Winslow JT, Mori Y. Philos Trans R Soc London B Biol Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AS, Wang Z. Biol Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraser ON, Stahl D, Aureli F. Proc Natl Acad Sci USA. 2008;105:8557–8562. doi: 10.1073/pnas.0804141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston SD, de Waal FBM. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- 25.de Waal FB. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 26.Batson CD. In: The Social Neuroscience of Empathy. Decety J, Ickes W, editors. MIT Press; 2009. [Google Scholar]
- 27.Buchanan TW, Bagley SL, Stansfield RB, Preston SD. Soc Neurosci. 2012;7:191–201. doi: 10.1080/17470919.2011.588723. [DOI] [PubMed] [Google Scholar]
- 28.Palagi E, Norscia I, Demuru E. PeerJ. 2014;2:e519. doi: 10.7717/peerj.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe S, Ono K. Behav Processes. 1986;13:269–277. doi: 10.1016/0376-6357(86)90089-6. [DOI] [PubMed] [Google Scholar]
- 30.Bartal I Ben-Ami, Decety J, Mason P. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Andari E, et al. Proc Natl Acad Sci USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurlemann R, et al. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamm C, Decety J, Singer T. NeuroImage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Berridge KC, Kringelbach ML. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preston SD. Psychol Bull. 2013;139:1305–1341. doi: 10.1037/a0031755. [DOI] [PubMed] [Google Scholar]
- 37.Numan M, Young LJ. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schino G, Geminiani S, Rosati L, Aureli F. J Comp Psychol. 2004;118:340–346. doi: 10.1037/0735-7036.118.3.340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


