Abstract
The distribution of phospholipid types between the two leaflets of a membrane bilayer is a controlled feature of membrane structure. One of the two membrane catalytic activities governing this distribution randomizes the composition of the two leaflets—the phospholipid scramblases. Two proteins (Xkr8 and TMEM16F) required for the activation of these activities have been identified. One of these proteins (TMEM16F) is quite clearly a scramblase itself and provides insight into the mechanism by which transbilayer phospholipid movement is facilitated.
Keywords: lipid asymmetry, TMEM16, Xkr8, phospholipid transport
Introduction
By nature of the forces that stabilize them, membrane bilayers with a hydrophobic core require that their two leaflets have the same area. In primitive bilayers formed with molecules such as fatty acids,1 rapid exchange of components between the two leaflets ensured that this condition was always met at the expense of homogenizing the composition of the two leaflets. Modern bilayers are constructed of phospholipids, and phospholipid headgroups are ions. As a result, they do not spontaneously exchange across the membrane bilayer at a physiologically relevant rate. Nevertheless, phospholipids do cross bilayers in all living organisms because membranes contain proteins that induce or facilitate such movement. While there is still much to be learned about phospholipid movements in different organisms and organelles, the mammalian plasma membrane provides an insight into two different modes of transbilayer phospholipid movement. The first kind of phospholipid movement is seen in the plasma membranes of normal growing cells: some types of phospholipids (eg, phosphatidylserine [PS]), when present in the outer leaflet facing the environment, are rapidly captured and transferred to the inner leaflet, while other types (eg, phosphatidylcholine) are not. This type of specific and active transport of phospholipids is catalyzed by ATP-dependent phospholipid translocases, a subfamily of the P-type ATPases that appeared in the earliest eukaryotes.2,3 The second kind of phospholipid movement is seen in the plasma membranes of apoptotic cells or activated platelets, or in the endoplasmic reticulum (ER) or prokaryote cell membrane: in this case, almost all phospholipids move rapidly across the bilayer from either leaflet to the other. This nonspecific, bidirectional, and energy-independent phospholipid movement is catalyzed by phospholipid scramblase, an activity present in both eukaryotes and prokaryotes.4,5
Plasma Membrane Phospholipid Scramblases in Living Cells
A general requirement for a phospholipid scramblase activity arises in the ER in eukaryotes, or the cell membrane in prokaryotes, because the enzymes that fabricate phospholipids and the precursors from which they are constructed are located in the cytoplasm, so that newly formed phospholipids are inserted into the cytoplasmic leaflet of the membrane. The resulting imbalance in leaflet area would be profoundly destabilizing if phospholipids could not exchange freely from the cytoplasmic to the exoplasmic side. Phospholipid scramblase activities that catalyze such exchange have been demonstrated in both eukaryotes6,7 and prokaryotes.8
A second membrane with demonstrated scramblase activity is the plasma membrane in animal and particularly vertebrate cells. There are two prominent physiological functions, blood clotting9 and the removal of apoptotic cells,10,11 that depend on these phospholipid rearrangements and have been the source of most of our information about scramblase activities. Studies of scrambling in these cells were the source of the general impression that phospholipid movement is bidirectional and not specific for any particular phospholipid,12 although there is an upper limit to the size of the headgroups whose transbilayer movement is accelerated.13 These studies also imply that the trigger for the activation of these scramblases is probably variable. In the ER, no activating signal is known, as might be expected for an activity that is likely to be constitutive. In the coagulation pathway, scramblase is activated by cytoplasmic Ca2+ levels, although other mechanisms can contribute;14 in addition to platelets, Ca2+-activated scramblase is clearly present in all cells in the hematopoietic family, including lymphocytes and macrophages, but not in cells in other differentiation lineages.15 Phospholipid scramblase activity is activated in apoptotic cells in order to expose PS on the surface; the latter is a general signal for engulfment of apoptotic cells.16 The inducing signal in this case is not clear, but is probably not Ca2+,17 and is likely to involve caspase cleavage in some cases,18,19 but perhaps not always.20
Observation of the net scrambling of phospholipids inevitably requires a concentration gradient, so that no energy source for phospholipid movement has ever been needed or identified. In these measurements, the nonspecificity of scramblase activity has posed a mechanistic question: is scramblase activity fundamentally a channel activity, opening a diffusion path between the two leaflets, or is it a transporter, with scrambled phospholipids enclosed in a protein pocket that has alternating access to the two leaflets? One piece of negative evidence for the latter possibility was the absence of any large-scale conductance pathway associated with the opening of the scramblase link between the two sides of the membrane; given the large size and structural heterogeneity of the phospholipid headgroups that can traverse the bilayer by way of the scramblase, it is not easy to envision how small ions might be excluded from an open channel that was available to phospholipids.
Proteins that do Not Mediate Plasma Membrane Phospholipid Scrambling
Such detailed mechanistic questions were irrelevant in the absence of information about the protein machinery underlying the phospholipid scrambling activity that is so readily measurable in intact cells. Progress toward the identification of relevant proteins has encountered a variety of pitfalls. The relevant polypeptides were initially sought using membrane protein isolation and reconstitution, with several quite different outcomes. In the case of the ER scramblase, this strategy has substantially narrowed the collection of potential candidates without identifying a single molecule yet.21 While promising, other results from this approach have generated very interesting, if perhaps not as promising, conclusions. For example, the reconstitution of membrane proteins from human erythrocytes (which contain the Ca2+-activated scramblase activity) identified a protein (PLSCR1), which accelerated transbilayer phospholipid movements in vesicles in the presence of Ca2+.22 In fact, the elimination of this protein (still labeled as phospholipid scramblase in the NCBI Protein database) has no effect on phospholipid scrambling in hematopoietic cells,23 and it may not even be a membrane protein.24 The important lesson from this experience is that proteins may enhance phospholipid movement in the reconstituted vesicles, even though that activity has no part in the protein’s physiological function, and may even be unlikely to scramble phospholipids in vivo. An even more dramatic case of such behavior has been observed in the case of G protein coupled receptors, beginning with opsin.25 Purified opsin substantially enhances transbilayer phospholipid movement when reconstituted into vesicles, and a similar behavior is observed with β1-adrenergic receptors.25 While these interesting experiments have great promise as potential systems for studying the mechanisms of protein-enhanced phospholipid scrambling, there is no evidence (or any reason to think) that the G protein coupled receptors in plasma membranes of normal healthy cells support constitutive phospholipid scrambling. However, there is one important lesson from these experiments that should be emphasized—the ability to promote transbilayer movement of phospholipids seems to be an activity present, at least in embryonic form, in structurally very distinct proteins. In addition to the cautionary implications of this fact for interpreting experiments in simple biochemical and biophysical systems, these findings suggest that natural selection can find multiple materials ready to hand in physiological cases where the transbilayer movement of phospholipids is advantageous.
Xkr Family Proteins
In recent years, the confusing fog about the protein basis of phospholipid scramblase activity has been substantially lifted by the identification of two proteins that are involved in phospholipid scrambling at the plasma membrane of animal cells, one of which is clearly a scramblase itself. One of these plays a critical role in the scrambling of membrane phospholipids in animal cells as they enter apoptosis. The loss of lipid asymmetry in apoptotic cells results in the exposure of PS, which is the basic signal for the engulfment of apoptotic cells by their neighbors or by professional phagocytes.5 Many years ago, a gene in nematodes, CED-8, was identified as an important component of the apoptotic machinery,26 and subsequent careful examination showed that mutations in this gene result in the persistence of apoptotic cells because of the failure to expose PS.18,19 The overexpression of a protein from the same membrane protein family, Xkr8, was shown to increase PS exposure in apoptotic mammalian cells.18 This protein family is specific to the animal lineage, and its first member was originally identified as a membrane protein component of red cells, where it occurs as part of a heterodimer together with a membrane protease.27 The presence of multiple transmembrane domains in this protein has long suggested that it is a transport protein of some kind, but there is not yet any experimental evidence that it transports anything, including phospholipids. On the other hand, its connection to apoptosis is clear. Both CED-8 and Xkr8 contain caspase cleavage sites, and the ability of these proteins to promote PS exposure is linked to their cleavage by caspases.18,19 Several other members of the family (but not Xkr1, the red cell protein) can replace Xkr8, and those members of the family also contain caspase cleavage sites.28 The critical question is what exactly do these proteins do? One obvious possibility is that they are caspase-activated phospholipid scramblases; an alternative is that they sense the onset of apoptosis and transduce the caspase signal by direct or indirect interaction with some other protein that is the actual scramblase. Future investigations should soon resolve this issue.
TMEM16 Proteins
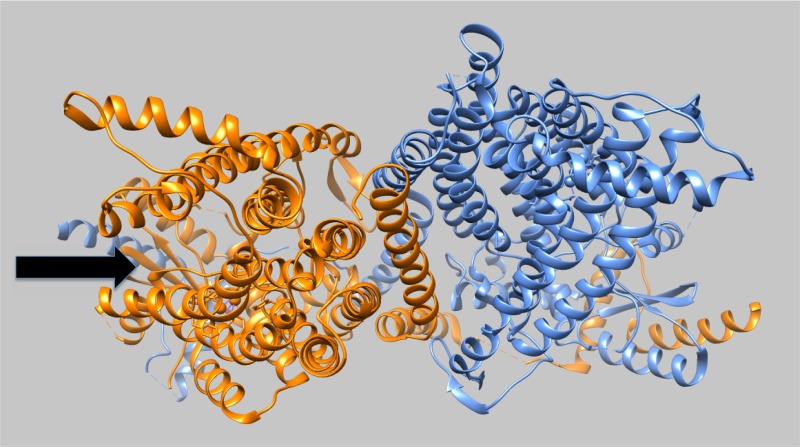
It has long been known that a Ca2+-induced phospholipid scramblase activity catalyzes the exposure of PS on the platelet surface, where it serves as the platform for the assembly of protease complexes that underlay clot formation.29 Moreover, a rare human mutation gives rise to Scott syndrome, a bleeding disorder resulting from the disappearance of this Ca2+-induced scramblase from platelets,30 as well as other hematopoietic cells, including erythrocytes and lymphocytes.5 Scott syndrome mutations are defects in TMEM16F, a member of the TMEM16 or anoctamin family of membrane proteins.31,32 At the time of this discovery, it was already clear that the TMEM16A member of this protein family is a Ca2+-activated chloride channel,33 showing that these proteins do mediate transbilayer ion movements. Remarkably, two fungal members of this family show robust Ca2+-activated scramblase activity when reconstituted into vesicles,34,35 and for one of these, a structure of the Ca2+-bound form at atomic resolution has been determined.35 This fungal phospholipid scramblase is a homodimer, with 10 transmembrane helices and a conserved Ca2+-binding site in each of the two monomers. A striking feature of the structure is the presence of a relatively hydrophilic trench in each monomer that faces the hydrophobic membrane core and extends from one side of the membrane to the other (Fig. 1). Domain swapping experiments have shown that the substitution of a helix that lines this trench into a Ca2+-activated chloride channel member of the family can convert the latter into a phospholipid scramblase.36 These data provide relatively direct evidence that this trench forms the pathway for phospholipid headgroup transfer across the membrane core. The dimensions of this trench imply that as phospholipids exchange between the two leaflets, the hydrophobic fatty acid side chains of those phospholipids probably never leave the hydrophobic core of the membrane. The general hydrophilic character of the trench, combined with the absence of a phospholipid bound anywhere along the trench, is consistent with the possibility that phospholipid scrambling does not depend on high affinity binding between a phospholipid and the scramblase. On the other hand, the absence of a Ca2+-free structure makes it difficult to see how Ca2+ binding activates the pathway for phospholipid exchange.
Figure 1.
TMEM16 structure. The structure of the Nectria haematococca TMEM16 dimer is shown as viewed from the membrane outer surface. The symmetry axis is tilted slightly toward the top of this figure to reveal the hydrophilic trench (arrow) on the protein surface that extends through the membrane core and forms the likely path taken by the phospholipid headgroups in this Ca2+-activated form of the scramblase. A similar groove is present in the other subunit but is obscured by the tilting of the viewing axis. Image created from PDB 4WIS35 using Chimera (https://www.cgl.ucsf.edu/chimera/).
The structure described above seems to settle the question of whether scramblases act as channels or as transporters in favor of the former: the TMEM16 family, at least, act as phospholipid channels. The issue of ion conductance through this protein has been the subject of a substantial number of investigations with very heterogeneous results.37–39 The structure of the fungal protein suggests that the relatively small and variable currents observed in the reconstitutes of TMEM16 proteins may represent a low level of leakage along the same pathway taken by phospholipids.35
Remarkably, this protein family is found throughout the eukaryote lineage, including in unicellular organisms for which blood coagulation is an irrelevant function. Moreover, phospholipid scrambling may have been the original function of these membrane proteins.36,40 It is not clear what might be the general physiological role played by Ca2+-activated phospholipid scrambling; one intriguing clue may be provided by the observation that the Scott defect not only impairs blood clotting but also interferes with bone formation.41 The mechanistic link between these two functions is currently obscure, but light shown on this problem may provide insight into the general function of this ancient activity.
Footnotes
ACADEMIC EDITOR: Tim Levine, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 543 words, excluding any confidential comments to the academic editor.
FUNDING: Author discloses no external funding sources.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: PW. Contributed to the writing of the manuscript: PW. Jointly developed the structure and arguments for the paper: PW. Made critical revisions and approved final version: PW. Author reviewed and approved of the final manuscript.
REFERENCES
- 1.Budin I, Debnath A, Szostak JW. Concentration-driven growth of model protocell membranes. J Am Chem Soc. 2012;134(51):20812–20819. doi: 10.1021/ja310382d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang X, Halleck MS, Schlegel RA, et al. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272(5267):1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 3.Sebastian TT, Baldridge RD, Xu P, et al. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821(8):1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomorski T, Menon AK. Lipid flippases and their biological functions. Cell Mol Life Sci. 2006;63(24):2908–2921. doi: 10.1007/s00018-006-6167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevers EM, Williamson PL. Phospholipid scramblase: an update. FEBS Lett. 2010;584(13):2724–2730. doi: 10.1016/j.febslet.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Backer JM, Dawidowicz EA. Reconstitution of a phospholipid flippase from rat liver microsomes. Nature. 1987;327(6120):341–343. doi: 10.1038/327341a0. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann A, Zachowski A, Devaux PF. Protein-mediated phospholipid translocation in the endoplasmic reticulum with a low lipid specificity. Biochemistry. 1990;29(8):2023–2027. doi: 10.1021/bi00460a010. [DOI] [PubMed] [Google Scholar]
- 8.Kubelt J, Menon AK, Muller P, et al. Transbilayer movement of fluorescent phospholipid analogues in the cytoplasmic membrane of Escherichia coli. Biochemistry. 2002;41(17):5605–5612. doi: 10.1021/bi0118714. [DOI] [PubMed] [Google Scholar]
- 9.Zwaal RF, Comfurius P, Bevers EM. Lipid-protein interactions in blood coagulation. Biochim Biophys Acta. 1998;1376(3):433–453. doi: 10.1016/s0304-4157(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 10.Fadok VA, deCathelineau A, Daleke DL, et al. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276(2):1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 11.Williamson P, Schlegel RA. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim Biophys Acta. 2002;1585(2–3):53–63. doi: 10.1016/s1388-1981(02)00324-4. [DOI] [PubMed] [Google Scholar]
- 12.Williamson P, Bevers EM, Smeets EF, et al. Continuous analysis of the mechanism of activated transbilayer lipid movement in platelets. Biochemistry. 1995;34(33):10448–10455. doi: 10.1021/bi00033a017. [DOI] [PubMed] [Google Scholar]
- 13.Dekkers DW, Comfurius P, Bevers EM, et al. Comparison between Ca2+-induced scrambling of various fluorescently labelled lipid analogues in red blood cells. Biochem J. 2002;362(pt 3):741–747. doi: 10.1042/0264-6021:3620741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Kruchten R, Mattheij NJ, Saunders C, et al. Both TMEM16F-dependent and TMEM16F-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation. Blood. 2013;121(10):1850–1857. doi: 10.1182/blood-2012-09-454314. [DOI] [PubMed] [Google Scholar]
- 15.Williamson P, Halleck MS, Malowitz J, et al. Transbilayer phospholipid movements in ABCA1-deficient cells. PLoS One. 2007;2:e729. doi: 10.1371/journal.pone.0000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlegel RA, Callahan MK, Williamson P. The central role of phosphatidylserine in the phagocytosis of apoptotic thymocytes. Ann N Y Acad Sci. 2000;926:217–225. doi: 10.1111/j.1749-6632.2000.tb05614.x. [DOI] [PubMed] [Google Scholar]
- 17.Williamson P, Christie A, Kohlin T, et al. Phospholipid scramblase activation pathways in lymphocytes. Biochemistry. 2001;40(27):8065–8072. doi: 10.1021/bi001929z. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki J, Denning DP, Imanishi E, et al. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341(6144):403–406. doi: 10.1126/science.1236758. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-Z, Mapes J, Lee E-S, et al. Caspase-mediated activation of Caenorhabditis elegans CED-8 promotes apoptosis and phosphatidylserine externalization. Nat Commun. 2013;4:2726. doi: 10.1038/ncomms3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182(5):1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanyal S, Frank CG, Menon AK. Distinct flippases translocate glycerophospholipids and oligosaccharide diphosphate dolichols across the endoplasmic reticulum. Biochemistry. 2008;47(30):7937–7946. doi: 10.1021/bi800723n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basse F, Stout JG, Sims PJ, et al. Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J Biol Chem. 1996;271(29):17205–17210. doi: 10.1074/jbc.271.29.17205. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Zhao J, Wiedmer T, et al. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood. 2002;99(11):4030–4038. doi: 10.1182/blood-2001-12-0271. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Efraim I, Zhou Q, Wiedmer T, et al. Phospholipid scramblase 1 is imported into the nucleus by a receptor-mediated pathway and interacts with DNA. Biochemistry. 2004;43(12):3518–3526. doi: 10.1021/bi0356911. [DOI] [PubMed] [Google Scholar]
- 25.Menon I, Huber T, Sanyal S, et al. Opsin is a phospholipid flippase. Curr Biol. 2011;21(2):149–153. doi: 10.1016/j.cub.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanfield GM, Horvitz HR. The ced-8 gene controls the timing of programmed cell deaths in C elegans. Mol Cell. 2000;5(3):423–433. doi: 10.1016/s1097-2765(00)80437-2. [DOI] [PubMed] [Google Scholar]
- 27.Redman CM, Lee S. A historical perspective on the discovery of the Kell blood group carriers. Transfusion. 2013;53(11 suppl 2):2831–2833. doi: 10.1111/trf.12405. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki J, Imanishi E, Nagata S. Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J Biol Chem. 2014;289(44):30257–30267. doi: 10.1074/jbc.M114.583419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Versteeg HH, Heemskerk JW, Levi M, et al. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 30.Rosing J, Bevers EM, Comfurius P, et al. Impaired factor X and prothrombin activation associated with decreased phospholipid exposure in platelets from a patient with a bleeding disorder. Blood. 1985;65(6):1557–1561. [PubMed] [Google Scholar]
- 31.Suzuki J, Umeda M, Sims PJ, et al. Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010;468(7325):834–838. doi: 10.1038/nature09583. [DOI] [PubMed] [Google Scholar]
- 32.Castoldi E, Collins PW, Williamson PL, et al. Compound heterozygosity for 2 novel TMEM16F mutations in a patient with Scott syndrome. Blood. 2011;117(16):4399–4400. doi: 10.1182/blood-2011-01-332502. [DOI] [PubMed] [Google Scholar]
- 33.Flores CA, Cid LP, Sepulveda FV, et al. TMEM16 proteins: the long awaited calcium-activated chloride channels? Braz J Med Biol Res. 2009;42(11):993–1001. doi: 10.1590/s0100-879x2009005000028. [DOI] [PubMed] [Google Scholar]
- 34.Malvezzi M, Chalat M, Janjusevic R, et al. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nat Commun. 2013;4:2367. doi: 10.1038/ncomms3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner JD, Lim NK, Schenck S, et al. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 36.Yu K, Whitlock JM, Lee K, et al. Identification of a lipid scrambling domain in ANO6/TMEM16F. eLife. 2015;4:e06901. doi: 10.7554/eLife.06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins) Physiol Rev. 2014;94(2):419–459. doi: 10.1152/physrev.00039.2011. [DOI] [PubMed] [Google Scholar]
- 38.Scudieri P, Caci E, Venturini A, et al. Ion channel and lipid scramblase activity associated with expression of TMEM16F/ANO6 isoforms. J Physiol. 2015;593(17):3829–3848. doi: 10.1113/JP270691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picollo A, Malvezzi M, Accardi A. TMEM16 proteins: unknown structure and confusing functions. J Mol Biol. 2015;427(1):94–105. doi: 10.1016/j.jmb.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bevers EM, Williamson P. Getting to the outer leaflet: the physiology of PS exposure. Physiol Rev. 2015 doi: 10.1152/physrev.00020.2015. [DOI] [PubMed] [Google Scholar]
- 41.Ehlen HW, Chinenkova M, Moser M, et al. Inactivation of anoctamin-6/Tmem16f, a regulator of phosphatidylserine scrambling in osteoblasts, leads to decreased mineral deposition in skeletal tissues. J Bone Miner Res. 2013;28(2):246–259. doi: 10.1002/jbmr.1751. [DOI] [PubMed] [Google Scholar]