Abstract
Postoperative cognitive dysfunction (POCD) is a decline in memory following anaesthesia and surgery in elderly patients. While often reversible, it consumes medical resources, compromises patient well-being, and possibly accelerates progression into Alzheimer’s disease. Anesthetics have been implicated in POCD, as has neuroinflammation, as indicated by cytokine inflammatory markers. Photobiomodulation (PBM) is an effective treatment for a number of conditions, including inflammation. PBM also has a direct effect on microtubule disassembly in neurons with the formation of small, reversible varicosities, which cause neural blockade and alleviation of pain symptoms. This mimics endogenously formed varicosities that are neuroprotective against damage, toxins, and the formation of larger, destructive varicosities and focal swellings. It is proposed that PBM may be effective as a preconditioning treatment against POCD; similar to the PBM treatment, protective and abscopal effects that have been demonstrated in experimental models of macular degeneration, neurological, and cardiac conditions.
Keywords: photobiomodulation, PBM, postoperative cognitive dysfunction, POCD, cytoskeleton, neuroprotection
Introduction
Postoperative cognitive dysfunction (POCD) is a neurodegenerative condition, acquired after surgery and anaesthesia,1,2 and is similar to Alzheimer’s disease (AD) in symptoms and risk factors such as age and education level.1,3 POCD has become a significant problem in the health-care system, in terms of both patient outcome and increased resources expended. As yet, there are a few effective therapeutic interventions. Photobiomodulation (PBM) is the use of (nonthermal) visible and infrared light to promote therapeutic benefits.4–6 Recently, PBM has been shown to be effective against neurodegenerative disorders, including AD,7 Parkinson’s disease (PD),8 and depression,9 in both animal models and clinically. The concept of preconditioning in health with laser treatments has been explored over the past few years with increasing evidence of its effectiveness.10 This paper reviews the effects of PBM treatment on the cytoskeleton as a mechanism behind preconditioning and its proposed use for preconditioning and neuroprotection against POCD. Cytoskeleton modulation, as well as the parallel between the evoked PBM response and endogenous mechanisms of neuroprotection in hibernation, cortical spreading depression (CSD), N-methyl-d-aspartate (NMDA) poisoning, and ischemic preconditioning, is reviewed. These mechanisms involve interaction between a number of proteins and signaling molecules, including TWIK-related spinal cord potassium channels (TRESK) and transient receptor potential vanilloid 1 (TRPV1) ion channels. These proteins may interact with the cytoskeleton,11,12 post-synaptic density protein 95 (PSD-95), cypin, and prion protein (PrPC), which together organize cytoskeleton structure.13–15 This review discusses the role of the cytoskeleton in allostasis in response to redox stress and cellular stress,15,16 which results in neuroinflammation17 and protein interactions of the axonal and synaptic densities.18 PBM has been shown to have a direct effect on the cytoskeleton, which is directly involved in neural blockade, in pain modulation19 and most probably in the preconditioning effects of PBM, which may also be important in preconditioning against POCD. The emphasis is on the neuroprotective role of small, reversible axonal varicosities that are protective against the large destructive neural varicosities seen in neurodegenerative disease and sympathetically dysregulated pain.
Postoperative Cognitive Dysfunction
POCD is also known, in the literature, as postoperative cognitive deficit, postoperative cognitive decline, perioperative cognition deficit, and postoperative cognitive change. It is a widely recognized clinical condition, involving the loss of cognition following anaesthesia and surgery. Although POCD has been extensively reviewed,1,20–24 it has no universally accepted definition. In fact, there is no International Statistical Classification of Disease code for POCD, Diagnostic and Statistical Manual of Mental Disorders code, gold standard diagnostic criteria, and recognized biomarker.22 However, the perception of POCD as a problem and a consequence of anaesthesia and surgery has been recognized since 1860 when Bigelow first used anesthetics.25 POCD has been increasingly documented since 195526 and has been well described as an objective diagnosis.27 An operational understanding of POCD is its manifestation as an acute but often subtle deterioration in cognition, with a loss of the ability to perform tasks involved with everyday living. It may affect a spectrum of cognitive abilities, including memory, speed of information processing, orientation, concentration, psychomotor ability, fine motor coordination, and attention span. POCD is observed in patients as the inability to accomplish simple cognitive tasks, such as crosswords,1 and is diagnosed using a variety of neuropsychological tests. Definitive diagnosis requires that the tests be performed preoperatively, in order to obtain a baseline from which a decline can be determined. Postoperative tests are best performed one week after surgery, after any postoperative delirium has passed, and after the cessation of any drugs and pain that might cause interference in the testing. Most studies20,27,28 agree that the major factors that influence POCD are increasing age (>60 years, although some studies use >65 years or even >70 years), preoperative cognitive condition, and education. Cognitive reserve and trajectory are perhaps the most important factors that influence the risk of POCD.29 Additional factors include length and complexity of the surgery (with cardiac surgery possibly being more risky than noncardiac surgery),20,28 a history of alcohol abuse,30 previous stroke,28 diabetes mellitus, hypertension, atherosclerosis,31 and postoperative complications, especially respiratory complications and postoperative infections.27 A recent study has also identified gender as a factor, with females being at greater risk than males,32 as is the case with AD.33 Although POCD in the very young is less studied, most evidence, such as that obtained from twin studies34 and cohort studies,35 suggests that it is much less of a problem. However, Yin et al determined that propofol could impair short-term memory in children.36
Although there have been numerous studies that have reported POCD, many of these are anecdotal, are case studies, or are poorly controlled and inadequately tested. Some clinicians and researchers consider that there is a lack of statistical evidence to separate POCD from normal cognitive decline and reviews of case-controlled studies using stringent criteria have shown mixed results in the past.22 For example, a review of 25 randomized controlled trials did not demonstrate unequivocal POCD response in patients37 and a meta-analysis of 26 randomized controlled trials found no evidence of POCD.38 Part of the difficulty in the study of POCD is the variety of testing regimes and diagnostic tools that have been used in various studies and the consequent inability to compare between studies. Other difficulties include the lack of appropriate control groups in many studies and the difficulty in determining the normal cognitive trajectory of surgery patients in the studies.22 In addition, many of the studies in the past have been small, lacked power, and were retrospective. Despite these problems, there is compelling evidence that POCD exists as a genuine phenomenon21,37,39 with a strong public and medical awareness of the consequences of the disorder. Recent prospective studies of POCD have indicated that the risk of POCD posed by anaesthesia/surgery was 1.3540 and 1.9941 compared with the general population. In recent years, a number of prospective, long-term, and cohort studies have been initiated in order to provide more definitive information and predictions for POCD.
The general acceptance of POCD as a real and measurable disorder has resulted in increased attention and research into the implications of POCD. Each day, millions of people around the world undergo anaesthesia and surgery. Increasing life expectancy and the consequent increase in the elderly population, the advances in surgical procedures, the decline in mortality rates, and the shortening of postoperative recovery times point to an increasing number of surgical procedures performed on the elderly, the population most at risk of POCD. For example, statistics from the Australian Institute of Health and Welfare (http://www.aihw.gov.au/) indicate that in 2010, 32% of all anesthetics were given to >65 years old (13.5% of population). With the predicted percentage of >65 years old in the population in 2051 increasing to 24.2%, anesthetics given to >65 years old is predicted to be 48% of all anesthetics administered. In addition to being major recipients of surgical procedures, elderly patients are at greater risk of cognitive decline and dementia, pointing to an increasingly important role for POCD in the postoperative recovery of elderly patients.
The reported incidence of POCD varies widely with different studies, most probably reflecting methodological differences. Incidence can range from 10% to 40% after one week and up to 15% after three months postoperatively in noncardiac surgery. The International Study of POCD (ISPOCD) has concluded that 26% of patients older than 60 years developed POCD at one week postoperatively and 10% had POCD at three months.27 Although it has been commonly accepted that cardiopulmonary bypass surgery has a higher risk of POCD than noncardiac surgery,20,28 this might in fact be due to the generally less rigorous criteria used in many cardiac surgery studies,22 the differences in diagnostic criteria21 or to specific factors common to cardiac surgery. Evered et al42 found that at three months, the number of patients with POCD were independent of whether the surgery was cardiac or total hip replacement and, in general, the number of patients with POCD at three months are similar in both groups.23
POCD may be short-lived and reversible or may last for months or possibly years, with the potential to affect clinical outcomes for up to five years postoperatively.43 While up to 47% of elderly patients could demonstrate some cognitive decline after 24 hours, this decreases to much lower levels by the time of discharge.21 Early POCD, lasting up to three months, may in fact be a common problem, affecting not only up to 10% of elderly surgery patients but also young patients, but in whom recovery is much faster.21 Recovery from POCD sets this condition apart from other neurodegenerative diseases (AD, PD, etc.) and the recovery is similar (albeit much slower) to the recovery in cognitive decline that occurs within hours after CSD that accompanies migraine with aura and cluster headaches.44
POCD may also be progressive, with some patients who do not show early POCD at one week, progressing to POCD at three months. POCD might also be cumulative, with more episodes of anaesthesia/surgery leading to a greater incidence of POCD.31 Even if there is complete recovery from POCD, the effects of short-term POCD impact on patients’ quality of life and the ability to continue in employment. There is, therefore, a socioeconomic burden, including increased hospital stays, increased out of hospital care, job loss, and dependence on social payments.45 There may also be an increase in mortality, with POCD patients 1.63 times as likely to die as non-POCD patients.45
Persistent POCD is more contentious. Some studies have shown evidence of persistent POCD in a small number of patients. The ISPOCD showed that after one to two years, 1% of patients showed persistent POCD.46 Some early studies indicated that dementia was still apparent after five years,47 with one study showing high levels of long-term POCD (42%) at five years.48 The ISPOCD long-term study found, however, no significant relationship with dementia after 11 years,49 and other studies have shown little evidence of cognitive decline after a number of years when compared with nonsurgery patients.50–52
The cause or causes of POCD have remained elusive, despite intensive research over the past 25 years. As with other forms of dementia, the cause of POCD is almost certainly multifactorial. Part of the difficulty in determining etiological factors involved in POCD is in the separation of anaesthesia, surgery, and perioperative care; it is not usual to give anaesthesia without surgery and surgery is most usually performed under anesthetic. The time in hospital may also be a factor, the so-called hospital stay syndrome.53 Other contributing factors to POCD may include perioperative conditions, inflammation, pain, and comorbidities, although a number of specific factors, such as changes in cerebral blood flow, cardiopulmonary bypass, hypoxemia, and microemboli, have been all but discarded as sole causes.23 In reviewing available evidence, Krenk et al21 suggest a multifactorial pathogenesis with the potential involvement of postoperative sleep disturbance (exacerbated by opioid analgesia), inflammatory stress response, pain, and environmental factors. Fast-track hip and knee replacements, which included patient education and preparation, as well as shorter hospital stays, were shown to result in decreased short-term POCD, but not long-term POCD.54
POCD shows some similarities with other forms of dementia, such as AD, which it mimics in a number of ways3,55,56 and POCD may in fact be triggered by AD pathways.55 Mild cognitive impairment (MCI) is a subjective decline in cognition and can be a precursor to AD, with a substantial minority with MCI (depending on age) progressing to AD.57 MCI is prevalent in the elderly population with between 14% and 18% of people over 75 showing symptoms.23 Since a substantial proportion of elderly patients undergoing surgery will have MCI, it is possible that anesthetics and surgery could aggravate or unmask MCI and lead to progression to POCD and ultimately to AD. Aging of proteins and an increase in misfolded and unrectified proteins lead to an increase in protein-folding neurodegenerative diseases (such as AD, PD, Huntington’s disease (HD), and prion diseases) and are most probably also linked with POCD.13,58 There is currently a great deal of research into the link between POCD, anaesthesia/surgery, and dementia, especially AD, since any connection would indicate a far greater and longer lasting impact of POCD.
The exact mechanism of action of anaesthesia is still unclear. General anesthetics have a number of common receptors in the central nervous system, including either blocking NMDA receptors (eg, ketamine and nitrous oxide)59 or enhancing gamma-aminobutyric acid type A (GABAA) receptors.60 The fact that these receptors are known to affect memory61,62 raises the possibility of a direct link between anesthetics and POCD. Cell culture studies indicate that a number of anesthetics cause apoptosis39 and mounting evidence from animal studies has strengthened the link between anesthetics and dementia including POCD.55,63–65 For example, anaesthesia has been shown to cause cognitive deficit and neurodegeneration in developing (rat) brains,66–68 and vanilloid anaesthesia has been shown to lead to long-term memory impairment in adult and aged rats69 and mice,70 as well as a transient decrease in the expression of hippocampal neuronal nitric oxide synthase (nNOS) and PSD-95 in aged rats, together with cognitive impairment.71 Anaesthesia and cognitive deficit in animal studies has been linked with NMDA receptor expression,72 disruption of calcium homeostasis,73 and neuroinflammation (see the following sections). Although many studies emphasize the potential link between anesthetics and cognitive decline, Callaway et al found no link between sevoflurane and long-term cognitive impairment in aged rats74 and found that the effects of desflurane were dose dependant and not long lasting.75
A number of neurodegenerative diseases (AD, PD, HD, tauopathies) have in common the disruption of the cytoskeleton, with the disassembly of microtubules (MTs) and the concomitant accumulation of tau fibrils and β-amyloid (Aβ). Anesthetics are known to interact with the cytoskeleton,64,76–79 can bind to tubulin and cause MT disassembly,80 and so are appropriate targets as potential causative agents of POCD. Craddock et al80 have identified multiple (32) binding sites for volatile anesthetics on α- and β-tubulin and consider that anesthetics are prime candidates as causative agents of POCD, via altered tubulin and phosphorylation of tau, leading to MT instability. Animal studies have also suggested that anesthetics (propofol, halothane, sevoflurane, and isoflurane) can increase AD β-amyloid24,63,81–83 and increase tau phosphorylation70,84,85 with the anesthetic sevoflurane shown to produce transient hyperphosphorylation of tau in mice on a single application and persistent tau hyperphosphorylation and memory impairment with repeated exposure.84 The anesthetic propofol was also shown to induce tau hyperphosphorylation in a mouse hippocampus model of AD.86 On the other hand, exposure of presymptomatic AD mice to anesthetics (halothane, isoflurane) did not accelerate the progression of the disease but, on the contrary, appeared to result in the preconditioning against neurodegeneration, due to increased phosphorylation of tau.87
Despite anecdotal and some epidemiological evidence of a link between anesthetics and AD,88 PD,89–91 and POCD,92 clinical evidence does not, on the whole, support the animal studies. Large studies such as the ISPOCD93 as well as meta-analyses94,95 have found no link between anesthetics and POCD or AD, including comparisons between general and regional anesthetics.93,96 However, an expert group attending the British Journal of Anaesthesia Salzburg Seminar in 2012 reviewed the available data on POCD and concluded that there was mounting evidence to indicate that general anaesthesia can negatively affect cognition especially in the elderly (as well as the very young).70,97 Although there is little direct evidence that the type of anesthetic is a risk factor,37,70 it is possible that the route of anesthetic and depth of anaesthesia may have an impact on POCD. The use of the bispectral index to guide anesthetic titration has been found to reduce the occurrence of POCD in some studies.98,99 There are also indications that it is not simply anesthetic use that leads to POCD. Surgery-induced nociception without anesthetics was shown to induce POCD in mice.100
There are a number of studies that have shown that surgery/anesthetics can lead to increases in the biomarkers for AD,85,101 including β-amyloid102,103 and phosphorylated tau,101,103 strengthening the case for some involvement of anesthetics with the risk of AD.89,90 The presence of brain β-amyloid has in fact been found to be a good predictor of POCD risk in cognitively normal patients.104 A consensus statement issued from an international workshop on anesthetics and AD105 concluded that there was sufficient evidence to warrant further investigations into the onset and progression of AD and neurodegeneration after anaesthesia and surgery and that clinical trials should be emphasized, which are led by anesthetists. Anesthetic delivery to patients undergoing surgery has always been a highly individualized process. This individual approach is amplified in elderly and other at-risk patients. There as yet have been no studies of POCD in groups that require different anesthetic regimes, such as redheaded women.106 The focus of research on POCD is moving from anaesthesia and surgical techniques that are common to all patients and moving toward individual patient-centered factors.2
The molecular mechanism of POCD (as with other neurodegenerative diseases) has been difficult to pin down. Induced POCD in mice has been shown to reduce NMDA receptor B levels,100 which, along with PSD-95, is implicated in synaptic plasticity and learning.107 Recently, aspartic acid, an agonist and activator of NMDA receptors which implicated in AD,108 has been identified as a possible biomarker of POCD in aged rats.109 POCD was found to be linked with endogenous melatonin levels and possibly circadian rhythms in patients who had undergone abdominal surgery.110 Proteomics has provided a window into possible molecular mechanisms of POCD and other neurodegenerative diseases. Li et al, in a study of aged rats with cognitive dysfunction following anesthetic and surgery, identified 21 proteins that were altered (upregulated or downregulated) following surgery/anaesthesia.111 Four of these proteins were involved in oxidative stress, seven proteins with mitochondrial energy production, and three proteins were implicated in neuroinflammation. Kalenka et al112 found that 17 proteins differentially expressed in rat hippocampus after isoflurane anaesthesia, including proteins involved in stress response and cytoskeleton integrity. In a clinical proteomic study, 58 separate polypeptides were found to have changed expression in patients identified with POCD following surgery.113 Interestingly, in a proteomic study of twins with no symptoms of AD, mitogen-activated protein kinase (MAPK) was found to be related to early cognitive decline over a 10-year period.114
Neuroinflammation may play a role in POCD,2,115–118 as pain119 and alleviation of each may reduce short-term POCD.118,120 Injury and insult lead to the formation of an inflammasome, which initiates an inflammatory cascade involving inflammatory cytokines, including interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and nuclear factor kappa enhancer of activated B cells (NF-κB), regulated by alpha-melanocyte-regulating hormone (α-MSH). The involvement of the inflammatory response in POCD is suggested by a number of animal studies. Anaesthesia alone and anaesthesia combined with surgery can induce IL-1β121–125 and TNF-α123 in mouse and rat models of POCD. Isoflurane without surgery has also been shown to increase TNF-α, IL-6, and IL-1β in mice.126 Li et al,111 identified three proteins that were involved in neuroinflammation.
The link between inflammatory cytokines and POCD is also suggested by clinical studies, which parallels the suggested association between inflammatory cytokines and AD.127 High-mobility group box 1 and IL-6 were found to be significantly correlated with POCD in patients who had undergone major surgery,128 while Ji et al129 found that IL-1β (but not IL-6) was associated with POCD in total hip replacement surgery. Inflammation markers 1L-6, IL-1β, TNF-α, S-100B, and tau were also found to increase after surgery.103 Recent studies have also shown some link between POCD in patients and levels of insulin-like growth factor 1 (IGF-1) and IGF-1 binding protein 7, both were believed to be important in memory consolidation and AD.17 IL-6 has been suggested as playing a crucial role in the neuroinflammatory response leading to POCD.130 Inflammatory cytokines have also been associated with POCD after cardiac surgery131 and levels of S-100, an indicator of traumatic brain injury, was found to be an indicator of POCD.132,133 In addition, in a meta-analysis of studies investigating the inflammatory response of patients, IL-6 and S-100B were identified as being correlated with POCD.134 The inflammatory cascade is, in part, controlled by the melanocortin system including α-MSH, which downregulates inflammatory cytokines.135 Melatonin is important as a risk factor of AD136 and possibly POCD.110 The melanocortin system also has a PrPC regulatory involvement137 and Mariante et al138 contend that PrPC is involved in a regulatory loop of inflammatory processes linked with systemic or cellular stress.
There is a need to identify patients at risk of POCD, but as yet, no common genomic indicators of POCD have been unambiguously identified. This includes apolipoprotein E (ApoE), which has been associated with POCD in some studies139 but not in others,140,141 although a recent prospective study has shown that patients carrying the ApoE4 genotype (the highest genetic risk factor for AD)142 had an increased risk of POCD.143 A review of the literature has identified a number of potential markers of POCD,144 including C-reactive protein (CRP), P-selectin (SELP), complement component 3 (C3F), inducible NOS (iNOS), and cytochrome P450. The presence of brain β-amyloid has also been found to be a good predictor of POCD risk in cognitively normal patients104 and a link has also been established between Aβ42/tau ratio (an indicator of AD) in the cerebrospinal fluid of patients prior to surgery and POCD.129,145 Proteomic studies have suggested that fibrinopeptide A is a potential biomarker.113
Neuroprotection by preconditioning is the use of sublethal insult to provoke a protective response and has had some success in the prevention of ischemic stroke, AD, and PD in animal models.146 Neuroprotection against POCD has had mixed success. Bilotta et al,37 based on a review of clinical trials, suggest that neuroprotection against POCD could be achieved with a number of drugs, such as atorvastatin. There is some evidence that amantadine, which increases glial-cell-line-derived neurotrophic factor and decreases neuroinflammation, might reduce the effect of POCD.147 IL-6 receptor antagonists have also been found to act as a preventative measure against POCD.130 Remote ischemic preconditioning, however, was not found to be effective as neuroprotection against POCD;148 nor was propofol (used to suppress electroencephalogram bursts),149 reduction in C5 complement,150 platelet activating factor antagonist151 or the corticosteroid dexamethasone.152 Presurgical cognitive intervention has been shown to have some effect on reducing POCD.153 Interestingly, the administration of melatonin prior to isoflurane anaesthesia in rats was shown to reduce cognitive impairment.154
PBM has been shown to have an effect on neurodegenerative diseases in animal models, including AD, PD, and depression.7,9,155,156 Purushothuman et al7 propose that the ability of PBM to reduce hyperphosphorylation of tau is neuroprotective in AD. It has also been shown that laser light is absorbed by β-amyloid,157 and Grillo et al158 have shown a decrease in β-amyloid with PBM. It is therefore proposed that the success of PBM in the preconditioning against AD and the treatment of PD suggest that it might also be an effective preconditioning agent against POCD.
Photobiomodulation
PBM has been defined as a “nonthermal process involving endogenous chromophores that elicit photophysical (linear and nonlinear effects) and photochemical events at various scales, resulting in beneficial photobiological responses.”4 This is most often low-level laser therapy (LLLT) but may also be a noncoherent light-emitting diode (LED). Light was used in 1903 as a therapy for skin lesions, with an article published by Finsen in the Lancet in 1903159 reporting the striking results of the use of red light treatment to prevent the disfigurement of smallpox scars, providing that the intervention was at an early stage of the disease. Additionally, Finsen was awarded the Nobel Prize in 1903 for the use of ultraviolet (UV) light for the treatment of lupus vulgaris. Phototherapy as a treatment fell from favor until 1968 when Mester et al first showed that laser could stimulate wound healing and hair growth in mice.160 Another early use of laser therapy was the treatment of wound and skin lesions (radiation ulcers following the Chernobyl nuclear accident using argon lasers (450–530 nm).161 Over the past 45 years, PBM in the visible to infrared wavelengths (between 400 and 1072 nm) has become increasingly accepted as a therapeutic intervention, with randomly controlled clinical trials as well as animal models demonstrating a significant role for LLLT in the treatment of many conditions in veterinary as well as human patients. It has also become apparent that there is a biphasic dose response for LLLT, following the Arndt–Schulz curve,162 where increasing dose corresponds to an increasing effect up to a maximum (a dose window), after which further increasing dose evokes a negative response. PBM is currently used to treat a variety of radiation and chemotherapy-induced ulcers,163 as well as oral and other wounds4,164 and wound infection.165 The use of PBM therapy can protect against damage to the skin by UV light as well as a number of other skin conditions, including vitiligo, psoriasis, and herpes simplex.166 LLLT is used for sports injuries,167 tendon repair,168 remodeling collagen fibers in tendon injuries,169 for lymphedema management,170 and for acceleration of tooth movement during orthodontics.171 PBM has been used in the treatment of cardiac disease and cardiac protection in animal models via the modulation of iNOS and induction of mesenchymal stem cells.172–175
PBM has also been successfully used in the treatment of both acute and chronic pain in the periphery176,177 and in centrally mediated pain states including chronic neck pain.178–181 The ability of photons introduced as LLLT to modify bioelectrical signaling in peripheral nerves has been unequivocally demonstrated in animal and human models.19,182 This is of primary importance in pain treatment as suppression of action potentials in nociceptors is one of the mechanisms for the direct analgesic effects of LLLT.177 Nociceptors are selectively affected by laser irradiation, and it has been proposed that this effect underpins the pain-relieving effects of LLLT in the treatment of acute and chronic pain182 and the basis of the local anesthetic effect of LLLT, which can be effective as a pain block in such things as dental extraction.183
Most recently, there has been increasing evidence from animal studies for the use of PBM in cognitive and neurodegenerative diseases, such as depression,9,184 traumatic brain injury,185 AD,7,158,186–189 and PD.8,155,156,189,190 PBM has the added benefit of a wide dose window to achieve the effect and no identified harmful effects, within the correct dose parameters and following the contraindication recommendations of not directing PBM into eyes, over a carcinoma site or over a fetus.191
LLLT has also been shown to have a role in neuro-protection190 and preconditioning against such conditions as muscle fatigue, inflammation, and pain, as reviewed by Agrawal et al,10 macular degeneration,192,193 preconditioning in cardiac protection,172 PD190 and AD.7,186 In addition to targeting the site of the disease, this preconditioning and protection can also involve an abscopal (indirect) effect, where the effect is elicited by irradiating an area of the body remote from the site of disease or injury.189,194 This has been shown to occur in patients with macular degeneration, where the nonirradiated eye experienced the same protection as the irradiated eye.193 The abscopal effect has also been shown for cardiac disease in rats, where LLLT to a remote site (tibia) elicited a response in protection against cardiac infarct,173 upregulating iNOS and mobilizing c-kit+ cells to be recruited to the heart damage site.172,174 This abscopal effect has been shown to be at least as effective as PBM at the site of injury.174 Tibial bone marrow as a target also improved cognition in a mouse model of AD.188 LLLT delivered to the skull in mice was also shown to improve AD β-amyloid and cognition.186 Johnstone et al8,155 have shown neuroprotection in a rat model of PD, where remote preconditioning produced a similar effect on trans-cranial LLLT. They propose a systemic effect with circulating cellular or molecular factors to induce the abscopal neuroprotective effect. Keszler et al suggest that direct application of LLLT to patients’ hearts may not be necessary for the protection against cardiac ischemia due to this systemic effect.175
Current known mechanisms of LLLT action have been well reviewed4,195,196 and include roles for cytochrome-c-oxidase and mitochondrial energy production,196 retrograde mitochondrial signaling,197 NOS modulation,173,181,196,198,199 electron transfer via a redox reaction200 resulting in antioxidant enzyme activity,201,202 restoration of balance between pro- and antioxidant mediators by increasing peroxisome proliferator-activated receptor expression and glutathione concentration,203 modulation of hypoxia-inducible factor 1α (HIF-1α),204 reduction in TNF-α,205 modulation of inflammatory cytokines and ILs, NF-κB,206,207 IL-6, and IL-1β,208 modulation of growth factors IGF-1, and transforming growth factor beta-1 (TGF-β1),201 modulation of opioid and its precursor molecule proopiomelanocortin (the melanocortin signaling system),209 and cytokine abscopal effects.155 LLLT is known to downregulate the inflammatory process210 by increasing antioxidants and decreasing oxidative stress,211 via the mechanisms described earlier and by increasing super-oxide dismutase.201,203 PBM also directly affects the cell signaling molecule MAPK.167,212
In addition to the photon receptors for the mechanisms described earlier, which includes the known chromophores of melanin, flavins, porphyrins, and cytochrome C oxidase,196 there may be a second group of interactions where physical perturbations by photons cause conformational changes in receptor proteins4,194,213 especially in redox-sensitive proteins. This perturbation involves a molecular switching mechanism214 which includes the receptor tyrosine kinases,195,215 ion channels such as TRPV1 channels, which can respond to visible and infrared light,216,217 and potassium channels.218 Various opsin proteins, which belong to the G-protein-coupled receptor family, also act as photoreceptors. These include rhodopsin molecules in rod cells of the retina and in the skin,219 photopsins in cone cells of the retina, melanopsins in retinal ganglion cells, encephalopsins (OPN3) in the brain,220 and neuropsin (OPN5) in spinal tissue (eye, brain, testes, spinal cord).221 Light also regulates neuronal activity in the eye by direct allosteric modulation of GABA and NMDA receptor proteins, which directly influence neuronal signaling, depending on the redox state of the receptor.222,223 This group of interactions with receptors would involve physical perturbation of the molecular structure in the skin and neural membranes to facilitate the physiological function.4,194
There has been less attention to the role of cytoskeleton modulation as a primary LLLT mechanism. Evidence for the role of LLLT in cytoskeleton modulation, pain attenuation, and neurotransmission blockade has been demonstrated by Chen et al224 and Chow et al.19,177 As the cytoskeleton is both a receptor and an initiator of signal transduction, cytoskeleton modulation by PBM is a candidate for the observed abscopal effects of LLLT.
MT and Cytoskeleton Modulation
The cytoskeleton is an important component of all cells and consists of the MT network, neurofilaments, and actin filaments. MTs provide structural support, connect targets, and act as a track to direct vesicle and organelle traffic within the cell. MTs are composed of α- and β-tubulin dimers and have dynamic instability, where they grow and shrink, switching between assembly (rescue) and disassembly (catastrophe) according to the need, and are thus in equilibrium with unpolymerized α- and β-tubulin. This process allows rapid reorganization of the MT cytoskeleton. In neurons, MTs are found in the dendrites, cell body, and axon. In dendrites, MTs are short and have a mixed polarity. In axons, MTs form bundles of various lengths but with the same polarity,225 which is critical for neurite polarity and neurite growth226 as well as anterograde and retrograde transport.
The control of this dynamic instability is very complex and as yet poorly understood but appears to be regulated in part by multiple posttranslational modifications to the tubulin protein (eg, tyrosination, polyglutamylation, acetylation, SUMOylation)225 and in part by MT-associated proteins (MAPs),227 which bind either tubulin or assembled MTs and are thus either stabilizing or destabilizing for MTs. MAPs, such as tau (in axons) and MAP2 (in dendrites), bind directly to MTs and form transient interactions that stabilize the MTs into the parallel arrays seen as bundles. Other MAPs can promote assembly (eg, MAP4) or disassembly (eg, stathmin) of MTs. Phosphorylation of tau is necessary for MT stabilization, but under normal conditions tau phosphorylation is limited.228 With increased phosphorylation, the extent of binding to MT decreases. A number of neurodegenerative diseases (such as AD, PD, and other tauopathies)229,230 are characterized by hyperphosphorylation of tau, where up to 100% of the available sites in the protein are phosphorylated. This destabilizes MTs and leads to the formation of intracellular aggregates (neurofibrillary tangles).229 An example of this is children in Mexico City, who are exposed to heavy pollution, can develop hyperphosphorylation of tau and protein changes (aggregates) in the brain, particularly if they have ApoE variant gene, which is associated with adult AD.231 Tau may also provide a link with the plasma membrane and play a role in signal transduction.232
Other molecules that may influence MT dynamics include PrPC and PSD-95. PrPC is known to bind to tubulin, stathmin, and tau233–235 and has been proposed as a major player in MT assembly/disassembly.236 Schmitz et al237,238 have shown that PrPC plays a direct role in the organization of the cytoskeleton, as well as cognition and behavior, as a result of its relationship with neurofilaments and MTs. Dysregulation (upregulation) of PrPC expression leads to hyperphosphorylation of tau and malformed stumpy neurites.239 PrPC overexpression is also believed to be involved in the β-amyloid formation and cognitive dysfunction of AD via its interaction with nicotinamide adenine dinucleotide phosphate (NADPH) oxidase at the membrane, inflammatory cytokines, and the subsequent alteration of actin filaments.240 Interaction between PSD-95 and cypin has also been proposed to regulate MT organization in dendrites.14
MTs are central in cellular signaling and a major target of signaling pathways to maintain the balance in their dynamic instability and thus control cellular (neuronal) function. They are also an effector of downstream signaling, interacting with other signaling molecules such as NFκB, extracellular signal-regulated kinase 2, and MAPK and organizing signal pathways.241 Linden et al242 have suggested that PrPC, with its membrane scaffolding connection to the extracellular matrix, links α-tubulin, β-tubulin, and MT and is thus involved in multicomponent signal transduction with a wide range of allosteric effects in physiology and pathophysiology. PrPC acts as a redox sensor molecule for oxidative stress and triggers downstream processes.243 Goswami11 has suggested that a component of MT signaling is centered around TRPV1 channels, which are redox sensors for infrared stimuli.244 Potassium leak channel TRESK may interact with cytoskeleton12 and is believed to be one of the two-pore domain potassium (K2P) ion channels that are important as targets for anaesthesia.245
MTs act as the scaffold for anterograde and retrograde axonal transport of organelles, vesicles, and proteins using kinesin and dynein motor proteins. The normal functioning of neurons depends on the integrity of the cytoskeleton for fast axonal flow. Because MTs are subject to constant catastrophe and rescue and because MT bundles are of different lengths, axons can normally cope with intermittent disruptions to the MT cytoskeleton. Varicosities or focal swellings form when complete breakage of the MT cytoskeleton leads to a buildup of cargo at the breakage point.246 Disruption of the cytoskeleton and varicosity formation has a profound effect on the bioelectrical function of nerves. Mitochondria, which deliver the adenosine triphosphate (ATP) required for many enzymes and the generation of action potentials, are not able to move along the cytoskeleton. Ion channels such as TREK247 and other signaling molecules such as nerve growth factor (NGF) and brain derived growth factor (BDGF) are also not able to move along the MT in retrograde or anterograde cargo transport, which has marked effects on signal transduction.248
Assembly and disassembly of the neural (synaptic) proteins is also observed as a common process during hibernation in mammals,249 where it is involved in reversible neuroplasticity and resistance to neural damage. During hibernation, cell bodies and dendritic spines shrink, synapses are lost, and synaptic proteins and MTs250 are disassembled. These proteins are stored in the axon until required for reassembly, rather than being degraded and then resynthesized de novo.249 Proteome variations during hibernation and arousal indicate that cytoskeleton changes are the dominant protein changes.251 MT disassembly is regulated by tau phosphorylation, which, in this case, does not form the fibrils that are typical of the tau hyperphosphorylation seen in AD.252 This regular disassembly/reassembly of proteins leads to some memory loss in hibernating ground squirrels when compared with non-hibernating squirrels.249 Human hypothermia with circulatory arrest and subsequent resuscitation can also commonly accompanied by some memory loss,253 similar to POCD. Using this hibernation evidence, Arendt and Bullmann have proposed a model for cytoskeleton modulation in the process for neuroplasticity in the hippocampus and other cortical synapses.254
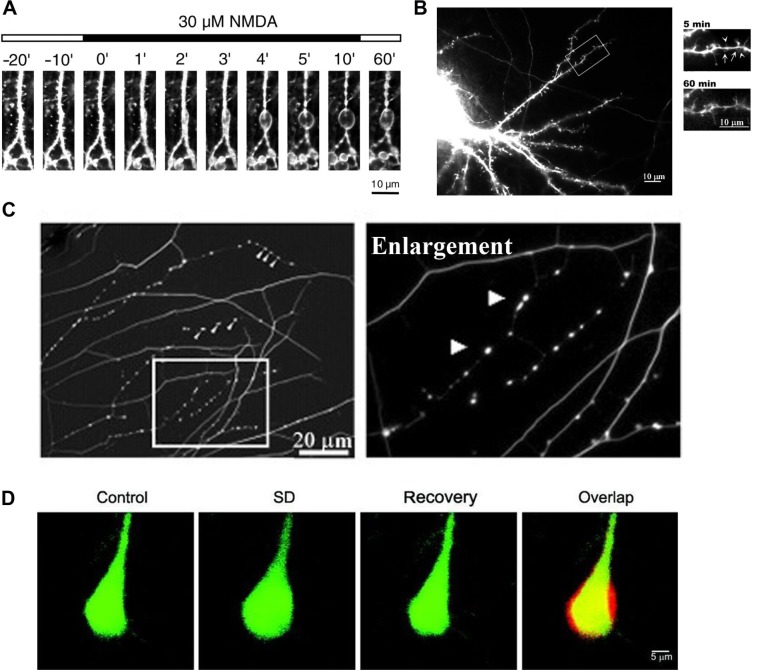
The appearance of varicosities in axons appears to be an endogenous mechanism that protects nerves from damage, occurring as a response to multiple stimuli and stressors, including mechanical stress, axonal damage, heat and cold, toxins, and anesthetics. Originally considered as only a sign of neuropathology, it is now apparent that varicosities are reversible and neuroprotective.14,255 There are numerous examples of neuroprotective varicosities. In the central nervous system, sublethal hypoxia can lead to reversible dendritic beading, which can be blocked by NMDA antagonists.256 Ikegaya et al255 have shown that small reversible dendritic varicosities are produced endogenously as a response to a stressor and can act as a neuroprotection against greater damage to the neuron (Fig. 1A). Prevention of this response led to increased neuronal damage, collapse of normal neural function, and cell death. This same response has been demonstrated more recently by Tseng and Firestein,14 where a toxic assault (NMDA poisoning) resulted in the production of small protective varicosities (Fig. 1B), which, as long as the response was early, rapid and reversible, prevented the formation of larger, destructive neuronal swellings and neuronal death. Varicosity formation depends on the induction of nNOS and the interaction between PSD-95, cypin, and tau. Increased cypin and decreased PSD-95 resulted in an increased number of small protective varicosities, while decreased cypin and increased PSD-95 resulted in the opposite14
Figure 1.
Formation of neuroprotective endogenous varicosities: (A) confocal laser microscopy images of formation of dendritic varicosities in rat hippocampus neurons treated with 30 μM NMDA;255 (B) immunofluorescent images of formation of dendritic varicosities (arrows) in rat embryo hippocampus neurons, immediately after exposure to 30 μM NMDA (5 minutes) and reversal of varicosities after recovery (60 minutes);14 (C) immunohistochemistry image stained for tubulin, showing varicosity formation in embryonic DRG neurons in response to resiniferatoxin activation of TRPV1;18 (D) two-photon laser scanning images, showing the transient increase in mouse neuron volume before (control), during spreading depression (SD) and after recovery from SD, including a merged image (overlap) showing the overlap (yellow), before volume (green), and during CSD (red).259
This was suggested as a pathway in which MT cytoskeleton is regulated by sublethal changes to dendrites. PSD-95 (as well as nNOS) is also implicated in the remyelination process of regeneration of peripheral axons in a rat injury model.257 The dopamine metabolite N-arachidonoyl-dopamine applied to dorsal root ganglia (DRG) neurites resulted in varicosity formation (Fig. 1C) implicating TRPV1 in the formation process.18 Endogenous dopamine metabolites are relevant to anesthetic induced responses.258
Neuroprotective cytoskeleton modulation is also present during the CSD associated with migraine with aura and cortical trauma (involving TRESK polymorphisms), where neurons undergo a transient volume increase259 (Fig. 1D). These are seen as part of the neuroprotective process that protects the cortex, as an adaptive response to cortical injury and to provide tolerance to subsequent ischemic episodes.260,261 nNOS increases during CSD,260 and the genes upregulated in this neuroprotective response are iNOS and HIF-1α.262 This is an example of an immune memory process, as reviewed by Szentivanyi et al,263 and may be a similar mechanism to that involved in peripheral nerve injury and varicosity formation. Reversible varicosity formation has also been noted for a number of conditions, such as ischemia264,265 and toxic assault,266–268 depending on the severity and/or duration of the stimulus.
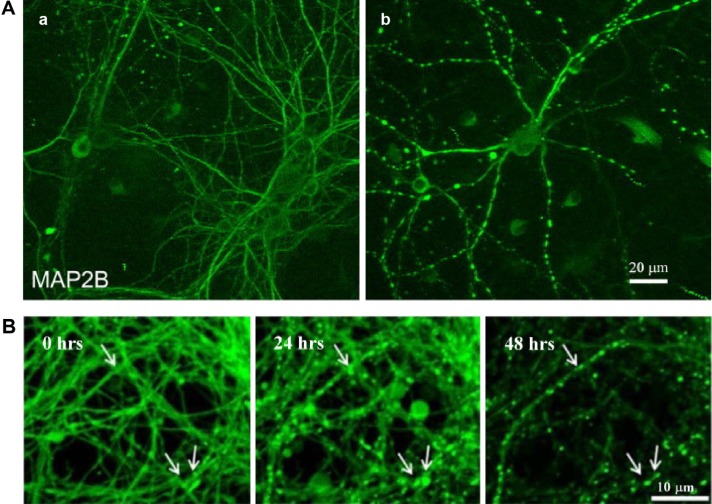
Preconditioning of neurons can also involve the formation of small protective varicosities. Subjecting cell cultures of rat neurons to ischemic preconditioning269 resulted in the formation of small varicosities in dendrites via a PSD-95 pathway (Fig. 2A). These were also reversible within four hours and may have had a role in neuroprotection against NMDA receptor-mediated toxicity. The use of black widow spider venom to speed recovery from botulism neurotoxin resulted in rapid varicosity formation (Fig. 2B) that (under sublethal conditions) were reversible within 48 hours.270
Figure 2.
Formation of preconditioning neuroprotective varicosities: (A) immunofluorescent images of neuronal cultures, showing control (a) and the formation of varicosities (b) following ischemic preconditioning using nonharmful oxygen and glucose deprivation for 30 minutes;269 (B) confocal laser microscopy images of stem cell-derived neurons stained with calcein green, showing the formation of varicosities (arrows) within 22 minutes of the application of black widow venom still apparent after 24 hours, but reducing after 48 hours.270
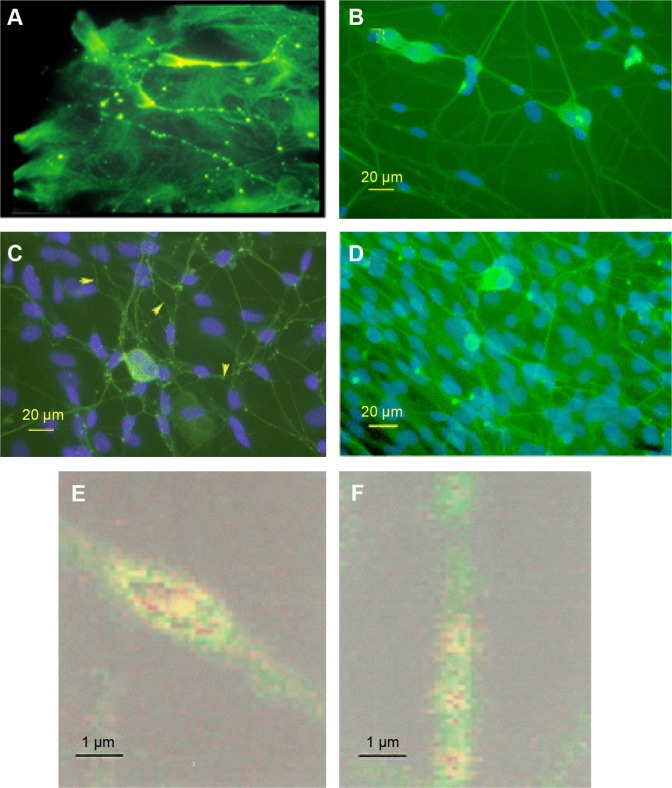
In a strikingly similar process to endogenously induced varicosities, PBM has also been shown to cause MT disruption and varicosity formation in the cytoskeleton of neurons.19,224 This has been demonstrated in cultured rat and murine DRG neurons for a number of wavelengths, including 650 (Chow, unpublished), 808,19 830,224 and 1064 nm (Chan, unpublished). This MT disruption leads to a pain blockade effect. Immunohistochemistry of DRG neuronal cultures shows the interruption of cytoskeletal integrity within 5–10 minutes following 30 or 60 seconds of laser irradiation. This effect can be seen with confocal microscopy as the formation of varicosities along the axon (Fig. 3A and B) and disruption of fast axonal flow (Fig. 3E). Specifically, β-tubulin from the MTs accumulates in the varicosities as do mitochondria, from which ATP is rapidly depleted. Importantly, this disruption is temporary and reversible, with the axon returning to its previous state within 24 hours (Fig. 3C). Other effects of LLLT on unmyelinated nerve fibers include the fragmentation of the neurite in the growth cone,224 a decrease in the number of neurofilaments, and increases in the number of MTs.271
Figure 3.
Confocal laser microscopy of axonal varicosities (arrows) produced by LLLT in cultured rat DRG neurons at wavelengths of 1064 nm (A) (Chan, unpublished) and 830 nm (B–F);19 (B) varicosity formation after 120 seconds of LLLT; (C) control; (D) reversal of varicosities 24 hours after irradiation; (E) magnified image of an axon showing a single varicosity formed after 30-second irradiation with mitochondria stained red; (F) control.
Interestingly, MT disassembly is also evident in cells, including neurons and lymphocytes, as a response to local77,79 and general272 anesthetics. This is characterized by the formation of varicosities in axons, in response to local anesthetics79 (Fig. 4A and B) and cell shape changes in macrophages273 and the formation of blebs in 3T3 cells (Fig. 4C).77
Figure 4.
Varicosities and MT changes due to anesthetics: (A) light photomicrographs of the effect of 2 × 10–3 M procaine on varicosity formation in cultured neurites from time zero to (a) two hours (b), three hours (c), and four hours (d);79 (B) scanning electron micrograph of swellings (S) in the neurite in response to 1 × 10−3 M procaine;79 (C) electron micrograph showing the formation of blebs on 3T3 cell surface, due to the disruption of membrane-associated MT and microfilaments after treatment with 0.6 mM tetracaine.77
There is a question as to the primary target of LLLT in neurons which will cause the cytoskeletal disruption and varicosity formation. Several of the proteins involved in the dynamic instability of MTs (including PrPC and PSD-95) have the capability to undergo conformational change, which could lead to MT instability. PBM could induce such a structural change by direct absorption of the light energy by the proteins or by the redox-sensing proteins responding to reactive oxygen species (ROS), such as nitric oxide. A potential mechanism for neural protection could also be postulated based on TRESK ion channels. The volume increase following cytoskeleton modulation and varicosity formation caused by LLLT will result in neural membrane stretch. Membrane tension is known to reversibly increase TRESK K+ currents in the DRG.274 This has a dampening effect on excessive neuron activation following injury and inflammation by reducing neural excitability.275 The inflammatory response is reduced by the downregulation of the calcium-activated cell stress cascade, including the unfolded protein response. This would have the effect of producing a preconditioning effect to allow the neuron a more efficient response due to immune memory.262 TRESK is phosphorylated by MT affinity-regulating kinase276 and also responsible for the phosphorylation of tau.277 In addition, TRESK has a physical link with tubulin and possibly MTs, at least in vitro.12 This suggests a (hypothetical) scaffold of TRESK/PrPC/MT, which could react to photons (PBM) either directly or via another mechanism to facilitate MT disassembly and varicosity formation.
A number of chemicals are known to destabilize MT in a similar manner to PBM. Drugs such as colchicine and nocodazole bind to tubulins and, therefore, prevent assembly into MTs. Taxol and other taxane drugs bind to and stabilize MTs preventing depolymerization, while demecolcine depolymerizes MTs.278,279 As previously noted, anesthetics are also known to interact with the cytoskeleton76–78 and can bind to tubulin and cause MT disassembly.80 Halo-thane interferes with MT reassembly in peripheral nerves in animal models,280 and chronic exposure can cause behavioral impairment and neuronal damage including reduced dendritic branching.281 Propofol causes reversible retraction of neurites in cultured rat neurones, mediated by GABAAR. Isoflurane can affect MT and neuronal filaments in astrocytes.64 The anesthetic sevoflurane has been shown to produce transient hyperphosphorylation of tau in mice on a single application, while repeated anesthetic led to a persistent tau hyperphosphorylation and a significant memory impairment (POCD).84 The anesthetic propofol was also shown to induce tau hyperphosphorylation in a mouse hippocampus model of AD.86
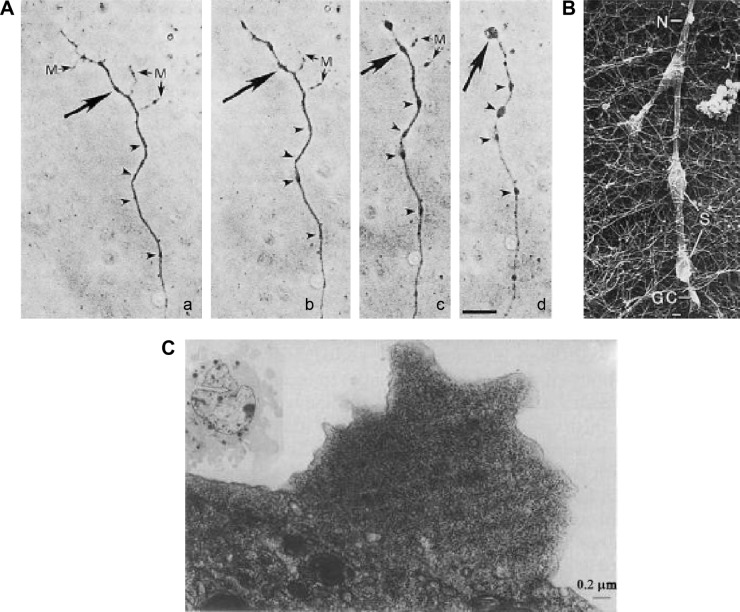
Although small, reversible varicosities are seen as a response to stress and are neuroprotective, continuation of the assault or continued dysregulation of cytoskeleton assembly/disassembly results in destructive cytoskeleton breakdown. Axonal trauma can trigger major MT breakage, inhibiting cargo transport to a greater extent than can be accommodated by normal catastrophe and rescue.282 Damage by trauma may not be readily repaired by the normal endogenous mechanisms, leading to a more long-term impact on neuron function and possibly damaged MTs, further accelerating the problem.65 This is exemplified by traumatic peripheral axonal injury (dynamic stretch injury)246 that results in axonal swellings (Fig. 5A). These axonal swellings are also seen in head injury trauma (Fig. 5B).246 In addition to the pathological conditions in the peripheral axon, the same physiological mechanism can occur in the axonal synapse and hippocampus, which involves synaptic plasticity and long-term potentiation in memories and learning, both linked with PSD-95.107,283
Figure 5.
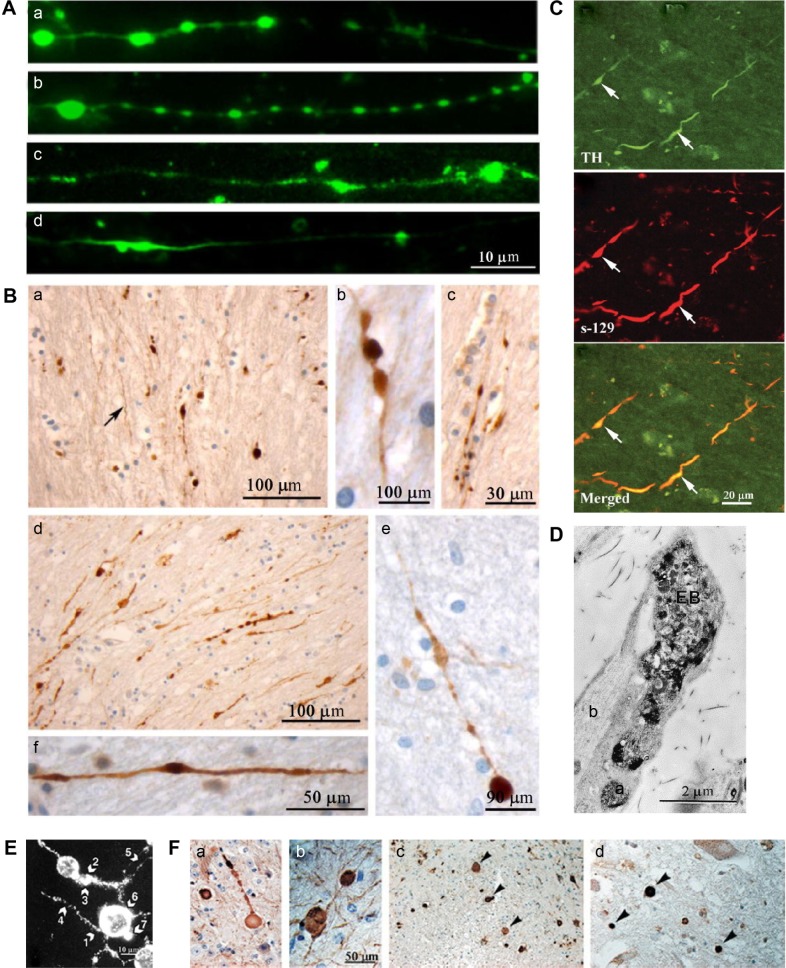
Pathological varicosities: (A) immunofluorescent images of axonal swellings produced during dynamic stretch injury of cultured neurons, stained for tubulin (a), tau (b), amyloid precursor protein (c), and neurofilament (d);246 (B) immunohistochemical stain against amyloid precursor protein, showing axonal varicosities in the corpus callosum of traumatic brain injury cases, caused by motor vehicle collision (a, e, f), falls (b, c), and blunt force trauma (d);246 (C) confocal laser microscopy images of putamen tissue from Parkinson’s disease cases, showing varicosities, stained for tyrosine hydroxylase (TH), α-synuclein (s-129), with a merged image;292 (D) electron micrograph of TH immune reactivity showing an axonal (synaptic) varicosity in rat DRG as a result of sensory and sympathetic interactions;284 (E) immunostained image of varicosity formation in a neuronal cell culture after exposure to prion protein peptide 106–126, showing varicosities (arrows 1–5);239 (F) immunohistochemical stains showing varicosities and spheroids in a mouse model of Alzheimer’s disease, stained for neurofilament (a, b, c) and the spinal cord of an early onset Alzheimer’s disease case, stained for amyloid precursor protein (d).289
Chung et al284 demonstrated pathological varicosities in sympathetic chronic pain when somatosensory nerves communicate with sympathetic nerves in the DRG (Fig. 5D). Focal swellings or spheroids are also evident in ischemia,285 epilepsy,286 and brain tumor.287 Varicosities (also called focal swellings, beading, or spheroids) are hallmarks and often early indicators of neurodegenerative diseases,288 such as AD182,285,289–291 (Fig. 5F), PD292,293 (Fig. 5C), prion disease,294 multiple sclerosis,295 Wallerian degeneration,296 rett syndrome,297 and children exposed to high levels of air pollution, who show signs of early AD.231 Overexpression of PrPC, which imitates prion disease,239 results in small contorted stumpy neurites with obvious swellings (Fig. 5E).
In summary, PBM may work well in a number of complimentary ways to promote neuroprotection. PBM produces cytoskeleton modulation and neuroprotective varicosities that inhibit or reduce cargo transport and fast axonal flow, in the same way as has been demonstrated for pain blockade.19,182 These varicosities mimic endogenous varicosities, and thus PBM may stimulate the body’s own neuroprotective mechanism. Small reversible varicosities have been previously suggested as a neuroprotective mechanism in animal models14,255 and have been invoked as part of neuroprotection against ischemia.269 This PBM stimulation may operate via photon activation of redox signaling (mitochondrial or NADPH at the cell membrane) or via direct protein conformational changes (possibly in TRPV1)18 and cell signaling to the cyto-skeleton via a (hypothetical) TRESK-PrPC-tau-tubulin scaffold and would include the molecules PSD-95, cypin, and MAPK (also known to be modulated by PBM)212,298 and the transient phosphorylation of tau.14 This immune memory effect262 could protect neurons against anesthetic attack on the cytoskeleton. PBM also has the effect of modulating the inflammatory response, by the regulation of the expression of iNOS196,199 and HIF-1a,204 the downregulation of the inflammatory cytokines IL-6, IL-1b, and TNF-α,208 and the upregulation of growth factor IGF-1,201 all suspected to be involved in POCD. PBM also modulates the cellular redox balance by decreasing oxidative stress and increasing levels of antioxidants,203,211 as well as the upregulation of mitochondrial function, biomarkers of which were found to be important in POCD.111
Conclusion
Despite early equivocal studies, POCD is recognized as a significant problem in the modern health-care system, affecting elderly patients undergoing anesthetics and surgery. Although most POCD appears to be reversible within weeks or months, it nonetheless has an effect on the quality of life of patients and an impact on health-care resources. There is also a possibility of long-term effects of POCD, including AD, in certain patients. The impact of POCD will increase into the future as medical and surgical procedures continue to improve and surgery becomes lengthier and more common. With an aging population, the patients most vulnerable to POCD are also the group with the greatest increase in surgical procedures.
The cause of POCD appears multifactorial but may involve similar mechanisms to AD, with which it shares some characteristics and common molecular markers. Anesthetic use and neuroinflammation are implicated, with many markers for neuroinflammation apparent in animal and clinical studies.
MTs, and the cytoskeleton generally, have a role in signal transduction, both as an initiator and a conduit. The dynamic stability of MTs, together with their function in directing neurite growth and in cellular signaling, gives the cytoskeleton a role in the stability of the neuron and they therefore have an allosteric role more generally in the nervous system. This would include reaction to the stimuli of injury and inflammation, including anaesthesia and surgery, which is in addition to any direct effect that anesthetics have on the cytoskeleton of neurons. Assembly and disassembly of the cytoskeleton is central to neuroplasticity and involves molecular switching. Disassembly and subsequent reassembly of MT is responsible for the neuroprotective effect in hibernation, in the CSD-associated migraine with aura, in the cortical adaptive response to injury, and in neuroprotection against toxic assault, such as NMDA and possibly anesthetics. Given the role of MT in such neurodegenerative diseases as AD, PD, and tauopathies, it is not unreasonable to suggest that similar mechanisms could be important in POCD.
Since there is no available treatment for POCD, preconditioning and neuroprotection would appear to be the optimum intervention for its prevention. Although neuroprotective drugs and cytokine antagonists have shown some success in animal models, it is suggested that PBM would be a viable option in preconditioning against POCD. PBM has been shown to directly affect MT and to cause small, reversible varicosities that affect cellular signaling, fast axonal transport, and pain blockade. This could occur via photoreceptors at the membrane such as opsins (neuropsin), NADPH, or TRPV1, which could in turn interact with tau and the MTs via ROS or via signal transduction involving PrPC and/or PSD-95. The varicosities produced by PBM mimic the endogenously produced varicosities that are known to be neuroprotective against the large, destructive varicosities, swelling, and greater damage to the neuron. Thus, PBM-generated varicosities act to precondition neurons against damage in an analogous mechanism to the varicosities produced during ischemic preconditioning. PBM is known as a preconditioning treatment in other diseases and conditions, such as macular degeneration, cardiovascular disease, and muscle performance. Taken together with the success of PBM in the prevention and treatment of animal models of neurodegenerative disease, it is proposed that the use of PBM preoperatively would have a preconditioning role for the prevention of POCD in patients undergoing surgery, especially in elderly, vulnerable patients.
Since POCD is not responsive to treatment, there is a need to identify patients at risk of POCD, including identifying MCI and serum markers of POCD risk. This would enable patients who would benefit from PBM preconditioning to be identified, especially those elderly patients with patterns of vulnerability to POCD, AD, and other forms of dementia. This would, however, not preclude the more widespread use of PBM on elderly surgical patients. PBM has the benefit of no identified harmful effects within the correct dose parameters and following contraindication recommendations. Elderly surgical patients who would most benefit from PBM preconditioning could include patients with conditions known or suspected to be related to POCD or AD (including preexisting cognitive decline, MCI, type I and type II diabetes, alcohol abuse, hypertension, and atherosclerosis); patients with markers and potential markers of AD and POCD (including β-amyloid, ApoE4, Ab42/tau ratio, CRP, SELP, C3F, iNOS, cytochrome P450, aspartic acid, and melatonin); patients with melanocortin signaling variations (such as redheaded women); patients with photophobia, CSD migraine with aura, and cluster headaches; and other patients with TRESK polymorphisms.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1337 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: BTB is an agent for Irradia AB, a company that manufactures therapeutic laser instruments. ADL, RC, and EV hold a patent (PCT/AU2015/00688) that is relevant to this manuscript. Permissions for use of all copyrighted images have been obtained.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to antiplagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: ADL. Contributed to the writing of the manuscript: ADL, RC, BTB, and EV. Agreed with the manuscript results and conclusions: ADL, RC, BTB, and EV. Jointly developed the structure and arguments for the paper: ADL and BTB. Made critical revisions and approved the final version: ADL, RC, BTB, and EV. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Hanning CD. Postoperative cognitive dysfunction. Br J Anaesth. 2005;95(1):82–87. doi: 10.1093/bja/aei062. [DOI] [PubMed] [Google Scholar]
- 2.van Harten AE, Scheeren TWL, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67(3):280–293. doi: 10.1111/j.1365-2044.2011.07008.x. [DOI] [PubMed] [Google Scholar]
- 3.Fodale V, Santamaria LB, Schifilliti D, Mandal PK. Anaesthetics and postoperative cognitive dysfunction: a pathological mechanism mimicking Alzheimer’s disease. Anaesthesia. 2010;65(4):388–395. doi: 10.1111/j.1365-2044.2010.06244.x. [DOI] [PubMed] [Google Scholar]
- 4.Khan I, Arany P. Biophysical approaches for oral wound healing: emphasis on photobiomodulation. Adv Wound Care (New Rochelle) 2015;4(12):724–737. doi: 10.1089/wound.2014.0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamblin MR, Pires de Sousa MV, Arany PR, Carroll JD, Patthoff D. Low level laser (light) therapy and photobiomodulation: the path forward; Paper presented at: SPIE 9309, Mechanisms for Low-Light Therapy X; 2015. [Google Scholar]
- 6.Karu T. Is it time to consider photobiomodulation as a drug equivalent? Photomed Laser Surg. 2013;31(5):189–191. doi: 10.1089/pho.2013.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purushothuman S, Johnstone D, Nandasena C, Mitrofanis J, Stone J. Photo-biomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex—evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6(1):2. doi: 10.1186/alzrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone D, Coleman K, Moro C, et al. The potential of light therapy in Parkinson’s disease. Chrono Physiol Ther. 2014;4:1–14. [Google Scholar]
- 9.Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230(0):13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal T, Gupta GK, Rai V, Carroll JD, Hamblin MR. Pre-conditioning with low-level laser (Light) therapy: light before the storm. Dose Response. 2014;12(4):619–649. doi: 10.2203/dose-response.14-032.Agrawal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami C. TRPV1—tubulin complex: involvement of membrane tubulin in the regulation of chemotherapy-induced peripheral neuropathy. J Neurochem. 2012;123(1):1–13. doi: 10.1111/j.1471-4159.2012.07892.x. [DOI] [PubMed] [Google Scholar]
- 12.Enyedi P, Veres I, Braun G, Czirják G. Tubulin binds to the cytoplasmic loop of TRESK background K+ channel in vitro. PLoS One. 2014;9(5):e97854. doi: 10.1371/journal.pone.0097854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurode-generative disease and aging. Genes Dev. 2008;22(11):1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng CY, Firestein BL. The role of PSD-95 and cypin in morphological changes in dendrites following sublethal NMDA exposure. J Neurosci. 2011;31(4):15468–15480. doi: 10.1523/JNEUROSCI.2442-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goggin K, Beaudoin S, Grenier C, Brown AA, Roucou X. Prion protein aggresomes are poly (A)+ ribonucleoprotein complexes that induce a PKR-mediated deficient cell stress response. Biochim Biophys Acta. 2008;1783(3):479–491. doi: 10.1016/j.bbamcr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Iram A, Naeem A. Protein folding, misfolding, aggregation and their implications in human diseases: discovering therapeutic ways to amyloid-associated diseases. Cell Biochem Biophys. 2014;70(1):51–61. doi: 10.1007/s12013-014-9904-9. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Chen Z, Liang B, et al. The change of circulating insulin like growth factor binding protein 7 levels may correlate with postoperative cognitive dysfunction. Neurosci Lett. 2015;588(0):125–130. doi: 10.1016/j.neulet.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Goswami C, Schmidt H, Hucho F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J. 2007;274(3):760–772. doi: 10.1111/j.1742-4658.2006.05621.x. [DOI] [PubMed] [Google Scholar]
- 19.Chow R, David M, Armati P. 830 nm laser irradiation induces varicosity formation, reduces mitochondrial membrane potential and blocks fast axonal flow in small and medium diameter rat dorsal root dorsal root ganglion: implications for the analgesic effects of 830 nm laser. J Peripher Nerv Syst. 2007;12:28–39. doi: 10.1111/j.1529-8027.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- 20.Newfield P. Postoperative cognitive dysfunction. F1000 Med Rep. 2009;1:14. doi: 10.3410/M1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54(8):951–956. doi: 10.1111/j.1399-6576.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 22.Avidan MS, Evers AS. Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. J Alzheimer Dis. 2011;24(2):201–216. doi: 10.3233/JAD-2011-101680. [DOI] [PubMed] [Google Scholar]
- 23.Silbert B, Evered L, Scott DA. Cognitive decline in the elderly: is anaesthesia implicated? Best Pract Res Clin Anaesthesiol. 2011;25(3):379–393. doi: 10.1016/j.bpa.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Hussain M, Eckenhoff RG, Seitz DP. General anesthetic and the risk of dementia in elderly patients: current insights. Clin Interv Aging. 2014;9:1619–1628. doi: 10.2147/CIA.S49680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigelow H. Insensibility during surgical operations produced by inhalation. N Engl J Med. 1846;35:309–317. [PMC free article] [PubMed] [Google Scholar]
- 26.Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269(6884):259–263. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- 27.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351(9106):857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 28.Monk T, Weldon B, Garvan C, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 29.Evered LA, Silbert B, Scott DA. The impact of the peri-operative period on cognition in older individuals. J Pharm Pract Res. 2015;45(1):93–99. [Google Scholar]
- 30.Hudetz J, Iqbal Z, Gandhi S, et al. Postoperative cognitive dysfunction in older patients with a history of alcohol abuse. Anesthesiology. 2007;106(3):423. doi: 10.1097/00000542-200703000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population-based case-control study. Alzheimers Dement. 2014;10(2):196–204. doi: 10.1016/j.jalz.2013.05.1766. [DOI] [PubMed] [Google Scholar]
- 32.Kotekar N, Kuruville CS, Murthy V. Post-operative cognitive dysfunction in the elderly: a prospective clinical study. Indian J Anaesth. 2014;58(3):263–268. doi: 10.4103/0019-5049.135034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alzheimer’s Association 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12(3):246–253. doi: 10.1375/twin.12.3.246. [DOI] [PubMed] [Google Scholar]
- 35.Hansen TG, Pedersen JK, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114(5):1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 36.Yin J, Wang SL, Liu XB. The effects of general anaesthesia on memory in children: a comparison between propofol and sevoflurane. Anaesthesia. 2014;69(2):118–123. doi: 10.1111/anae.12504. [DOI] [PubMed] [Google Scholar]
- 37.Bilotta F, Gelb AW, Stazi E, Titi L, Paoloni FP, Rosa G. Pharmacological perioperative brain neuroprotection: a qualitative review of randomized clinical trials. Br J Anaesth. 2013;110(suppl 1):i113–i120. doi: 10.1093/bja/aet059. [DOI] [PubMed] [Google Scholar]
- 38.Guay J. General anaesthesia does not contribute to long-term post-operative cognitive dysfunction in adults: a meta-analysis. Indian J Anaesth. 2011;55(4):358–363. doi: 10.4103/0019-5049.84850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hudson AE, Hemmings HC. Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107(1):30–37. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sztark F, Le Goff M, André D, Ritchie K, Dartigues JF, Helmer C. Exposure to general anaesthesia could increase the risk of dementia in elderly: 18AP1-4. Eur J Anaesthesiol. 2013;30:245. [Google Scholar]
- 41.Chen PL, Yang CW, Tseng YK, et al. Risk of dementia after anaesthesia and surgery. Br J Psychiatry. 2014;204(3):188–193. doi: 10.1192/bjp.bp.112.119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179–1185. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- 43.Newman M, Grocott H, Mathew J, et al. Neurologic Outcome Research Group and the Cardiothoracic Anesthesia Research Endeavors (CARE) Investigators of the Duke Heart Center Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32:2874. doi: 10.1161/hs1201.099803. [DOI] [PubMed] [Google Scholar]
- 44.Meyer JS, Thornby J, Crawford K, Rauch GM. Reversible cognitive decline accompanies migraine and cluster headaches. Headache. 2000;40(8):638–646. doi: 10.1046/j.1526-4610.2000.040008638.x. [DOI] [PubMed] [Google Scholar]
- 45.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, ISPOCD Group Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 46.Abildstrom H, Rasmussen LS, Rentowl P, et al. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. Acta Anaesthesiol Scand. 2000;44(10):1246–1251. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]
- 47.Stygall J, Newman SP, Fitzgerald G, et al. Cognitive change 5 years after coronary artery bypass surgery. Health Psychol. 2003;22(6):579–586. doi: 10.1037/0278-6133.22.6.579. [DOI] [PubMed] [Google Scholar]
- 48.Newman MF, Kirchner JL, Phillips-Bute B, et al. Neurological Outcome Research Group and the Cardiothoracic Anesthesiology Research Endeavors Investigators Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 49.Steinmetz J, Siersma V, Kessing LV, Rasmussen LS, ISPOCD Group Is postoperative cognitive dysfunction a risk factor for dementia? A cohort follow-up study. Br J Anaesth. 2013;110(suppl 1):i92–i97. doi: 10.1093/bja/aes466. [DOI] [PubMed] [Google Scholar]
- 50.Selnes OA, Royall RM, Grega MA, et al. Cognitive changes 5 years after coronary artery bypass grafting: Is there evidence of late decline? Arch Neurol. 2001;58(4):598–604. doi: 10.1001/archneur.58.4.598. [DOI] [PubMed] [Google Scholar]
- 51.Selnes OA, Grega MA, Bailey MM, et al. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63(5):581–590. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 52.Avidan MS, Searleman AC, Storandt M, et al. Long-term cognitive decline in older subjects was not attributable to non-cardiac surgery or major illness. Anesthesiology. 2009;111(5):964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krenk L, Kehlet H, Bæk Hansen T, Solgaard S, Soballe K, Rasmussen LS. Cognitive dysfunction after fast-track hip and knee replacement. Anesth Analg. 2014;118(5):1034–1040. doi: 10.1213/ANE.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 55.Xie Z, Tanzi RE. Alzheimer’s disease and post-operative cognitive dysfunction. Exp Gerontol. 2006;41(4):346–359. doi: 10.1016/j.exger.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Daiello L, Daniels A, Lareau C, Robidoux K, Luo W. Association of unrecognized preoperative cognitive impairment with postoperative cognitive and functional outcomes in elderly people awaiting hip fracture repair. Alzheimers Dement. 2013;9(4, suppl):777. [Google Scholar]
- 57.Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67(7):1201–1207. doi: 10.1212/01.wnl.0000238517.59286.c5. [DOI] [PubMed] [Google Scholar]
- 58.Moreno J, Radford H, Peretti D, et al. Sustained translational repression by elF2alpa-P mediates prion neurodegeneration. Nature. 2012;485(7399):507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sleigh J, Harvey M, Voss L, Denny B. Ketamine–more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care. 2014;4(2):76–81. [Google Scholar]
- 60.Garcia PS, Kolesky SE, Jenkins A. General anesthetic actions on GABA(A) receptors. Curr Neuropharmacol. 2010;8(1):2–9. doi: 10.2174/157015910790909502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Driesen NR, McCarthy G, Bhagwagar Z, et al. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38(13):2613–2622. doi: 10.1038/npp.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim DH, Kim JM, Park SJ, et al. GABA(A) receptor blockade enhances memory consolidation by increasing hippocampal BDNF levels. Neuropsychopharmacology. 2012;37(2):422–433. doi: 10.1038/npp.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie Z, Culley DJ, Dong Y, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid β-protein level in vivo. Ann Neurol. 2008;64(6):618–627. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Culley DJ, Cotran EK, Karlsson E, et al. Isoflurane affects the cytoskeleton but not survival, proliferation, or synaptogenic properties of rat astrocytes in vitro. Br J Anaesth. 2013;110(suppl 1):i19–i28. doi: 10.1093/bja/aet169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dikranian K, Cohen R, Mac Donald C, et al. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp Neurol. 2008;211:551–560. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2010;17(2):179–188. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stratmann G, Sall Jeffrey W, May Laura DV, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110(4):834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 69.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100(2):309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 70.Jevtovic-Todorovic V, Absalom AR, Blomgren K, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111(2):143–151. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan XB, Ouyang W, Li G, Duan KM. Involvement of neuronal nitric oxide synthase in cognitive impairment in isoflurane-treated rats. Neurosci Lett. 2012;506(2):240–244. doi: 10.1016/j.neulet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 72.Mawhinney LJ, de Rivero Vaccari JP, Alonso OF, et al. Isoflurane/nitrous oxide anesthesia induces increases in NMDA receptor subunit NR2B protein expression in the aged rat brain. Brain Res. 2012;1431(0):23–34. doi: 10.1016/j.brainres.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei H, Liang G, Yang H, et al. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108(2):251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 74.Callaway JK, Jones Nigel C, Royse Alistair G, Royse Colin F. Sevoflurane anesthesia does not impair acquisition learning or memory in the morris water maze in young adult and aged rats. Anesthesiology. 2012;117(5):1091–1101. doi: 10.1097/ALN.0b013e31826cb228. [DOI] [PubMed] [Google Scholar]
- 75.Callaway JK, Jones NC, Royse AG, Royse CF. Memory impairment in rats after desflurane anesthesia is age and dose dependent. J Alzheimer Dis. 2015;44(3):995–1005. doi: 10.3233/JAD-132444. [DOI] [PubMed] [Google Scholar]
- 76.Nicolson GL, Smith JR, Poste G. Effects of local anesthetics on cell morphology and membrane-associated cytoskeletal organization in BALB/3T3 cells. J Cell Biol. 1976;68(2):395–402. doi: 10.1083/jcb.68.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poste G, Papahadjopoulos D, Nicolson GL. Local anesthetics affect transmembrane cytoskeletal control of mobility and distribution of cell surface receptors. Proc Natl Acad Sci U S A. 1975;72(11):4430–4434. doi: 10.1073/pnas.72.11.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allison AC, Nunn JF. Effects of general anaesthetics on microtubules: a possible mechanism of anaesthesia. Lancet. 1968;292(7582):1326–1329. doi: 10.1016/s0140-6736(68)91821-7. [DOI] [PubMed] [Google Scholar]
- 79.Anderson PL, Bamburg JR. Effects of local anesthetics on nerve growth in culture. Dev Neurosci. 1981;4:273–290. doi: 10.1159/000112767. [DOI] [PubMed] [Google Scholar]
- 80.Craddock TJA, St. George M, Freedman H, et al. Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: implications for side effects of general anesthesia. PLoS One. 2012;7(6):e37251. doi: 10.1371/journal.pone.0037251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases β-amyloid protein levels. Arch Neurol. 2009;66(5):620–631. doi: 10.1001/archneurol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-β oligomerization and cytotoxicity. Anesthesiology. 2004;101(3):703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 83.Mandal PK, Williams JP, Mandal R. Molecular understanding of Ab peptide interaction with isoflurane, propofol, and thiopental: NMR spectroscopic study. Biochemistry. 2007;46(3):762–771. doi: 10.1021/bi062184l. [DOI] [PubMed] [Google Scholar]
- 84.Le Freche SH, Brouillette J, Fernandez-Gomez F-J, et al. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology. 2012;116(4):779–787. doi: 10.1097/ALN.0b013e31824be8c7. [DOI] [PubMed] [Google Scholar]
- 85.Whittington R, Planel E. Is general anaesthesia a risk factor for Alzheimer’s? Clues from biomarkers. Alzheimer Dement. 2012;8(4, suppl):425. [Google Scholar]
- 86.Whittington RA, Virág L, Marcouiller F, et al. Propofol directly increases tau phosphorylation. PLoS One. 2011;6(1):e16648. doi: 10.1371/journal.pone.0016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang JX, Mardini F, Caltagarone BM, et al. Anesthesia in presymptomatic Alzheimer’s disease: a study using the triple-transgenic mouse model. Alzheimer Dement. 2011;7(5):521.e–531.e. doi: 10.1016/j.jalz.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papon M-A, Whittington RA, El-Khoury NB, Planel E. Alzheimer’s disease and anesthesia. Front Neurosci. 2010;4:272. doi: 10.3389/fnins.2010.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mastrangelo G, Comiati V, dell’Aquila M, Zamprogno E. Exposure to anesthetic gases and Parkinson’s disease: a case report. BMC Neurol. 2013;13:194. doi: 10.1186/1471-2377-13-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zorzon M, Capus L, Pellegrino A, Cazzato G, Zivadinov R. Familial and environmental risk factors in Parkinson’s disease: a case-control study in north-east Italy. Acta Neurol Scand. 2002;105(2):77–82. doi: 10.1034/j.1600-0404.2002.1o040.x. [DOI] [PubMed] [Google Scholar]
- 91.Peretz C, Alexander BH, Nagahama SI, Domino KB, Checkoway H. Parkinson’s disease mortality among male anesthesiologists and internists. Mov Disord. 2005;20(12):1614–1617. doi: 10.1002/mds.20606. [DOI] [PubMed] [Google Scholar]
- 92.Royse CF, Andrews DT, Newman SN, et al. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia. 2011;66(6):455–464. doi: 10.1111/j.1365-2044.2011.06704.x. [DOI] [PubMed] [Google Scholar]
- 93.Rasmussen LS, Johnson T, Kuipers HM, et al. ISPOCD2 (International Study of Postoperative Cognitive Dysfunction) Investigators Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47(3):260–266. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 94.Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and postoperative delirium: a systematic review with meta-analysis. J Alzheimers Dis. 2010;22(suppl 3):67–79. doi: 10.3233/JAD-2010-101086. [DOI] [PubMed] [Google Scholar]
- 95.Seitz DP, Reimer CL, Siddiqui N. A review of epidemiological evidence for general anesthesia as a risk factor for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47(0):122–127. doi: 10.1016/j.pnpbp.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 96.Silbert BS, Evered LA, Scott DA. Incidence of postoperative cognitive dysfunction after general or spinal anaesthesia for extracorporeal shock wave lithotripsy. Br J Anaesth. 2014;113(5):784–791. doi: 10.1093/bja/aeu163. [DOI] [PubMed] [Google Scholar]
- 97.Hemmings HC, Jevtovic-Todorovic V. Special issue on anaesthetic neurotoxicity and neuroplasticity. Br J Anaesth. 2013;110(suppl 1):i1–i2. doi: 10.1093/bja/aet195. [DOI] [PubMed] [Google Scholar]
- 98.Ballard C, Jones E, Gauge N, et al. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One. 2012;7(6):e37410. doi: 10.1371/journal.pone.0037410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chan MTV, Cheng BCP, Lee TMC, Gin T, CODA Trial Group BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25(1):33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 100.Zhang X, Xin X, Dong Y, et al. Surgical incision-induced nociception causes cognitive impairment and reduction in synaptic NMDA receptor 2B in mice. J Neurosci. 2013;33(45):17737–17748. doi: 10.1523/JNEUROSCI.2049-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palotás A, Reis HJ, Bogáts G, et al. Coronary artery bypass surgery provokes Alzheimer’s disease-like changes in the cerebrospinal fluid. J Alzheimer Dis. 2010;21(4):1153–1164. doi: 10.3233/jad-2010-100702. [DOI] [PubMed] [Google Scholar]
- 102.Reinsfelt B, Westerlind A, Blennow K, Zetterberg H, Ricksten SE. Open-heart surgery increases cerebrospinal fluid levels of Alzheimer-associated amyloid β. Acta Anaesthesiol Scand. 2013;57(1):82–88. doi: 10.1111/j.1399-6576.2012.02769.x. [DOI] [PubMed] [Google Scholar]
- 103.Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115(4):727–732. doi: 10.1097/ALN.0b013e31822e9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brzezinski M, Gregersen M, Aparici CM, et al. Brain β-amyloid in cognitively normal subjects is a predictor of early postoperative cognitive dysfunction. Alzheimer Dement. 2014;10(4, suppl):898–899. [Google Scholar]
- 105.Baranov D, Bickler PE, Crosby GJ, et al. First International Workshop on Anesthetics and Alzheimer’s Disease. Consensus statement: first international workshop on anesthetics and Alzheimer’s disease. Anesth Analg. 2009;108(5):1627–1630. doi: 10.1213/ane.0b013e318199dc72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liem EB, Lin CM, Suleman MI, et al. Anesthetic requirement is increased in redheads. Anesthesiology. 2004;101(2):279–283. doi: 10.1097/00000542-200408000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Migaud M, Charlesworth P, Dempster M, et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396(6710):433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 108.Blanke ML, Van Dongen AM. Activation mechanisms of the NMDA Receptor. In: Van Dongen AM, editor. Biology of the NMDA Receptor. Boca Raton, FL: CRC Press; 2009. pp. 283–312. [PubMed] [Google Scholar]
- 109.Hu R, Tong DH, Liao Q, Hu Z, Ouyang W. Aspartic acid in the hippocampus: a biomarker for postoperative cognitive dysfunction. Neural Regen Res. 2014;9(2):143–152. doi: 10.4103/1673-5374.125343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu Y, Wang J, Wu A, Yue Y. Do fluctuations in endogenous melatonin levels predict the occurrence of postoperative cognitive dysfunction (POCD)? Int J Neurosci. 2014;124(11):787–791. doi: 10.3109/00207454.2014.882919. [DOI] [PubMed] [Google Scholar]
- 111.Li Y, Wang S, Ran K, Hu Z, Liu Z, Duan K. Differential hippocampal protein expression between normal aged rats and aged rats with postoperative cognitive dysfunction: a proteomic analysis. Mol Med Rep. 2015;12:2953–2960. doi: 10.3892/mmr.2015.3697. [DOI] [PubMed] [Google Scholar]
- 112.Kalenka A, Gross B, Maurer MH, Thierse H-J, Feldmann REJ. Isoflurane anesthesia elicits protein pattern changes in rat hippocampus. J Neurosurg Anesthesiol. 2010;22(2):144–154. doi: 10.1097/ANA.0b013e3181cb7cb8. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Q, Li SZ, Feng CS, et al. Serum proteomics of early postoperative cognitive dysfunction in elderly patients. Chin Med J. 2012;125(14):2455–2461. [PubMed] [Google Scholar]
- 114.Kiddle SJ, Steves CJ, Mehta M, et al. Plasma protein biomarkers of Alzheimer’s disease endophenotypes in asymptomatic older twins: early cognitive decline and regional brain volumes. Transl Psychiatry. 2015;5:e584. doi: 10.1038/tp.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79(0):1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 116.Riedel B, Browne K, Silbert B. Cerebral protection: inflammation, endothelial dysfunction, and postoperative cognitive dysfunction. Curr Opin Anaesthesiol. 2014;27(1):89–97. doi: 10.1097/ACO.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 117.Hu Z, Ou Y, Duan K, Jiang X. Inflammation: a bridge between postoperative cognitive dysfunction and Alzheimer’s disease. Med Hypotheses. 2010;74(4):722–724. doi: 10.1016/j.mehy.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 118.Degos V, Vacas S, Han Z, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118(3):527–536. doi: 10.1097/ALN.0b013e3182834d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oh Y-S, Kim D-W, Chun H-J, Yi HJ. Incidence and risk factors of acute postoperative delirium in geriatric neurosurgical patients. J Korean Neurosurg Soc. 2008;43(3):143–148. doi: 10.3340/jkns.2008.43.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24(2–3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 121.Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1b in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fidalgo AR, Cibelli M, White JPM, Nagy I, Maze M, Ma D. Systemic inflammation enhances surgery-induced cognitive dysfunction in mice. Neurosci Lett. 2011;498(1):63–66. doi: 10.1016/j.neulet.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 123.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106(3):436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 124.Cao L, Li L, Lin D, Zuo Z. Isoflurane induces learning impairment that is mediated by interleukin 1b in rodents. PLoS One. 2012;7(12):e51431. doi: 10.1371/journal.pone.0051431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61(8):1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu X, Lu Y, Dong Y, et al. The inhalation anesthetic isoflurane increases levels of proinflammatory cytokine TNF-α, IL-6 and IL-1b. Neurobiol Aging. 2012;33(7):1364–1378. doi: 10.1016/j.neurobiolaging.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tan ZS, Beiser AS, Vasan RS, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham study. Neurology. 2007;69:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 128.Lin G-X, Wang T, Chen M-H, Hu Z, Ouyang W. Serum high-mobility group box 1 protein correlates with cognitive decline after gastrointestinal surgery. Acta Anaesthesiol Scand. 2014;58(6):668–674. doi: 10.1111/aas.12320. [DOI] [PubMed] [Google Scholar]
- 129.Ji M-H, Yuan H-M, Zhang G-F, et al. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J Anesth. 2013;27(2):236–242. doi: 10.1007/s00540-012-1506-3. [DOI] [PubMed] [Google Scholar]
- 130.Jiang P, Ling Q, Liu H, Tu W. Intracisternal administration of an interleukin-6 receptor antagonist attenuates surgery-induced cognitive impairment by inhibition of neuroinflammatory responses in aged rats. Exp Ther Med. 2015;9(3):982–986. doi: 10.3892/etm.2014.2149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Ramlawi B, Rudolph JL, Mieno S, et al. C-Reactive protein and inflammatory response associated to neurocognitive decline following cardiac surgery. Surgery. 2006;140(2):221–226. doi: 10.1016/j.surg.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 132.Linstedt U, Meyer O, Kropp P, Berkau A, Tapp E, Zenz M. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand. 2002;46(4):384–389. doi: 10.1034/j.1399-6576.2002.460409.x. [DOI] [PubMed] [Google Scholar]
- 133.Li YC, Xi CH, An YF, Dong WH, Zhou M. Perioperative inflammatory response and protein S-100b concentrations—relationship with post-operative cognitive dysfunction in elderly patients. Acta Anaesthesiol Scand. 2012;56(5):595–600. doi: 10.1111/j.1399-6576.2011.02616.x. [DOI] [PubMed] [Google Scholar]
- 134.Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One. 2013;8(11):e79624. doi: 10.1371/journal.pone.0079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Getting SJ. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther. 2006;111(1):1–15. doi: 10.1016/j.pharmthera.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 136.Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol. 2010;8(3):218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cagampang FRA, Whatley SA, Mitchell L, Powell JF, Campbell IC, Coen CW. Circadian regulation of prion protein messenger RNA in the rat forebrain: a widespread and synchronous rhythm. Neuroscience. 1999;91(4):1201–1204. doi: 10.1016/s0306-4522(99)00092-5. [DOI] [PubMed] [Google Scholar]
- 138.Mariante RM, Nobrega A, Martins RAP, Areal RB, Bellio M, Linden R. Neuroimmunoendocrine regulation of the prion protein in neutrophils. J Biol Chem. 2012;287:35506–35515. doi: 10.1074/jbc.M112.394924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cao L, Wang K, Gu T, Du B, Song J. Association between APOE epsilon 4 allele and postoperative cognitive dysfunction: a meta-analysis. Int J Neurosci. 2013;124(7):478–485. doi: 10.3109/00207454.2013.860601. [DOI] [PubMed] [Google Scholar]
- 140.Ancelin M-L, De Roquefeuil G, Scali J, et al. Long-term post-operative cognitive decline in the elderly: the effects of anesthesia type, apolipoprotein E genotype, and clinical antecedents. J Alzheimer Dis. 2010;22:105. doi: 10.3233/JAD-2010-100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McDonagh DL, Mathew JP, White WD, et al. Neurologic Outcome Research Group Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112(4):852–859. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shoair OA, Grasso MP, Lahaye LA, Daniel R, Biddle CJ, Slattum PW. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study. J Anaesthesiol Clin Pharmacol. 2015;31(1):30–35. doi: 10.4103/0970-9185.150530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hogan KJ. Hereditary vulnerabilities to post-operative cognitive dysfunction and dementia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47(0):128–134. doi: 10.1016/j.pnpbp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 145.Xie Z, McAuliffe S, Swain CA, et al. Cerebrospinal fluid Aβ to tau ratio and postoperative cognitive change. Ann Surg. 2013;258(2):364–369. doi: 10.1097/SLA.0b013e318298b077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stetler RA, Leak RK, Gan Y, et al. Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol. 2014;0:58–83. doi: 10.1016/j.pneurobio.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang J, Tan H, Jiang W, Zuo Z. Amantadine alleviates postoperative cognitive dysfunction possibly by increasing glial cell line-derived neurotrophic factor in rats. Anesthesiology. 2014;121(4):773–785. doi: 10.1097/ALN.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meybohm P, Renner J, Broch O, et al. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double-blind randomized controlled pilot study. PLoS One. 2013;8(5):e64743. doi: 10.1371/journal.pone.0064743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roach G, Newman M, Murkin J, et al. Ineffectiveness of burst suppression therapy in mitigating perioperative cerebrovascular dysfunction. Multicenter study of perioperative ischemia (McSPI) research group. Anesthesiology. 1999;90(5):1255–1264. doi: 10.1097/00000542-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 150.Mathew JP, Shernan SK, White WD, et al. Preliminary report of the effects of complement suppression with pexelizumab on neurocognitive decline after coronary artery bypass graft surgery. Stroke. 2004;35(10):2335–2339. doi: 10.1161/01.STR.0000141938.00524.83. [DOI] [PubMed] [Google Scholar]
- 151.Taggart DP, Browne SM, Wade DT, Halligan PW. Neuroprotection during cardiac surgery: a randomised trial of a platelet activating factor antagonist. Heart. 2003;89(8):897–900. doi: 10.1136/heart.89.8.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ottens TH, Dieleman JM, Sauër A-MC, et al. DExamethasone for Cardiac Surgery (DECS) Study Group Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121(3):492–500. doi: 10.1097/ALN.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 153.Saleh AJ, Tang G-X, Hadi SM, et al. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med Sci Monit. 2015;21:798–805. doi: 10.12659/MSM.893359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Y, Ni C, Tang Y, et al. Melatonin attenuates isoflurane-induced acute memory impairments in aged rats. Basic Clin Pharmacol Toxicol. 2013;113(4):215–220. doi: 10.1111/bcpt.12079. [DOI] [PubMed] [Google Scholar]
- 155.Johnstone D, Massri N, Moro C, et al. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism—an abscopal neuro-protective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 156.Reinhart F, Massri NE, Darlot F, et al. 810 nm near-infrared light offers neuro-protection and improves locomotor activity in MPTP-treated mice. Neurosci Res. 2015;92:86–90. doi: 10.1016/j.neures.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 157.Hanczyc P, Samoc M, Norden B. Multiphoton absorption in amyloid protein fibres. Nat Photonics. 2013;7(12):969–972. [Google Scholar]
- 158.Grillo SL, Duggett NA, Ennaceur A, Chazot PL. Non-invasive infra-red therapy (1072 nm) reduces β-amyloid protein levels in the brain of an Alzheimer’s disease mouse model, TASTPM. J Photochem Photobiol B. 2013;123(0):13–22. doi: 10.1016/j.jphotobiol.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 159.Finsen N. The red-light treatment of small-pox. Lancet. 1903;6:1297–1298. doi: 10.1136/bmj.1.2214.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mester E, Szende B, Gärtner P. The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl) 1968;9(5):621–626. [PubMed] [Google Scholar]
- 161.Peter RU, Braun-Falco O, Birioukov A, et al. Chronic cutaneous damage after accidental exposure to ionizing radiation: the Chernobyl experience. J Am Acad Dermatol. 1994;30(5):19–23. doi: 10.1016/s0190-9622(08)81501-0. [DOI] [PubMed] [Google Scholar]
- 162.Huang Y-Y, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in Low Level Light Therapy—an update. Dose-Response. 2011;9:602–619. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bensadoun R-J, Nair RG. Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol. 2012;24(4):363–370. doi: 10.1097/CCO.0b013e328352eaa3. [DOI] [PubMed] [Google Scholar]
- 164.Ahmed A. To evaluate the safety and efficiency of low level laser therapy (LLLT) in treating decubitus ulcers: a review; Paper presented at: Proceedings. SPIE 9309, Mechanisms for Low-Light Therapy; 2015. [Google Scholar]
- 165.Percival SL, Francolini I, Donelli G. Low-level laser therapy as an antimicrobial and antibiofilm technology and its relevance to wound healing. Future Microbiol. 2015;10(2):255–272. doi: 10.2217/fmb.14.109. [DOI] [PubMed] [Google Scholar]
- 166.Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32(1):41–52. [PMC free article] [PubMed] [Google Scholar]
- 167.Liu X-G, Liu TC, Jiao J-L, Li C-Z, Xu XY. Photobiomodulation on sports injuries. Proc SPIE. 2003;5254:444–451. [Google Scholar]
- 168.Chen M-H, Huang Y-C, Sun J-S, Chao Y-H, Chen MH. Second messengers mediating the proliferation and collagen synthesis of tenocytes induced by low-level laser irradiation. Lasers Med Sci. 2015;30(1):263–272. doi: 10.1007/s10103-014-1658-5. [DOI] [PubMed] [Google Scholar]
- 169.Neves MAI, Pinfildi CE, Wood VT, et al. Different power settings of LLLT on the repair of the calcaneal tendon. Photomed Laser Surg. 2011;29(10):663–668. doi: 10.1089/pho.2010.2919. [DOI] [PubMed] [Google Scholar]
- 170.Smoot B, Chiavola-Larson L, Lee J, Manibusan H, Allen D. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymph-edema: a systematic review and meta-analysis. J Cancer Surviv. 2015;9(2):287–304. doi: 10.1007/s11764-014-0411-1. [DOI] [PubMed] [Google Scholar]
- 171.Ge MK, He WL, Chen J, et al. Efficacy of low-level laser therapy for accelerating tooth movement during orthodontic treatment: a systematic review and meta-analysis. Lasers Med Sci. 2015;30(5):1609–1618. doi: 10.1007/s10103-014-1538-z. [DOI] [PubMed] [Google Scholar]
- 172.Oron U. Low-energy laser irradiation reduces formation of scar tissue after myocardial infarction in rats and dogs. Circulation. 2001;103:296–301. doi: 10.1161/01.cir.103.2.296. [DOI] [PubMed] [Google Scholar]
- 173.Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angio-genesis. Lasers Surg Med. 2006;38(7):682–688. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 174.Tuby H, Maltz L, Oron U. Induction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Lasers Surg Med. 2011;43:401–409. doi: 10.1002/lsm.21063. [DOI] [PubMed] [Google Scholar]
- 175.Keszler A, Brandal G, Baumgardt S, et al. Far red/near infrared light-induced protection against cardiac ischemia and reperfusion injury remains intact under diabetic conditions and is independent of nitric oxide synthase. Front Physiol. 2014;5:305. doi: 10.3389/fphys.2014.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bjordal JM, Johnson MI, Lopes-Martins RA, Bogen B, Chow R, Ljunggren AE. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8(1):51. doi: 10.1186/1471-2474-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chow R, Armati P, Laakso EL, Bjordal JM, Baxter GD. Inhibitory effects of laser irradiation on peripheral mammalian nerves and relevance to analgesic effects: a systematic review. Photomed Laser Surg. 2011;29(6):365–381. doi: 10.1089/pho.2010.2928. [DOI] [PubMed] [Google Scholar]
- 178.Chow RT, Barnsley L. Systematic review of the literature of low-level laser therapy (LLLT) in the management of neck pain. Lasers Surg Med. 2005;37(1):46–52. doi: 10.1002/lsm.20193. [DOI] [PubMed] [Google Scholar]
- 179.Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124:204–210. doi: 10.1016/j.pain.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 180.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897–1908. doi: 10.1016/S0140-6736(09)61522-1. [DOI] [PubMed] [Google Scholar]
- 181.Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J Mol Cell Cardiol. 2009;47(2):256–263. doi: 10.1016/j.yjmcc.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yan W, Chow R, Armati PJ. Inhibitory effects of visible 650-nm and infrared 808-nm laser irradiation on somatosensory and compound muscle action potentials in rat sciatic nerve: implications for laser-induced analgesia. J Peripher Nerv Syst. 2011;16(2):130–135. doi: 10.1111/j.1529-8027.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- 183.Chan A, Armati P, Moorthy AP, Pulsed ND. YAG laser induces pulpal analgesia: a randomized clinical trial. J Dent Res. 2012;91(7 suppl):79S–84S. doi: 10.1177/0022034512447947. [DOI] [PubMed] [Google Scholar]
- 184.Schiffer F, Johnston AL, Ravichandran C, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5(46):9081–9085. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Naeser M. Improved cognitive function after transcranial light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photomed Laser Surg. 2011;29(5):351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Purushothuman S, Johnstone DM, Nandasena C, et al. Near infrared light mitigates cerebellar pathology in transgenic mouse models of dementia. Neurosci Lett. 2015;591:155–159. doi: 10.1016/j.neulet.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 187.De Taboada L, Yu J, El-Amouri S, et al. Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J Alzheimer Dis. 2011;23(3):521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 188.Farfara D, Tuby H, Trudler D, et al. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2015;55(2):430–436. doi: 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- 189.Johnstone DM, Moro C, Stone J, Benabid A-L, Mitrofanis J. Turning on lights to stop neurodegeneration: the potential of near infrared light therapy in Alzheimer’s and Parkinson’s disease. Front Neurosci. 2016;9:500. doi: 10.3389/fnins.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moro C, Massri NE, Torres N, et al. Photobiomodulation inside the brain: a novel method of applying near-infrared light intracranially and its impact on dopaminergic cell survival in MPTP-treated mice. J Neurosurg. 2014;120(3):670–683. doi: 10.3171/2013.9.JNS13423. [DOI] [PubMed] [Google Scholar]
- 191.Cotler H, Chow R, Hamblin M, Carroll J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2015;2(5):00068. doi: 10.15406/mojor.2015.02.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ivandic BT, Ivandic T. Low level laser therapy improves vision in patients with age-related macular degeneration. Photomed Laser Surg. 2008;26(3):241–245. doi: 10.1089/pho.2007.2132. [DOI] [PubMed] [Google Scholar]
- 193.Rutar M, Natoli R, Albarracin R, Valter K, Provis J. 670-nm light treatment reduces complement propagation following retinal degeneration. J Neuroinflammation. 2012;9:257. doi: 10.1186/1742-2094-9-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liebert A, Bicknell B, Adams R. Protein conformational modulation by photons: a mechanism for laser treatment effects. Med Hypotheses. 2014;82(3):275–281. doi: 10.1016/j.mehy.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 195.Wu S, Xing D. Intracellular signaling cascades following light irradiation. Laser Photonics Rev. 2014;8(1):115–130. [Google Scholar]
- 196.Chung H, Dai T, Sharma S, Huang Y-Y, Carroll J, Hamblin M. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Karu T. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;28:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 198.Poyton RO, Ball KA. Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome C oxidase. Discov Med. 2011;11:154–159. [PubMed] [Google Scholar]
- 199.Moriyama Y, Nguyen J, Akens M, Moriyama EH, Lilge L. In vivo effects of low level laser therapy on inducible nitric oxide synthase. Lasers Surg Med. 2009;41(3):227–231. doi: 10.1002/lsm.20745. [DOI] [PubMed] [Google Scholar]
- 200.Friedmann H, Lubart R. Nonlinear photobiostimulation: the mechanism of visible and infrared laser-induced stimulation and reduction of neural excitability and growth. Laser Ther. 1993;5(1):30–42. [Google Scholar]
- 201.Luo L, Sun Z, Zhang L, Li X, Dong Y, Liu TC. Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-beta1 in skeletal muscle during the repair process. Lasers Med Sci. 2013;28(3):725–734. doi: 10.1007/s10103-012-1133-0. [DOI] [PubMed] [Google Scholar]
- 202.Lavi R. Detailed analysis of reactive oxygen species induced by visible light in various cell types. Lasers Surg Med. 2010;42:473–480. doi: 10.1002/lsm.20919. [DOI] [PubMed] [Google Scholar]
- 203.de Lima F, Albertini R, Dantas Y, et al. Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem Photobiol. 2013;89(1):179–188. doi: 10.1111/j.1751-1097.2012.01214.x. [DOI] [PubMed] [Google Scholar]
- 204.Hsieh Y-L, Chou L-W, Chang P-L, Yang C-C, Kao M-J, Hong CZ. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1a (HIF-1a) J Comp Neurol. 2012;520(13):2903–2916. doi: 10.1002/cne.23072. [DOI] [PubMed] [Google Scholar]
- 205.Aimbire F, Albertini R, Pacheco MTT, et al. Low-level laser therapy induces dose-dependent reduction of TNFa levels in acute inflammation. Photomed Laser Surg. 2006;24(1):33–37. doi: 10.1089/pho.2006.24.33. [DOI] [PubMed] [Google Scholar]
- 206.Rizzi C, Mauriz J, Correa D, et al. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kB signalling pathway in traumatized muscle. Lasers Surg Med. 2006;38(7):704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 207.Chen AC-H, Arany PR, Huang Y-Y, et al. Low level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6(7):e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gao X, Xing D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci. 2009;16(1):1–16. doi: 10.1186/1423-0127-16-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Laakso L, Cramond T, Richardson C, Galligan JP. Plasma ACTH and betaendorphin levels in response to low level laser therapy (LLLT) in myofascial trigger points. Laser Ther. 1994;6:133–142. [Google Scholar]
- 210.Bjordal JM, Lopes-Martins RAB, Joensen J, Iversen VV. The anti-inflammatory mechanism of low level laser therapy and its relevance for clinical use in physiotherapy. Phys Ther Rev. 2010;15(4):286–293. [Google Scholar]
- 211.Fillipin LI, Mauriz JL, Vedovelli K, et al. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized achilles tendon. Lasers Surg Med. 2005;37(4):293–300. doi: 10.1002/lsm.20225. [DOI] [PubMed] [Google Scholar]
- 212.Cheng Y-J, Ou H-C, Lee CH. Low level laser therapy prevent endothelial cells from TNF-alpha/cycloheximide-induced apoptosis by modulating P38 MAPK/NF-kB pathway. Physiotherapy. 2015;101(Sup 1):e236. [Google Scholar]
- 213.Ignatov YD, Vislobokov AI, Vlasov TD, Kolpakova ME, Mel’nikov KN, Petrishchev IN. Effects of helium-neon laser irradiation and local anesthetics on potassium channels in pond snail neurons. Neurosci Behav Physiol. 2005;35(8):871–875. doi: 10.1007/s11055-005-0137-7. [DOI] [PubMed] [Google Scholar]
- 214.Lavi R, Ankri R, Sinyakov M, et al. The plasma membrane is involved in the visible light-tissue interaction. Photomed Laser Surg. 2012;30(1):14–19. doi: 10.1089/pho.2011.3083. [DOI] [PubMed] [Google Scholar]
- 215.Duan R, Liu TC, Li Y, Guo H, Yao LB. Signal transduction pathways involved in low intensity He-Ne laser-induced respiratory burst in bovine neutrophils: a potential mechanism of low intensity laser biostimulation. Lasers Surg Med. 2001;29(2):174–178. doi: 10.1002/lsm.1106. [DOI] [PubMed] [Google Scholar]
- 216.Takahashi N, Mori Y. TRP channels as sensors and signal integrators of redox status changes. Front Pharmacol. 2011;2:1–11. doi: 10.3389/fphar.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang L, Zhang D, Schwarz W. TRPV channels in mast cells as a target for low-level-laser therapy. Cells. 2014;3(3):662. doi: 10.3390/cells3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Karu T, Kurchikov A, Letokhov V, Mokh V. He-Ne laser radiation influences single-channel ionic currents through cell membranes: a patch-clamp study. Lasers Life Sci. 1996;71(1):35–48. [Google Scholar]
- 219.Wicks NL, Chan JW, Najera JA, Ciriello JM, Oancea E. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol. 2011;21(22):1906–1911. doi: 10.1016/j.cub.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nissilä J, Mänttäri S, Särkioja T, et al. Encephalopsin (OPN3) protein abundance in the adult mouse brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198(11):833–839. doi: 10.1007/s00359-012-0754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue1. FEBS Lett. 2003;554(3):410–416. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- 222.Leszkiewicz DN, Kandler K, Aizenman E. Enhancement of NMDA receptor-mediated currents by light in rat neurones in vitro. J Physiol. 2000;524(2):365–374. doi: 10.1111/j.1469-7793.2000.t01-1-00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Leszkiewicz DN, Aizenman E. Reversible modulation of GABAA receptor-mediated currents by light is dependent on the redox state of the receptor. Eur J Neurosci. 2003;17(10):2077–2083. doi: 10.1046/j.1460-9568.2003.02656.x. [DOI] [PubMed] [Google Scholar]
- 224.Chen M-Y, Shimada K, Fujita K, Ishill J, Hirata T, Fujisawa H. Neurite elongation from cultured dorsal root ganglion cells is inhibited by Ga-Al-As diode laser irradiation. Lasers Life Sci. 1993;5(4):237–242. [Google Scholar]
- 225.Song Y, Brady ST. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25(3):125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180(3):619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.de Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. Int J Biochem Cell Biol. 2012;44(2):266–274. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 228.Johnson GVW, Stoothoff WH. Tau phosphorylation in neuronal cell function and dysfunction. J Cell Sci. 2004;117(24):5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 229.Buée L, Troquier L, Burnouf S, et al. From tau phosphorylation to tau aggregation: what about neuronal death? Biochem Soc Trans. 2010;39:967–972. doi: 10.1042/BST0380967. [DOI] [PubMed] [Google Scholar]
- 230.Lei P, Ayton S, Finkelstein DI, Adlard PA, Masters CL, Bush AI. Tau protein: relevance to Parkinson’s disease. Int J Biochem Cell Biol. 2010;42(11):1775–1778. doi: 10.1016/j.biocel.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 231.Calderón-Garcidueñas L, Kavanaugh M, Block M, et al. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimer Dis. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- 232.Samsonov A, Yu J-Z, Rasenick M, Popov SV. Tau interaction with microtubules in vivo. J Cell Sci. 2005;117(25):6129–6141. doi: 10.1242/jcs.01531. [DOI] [PubMed] [Google Scholar]
- 233.Nieznanski K, Nieznanska H, Skowronek KJ, Osiecka KM, Stepkowski D. Direct interaction between prion protein and tubulin. Biochem Biophys Res Commun. 2005;334(2):403–411. doi: 10.1016/j.bbrc.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 234.Monnet C, Gavard J, Mège R-M, Sobel A. Clustering of cellular prion protein induces ERK1/2 and stathmin phosphorylation in GT1–7 neuronal cells. FEBS Lett. 2004;576(1–2):114–118. doi: 10.1016/j.febslet.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 235.Osiecka KM, Nieznanska H, Skowronek KJ, Jozwiak J, Nieznanski K. Tau inhibits tubulin oligomerization induced by prion protein. Biochim Biophys Acta. 2011;1813(10):1845–1853. doi: 10.1016/j.bbamcr.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 236.Osiecka KM, Nieznanska H, Skowronek KJ, Karolczak J, Schneider G, Nieznanski K. Prion protein region 23–32 interacts with tubulin and inhibits microtubule assembly. Proteins. 2009;77(2):279–296. doi: 10.1002/prot.22435. [DOI] [PubMed] [Google Scholar]
- 237.Schmitz M, Greis C, Ottis P, et al. Loss of prion protein leads to age-dependent behavioral abnormalities and changes in cytoskeletal protein expression. Mol Neurobiol. 2014;50(3):923–936. doi: 10.1007/s12035-014-8655-3. [DOI] [PubMed] [Google Scholar]
- 238.Schmitz M, Zafar S, Silva CJ, Zerr I. Behavioral abnormalities in prion protein knockout mice and the potential relevance of PrPC for the cytoskeleton. Prion. 2014;8(6):381–386. doi: 10.4161/19336896.2014.983746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pietri M, Caprini A, Mouillet-Richard S, et al. Overstimulation of PrPC signaling pathways by prion peptide 106–126 causes oxidative injury of bioaminergic neuronal cells. J Biol Chem. 2006;281(38):28470–28479. doi: 10.1074/jbc.M602774200. [DOI] [PubMed] [Google Scholar]
- 240.Walsh KP, Kuhn TB, Bamburg JR. Cellular prion protein: a co-receptor mediating neuronal cofilin-actin rod formation induced by β-amyloid and proinflammatory cytokines. Prion. 2014;8(6):375–380. doi: 10.4161/pri.35504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gundersen GG, Cook TA. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11(1):81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 242.Linden R, Cordeiro Y, Lima L. Allosteric function and dysfunction of the prion protein. Cell Mol Life Sci. 2012;69:1105–1124. doi: 10.1007/s00018-011-0847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sauer H, Wefer K, Vetrugno V, et al. Regulation of intrinsic prion protein by growth factors and TNF-a: the role of intracellular reactive oxygen species. Free Radic Biol Med. 2003;35(6):586–594. doi: 10.1016/s0891-5849(03)00360-5. [DOI] [PubMed] [Google Scholar]
- 244.Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal. 2014;21:971–986. doi: 10.1089/ars.2013.5616. [DOI] [PubMed] [Google Scholar]
- 245.Steinberg E, Wafford K, Brickley S, Franks N, Wisden W. The role of K2P channels in anaesthesia and sleep. Pflugers Arch. 2014;467(5):907–916. doi: 10.1007/s00424-014-1654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tang-Schomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol. 2012;233(1):364–372. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bearzatto B, Lesage F, Reyes R, Lazdunski M, Laduron PM. Axonal transport of TREK and TRAAK potassium channels in rat sciatic nerves. Neuroreport. 2000;11(5):927–930. doi: 10.1097/00001756-200004070-00006. [DOI] [PubMed] [Google Scholar]
- 248.Ibáñez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17(11):519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 249.von der Ohe CG, Garner CC, Darian-Smith C, Heller HC. Synaptic protein dynamics in hibernation. J Neurosci. 2007;27(1):84–92. doi: 10.1523/JNEUROSCI.4385-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Emirbekov E, Pashaeva M. Expression of cytoskeleton proteins in hypothalamic cells in winter sleeping ground squirrels Citellus pygmaeus Pallas during hibernation. Neurochem J. 2014;8(3):178–183. [Google Scholar]
- 251.Hindle AG, Martin SL. Cytoskeletal regulation dominates temperature-sensitive proteomic changes of hibernation in forebrain of 13-lined ground squirrels. PLoS One. 2013;8(8):e71627. doi: 10.1371/journal.pone.0071627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Arendt T, Stieler J, Holzer M. Brain hypometabolism triggers PHF-like phosphorylation of tau, a major hallmark of Alzheimer’s disease pathology. J Neural Transm. 2015;122(4):531–539. doi: 10.1007/s00702-014-1342-8. [DOI] [PubMed] [Google Scholar]
- 253.Walpoth BH, Walpoth-Aslan BN, Mattle HP, et al. Outcome of survivors of accidental deep hypothermia and circulatory arrest treated with extracorporeal blood warming. N Engl J Med. 1997;337(21):1500–1505. doi: 10.1056/NEJM199711203372103. [DOI] [PubMed] [Google Scholar]
- 254.Arendt T, Bullmann T. Neuronal plasticity in hibernation and the proposed role of the microtubule-associated protein tau as a “master switch” regulating synaptic gain in neuronal networks. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):478–489. doi: 10.1152/ajpregu.00117.2013. [DOI] [PubMed] [Google Scholar]
- 255.Ikegaya Y, Kim JA, Baba M, Iwatsubo T, Nishiyama N, Matsuki N. Rapid and reversible changes in dendrite morphology and synaptic efficacy following NMDA receptor activation: implication for a cellular defense against excitotoxicity. J Cell Sci. 2001;114(22):4083–4093. doi: 10.1242/jcs.114.22.4083. [DOI] [PubMed] [Google Scholar]
- 256.Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3(3):215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- 257.Gao S, Fei M, Cheng C, et al. Spatiotemporal expression of PSD-95 and nNOS after rat sciatic nerve injury. Neurochem Res. 2008;33(6):1090–1100. doi: 10.1007/s11064-007-9555-y. [DOI] [PubMed] [Google Scholar]
- 258.Adachi YU, Yamada S, Satomoto M, Higuchi H, Watanabe K, Kazama T. Isoflurane anesthesia induces biphasic effect on dopamine release in the rat striatum. Brain Res Bull. 2005;67(3):176–181. doi: 10.1016/j.brainresbull.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 259.Zhou N, Gordon GR, Feighan D, MacVicar BA. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb Cortex. 2010;20(11):2614–2624. doi: 10.1093/cercor/bhq018. [DOI] [PubMed] [Google Scholar]
- 260.Shen PJ, Gundlach AL. Prolonged induction of neuronal NOS expression and activity following cortical spreading depression (SD): implications for SD- and NO-mediated neuroprotection. Exp Neurol. 1999;160(2):317–332. doi: 10.1006/exnr.1999.7218. [DOI] [PubMed] [Google Scholar]
- 261.Shen PJ, Gundlach AL. Differential spatiotemporal alterations in adrenoceptor mRNAs and binding sites in cerebral cortex following spreading depression: selective and prolonged up-regulation of a 1B-adrenoceptors. Exp Neurol. 1998;154(2):612–627. doi: 10.1006/exnr.1998.6915. [DOI] [PubMed] [Google Scholar]
- 262.Rana G, Donizetti A, Virelli G, et al. Cortical spreading depression differentially affects lysine methylation of H3 histone at neuroprotective genes and retrotransposon sequences. Brain Res. 2012;1467:113–119. doi: 10.1016/j.brainres.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 263.Szentivanyi A, Berczi I, Nyanteh H, Goldman A. Some evolutionary, morphoregulatory and functional aspects of the immune-neuroendocrine circuitry. Immune Neuroendocr Circuitry. 2003;3:31–61. [Google Scholar]
- 264.Zhang S, Boyd J, Delaney K, Murphy TH. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci. 2005;25(22):5333–5338. doi: 10.1523/JNEUROSCI.1085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Murphy TH, Li P, Betts K, Liu R. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28(7):1756–1772. doi: 10.1523/JNEUROSCI.5128-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Greenwood SM, Mizielinska SM, Frenguelli BG, Harvey J, Connolly CN. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J Biol Chem. 2007;282(36):26235–26244. doi: 10.1074/jbc.M704488200. [DOI] [PubMed] [Google Scholar]
- 267.Ahlgren H, Bas-Orth C, Freitag HE, Hellwig A, Ottersen OP, Bading H. The nuclear calcium signaling target ATF3 protects against dendrotoxicity and facilitates the recovery of synaptic transmission after an excitotoxic insult. J Biol Chem. 2014;289(14):9970–9982. doi: 10.1074/jbc.M113.502914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Waataja JJ, Kim HJ, Roloff AM, Thayer SA. Excitotoxic loss of post-synaptic sites is distinct temporally and mechanistically from neuronal death. J Neurochem. 2008;104(2):364–375. doi: 10.1111/j.1471-4159.2007.04973.x. [DOI] [PubMed] [Google Scholar]
- 269.Meller R, Thompson SJ, Lusardi TA, et al. Ubiquitin-proteasome mediated synaptic reorganization—a novel mechanism underlying rapid ischemic tolerance. J Neurosci. 2008;28(1):50–59. doi: 10.1523/JNEUROSCI.3474-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mesngon M, McNutt P. Alpha-latrotoxin rescues SNAP-25 from BoNT/A-mediated proteolysis in embryonic stem cell-derived neurons. Toxins. 2011;3(5):489–503. doi: 10.3390/toxins3050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wesselmann U, Kerns JM, Rymer WZ. Laser effects on myelinated and nonmyelinated fibers in the rat peroneal nerve: a quantitative ultrastructural analysis. Exp Neurol. 1994;129(2):257–265. doi: 10.1006/exnr.1994.1168. [DOI] [PubMed] [Google Scholar]
- 272.Hinkley RE., Jr Microtubule—macrotubule transformations induced by volatile anesthetics. Mechanism of macrotubule assembly. J Ultrastruct Res. 1976;57(3):237–250. doi: 10.1016/s0022-5320(76)80113-x. [DOI] [PubMed] [Google Scholar]
- 273.Rabinovitch M, DeStefano MJ. Cell shape changes induced by cationic anesthetics. J Exp Med. 1976;143:290–304. doi: 10.1084/jem.143.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Callejo G, Giblin JP, Gasull X. Modulation of TRESK background K+ channel by membrane stretch. PLoS One. 2013;8(5):e64471. doi: 10.1371/journal.pone.0064471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tulleuda A, Cokic B, Callejo G, Saiani B, Serra J, Gasull X. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain. 2011;7:30. doi: 10.1186/1744-8069-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Braun G, Nemcsis B, Enyedi P, Czirjak G. TRESK background K+ channel is inhibited by PAR-1/MARK microtubule affinity-regulating kinases in xenopus oocytes. PLoS One. 2011;6(12):e28119. doi: 10.1371/journal.pone.0028119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Matenia D, Mandelkow EM. The tau of MARK: a polarized view of the cyto-skeleton. Trends Biochem Sci. 2009;34(7):332–342. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 278.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 279.Correia JJ, Lobert S. Molecular mechanisms of microtubule acting cancer drugs. In: Fojo T, editor. The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. Totowa, NJ: Springer; 2008. pp. 21–46. [Google Scholar]
- 280.Livingston A, Vergara GA. Effects of halothane on microtubules in the sciatic nerve of the rat. Cell Tissue Res. 1979;198(1):137–144. doi: 10.1007/BF00234841. [DOI] [PubMed] [Google Scholar]
- 281.Levin ED, Uemura E, Bowman RE. Neurobehavioral toxicology of halothane in rats. Neurotoxicol Teratol. 1991;13(4):461–470. doi: 10.1016/0892-0362(91)90096-f. [DOI] [PubMed] [Google Scholar]
- 282.Dulla CG, Coulter DA, Ziburkus J. From molecular circuit dysfunction to disease: case studies in epilepsy, traumatic brain injury, and Alzheimer’s disease. Neuroscientist. 2015 doi: 10.1177/1073858415585108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Nagura H, Ishikawa Y, Kobayashi K, et al. Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice. Mol Brain. 2012;5:43–63. doi: 10.1186/1756-6606-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chung JM, Chung K, Yoon YW. Sprouting sympathetic fibers form synaptic varicosities in the dorsal root ganglion of the rat with neuropathic injury. Brain Res. 1997;751(2):275–280. doi: 10.1016/s0006-8993(96)01408-4. [DOI] [PubMed] [Google Scholar]
- 285.Hori N, Carpenter DO. Functional and morphological changes induced by transient in vivo ischemia. Exp Neurol. 1994;129(2):279–289. doi: 10.1006/exnr.1994.1170. [DOI] [PubMed] [Google Scholar]
- 286.Swann JW, Al-Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10(5):617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 287.Goel S, Wharton SB, Brett LP, Whittle IR. Morphological changes and stress responses in neurons in cerebral cortex infiltrated by diffuse astrocytoma. Neuropathology. 2003;23(4):262–270. doi: 10.1046/j.1440-1789.2003.00510.x. [DOI] [PubMed] [Google Scholar]
- 288.Takeuchi H, Mizuno T, Zhang G, et al. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280(11):10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- 289.Wirths O, Weis J, Kayed R, Saido TC, Bayer TA. Age-dependent axonal degeneration in an Alzheimer mouse model. Neurobiol Aging. 2007;28(11):1689–1699. doi: 10.1016/j.neurobiolaging.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 290.Dudas B, Rose M, Cornelli U, Saido TC, Bayer TA. Neuroprotective properties of glycosaminoglycans: potential treatment for neurodegenerative disorders. Neurodegener Dis. 2008;5(3–4):200–205. doi: 10.1159/000113702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Dickson TC, King CE, McCormack GH, Vickers JC. Neurochemical diversity of dystrophic neurites in the early and late stages of Alzheimer’s disease. Exp Neurol. 1999;156(1):100–110. doi: 10.1006/exnr.1998.7010. [DOI] [PubMed] [Google Scholar]
- 292.Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135(pt 7):2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mattila PM, Rinne JO, Helenius H, Röyttä M. Neuritic degeneration in the hippocampus and amygdala in Parkinson’s disease in relation to Alzheimer pathology. Acta Neuropathol. 1999;98(2):157164. doi: 10.1007/s004010051064. [DOI] [PubMed] [Google Scholar]
- 294.Fuhrmann M, Bittner T, Mitteregger G, et al. Loss of the cellular prion protein affects the Ca2+ homeostasis in hippocampal CA1 neurons. J Neurochem. 2006;98(6):1876–1885. doi: 10.1111/j.1471-4159.2006.04011.x. [DOI] [PubMed] [Google Scholar]
- 295.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22 suppl 3):S22–S31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 296.Mi W, Beirowski B, Gillingwater TH, et al. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain. 2005;128(2):405–416. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- 297.Belichenko PV, Wright EE, Belichenko NP, et al. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol. 2009;514(3):240–258. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- 298.Liu TC, Liu R, Zhu L, Yuan J, Hu M, Liu S. Homeostatic photobiomodulation. Front Optoelectron China. 2009;2(1):1–8. [Google Scholar]