Abstract
Aquaporin-4 (AQP4) is a water channel expressed in astrocyte end-feet lining the blood-brain barrier. AQP4 deletion in mice is associated with improved outcomes in global cerebral ischemia produced by transient carotid artery occlusion, and focal cerebral ischemia produced by permanent middle cerebral artery occlusion (MCAO). Here, we investigated the consequences of 1-hour transient MCAO produced by intraluminal suture blockade followed by 23 hours of reperfusion. In nine AQP4+/+ and nine AQP4−/− mice, infarct volume was significantly reduced by an average of 39 ± 4 % at 24 hours in AQP4−/− mice, cerebral hemispheric edema was reduced by 23 ± 3 %, and Evans blue extravasation was reduced by 31 ± 2 % (mean ± SEM). Diffusion-weighted magnetic resonance imaging showed greatest reduction in apparent diffusion coefficient around the occlusion site after reperfusion, with remarkably lesser reduction in AQP4−/− mice. The reduced infarct volume in AQP4−/− mice following transient MCAO supports the potential utility of therapeutic AQP4 inhibition in stroke.
Keywords: AQP4, transient cerebral ischemia, brain edema, blood-brain barrier
INTRODUCTION
Aquaporin-4 (AQP4) is a bidirectional water transporting protein expressed in astrocytes throughout the central nervous system [6, 18]. AQP4 expression is greatest in the plasma membrane of perivascular end-feet in astrocytes lining the blood-brain barrier (BBB). AQP4 provides a major pathway for water entry into the brain across an intact BBB in cytotoxic brain edema, as produced by water intoxication [20]. AQP4 also appears to be involved in the removal of excess water from the brain in vasogenic edema, as produced by brain tumors, though the mechanisms involved are not clear because AQP4 functions as a water-selective transporter [19].
An early pathogenic event in ischemic brain injury is cytotoxic brain swelling in which water from the vasculature enters the brain across the BBB and accumulates in astrocytes [16]. Cytotoxic brain swelling in ischemia is the consequence of cellular dysfunction with consequent Na+/K+ pump failure, creating an osmotic gradient driving cellular water influx [22]. Downstream effects of cerebral edema and astrocyte swelling include increased extracellular space glutamate and K+ concentrations, BBB breakdown and inflammation, resulting in neuronal loss [3, 17]. AQP4 has been shown to be the primary water transport pathway across the BBB [25] as well as the astrocyte plasma membrane [23]. As such, its inhibition has been proposed to be of potential therapeutic utility in cytotoxic brain swelling [28].
Here, we tested the hypothesis AQP4 deletion in mice reduces brain swelling and infarct volume in a model of ischemic stroke produced by transient (1-h) middle cerebral artery occlusion (MCAO) followed by 23-h reperfusion. We previously reported greatly improved outcome in AQP4 knockout mice following focal ischemia produced by permanent MCAO [16], and more recently, improved outcome in AQP4 knockout mice following global ischemia produced by transient carotid artery occlusion [1, 10]. Also, several correlative studies reported reduced cerebral edema following ischemia with reduced AQP4 expression, including propofol and edaravone administration, protein kinase C activation [4], hypertonic saline administration [30], and endothelin-1 overexpression [13]. Together, these results provide a robust body of evidence supporting a neuroprotective effect of AQP4 down-regulation or deletion in cerebral ischemia. However, one recent study reported worse outcome in AQP4 deficiency in an ischemia-reperfusion model [31], though the results are difficult to interpret because the mice used manifest marked baseline abnormalities including BBB dysfunction [32], which are not seen in AQP4-deficient mice used in our studies [1, 10, 16, 21] or those generated by the Oslo group [7].
MATERIALS AND METHODS
Transgenic mice
AQP4 knockout (AQP4−/−) mice were generated by targeted gene disruption as described [14]. All experiments were performed on weight-matched littermates (25–30 g) produced by intercrossing of heterozygous mice in a CD1 genetic background. Protocols were approved by the University of California, San Francisco Committee on Animal Research.
Transient focal cerebral ischemia and reperfusion model
Adult male AQP4+/+ and AQP4−/− mice were subjected to transient focal cerebral ischemia by intraluminal middle cerebral artery (MCA) blockage with a 5-0 monofilament nylon suture, as described [29]. Mice were anesthetized with 2% isoflurane in 30% oxygen / 70% nitrous oxide using a facemask. Core body temperature was maintained at 37 ± 0.5 °C. Cannulation of the femoral artery allowed for monitoring of mean arterial blood pressure and arterial blood gases. A 9.5-mm 5-0 surgical monofilament nylon suture, blunted at the end, was introduced into the left internal carotid artery through the external carotid artery stump, and the left common carotid artery was temporarily occluded. After 1-h MCA occlusion blood flow was restored by the withdrawal of the nylon suture and the mice was sacrificed after 23-h reperfusion.
Regional cerebral blood flow (rCBF) was measured in anesthetized mice using a LASERFLO BPM2 blood perfusion monitor equipped with a flexible small-caliber probe of 0.7-mm diameter (VASAMEDICS, St. Paul, MN) [29] which gently touch the skull (1 mm anterior, 4 mm lateral to bregma), away from large pial vessels. Laser Doppler Flowmetry values were averaged over 5-s intervals and recorded before ischemia as a baseline, during occlusion, and after reperfusion. Neurological deficit was scored at 30 min after MCA occlusion to verify successful occlusion. A score ≥ 2 indicated successful occlusion [2].
Quantification of infarct volume and cerebral edema
Brains were removed after 1-h occlusion and 23-h reperfusion, and coronal brain slices were cut at 1, 3, 5 and 7 mm away from the frontal pole by using a mouse Brain Matrix (Harvard Apparatus, Holliston, MA). Brain slices were stained with 2% (weight/volume) 2,3,5-triphenyl- tetrazolium chloride (TTC) in 0.1 M PBS (pH 7.4) at 37 °C [24, 29]. Infarct areas and hemispheric contours at each level were outlined by an investigator blinded to the experimental groups using Image J software. Infarct volume and brain edema were determined by integrating the infarct area of different brain slices areas with the use of cylinder and cone rules [24].
Diffusion-weighted magnetic resonance imaging
Imaging was done on a 7-Tesla, 183-mm bore Oxford magnet equipped with SMIS console and Magnex self-shielded gradients, as described [12]. Diffusion-weighted images (DWI) were acquired from a 1.4-mm thick slice approximately at the level of the bregma. After surgical preparation and occlusion, mice were placed supine in an acrylic carriage with the surface coil fixed securely under the head. The head was taped to the carriage to minimize motion artifact. The time from the onset of occlusion and reperfusion to the start of the first image acquisition was less than 2 min. ADC maps were calculated on a pixel-by-pixel basis from each set of four DWIs [15]. A color scale was used to delineate regions of the ipsilateral hemisphere with reduced ADC relative to the contralateral hemisphere.
Evans blue extravasation
A quantitative assay of Evans Blue was used, as described [11]. 2.5 ml/kg of 4% Evans blue (Sigma, St. Louis, MO) in 0.9% saline was injected by tail vein. After one hour circulation, the mice were sacrificed and perfused transcardially with 200 ml of heparinized saline (10 U/ml heparin in 0.9% of saline). The cerebral hemispheres were separated and homogenized in 400 µl of N,N-dimethylformamide (Sigma, MO) and then incubated for 72 h in a water bath at 55 °C. The samples were centrifuged at 1500g for 20 min. The extracted Evans blue dye in the supernatant was quantified by absorbance at 620 nm. Results were expressed as µg of Evans blue per gram of brain hemisphere by comparison with solution standards.
Statistics analysis
Data are presented as the mean ± SEM, with comparisons between AQP4+/+ and AQP4−/− mice done by the Student t-test using Stat-View 4.0 software (Abacus Concepts, Berkeley, CA). P < 0.05 was considered statistically significant.
RESULTS
Physiological parameters
Physiological parameters of four AQP4+/+ and four AQP4−/− mice undergoing MCAO were shown at Table 1. There were no significant differences among the groups in rectal temperature, MABP, PO2, PCO2, and pH, and no differences in each parameter before, during and after MCA occlusion. Regional cerebral blood flow was reduced similarly by 93.5 % and 92.4 % in AQP4+/+ and AQP4−/− mice during occlusion, and was restored at 15 min after reperfusion to 44.9 % and 47.1 % of baseline values before occlusion. These results are typical of reported MCAO studies in mice [9, 29].
Table 1.
Physiological parameters of mice undergoing MCAO
| AQP4+/+ | AQP4−/− | |
|---|---|---|
| PTemperature (°C) | 37 ± 0.5 | 37 ± 0.5 |
| rpH | 7.3 ± 0.1 | 7.4 ± 0.1 |
| ePCO2 (mmHg) | 31.6 ± 3.2 | 31.6 ± 3.2 |
| -PO2 (mmHg) | 124.0 ± 10.3 | 122.0 ± 9.1 |
| oMABP(mmH2O) | 104.0 ± 3.6 | 97.6 ± 9.2 |
| crCBF (mlLD/min/100 mg) | 104.3 ± 2.7 | 104.8 ± 4.7 |
| cBaseline ADC (µm2/s) | 625 ± 42 | 640 ± 18 |
| lDuring occlusion (at 15 min after occlusion) | ||
| uTemperature (°C) | 37 ± 0.5 | 37 ± 0.5 |
| spH | 7.4 ± 0.1 | 7.4 ± 0.1 |
| iPCO2 (mmHg) | 35.3 ± 2.5 | 32.3 ± 3.7 |
| oPO2 (mmHg) | 97.6 ± 3.0 | 105.0 ± 10.0 |
| nMABP (mmH2O) | 114.3 ± 6.0 | 122.6 ± 12.2 |
| rCBF (mlLD/min/100mg) | 6.7 ± 0.6 | 8.0 ± 1.1 |
| After reperfusion (at 15 min) | ||
| Temperature (°C) | 37 ± 0.5 | 37 ± 0.5 |
| pH | 7.4 ± 0.0 | 7.4 ± 0.0 |
| PCO2 (mmHg) | 35.0 ± 4.5 | 32.6 ± 5.0 |
| PO2 (mmHg) | 117.6 ± 13.5 | 108.3 ± 10.1 |
| MABP (mmH2O) | 101.6 ± 10.4 | 102.6 ± 10.8 |
| rCBF (mlLD/min/100mg) | 46.8 ± 1.1 | 49.5 ± 6.6 |
Values are mean ± SEM, 4 mice per group. Differences not significant.
MABP, mean arterial blood pressure; rCBF, regional cerebral blood flow.
Reduced infarct volume and water content in AQP4−/− mice
MCA occlusion was carried out in nine AQP4+/+ and nine AQP4−/− mice in which the MCA was occluded for 1 h and brains were removed at 24 h (23 h after reperfusion) (Fig. 1A, top). Fig. 1A (bottom) shows TTC staining of brain sections cut at 1, 3, 5 and 7 mm from the frontal pole. There was significant reduction in infarct area in the slice at 3 mm. Total infarct volume determined from multiple slices in each mouse is summarized in Fig. 1B, with data for each mouse shown separately. There was a significant, 44 % reduction in total infarct volume from 39 ± 4 mm3 in AQP4+/+ mice to 22 ± 4 mm3 in AQP4−/− mice. The enlargement of the ipsilateral, ischemic hemisphere (resulting from brain swelling) was less in the AQP4−/− mice, which attributed to 23% less water (Fig. 1C).
Figure 1. Infarct volume and hemispheric water content at 23-h reperfusion after 1-h MCA occlusion in AQP4+/+ and AQP4−/− mice.
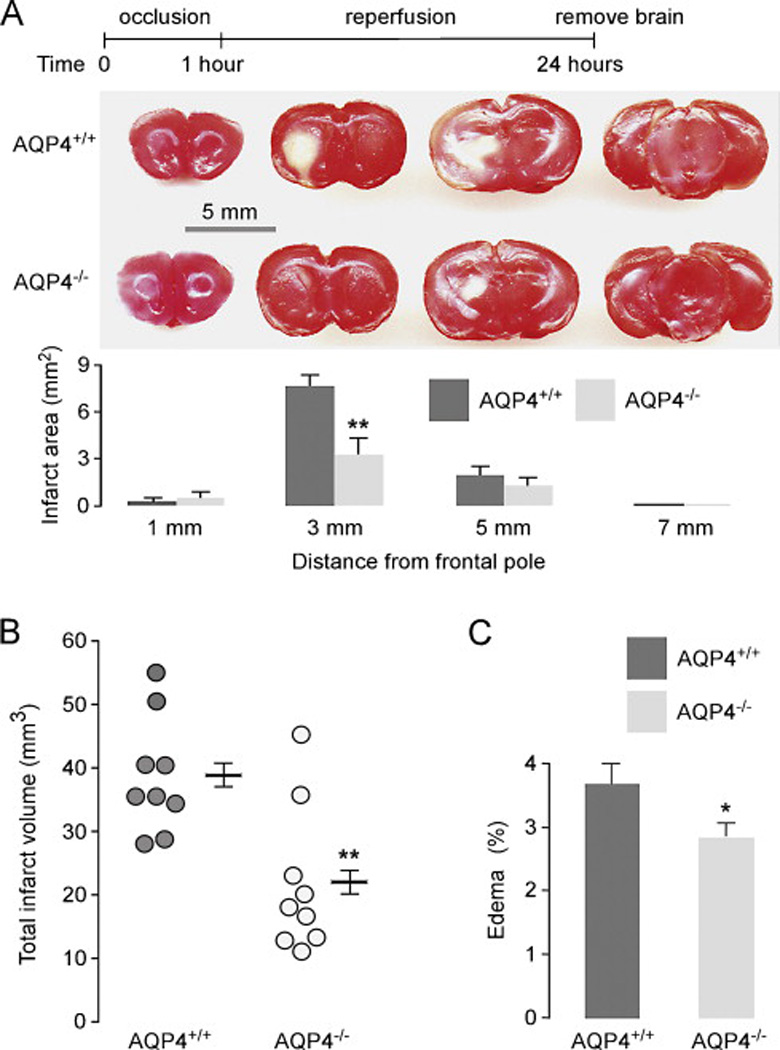
Brain slices of 2-mm thickness were sectioned at 1, 3, 5, and 7 mm distal from the frontal pole and stained with TTC. A. (top) Diagram of MCA occlusion model. (middle) Representative brain slices showing infarcted area (in white). (bottom) Infarct area (mean ± SEM, n=9, ** P < 0.01). B. Total infarct volume (from mice in A), **P < 0.01. C. Edema volume deduced from hemispheric enlargement comparing ipsilateral and contralateral brain of mice in A [24]. Edema volume = (Vi – Vc)/Vc*100%, where Vi is ipsilateral hemisphere volume and Vc is contralateral hemisphere volume. * P < 0.05.
Changes in ADC
Representative ADC maps (Fig. 2A) show reduced ADC on the ipsilateral (left) hemisphere corresponding to increased brain water in the intracellular space and an increase in the size of the intracellular compartment at the ischemic core and surrounding penumbra. The lesion area in the ipsilateral hemisphere was defined as the area in which ADC decreased by more than 20% of that in the contralateral hemisphere. As summarized in Fig. 2B, reduced ADC was seen in the first 15 min during occlusion and remained elevated over 60 min of reperfusion in the AQP4+/+ mice. Significantly less reduction in ADC was seen in AQP4−/− mice during the reperfusion period. Baseline ADC values were 625 ± 42 vs. 640 ± 18 µm2/s in AQP4+/+ and AQP4−/− mice, respectively (difference not significant). These values are in agreement with previous reported absolute ADC values in normal mouse brain [26, 27].
Figure 2. ADC at indicated times during ischemia and reperfusion in AQP4+/+ and AQP4−/−mice.
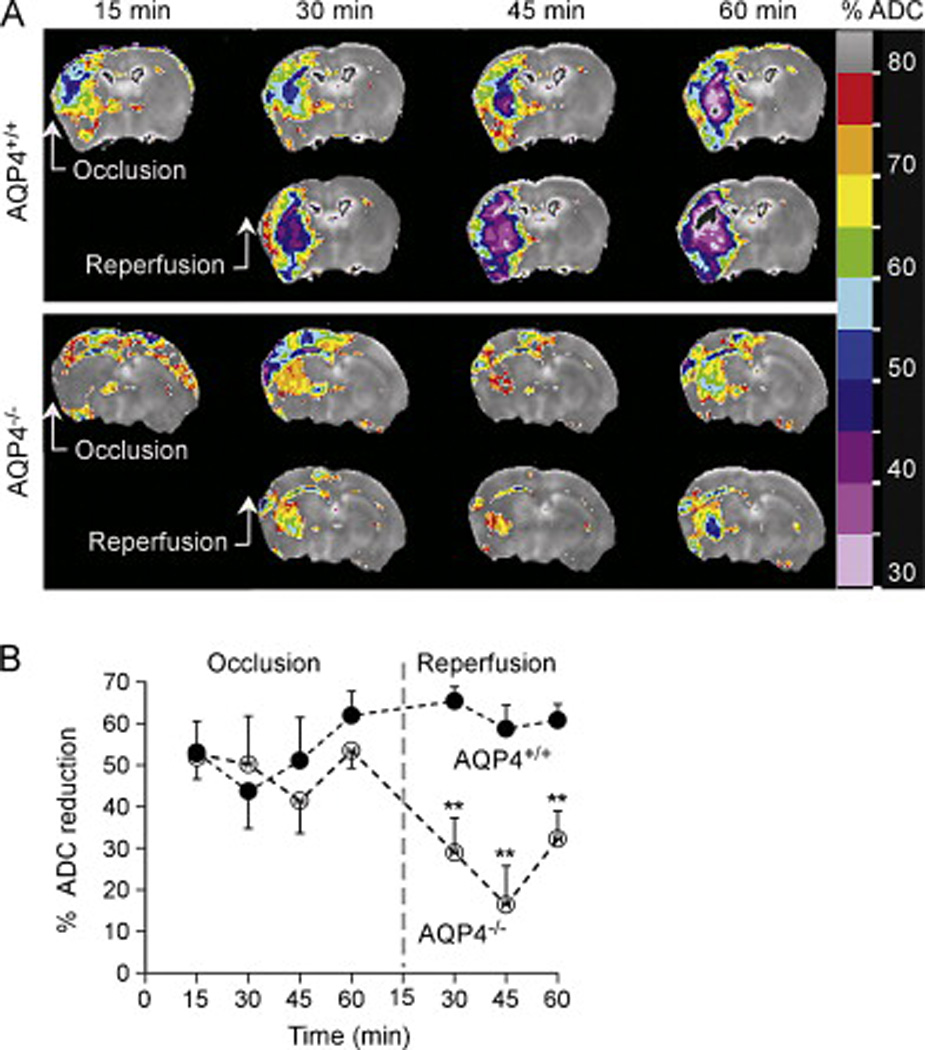
A. ADC maps calculated from three adjacent, 2-mm-thick slices, covering the majority of the brain. Representative of maps obtained from four AQP4+/+ and four AQP4−/− mice. B. Relative ADC values were calculated by normalizing to the contralateral hemisphere ADC values on a pixel-by-pixel basis, ** P < 0.01.
Reduced Evans blue extravasation in AQP4−/− mice
Blood-brain barrier permeability in AQP4+/+ and AQP4−/− mice was evaluated after 1-h occlusion and 23-h reperfusion by Evans Blue dye extravasation. Fig. 3 shows increased dye extravasation in the ipsilateral hemisphere of mice undergoing MCAO, with significantly reduced extravasation in the AQP4−/− mice. There was little increase in Evans blue dye in the contralateral hemisphere compared with that in control mice that did not undergo MCAO.
Figure 3. Evans Blue extravasation in AQP4+/+ and AQP4−/− mice at 23-h reperfusion after 1-h MCA occlusion.
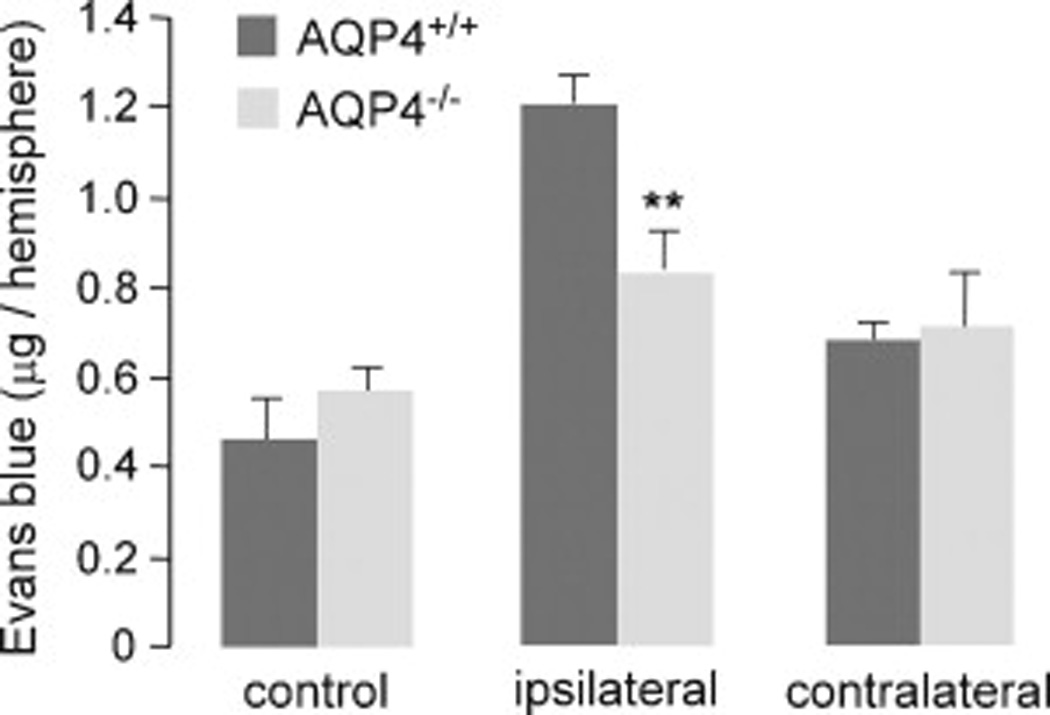
Evans blue dye was injected intraveneously 1 h before sacrifice. Dye extravasation was quantified following dye extraction from brain hemispheres after perfusion to remove intravascular dye (mean ± SEM, 4 mice per group, ** P < 0.01).
DISCUSSION
The principal finding of this study was significantly reduced infarct volume and brain swelling in AQP4-deficient mice following transient, 1-h focal cerebral ischemia produced by MCA occlusion, following by 23-h reperfusion. These results extend our original demonstration of improved outcome in AQP4-deficient mice following focal ischemia produced by permanent MCA occlusion [16]. In that study there was significantly improved neurological outcome at 24 h, with reduced infarct volume and brain swelling. Our results also extend data obtained in a mouse model of secondary AQP4 insufficiency produced by α-syntrophin knockout, where the knockout mice showed neuroprotection and reduced brain swelling following 90-min MCA occlusion and 23-h reperfusion [2].
Recently, we showed significant neuroprotection in AQP4-deficient mice in models of global cerebral ischemia produced by transient bilateral carotid artery occlusion (BCAO) alone (‘2-vessel occlusion’) [10], as well as transient BCAO done one day after bilateral vertebral artery cauterization (‘4-vessel occlusion’) [1]. In the 4-vessel occlusion model cerebral blood flow during BCAO was reduced by > 94%, which models cardiac arrest and resuscitation. In both the 2-vessel and 4-vessel BCAO models there was greatly improved survival and neurological outcome in AQP4-deficient mice, supporting the conclusion that reduced water permeability of the blood-brain barrier and astrocyte plasma membrane in AQP4-deficiency was responsible for reduced infarct volume and improved outcome. Measurements of intracranial pressure following BCAO and studies in brain slices from AQP4-deficient mice exposed to anoxia supported a mechanism in which early cytotoxic brain edema and astrocyte swelling in response to cerebral ischemia triggers a series of events including increased intracranial pressure, reduced extracellular space volume, BBB disruption, oxidative injury and inflammation, leading to cytotoxicity and infarction. We presume that similar neuroprotective mechanisms apply to account for the beneficial effects of AQP4 deletion in the transient focal ischemia studies done here. Another possible contributing mechanism, which will require further investigation, is that the endothelium in AQP4-deficient mice is less susceptible to ischemic injury than that in wild-type mice.
In addition to extending prior work, a motivation in carrying out the study reported here was a conflicting report published recently [31] in which mice were subjected to 5-min, 15-min or 40-min transient MCA occlusion followed by 24 or 72 h reperfusion. That study showed an increased mortality of up to 50% in AQP4-deficient mice during reperfusion. No mice died in the MCAO study here or the study done in alpha-syntrophin knockout mice [2]. The conflicting data of Zeng et al. [31] are difficult to explain; as reviewed previously [20], a number of unusual phenotypes reported in AQP4-deficient mice created by the Nanjing group may be related to marked baseline abnormalities, including BBB dysfunction [32] and sporadic hydrocephalus [5]. No differences were found in our AQP4+/+ and AQP4−/− mice in baseline phenotype, cerebrovascular anatomy, blood-brain barrier integrity, and brain histology [16, 21].
DWI was used here as a secondary measure of cerebral edema, as it is sensitive to early changes in brain tissue water distribution during acute ischemia [12, 26]. Hyperintensity on DWI is the result of a reduction in the ADC of water. The mechanisms underlying the ADC decrease following acute cerebral ischemia are thought to be related to an increase in the fraction of intracellular versus extracellular water following anoxic depolarization due to Na-K-ATPase pump failure, producing an increase in the tortuosity of the extracellular space [8]. Reduced ADC reflects intracellular edema and regional lesion severity [27]. The lesser reduction in ADC in AQP4-deficient mice compared to wild-type mice during reperfusion supports an important role of early brain water accumulation of the pathogenesis of cerebral infarction in ischemia.
In conclusion, AQP4 deficiency remarkably reduced infarct volume and brain edema in an established mouse model of transient focal cerebral ischemia produced by transient MCA occlusion followed by reperfusion. These results support prior studies showing improve outcomes in AQP4-deficient mouse models of focal cerebral ischemic produced by permanent MCA occlusion and global cerebral ischemia produced by transient carotid artery occlusion. Together, these results support the potential therapeutic utility of AQP4 inhibition or down-regulation in ischemic stroke.
Highlights.
Transient focal ischemia and reperfusion injury was produced by one-hour MCA occlusion followed by 23-hour reperfusion
Infarct volume and brain edema were greatly reduced in AQP4-deficient mice
The neuroprotective effect of AQP4 deletion suggests the therapeutic utility of AQP4 inhibition in stroke
Acknowledgments
This work was supported by grants DK35124, EY13574, EB00415, DK72517 and NS050173 from the National Institutes of Health and a grant from the Guthy-Jackson Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akdemir G, Ratelade J, Asavapanumas N, Verkman AS. Neuroprotective effect of aquaporin-4 deficiency in a mouse model of severe global cerebral ischemia produced by transient 4-vessel occlusion. Neurosci Lett. 2014;574:70–75. doi: 10.1016/j.neulet.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 4.Fazzina G, Amorini AM, Marmarou CR, Fukui S, Okuno K, Dunbar JG, Glisson R, Marmarou A, Kleindienst A. The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat. J Neurotrauma. 2010;27:453–461. doi: 10.1089/neu.2008.0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng X, Papadopoulos MC, Liu J, Li L, Zhang D, Zhang H, Verkman AS, Ma T. Sporadic obstructive hydrocephalus in Aqp4 null mice. Journal of neuroscience research. 2009;87:1150–1155. doi: 10.1002/jnr.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare O, Laake P, Klungland A, Thoren AE, Burkhardt JM, Ottersen OP, Nagelhus EA. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17815–17820. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris NG, Zilkha E, Houseman J, Symms MR, Obrenovitch TP, Williams SR. The relationship between the apparent diffusion coefficient measured by magnetic resonance imaging, anoxic depolarization, and glutamate efflux during experimental cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:28–36. doi: 10.1097/00004647-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Jing Z, Xing J, Chen X, Stetler RA, Weng Z, Gan Y, Zhang F, Gao Y, Chen J, Leak RK, Cao G. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014 doi: 10.1038/jcbfm.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katada R, Akdemir G, Asavapanumas N, Ratelade J, Zhang H, Verkman AS. Greatly improved survival and neuroprotection in aquaporin-4-knockout mice following global cerebral ischemia. FASEB J. 2014;28:705–714. doi: 10.1096/fj.13-231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim GW, Lewen A, Copin J, Watson BD, Chan PH. The cytosolic antioxidant, copper/zinc superoxide dismutase, attenuates blood-brain barrier disruption and oxidative cellular injury after photothrombotic cortical ischemia in mice. Neuroscience. 2001;105:1007–1018. doi: 10.1016/s0306-4522(01)00237-8. [DOI] [PubMed] [Google Scholar]
- 12.Kokubo Y, Matson GB, Derugin N, Hill T, Mancuso A, Chan PH, Weinstein PR. Transgenic mice expressing human copper-zinc superoxide dismutase exhibit attenuated apparent diffusion coefficient reduction during reperfusion following focal cerebral ischemia. Brain Res. 2002;947:1–8. doi: 10.1016/s0006-8993(02)02899-8. [DOI] [PubMed] [Google Scholar]
- 13.Lo AC, Chen AY, Hung VK, Yaw LP, Fung MK, Ho MC, Tsang MC, Chung SS, Chung SK. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:998–1011. doi: 10.1038/sj.jcbfm.9600108. [DOI] [PubMed] [Google Scholar]
- 14.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancuso A, Derugin N, Ono Y, Hara K, Sharp FR, Weinstein PR. Transient MRI-detected water apparent diffusion coefficient reduction correlates with c-fos mRNA but not hsp70 mRNA induction during focal cerebral ischemia in rats. Brain Res. 1999;839:7–22. doi: 10.1016/s0006-8993(99)01631-5. [DOI] [PubMed] [Google Scholar]
- 16.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 17.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 20.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadoun S, Tait MJ, Reza A, Davies DC, Bell BA, Verkman AS, Papadopoulos MC. AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience. 2009;161:764–772. doi: 10.1016/j.neuroscience.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 22.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solenov E, Watanabe H, Manley GT, Verkman AS. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol Cell Physiol. 2004;286:C426–C432. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- 24.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 25.Thiagarajah JR, Papadopoulos MC, Verkman AS. Noninvasive early detection of brain edema in mice by near-infrared light scattering. Journal of neuroscience research. 2005;80:293–299. doi: 10.1002/jnr.20439. [DOI] [PubMed] [Google Scholar]
- 26.Vajda Z, Pedersen M, Fuchtbauer EM, Wertz K, Stodkilde-Jorgensen H, Sulyok E, Doczi T, Neely JD, Agre P, Frokiaer J, Nielsen S. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13131–13136. doi: 10.1073/pnas.192457099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dorsten FA, Hata R, Maeda K, Franke C, Eis M, Hossmann KA, Hoehn M. Diffusion- and perfusion-weighted MR imaging of transient focal cerebral ischaemia in mice. NMR in biomedicine. 1999;12:525–534. doi: 10.1002/(sici)1099-1492(199912)12:8<525::aid-nbm597>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13:259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 1994;25:165–170. doi: 10.1161/01.str.25.1.165. [DOI] [PubMed] [Google Scholar]
- 30.Zeng HK, Wang QS, Deng YY, Fang M, Chen CB, Fu YH, Jiang WQ, Jiang X. Hypertonic saline ameliorates cerebral edema through downregulation of aquaporin-4 expression in the astrocytes. Neuroscience. 2010;166:878–885. doi: 10.1016/j.neuroscience.2009.12.076. [DOI] [PubMed] [Google Scholar]
- 31.Zeng XN, Xie LL, Liang R, Sun XL, Fan Y, Hu G. AQP4 knockout aggravates ischemia/reperfusion injury in mice. CNS Neurosci Ther. 2012;18:388–394. doi: 10.1111/j.1755-5949.2012.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Kong H, Hua X, Xiao M, Ding J, Hu G. Altered blood-brain barrier integrity in adult aquaporin-4 knockout mice. Neuroreport. 2008;19:1–5. doi: 10.1097/WNR.0b013e3282f2b4eb. [DOI] [PubMed] [Google Scholar]


