Abstract
♦ Background:
Fructo-oligosaccharides (FOS) exhibit soluble-fiber properties that beneficially affect bowel function and relieve constipation. The effects of FOS supplementation on constipation and biochemical parameters were examined in elderly continuous ambulatory peritoneal dialysis (CAPD) patients.
♦ Methods:
This randomized, double-blind, placebo-controlled, cross-over study was performed in elderly CAPD patients (5 males and 4 females) with chronic constipation. All subjects were randomly assigned to receive either 20 g FOS or placebo daily for 30 days. After a 14-day washout period, the patients were switched to the other substance for 1 more month. Before and after each treatment period, frequency of defecation, characteristics of feces, and colonic transit were evaluated. Biochemical parameters were also assessed.
♦ Results:
Fructo-oligosaccharides significantly increased the frequency of defecation (10.5 ± 2.0 vs 6.2 ± 1.4 times per week, p < 0.005) and changed the feces' appearance from type 1 (nut-like) to type 4 (sausage–like). The colonic transit determined by geometric center (GC) was augmented after FOS supplementation (3.9 ± 0.3 vs 3.2 ± 0.4, p < 0.05). Fructo-oligosaccharides had no effects on biochemical parameters. Fructo-oligosaccharides caused mild discomforts which were well tolerated after dose adjustment.
♦ Conclusions:
Fructo-oligosaccharide supplementation is effective, well tolerated, and can be an alternative to other laxatives in CAPD patients with constipation. Further studies are needed to better assess the biochemical effects of FOS in the chronic kidney disease population.
Keywords: Fructo-oligosaccharides, constipation, CAPD, colonic transit, elderly, nutritional status
Constipation is a common complaint of patients undergoing continuous ambulatory peritoneal dialysis (CAPD). Based on early reports, it is estimated that the prevalence of constipation in CAPD patients ranges from 16.0 to 28.9%, especially in the elderly (1,2). Although constipation is a more common problem in hemodialysis patients, peritoneal dialysis (PD) patients have worse quality of life once constipation occurs. Moreover, constipation in the PD population is believed to be a risk factor for developing peritonitis as a result of transmural migration of bacteria and rare colonic perforation (3–5). A widely accepted recommendation for the initial treatment of constipation includes high dietary fiber intake and bulk-forming agents such as psyllium (6). In subjects unresponsive to fiber, both osmotic and stimulant laxatives are effective. However, the latter tends to be recommended for occasional use as the long-term safety profile is unclear (6,7).
Fructo-oligosaccharides (FOS) are oligosaccharides that are indigestible and non-absorbable in the small intestine but are fermented in the colon by the resident microflora (8). Through the process of fermentation, FOS are metabolized to produce short-chain fatty acids which acidify the colonic content and selectively stimulate the growth of bifidobacteria. The proliferation of these colonic bacteria causes an increase in bacteria fecal mass and produces a bulky effect. In addition, the development of bifidobacteria is detrimental to the growth of harmful pathogens, and FOS is therefore considered a prebiotic. These beneficial properties help modulating bowel functions and improve defecation frequency (9–11).
Several studies have demonstrated that FOS can significantly increase defecation frequency and accelerate colonic transit time without significant side effects (12–15). Additionally, experimental studies have shown that FOS can reduce the levels of glucose, cholesterol, and serum urea nitrogen produced by bacterial growth and utilization (15). Change in colonic acidity also facilitates calcium absorption (9,16). Currently, no data are available for the use of FOS in CAPD patients with constipation. The present study was conducted to examine the effects of FOS on constipation and biochemical parameters including serum glucose, cholesterol, urea nitrogen, and calcium in elderly CAPD patients.
Methods
Patients
This randomized, double-blind, placebo-controlled cross-over study was conducted in CAPD patients aged more than 50 years old at King Chulalongkorn Memorial Hospital. Inclusion criteria were patients who underwent CAPD for more than 6 months and had chronic constipation which was defined according to the Rome II criteria (17). The experimental protocol was approved by the Ethical Committee of Research, Faculty of Medicine, Chulalongkorn University. Written informed consent was obtained from each patient. A 4-week washout period was undertaken for patients receiving antibiotics or medications that might potentially influence bowel habit. Patients with inadequate dialysis, acute and chronic inflammations, hepatobiliary diseases, and gastrointestinal diseases were excluded from the study.
Experimental Design
Each subject was given either FOS (20 g/d) or placebo (sucrose) for 30 days, followed by a 14-day washout period. Then the subjects were switched to receive the other experimental substance for another 30-day period. Fructo-oligosaccharides as well as the placebo were similarly prepared in a 10-g sachet. The subjects were instructed to dissolve each sachet of FOS or placebo in approximately 150 – 200 mL of water, stir thoroughly, and drink after breakfast and dinner. The frequency of defecation was recorded and the characteristics of feces were assessed following the Bristol Stool Form Scale (18) as follows: type 1, separate hard lumps like nuts, hard to pass; type 2, sausage shaped but lumps; type 3, like a sausage but with cracks on its surface; type 4, like a sausage or snake, smooth, and soft; type 5, soft blobs with clear-cut edges, passed easily; type 6, fluffy pieces with ragged edges, a mushy stool; type 7, watery, no solid pieces, entirely liquid. Standard colonic transit time was assessed by calculation of a geometric center (GC) at baseline and the end of each treatment period. In brief, gastrointestinal tract x-rays were performed after 48-h radiopaque marker administration (Sitzmark, Konsyl Pharmaceuticals, Maryland, USA). The images were reviewed according to the colonic regions of interest, which were divided into 5 segments as follows: segment 1, the ascending colon; segment 2, the transverse colon; segment 3, the descending colon; segment 4, the rectosigmoid colon; and segment 5, feces (19). Geometric center referred to the median point of the distribution of the radiopaque within the colon regions and was calculated from the following equation: GC = sum of counts in each region × region number/total counts (including counts excreted in stool).
Assessment of Serum Biochemical Parameters
Three-day food record and serum biochemical parameters were examined before and after each treatment period. The serum biochemical parameters studied included serum glucose, cholesterol, total protein, albumin, calcium, phosphorus, sodium, potassium, urea nitrogen, and creatinine.
Statistical Analysis
Descriptive statistics were expressed as mean ± standard of error (SEM). Comparisons of all parameters measured between before and after supplementation of either FOS or placebo were performed by paired sample t-test (SPSS for window version 11.5, SPSS Inc., Illinois, USA). The values of parameters measured after FOS supplementation were compared with those after placebo supplementation by cross-over analysis using t-test (NCSS97 for window, NCSS Company, Utah, USA). The differences were considered significant at p < 0.05.
Results
Baseline Characteristics
Thirteen subjects (8 male and 5 female) were enrolled in the study. However, 4 subjects were excluded: 2, both assigned to take the placebo, were found later not to have stopped taking other laxatives right at the beginning of the experimental period, 1 subject was switched to hemodialysis shortly after starting placebo, and 1 subject died after the crossover while taking FOS before outcome could be assessed. The patient who died was an elderly patient who was found dead at home without prior symptoms. Therefore, 9 subjects (5 male and 4 female) completed the study and were included in the final statistical analysis. The average age of the patients was 71.2 ± 6.5 (55 – 83) years. The diseases underlying the end-stage renal disease were diabetes (n = 5), hypertension (n = 3), and unknown (n = 1). The patients underwent CAPD for 17.8 ± 3.2 months (6 – 24 months). The drugs that were used in the patients and could alter bowel habit, including diuretics (100%), calcium channel blockers (55.6%), calcium supplements (55.6%), and iron (11.1%) were withdrawn at least 1 month before starting the experiment. Most of the patients (55.6 %) had daily fiber intake about one half of their total food intake and all of them drank less than 1,500 mL of water per day. At both 3 months and 1 month before entering the study, the stool characteristic of all patients was separate hard lumps, “like nuts” (type 1). The patients experienced pain and bleeding during defecation.
Effects of FOS on Constipation
The frequency of defecation was significantly increased after supplementation with FOS for 30 days, compared to the placebo and the baseline (10.5 ± 2.0 vs 6.2 ± 1.4 times per week, p < 0.005) (Figure 1). After FOS supplementation, the stool appearance changed from type 1 (nut-like) to type 4 (sausage-like, smooth, and soft). The patients defecated easily without pain.
Figure 1 —
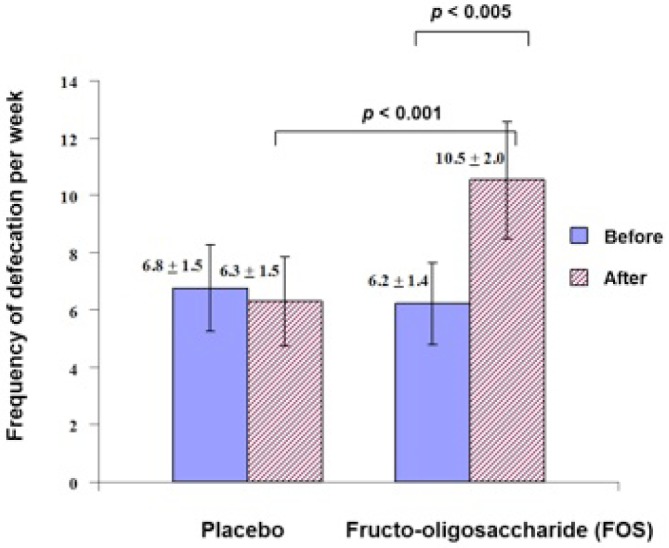
Effect of fructo-oligosaccharide (FOS) supplementation on the frequency of defecation per week in continuous ambulatory peritoneal dialysis (CAPD) patients. FOS = fructooligosaccharides; CAPD = continuous ambulatory peritoneal dialysis.
The effect of FOS on colonic transit was evaluated by the change of GC after 48 hours of radiopaque marker administration. The GC values significantly increased after FOS supplementation when compared with the baseline (Figure 2), indicating a faster colonic transit after FOS supplementation. In contrast, slower colonic transit was found as the GC was significantly decreased after placebo supplementation when compared with the baseline FOS-supplement period. When GC after FOS supplementation was compared with that after placebo, it was found that FOS supplementation resulted in increased GC (Figure 2).
Figure 2 —
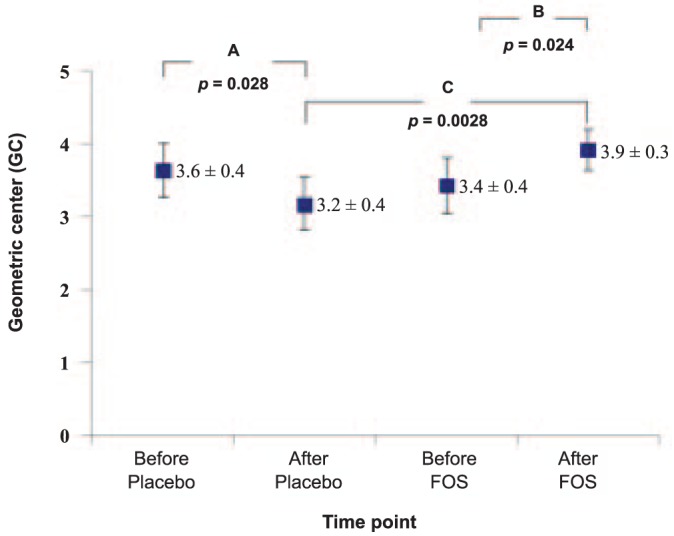
Effect of fructo-oligosaccharide (FOS) supplementation on geometric center after 48-hour administration of radiopaque markers in CAPD patients. A: Significant differences in GC between before and after placebo supplementation (p < 0.05) using paired sample t-test; B: Significant differences in GC between before and after FOS supplementation (p < 0.05) using paired sample t-test; C: Significant differences in GC after FOS supplementation (p < 0.01), compared with placebo using cross-over analysis using t-test. GC = geometric center; FOS = fructo-oligosaccharides; CAPD = continuous ambulatory peritoneal dialysis.
Effects of FOS on Biochemical Parameters
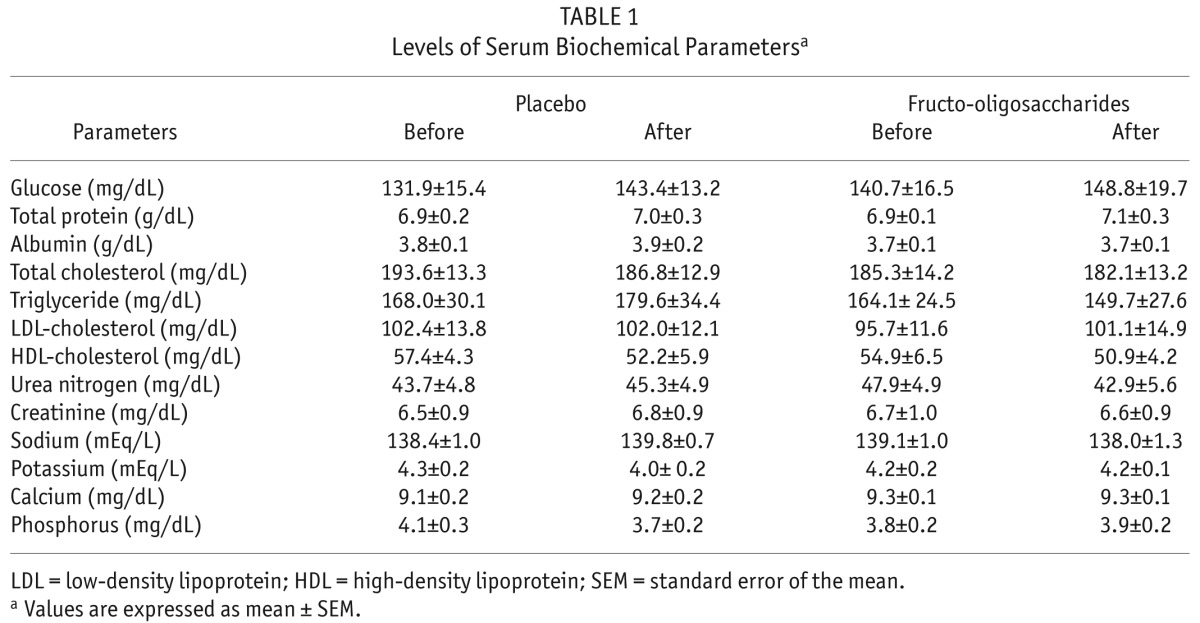
The levels of biochemical parameters measured in this study are shown in Table 1. Both FOS and placebo treatment had no effects on the levels of all biochemical parameters. Of note, there was slightly but not significantly decreased blood urea nitrogen after FOS supplementation (Table 1).
TABLE 1.
Levels of Serum Biochemical Parametersa
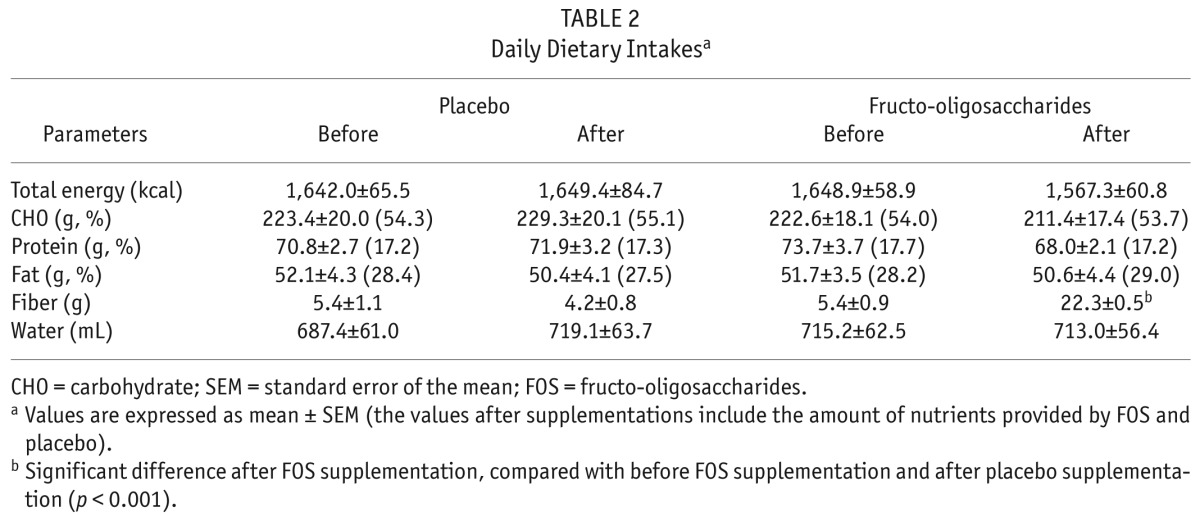
Average daily dietary nutrient intakes are presented in Table 2. At baselines before either treatment, total energy and macronutrient intakes were lower than the Kidney Disease Outcomes Quality Initiative nutrition work group recommended for patients with chronic kidney disease (20). The amount and percentages of total calories from carbohydrate, protein, and fat were not significantly changed throughout the study. The results showed that supplementation with FOS significantly increased the dietary fiber intake, compared with baseline (p < 0.001) and placebo (p < 0.001).
TABLE 2.
Daily Dietary Intakesa
Compliance and Adverse Events During FOS Supplementation
Subjects appropriately consumed both FOS and placebo, as compliance was higher than 95 percent. Some minor adverse events were reported by the patients during the first few days after starting FOS supplementation. Three patients experienced mild flatulence and abdominal discomfort which required a dose reduction to 10 g/day for the first 2 – 3 days. The dose was successfully increased to 20 g/day without any complaints. No significant differences in urine volume, residual renal function, peritoneal function assessed by peritoneal equilibrium test, and small-solute adequacy were found during the periods of placebo and FOS supplementation.
Discussion
The results in the present study showed that the frequency of defecation was significantly enhanced, the stool was softer and easily passed, and the colonic transit was faster, after FOS supplementation. These findings in elderly CAPD patients were in agreement with previous studies in the elderly without renal diseases (12,15). The effect of FOS in ameliorating constipation is believed to be due to their prebiotic properties. This trial showed for the first time that administration of FOS can successfully improve constipation in a CAPD population.
Like other non-absorbed carbohydrates that yield gas during the process of fermentation, supplementation of FOS could result in gastrointestinal tract–related adverse effects, including flatulence, bloating, and gastric discomfort. The severity of these effects appeared to be dose-dependent (8). In accordance with previous studies (20,21), FOS was well-tolerated with only mild gastrointestinal adverse effects reported in our study. However, we could not completely exclude the role of FOS as a cause of death in 1 subject. Given that there have been no serious adverse events reported with the use of FOS in the literature, and the death occurred in an elderly end-stage renal disease patient with no prior alarming symptoms, FOS related-death appears highly unlikely but could not be entirely excluded.
Lactulose, which is also an indigestible sugar and acts as an osmotic laxative, is hydrolyzed by a wide variety of colonic bacteria and produces more side effects than FOS (22). Another commonly used osmotic laxative in chronic kidney disease patients is polyethylene glycol, which is an inert polymer and is better tolerated than lactulose (23). This agent comes as a powder that needs to be mixed with water before it is used, whereas FOS is considered as a dietary supplement that can be incorporated into ordinary diet (15). This may be beneficial in patients with poor compliance or who require fluid restriction.
Non-digestible oligosaccharides are often cited as being important supplemental dietary fibers in the treatment and prevention of metabolic syndrome (24). The role of FOS in reducing plasma glucose and cholesterol has been studied widely; however, the results remain inconclusive (15,25). Short-chain fatty acids, mainly acetate and propionate, are produced and absorbed during the process of colonic fermentation. These short-chain fatty acids can reduce plasma-free fatty acids which might theoretically lower blood sugar and increase insulin sensitivity (26). The mechanism by which FOS reduces cholesterol levels remains to be investigated. It is proposed that FOS may increase fecal cholesterol excretion by stimulating bacterial growth, which in turn decreases the blood cholesterol level (15,27). In our study, FOS failed to show hypoglycemic and hypocholesterolemic effects as these parameters did not change throughout the study. The present findings supported the study of Alles et al. (28). They also found no reduction in abnormal high levels of glucose and lipid levels after FOS supplementation (15 g/d for 20 days). Using the same amount and duration of FOS supplementation (20 g/d for 4 weeks), the present results were in accordance with those found by Luo et al. in type 2 diabetic patients (26,29).
A significant decrease in serum urea nitrogen was observed in a study conducted among elderly people with normal renal function (15). Many other studies regarding FOS and nitrogen metabolism were extensively conducted on both normal renal function and renal-failure animal models (30,31). Colonic microflora is likely the cause of increased serum urea utilization, which in turn increases the fecal excretion of nitrogen waste (32). Interestingly, a decrease in serum urea nitrogen in our study was also observed after FOS supplementation. However, the difference was not statistically significant which was probably due to small sample size. Nonetheless, this finding is promising as it would imply that FOS may have a role in the management of chronic kidney disease by reducing serum urea nitrogen. Another physiologic benefit of the consumption of FOS is increased bioavailability of calcium. Short-chain fatty acids produced during fermentation decrease intestinal pH thereby increasing calcium solubility and absorption in the distal colon (33). This physiologic change has been confirmed by a few human trials (16,34,35). However, there were no significant changes in serum calcium level in the present study.
There were certain limitations in the present study including small sample size and short duration of treatment and follow-up. These limitations might be the main reasons why we did not find any significant changes in biochemical parameters. Long-term safety and efficacy were not evaluated in this study.
Conclusions
Supplementation of FOS at the level of 20 g/d for 30 days could significantly increase bowel frequency, soften stool, and accelerate colonic transit time in patients with CAPD. Fructo-oligosaccharides are well tolerated with only mild adverse events such as bloating and flatulence. Unlike other laxatives, FOS can be easily incorporated into an ordinary diet as a food ingredient. Moreover, FOS show a promising role in the reduction of serum urea nitrogen in CAPD patients. Further studies are needed to better assess the biochemical effects and long-term efficacy of FOS. In the present study, we have proven that FOS supplementation is effective, well tolerated, and can be an alternative to other laxatives in CAPD patients with constipation.
Disclosures
The authors declare no financial conflict of interest related to this study.
Acknowledgments
We would like to express our gratitude to Associate Professor Dr. Oranong Kangsadalampai for valuable suggestions and to Dr. Kathy Savage for reviewing the manuscript. We thank the staff of the CAPD clinic at King Chulalongkorn Memorial Hospital for their helpful cooperation and support in providing many facilities. This study was supported by CU. Graduate School Thesis Grant, Chulalongkorn University.
REFERENCES
- 1. Yasuda G, Takeshita Y, Kimura T, Tochikubo O, Ikeda Y, Tokita Y, et al. Constipation occurs less frequently in CAPD patients than in HD patients. Perit Dial Int 1995; 15(6):283. [PubMed] [Google Scholar]
- 2. Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, et al. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis 2002; 39(6):1292–9. [DOI] [PubMed] [Google Scholar]
- 3. Zhang J, Huang C, Li Y, Chen J, Shen F, Yao Q, et al. Health-related quality of life in dialysis patients with constipation: a cross-sectional study. Patient Prefer Adherence 2013; 7:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singharetnam W, Holley JL. Acute treatment of constipation may lead to transmural migration of bacteria resulting in gram-negative, polymicrobial, or fungal peritonitis. Perit Dial Int 1996; 16(4):423–5. [PubMed] [Google Scholar]
- 5. Afsar B, Elsurer R, Bilgic A, Sezer S, Ozdemir F. Regular lactulose use is associated with lower peritonitis rates: an observational study. Perit Dial Int 2010; 30(2):243–6. [DOI] [PubMed] [Google Scholar]
- 6. Liu LW. Chronic constipation: current treatment options. Can J Gastroenterol 2011; 25(Suppl B):22B–8B. [PMC free article] [PubMed] [Google Scholar]
- 7. Mimidis K, Mourvati E, Kaliontzidou M, Papadopoulos V, Thodis E, Kartalis G, et al. Efficacy of polyethylene glycol in constipated CAPD patients. Perit Dial Int 2005; 25(6):601–3. [PubMed] [Google Scholar]
- 8. Stone-Dorshow T, Levitt MD. Gaseous response to ingestion of a poorly absorbed fructo-oligosaccharide sweetener. Am J Clin Nutr 1987; 46(1):61–5. [DOI] [PubMed] [Google Scholar]
- 9. Roberfroid MB. Fructo-oligosaccharide malabsorption: benefit for gastrointestinal functions. Curr Opin Gastroenterol 2000; 16(2):173–7. [DOI] [PubMed] [Google Scholar]
- 10. Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr 2009; 63(11):1277–89. [DOI] [PubMed] [Google Scholar]
- 11. Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 2004; 17(2):259–75. [DOI] [PubMed] [Google Scholar]
- 12. Chen HL, Lu YH, Lin JJ, Ko LY. Effects of isomalto-oligosaccharides on bowel functions and indicators of nutritional status in constipated elderly men. J Am Coll Nutr 2001; 20(1):44–9. [DOI] [PubMed] [Google Scholar]
- 13. Cherbut C. Inulin and oligofructose in the dietary fibre concept. Br J Nutr 2002; 87(Suppl 2):S159–62. [DOI] [PubMed] [Google Scholar]
- 14. Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 1995; 108(4):975–82. [DOI] [PubMed] [Google Scholar]
- 15. Yen CH, Kuo YW, Tseng YH, Lee MC, Chen HL. Beneficial effects of fructo-oligosaccharides supplementation on fecal bifidobacteria and index of peroxidation status in constipated nursing-home residents—a placebo-controlled, diet-controlled trial. Nutrition 2011; 27(3):323–8. [DOI] [PubMed] [Google Scholar]
- 16. Sanwalka NJ, Khadilkar AV, Chiplonkar SA, Khadilkar VV, Mughal MZ. Galacto-fructo-oligosaccharide fortification of fermented non-dairy snack enhances calcium absorption in healthy adolescent girls. Int J Food Sci Nutr 2012; 63(3):343–52. [DOI] [PubMed] [Google Scholar]
- 17. Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003; 349(14):1360–8. [DOI] [PubMed] [Google Scholar]
- 18. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32:920–4. [DOI] [PubMed] [Google Scholar]
- 19. Hardy JG, Perkins AC. Validity of the geometric mean correction in the quantification of whole bowel transit. Nucl Med Commun 1985; 6(4):217–24. [DOI] [PubMed] [Google Scholar]
- 20. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35(6):S1–140. [DOI] [PubMed] [Google Scholar]
- 21. Bouhnik Y, Raskine L, Simoneau G, Paineau D, Bornet F. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: a dose-response relationship study in healthy humans. Nutr J 2006; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, et al. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol 2010; 22(2):199–206. [DOI] [PubMed] [Google Scholar]
- 23. Attar A, Lemann M, Ferguson A, Halphen M, Boutron MC, Flourie B, et al. Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999; 44(2):226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slavin JL. Dietary fiber and body weight. Nutrition 2005; 21(3):411–8. [DOI] [PubMed] [Google Scholar]
- 25. Giacco R, Clemente G, Luongo D, Lasorella G, Fiume I, Brouns F, et al. Effects of short-chain fructo-oligosaccharides on glucose and lipid metabolism in mild hypercholesterolaemic individuals. Clin Nutr 2004; 23(3):331–40. [DOI] [PubMed] [Google Scholar]
- 26. Luo J, Van Yperselle M, Rizkalla SW, Rossi F, Bornet FR, Slama G. Chronic consumption of short-chain fructo-oligosaccharides does not affect basal hepatic glucose production or insulin resistance in type 2 diabetics. J Nutr 2000; 130(6):1572–7. [DOI] [PubMed] [Google Scholar]
- 27. Bouhnik Y, Achour L, Paineau D, Riottot M, Attar A, Bornet F. Four-week short chain fructo-oligosaccharides ingestion leads to increasing fecal bifidobacteria and cholesterol excretion in healthy elderly volunteers. Nutr J 2007; 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alles MS, de Roos NM, Bakx JC, van de Lisdonk E, Zock PL, Hautvast GA. Consumption of fructooligosaccharides does not favorably affect blood glucose and serum lipid concentrations in patients with type 2 diabetes. Am J Clin Nutr 1999; 69(1):64–9. [DOI] [PubMed] [Google Scholar]
- 29. Luo J, Rizkalla SW, Alamowitch C, Boussairi A, Blayo A, Barry JL, et al. Chronic consumption of short-chain fructooligosaccharides by healthy subjects decreased basal hepatic glucose production but had no effect on insulin-stimulated glucose metabolism. Am J Clin Nutr 1996; 63(6):939–45. [DOI] [PubMed] [Google Scholar]
- 30. Younes H, Remesy C, Behr S, Demigne C. Fermentable carbohydrate exerts a urea-lowering effect in normal and nephrectomized rats. Am J Physiol 1997; 272(3 Pt 1s):G515–21. [DOI] [PubMed] [Google Scholar]
- 31. Younes H, Alphonse JC, Hadj-Abdelkader M, Remesy C. Fermentable carbohydrate and digestive nitrogen excretion. J Ren Nutr 2001; 11(3):139–48. [DOI] [PubMed] [Google Scholar]
- 32. Geboes KP, De Hertogh G, De Preter V, Luypaerts A, Bammens B, Evenepoel P, et al. The influence of inulin on the absorption of nitrogen and the production of metabolites of protein fermentation in the colon. Br J Nutr 2006; 96(6):1078–86. [DOI] [PubMed] [Google Scholar]
- 33. Cashman K. Prebiotics and calcium bioavailability. Curr Issues Intest Microbiol 2003; 4(1):21–32. [PubMed] [Google Scholar]
- 34. van den Heuvel EG, Muys T, van Dokkum W, Schaafsma G. Oligofructose stimulates calcium absorption in adolescents. Am J Clin Nutr 1999; 69(3):544–8. [DOI] [PubMed] [Google Scholar]
- 35. Coudray C, Bellanger J, Castiglia-Delavaud C, Remesy C, Vermorel M, Rayssignuier Y. Effect of soluble or partly soluble dietary fibres supplementation on absorption and balance of calcium, magnesium, iron and zinc in healthy young men. Eur J Clin Nutr 1997; 51(6):375–80. [DOI] [PubMed] [Google Scholar]


