Abstract
Synaptotagmin VII (Syt VII) is a Ca2+ sensing molecule that regulates lysosomal exocytosis in several cell types. In macrophages (MØ), Syt VII is required for efficient uptake of large particle loads, by promoting the delivery of lysosomal membrane to phagocytic cups. Here we compare the phagocytic capacity of bone marrow-derived MØs and dendritic cells (DC), and show that the requirement for Syt VII correlates with the unique ability of MØs for continuous phagocytosis. In contrast to MØs, Syt VII+/+ and Syt VII−/− immature DCs show similar levels of initial phagocytosis, followed by a marked decrease in particle uptake. [Ca2+]i chelation and PI-3 kinase inhibition reduce particle uptake by MØs, but are markedly less inhibitory in DCs. Thus, immature DCs appear to lack the Syt VII, Ca2+ and PI-3 kinase-dependent forms of phagocytosis that are present in MØs. Interestingly, expression of Syt VII is up-regulated during LPS-induced DC maturation, a stimulus that also induces Syt VII translocation from intracellular compartments to the plasma membrane. Syt VII−/− DCs show a delayed translocation of MHC class II to the cell surface during maturation, consistent with the possibility that Syt VII facilitates exocytosis and/or surface retention of molecules critical for antigen presentation.
Keywords: Antigen, Calcium, MHC class II, Phagocytosis, Presentation
Introduction
Macrophages (MØ) and dendritic cells (DC) originate from circulating bone marrow precursors, and acquire markedly distinctive characteristics after leaving the bloodstream and completing their differentiation in peripheral tissues. Thanks to their extraordinary capacity for endocytic uptake (Steinman et al., 1983), MØs are specialized in the clearance of particulate material such as dead cells and invading microorganisms. DCs, on the other hand, are largely dedicated to the presentation of internalized antigen, a critical function needed for the initiation and regulation of immune responses (Steinman and Hemmi, 2006; Trombetta and Mellman, 2005). Consistent with this view, MØ-deficient mice retain the capacity to initiate immune responses (Delemarre et al., 1990), while DC-depleted mice are strongly deficient (Jung et al., 2002). Reflecting these different roles, MØs and DCs are differentially distributed within lymphoid organs, with DCs accumulating in areas involved in naïve T cell activation that exclude MØs (Steinman et al., 1997). DCs are also unique in their ability to respond to inflammatory stimuli by undergoing maturation, a profound cellular reorganization that results in high surface levels of MHC-II and co-stimulatory molecules. Prior to maturation, immature DCs share with MØs a capacity for avid phagocytosis (Trombetta and Mellman, 2005).
MØs have a remarkable capacity for plasma membrane uptake and recycling, internalizing up to 200% of their surface area per hour of fluid phase endocytosis (Steinman et al., 1983). MØs can also internalize very large particles (Aderem and Underhill, 1999), suggesting that intracellular sources of membrane might be added to nascent phagocytic cups to facilitate particle engulfment. In support of this idea, MØs were found to undergo rapid increases in surface area during phagocytosis (Cox et al., 1999; Hackam et al., 1998; Holevinsky and Nelson, 1998). Subsequent studies showed that exocytosis of early endosomes (Bajno et al., 2000; Cox et al., 2000) and lysosomes (Braun et al., 2004; Czibener et al., 2006) is important for efficient particle uptake in MØs. The delivery of lysosomal membranes to phagocytic cups was linked to a Ca2+-dependent component of the phagocytic process, since it is mediated by synaptotagmin VII (Syt VII) (Czibener et al., 2006), a high affinity Ca2+ sensor protein that regulates SNARE-mediated membrane fusion with the plasma membrane (Andrews and Chakrabarti, 2005; Bhalla et al., 2005).
Syt VII belongs to a large family of membrane proteins capable of binding Ca2+ through their two cytosolic C2 domains (Chapman, 2002). In MØs, Syt VII is localized on peripheral domains of lysosomal compartments, which are rapidly mobilized to nascent phagosomes during particle uptake (Czibener et al., 2006). Syt VII is also present on the membrane of lysosomes and of non-synaptic secretory vesicles in several cell types, where it regulates Ca2+-triggered exocytosis (Andrews and Chakrabarti, 2005). In this study we examined the phagocytic behavior of MØs and DCs derived from the bone marrow of wild type or Syt VII-deficient mice, with the goal of assessing Syt VII function in DCs.
Materials and methods
Mice
Synaptotagmin VII KO mice (Syt VII−/−) were generated as previously described (Chakrabarti et al., 2003) and backcrossed 8 generations into a C57BL/6 background. Six to ten week old mice (Charles River Laboratories) were used in experiments. All mice were maintained at the Yale Animal Resources Center, New Haven.
Cell culture
Mouse bone marrow-derived MØs were prepared from C57BL/6 Syt VII+/+ (WT), and Syt VII−/− (KO) mice (Chakrabarti et al., 2003), seeded onto 96-well (100 ml of a 5 × 105/ml suspension) or 24-well (0.5 ml of a 1.5 × 105/ml suspension) plates 24 h prior to experiments, and incubated in MØ media (RPMI 10% FBS, 20% L cell-conditioned supernatant (vol/vol) and 1% pen/strep), at 37 °C/5% CO2. Mouse bone marrow-derived DCs were prepared and cultured from Syt VII+/+ and Syt VII−/− mice using the procedure developed by Inaba et al. (1992). In brief, bone marrow from mouse femurs and tibias were flushed into RPMI 5% FBS media and exposed to 0.8% ammonium chloride for red blood cell lysis. Cells were washed with RPMI 5% FBS and incubated with the mAbs GK1.5 anti-CD4, HO 2.2 anti-CD8, B21-2 anti-Ia, and RA3-3A1/6.1a and rabbit complement for 1 h at 37 °C/5% CO2. Recombinant mouse GM-CSF was produced as culture supernatant from J558L cells transfected with the mouse GM-CSF. Cells were washed and plated in 24-well plates at 1 × 106 cells/ml per well in DC Media (RPMI, 5% FBS, 50 μM β-ME, 1:100 GM-CSF supernatant, and 20 μg/ml gentamycin) for 5–6 days before being harvested.
DC purification
DCs were purified using monoclonal hamster anti-mouse CD11c antibodies conjugated to magnetic MicroBeads (CD11c-N418 Microbeads from Miltenyi Biotech). A total of 5–10 × 106 DCs were harvested and incubated with CD11c Microbeads for 20 min at 4 °C in MACS buffer (PBS without Ca2+ or Mg2+, 2 mM EDTA, 0.5% BSA). Excess CD11c Microbeads were washed away and the magnetically labeled DCs were passed through a MS MACS column (Miltenyi Biotech) held in a magnetic field to capture the labeled cells. After washing off the unlabeled cells with MACS buffer, CD11c-positive cells were eluted by removing the column from the magnetic field and using a plunger to dislodge the labeled cells into a falcon tube. DCs were then washed in PBS and prepared for subsequent experiments.
Antibodies, immunofluorescence, imaging and image analysis
For flow cytometry staining the following antibodies were diluted in 2% BSA: fluorescein isothiocyanate (FITC)-conjugated mouse anti-I-Ab (MHC II) mAb, phycoerythrin (PE)-conjugated rat anti-CD86 mAb, allophycocyanin (APC) or FITC-conjugated hamster anti-CD11c mAb (all from BD Pharmingen). For immunofluorescent staining, day 5–6 DCs were used. Cells were harvested and washed with RMPI 2% FBS and added at 1 × 105 cells per coverslip to Alcian blue-coated coverslips at 37 °C for 15 min before fixing with 2% paraformaldehyde (PFA) at 4 °C. After washing with PBS the cells were quenched with 50 mM ammonium chloride for 15 min, washed and permeabilized with 0.05% saponin for 20 min, before blocking in PBS 2% BSA solution for 30 min. MHC II was detected with supernatant from rat anti-mouse hybridoma, clone M5/114, followed by Alexa Fluor 568 or 488-conjugated goat anti-mouse or goat anti-rat secondary antibodies (Invitrogen). For detection of dextran-loaded lysosomes, MØs or DCs were incubated with 0.25 mg/ml lysine-fixable Rhodamine B/Texas Red/FITC dextran (10 kDa, Invitrogen) for 1 h at 37 °C, washed and chased in fresh medium for 2–3 h, followed by fixation in 2% paraformaldehyde. Coverslips were mounted in Prolong anti-fade medium (Invitrogen) and images were acquired through a 100 × objective in a Zeiss Axiovert microscope with a Hamamatsu Orca II cooled CCD camera controlled by Metamorph Software (Universal Imaging), or in a Zeiss LSM 510 laser scanning confocal microscope (Z stack images were acquired with optical sections of 0.5–0.8 μm, at 1 μm intervals).
Retroviral transduction and live imaging
Bone marrow-derived progenitor cells were transduced using retroviral vectors encoding SytVII-YFP (pLZRS-SytVII-YFP), as previously described (Sherer et al., 2003). SytVII-YFP was constructed by insertion of the SytVII coding region from SytVII-GFP (Martinez et al., 2000) into a modified pLZRS-YFP retroviral construct using XhoI and HindIII sites. For retroviral transduction of DCs 1 × 106 DC precursors were incubated with MHC II-GFP (I–Eα-GFP) or Syt VII-YFP viral supernatants one day post-isolation for 3–8 h. Transduced DCs were imaged on day 4 or 5 in MatTek glass coverslip dishes.
Phagocytosis assay by flow cytometry (FACS)
MØs were plated at 2–3 × 105 cells/well in 6-well non-tissue culture dishes overnight. Immature DCs were cultured in 24-well plates at 1 × 106 cells/well and were assayed by FACS analysis on day 5 for surface CD11c, to assess the percentage of DCs and for surface MHC II and CD86, to assess maturation state. Unopsonized Texas Red zymosan Bio-particles (Invitrogen) were incubated with MØs or DCs at different particle/cell ratios for different periods of time. MØs were washed and lifted from plates by incubation on ice with ice cold PBS without Ca2+ or Mg2+. DCs were harvested by pipetting, washed four times with PBS, and placed on ice for live CD11c-FITC staining and FACS analysis. For Ca2+ chelation assays, MØs and DCs were incubated in Hanks’ Balanced Salt Solution without Ca2+ and Mg2+ (HBSS−/−) containing 20–50 μM BAPTA-AM (Molecular Probes) and 5 mM EGTA for 30 min at room temperature, prior to incubation with zymosan. Upon addition of zymosan, BAPTA-AM was removed and replaced with fresh media. For PI 3-kinase inhibition assays, MØs and DCs were incubated in RPMI containing 5–100 nM wortmannin (Sigma) for 30 min at 37 °C prior to incubation with zymosan. FACS data was plotted and analyzed using Flowjo (Treestar Inc.) or Cellquest Pro (BD Biosciences) software.
DC maturation time course
To induce maturation, we disaggregated d4–5 DC clusters by gentle pipetting and added LPS (50 ng/ml). At various times over 12 h cells were harvested, placed on ice, and stained with indicated antibodies. FACS data were plotted and analyzed using Flowjo (Treestar Inc.) or Cellquest Pro (BD Biosciences) software.
Real-time quantitative PCR
A total of 5–10 × 106 DCs were purified as either immature or 24 h after inducing maturation. Qiagen RNeasy kit was used to isolate total RNA. cDNA was generated using oligo dT primers with SuperScript I IRNase H-reverse transcriptase (Invitrogen) according to manufacturer’s instructions. Real time PCR was performed with a Biorad iCycler with iQ SYBR Green Supermix according to manufacturer’s instructions (Biorad). Parameters: 1 cycle of 95 °C for 15 min; 35 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min; and 1 cycle of 72 °C for 5 min. Fluorescence data were collected during the 72 °C step at the end of each cycle. All determinations were performed at least in triplicate to achieve reproducibility. Nontemplate controls run with every assay had consistently no cycle threshold values before 35 cycles of PCR. Amplifications were carried out in a total volume of 20 μl by using iQ SYBR Green Supermix (Biorad). Oligonucleotide Primers: Syt7alpha fwd: 5′-CCG TCA GCC TTA GCG TCA C-3′; Syt7alpha rev: 5′-GCA GGC AAC TTG ATG GCT TTC-3′; Beta actin fwd: 5′-GGC TGT ATT CCC CTC CAT CG-3′; Beta actin rev: 5′-CCA GTT GGT AAC AAT GCC ATG T-3′.
Western blot
Approximately 20 × 106 DCs were purified in anti-CD11c+ microBeads as either immature or 24 h after inducing maturation. After purification they were lysed in 50 mM HEPES pH 7.0, 500 mM NaCl, 1% NP-40 and complete protease inhibitor cocktail (Roche). The lysates were left on ice for 30 min, spun for 30 min at 15,000g, and the protein concentration in the soluble material was assayed using a BCA Protein Assay Reagent Kit (Pierce). Samples were incubated with SDS sample buffer with 2% β-ME and boiled for 5 min. A total of 100 μg of protein was loaded into each SDS-PAGE well, with 20 μg of protein for each loading control well. After transfer membranes were blotted with affinity purified rabbit polyclonal antibodies against the NH2-terminal of Syt VII (Martinez et al., 2000), or a mouse anti-β-actin (Abcam) as a loading control.
Results
To directly compare the phagocytic ability of MØs and DCs, bone marrow cells extracted from the same mouse were subjected to distinct differentiation protocols, to generate M-CSF-dependent MØs, or GM-CSF-dependent CD11c+DCs (see Materials and Methods section). The CD11c+DC population generated by our procedure was largely immature, with low levels of surface-exposed MHC-II and CD86. Both cell types were incubated with Texas Red zymosan Bio-particles for increasing periods of time, washed, and analyzed by flow cytometry. The washes removed more than 95% of the extracellularly attached particles, as determined by an inside/outside immunofluorescence assay (Fig. 1A and B, right panels), confirming that the cell-associated fluorescence reflected the amount of internalized zymosan. At early time points both MØs and DCs avidly internalized a comparable number of particles. However, in MØs phagocytosis continued to increase over time for up to 4 h, while particle uptake by DCs effectively stopped by 2 h (Fig. 1B).
Fig. 1.
Early down-regulation of phagocytosis in immature DCs. (A, B) FACS analysis of zymosan uptake in MØs and DCs. Cells were incubated with Texas Red zymosan at 25 particles/cell for 0.5, 1, 2, 4, and 16 h. Extracellular zymosan was removed with extensive washes with ice cold PBS. Black line: background fluorescence of cells without exposure to zymosan. Samples were also gated by size to exclude any debris or residual particles. The images show that only very few externally attached particles remained after the washes, as detected with anti-zymosan antibodies (green). Bars = 10 μm. (C) Quantification of the geometric mean fluorescence of the FACS charts shown in A and B.
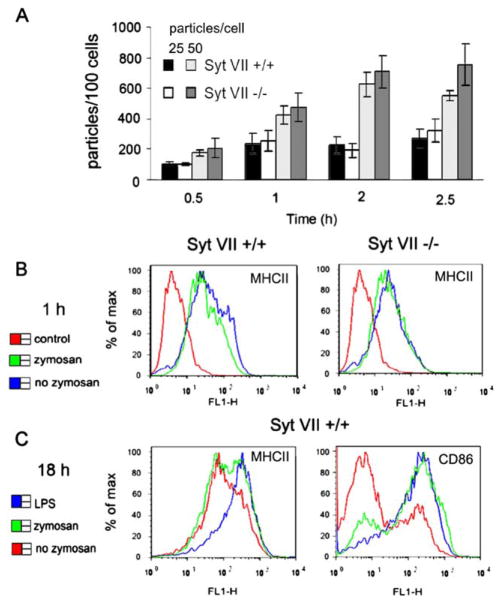
Earlier studies demonstrated that MØs from Syt VII-deficient mice (Syt VII−/−) have a marked defect in their capacity to phagocytose large particle loads (25–50 particles/cell) (Czibener et al., 2006). To determine if the same occurred with DCs, phagocytosis assays were performed comparing cells derived from the bone marrow of Syt VII+/+ and Syt VII−/− mice. DCs were exposed to 25 and 50 zymosan particles/cell for short periods, when phagocytosis was still active (Fig. 1B). The number of internalized particles was similar in both DC populations, indicating that Syt VII is dispensable for the uptake of large particle loads by DCs (Fig. 2A). Surface levels of MHC-II were similarly low in Syt VII+/+ and Syt VII−/− DCs exposed to zymosan for 1 h (Fig. 2B). After 18 h exposure to zymosan higher levels of surface MHC-II and CD86 were detected in part of the DC population, indicating that zymosan provides a maturation stimulus that is slower-acting than LPS (Fig. 2C).
Fig. 2.
Phagocytosis of large particle loads is not Syt VII dependent in immature DCs. (A) Texas Red zymosan was added to DCs at 25 or 50 particles/cell, for 0.5, 1, 2, or 2.5 h, and the number of internalized particles was determined microscopically after washing, fixation and staining with anti-zymosan to detect extracellular particles. The data represents the mean +/− SD of internalized particles per 100 DCs. Over 300 cells per coverslip were counted in triplicate. (B) DCs from Syt VII+/+ or Syt VII−/− mice were stained live on ice for surface MHC-II, after incubation with (green) or without (blue) unlabeled zymosan at 50 particles/cell for 1 h at 37 °C. An isotype control antibody is shown in red. (C) Zymosan induces maturation in part of the DC population after 18 h. DCs from Syt VII+/+ mice were incubated with unlabeled zymosan at 50 particles/cell (green) or LPS (50 ng/ml) (blue) for 18 h, harvested and stained live with antibodies to MHC-II and CD86. Surface levels of MHC-II and CD86 from unstimulated (no zymosan or LPS) DCs cultured in parallel for 18 h are shown for comparison (red). The results shown are representative of several independent experiments.
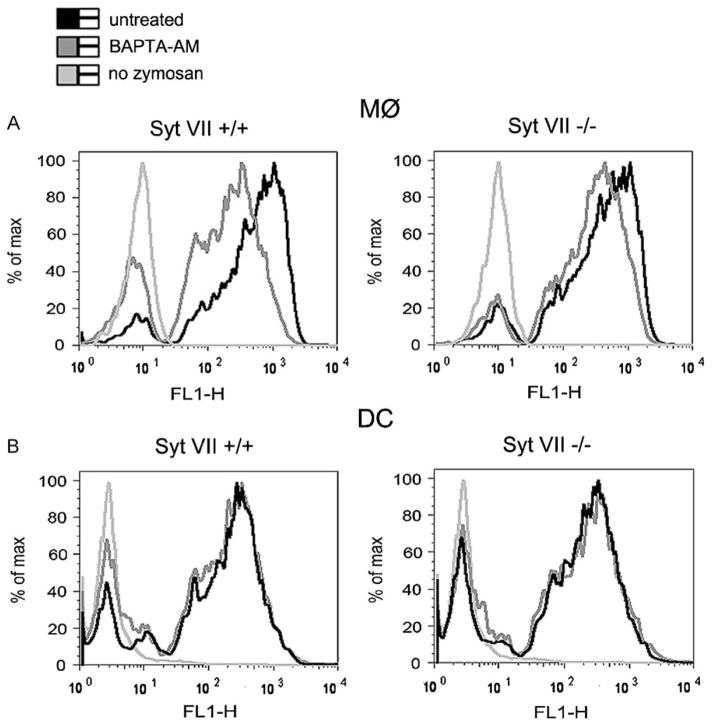
Particle uptake by Syt VII+/+ MØs is partially inhibited by preventing intracellular free Ca2+ ([Ca2+]i) elevations with intracellular Ca2+ chelators (Czibener et al., 2006). However, the same is not observed with the residual phagocytosis of Syt VII-deficient MØs (Czibener et al., 2006). This finding suggested that Syt VII might correspond to a Ca2+-dependent component of the molecular machinery regulating phagocytosis in these cells. Having determined that DCs do not require Syt VII for efficient zymosan uptake, we used a FACS assay to examine in parallel the effect of chelating [Ca2+]i on phagocytosis by bone marrow-derived MØs and DCs. As previously reported (Czibener et al., 2006), [Ca2+]i chelation inhibited phagocytosis in Syt VII+/+, but not Syt VII−/− MØs (Fig. 3A). Interestingly, under the same conditions [Ca2+]i chelation did not alter the phagocytic ability of immature DCs (Fig. 3B). Taken together, these results suggested that DCs lack a Syt VII and Ca2+-dependent component(s) of the phagocytic process.
Fig. 3.
Intracellular Ca2+ chelation inhibits zymosan uptake in a Syt VII-dependent manner by MØs, but not DCs. Fluorescent zymosan uptake was analyzed by FACS in Syt VII+/+ or Syt VII−/− MØs (A) and DCs (B) either with or without pretreatment of 20 μM BAPTA-AM. Zymosan was added at 25 particles/cell for 1 h. The results shown are representative of at least three independent experiments.
Prior studies in MØs found that inhibition of PI 3-kinase affected pseudopod extension during the internalization of large particles. This observation suggested that PI 3-kinase might be required for the exocytosis of intracellular compartments involved in donating membrane for phagocytic cup formation (Cox et al., 1999). Since this limitation in the ability to take up large particle loads is similar to what is observed in Syt VII-deficient MØs, we compared the requirement for PI 3-kinase activity in zymosan phagocytosis by MØs and DCs. After treatment with the irreversible PI 3-kinase inhibitor wortmannin, MØs or DCs were incubated with 25 fluorescent zymosan particles/cell for 1 h, washed, and the cell-associated fluorescence determined by flow cytometry. As expected (Cox et al., 1999), wortmannin significantly inhibited particle uptake in MØs (Fig. 4A and B, MØ). In sharp contrast, when DCs prepared from the same mouse were tested under the same conditions, wortmannin was markedly less inhibitory (Fig. 4A and B, DC). These results confirm that early phagocytosis of MØs and DCs have markedly different properties, including different requirements for Syt VII, Ca2+ and PI 3-kinase activity.
Fig. 4.
PI 3-kinase inhibition reduces zymosan uptake by MØs, but not by DCs. (A) FACS profiles of fluorescent zymosan uptake in MØs and DCs left untreated or pretreated with wortmannin (WM) at 5, 50, or 100 nM for 30 min. Zymosan was added at 25 particles/cell for 1 h. The “no zymosan” population reflects autofluorescence of cells without zymosan. (B) Quantification of percent phagocytosis in WM-treated versus untreated, determined from the geometric mean fluorescence of the FACS profiles shown in A. The data represents the mean +/− SD of triplicate experiments. The results shown are representative of at least three independent experiments.
In MØs, Syt VII localizes to peripheral domains of the highly tubular late endosomal/lysosomal compartment (Czibener et al., 2006). Given the apparent lack of Syt VII involvement in phagocytosis by DCs, we examined its sub-cellular localization in these cells. DCs were retrovirally transduced with Syt VII-YFP, and the lysosomal compartment was loaded with fluorescent dextran. As previously reported for DCs (Chow et al., 2002), the lysosomal marker was detected in a large perinuclear compartment (Fig. 5A), which also contained MHC-II and extended in a tubular fashion towards the cell periphery after exposure to a maturation stimulus (Fig. 5B). Examination of sequential confocal sections revealed that Syt VII-YFP was targeted to discrete, peripheral domains of the dextran-loaded lysosomes, in a pattern very similar to what was previously observed in MØs (Czibener et al., 2006) (Fig. 5A). When the transduced immature DCs were exposed to LPS, Syt VII-YFP redistributed to the cell surface in a pattern similar to that observed for MHC-II (Fig. 5C) (Chow et al., 2002).
Fig. 5.
Syt VII-YFP translocates from peripheral domains of lysosomal compartments to the plasma membrane during DC maturation. (A) Immature DCs were transduced with Syt VII-YFP (green) and loaded with Texas red (TR) dextran (red) prior to fixation. Images are 0.8 μm optical slices from the middle and top of a confocal Z-stack. Arrows point to peripheral domains of dextran loaded lysosomes containing Syt VII-YFP. (B) Live confocal image of a maturing (1 h LPS) DC loaded with TR-dextran (red) and transduced with MHC-II-GFP (green). (C) Live confocal images of DCs transduced with either MHC-II-GFP (green) [left] or Syt VII-YFP (green) [middle], loaded with TR-dextran (red), and induced to mature for 24 h with LPS/cluster disruption. Right panel: FACS profile comparing surface levels of endogenous Syt VII in immature DCs, or DCs induced to mature for 24 h with LPS/cluster disruption. Anti-Syt VII NH2-terminal antibodies were added at 4 °C to detect surface exposed Syt VII. The background control (black) represents secondary antibody alone. About 36% of LPS-treated cells had surface levels of Syt VII significantly above that of immature cells. The results shown are representative of several independent assays. Bars = 5 μm.
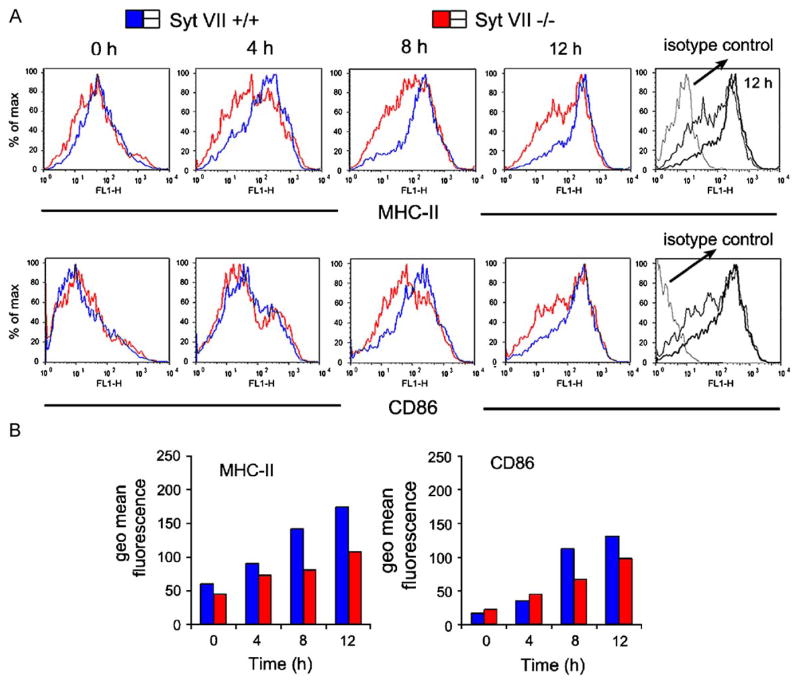
These results suggested that Syt VII might be playing a role in exocytic events involved in delivering MHC-II to the cell surface from lysosomal compartments. This possibility was reinforced by quantitative RT-PCR and Western blot assays, which revealed a marked up-regulation of Syt VII transcripts following LPS-induced maturation (Fig. 6). To directly examine this issue, immature DCs from Syt VII+/+ or Syt VII−/− mice were analyzed for surface-exposed MHC-II at increasing periods of time after incubation with LPS. The expected increase in surface MHC-II during LPS-induced maturation was observed in both cell types. However, the kinetics of the process was delayed in Syt VII−/− DCs, with only part of the population reaching the high surface MHC-II levels typical of wild type cells after 12 h (Fig. 7A and B). A less pronounced delay was also detected in surface expression of the co-stimulatory molecule CD86 (Fig. 7A and B).
Fig. 6.
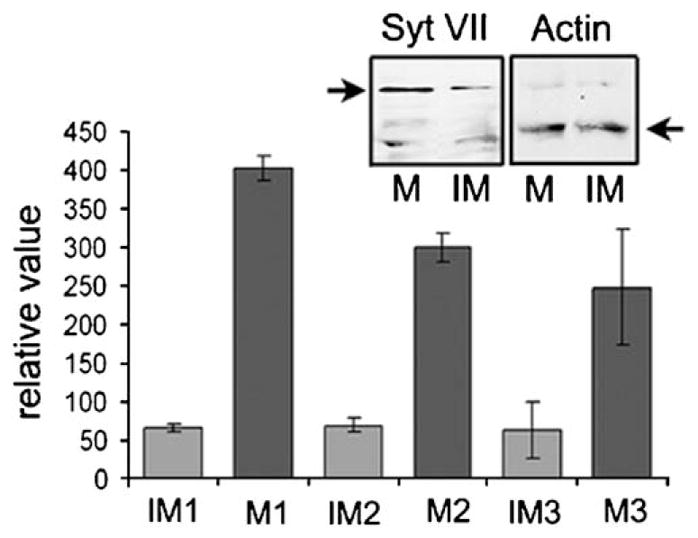
Syt VII is up-regulated in mature DCs. Syt VII mRNA transcript levels were measured using real-time qPCR on three independent preparations of CD11c+ purified immature and mature DCs. Values were normalized using β-actin transcript levels. The data shown represents +/− SD of triplicate determinations. Inset: 100 μg of whole cell lysates from CD11c+ purified immature (IM) and mature (M) DCs were run on SDS-PAGE and transferred to membrane. Western blotting using affinity purified polyclonal antibody to the amino-terminus of Syt VII detected a 66 kDa band which corresponds to the Syt VII α-isoform. Anti-β-actin antibodies (Abcam) were used as a loading control.
Fig. 7.
MHC-II surface transport is delayed in Syt VII-deficient cells. (A) Immature Syt VII+/+ or Syt VII−/− DCs were stimulated with LPS and cluster disruption and assessed for MHC-II and CD86 surface expression by FACS at indicated time points up to 12 h. Top profiles compare MHC-II levels, and bottom profiles compare CD86 levels. Syt VII+/+ DCs (blue), Syt VII−/− DCs (red). For clarity, the isotype control antibody pattern is only shown for the 12 h time point (right panels, grey). (B) Quantification of the geometric mean fluorescence of the charts shown in A. The results shown are representative of three independent experiments.
Discussion
Both MØs and DCs express a large array of phagocytic receptors, and can avidly phagocytose particles and pathogens. However, it is clear that phagocytosis in these two cell types leads to very different outcomes. MØs are specialized in the scavenging and digestion of particulate material, while DCs have unique mechanisms for preserving ingested material for efficient antigen presentation. Consistent with these different roles, phagosome acidification and degradation are significantly reduced in immature DCs, when compared to MØs (Savina and Amigorena, 2007; Trombetta and Mellman, 2005). In addition, only DCs undergo maturation, a profound cellular reorganization that results in nearly complete down-regulation of macropinocytic/phagocytic uptake (Garrett et al., 2000; Sallusto and Lanzavecchia, 1994). When we compared the phagocytic ability of MØs and DCs generated from the same mice, we also detected an abrupt reduction in particle uptake in DCs after a short 1–2 h period, while in macrophages phagocytosis proceeded at a similar rate for several hours. These assays allowed us to identify an early assay period, within the first two hours of exposure to zymosan particles, when phagocytosis was equally active in MØs and immature DCs.
In a recent study, we found that phagocytosis of large particle loads in MØs requires the lysosomal Ca2+ sensor Syt VII, which mediates the delivery of lysosomal membranes to nascent phagosomes (Czibener et al., 2006). In contrast, in this work we found that Syt VII deficiency does not alter particle uptake by immature DCs. In MØs, [Ca2+]i chelation inhibits phagocytosis in Syt VII+/+, but not Syt VII−/− MØs, suggesting that Syt VII specifically regulates a Ca2+-dependent component of phagocytosis. In contrast, [Ca2+]i chelation in DCs has no effect in the efficiency of particle uptake, regardless of the presence or not of Syt VII in the cells. Taken together, these observations suggest that DCs may lack Ca2+/Syt VII-dependent regulatory components of phagocytosis. Consistent with this idea, we found that phagocytosis of large particle loads by MØs is sensitive to PI 3-kinase inhibition, but the same is not true for DCs. Class I PI 3-kinases were previously shown to specifically interfere with the uptake of large particles by MØs (Cox et al., 1999; Vieira et al., 2001).
These intriguing differences between the phagocytic response of MØs and DCs suggested that Syt VII might play a functional role in DCs only after phagocytosis is down-regulated. This possibility was reinforced by the strong up-regulation observed in Syt VII expression, when DCs were induced to mature by exposure to LPS. Since Syt VII regulates the exocytosis of lysosomes in several cell types (Andrews and Chakrabarti, 2005; Martinez et al., 2000), we investigated whether it might be involved in the transport of MHC-II molecules from late endosomes/lysosomes to the plasma membrane during DC maturation. When transduced into immature DCs, Syt VII was targeted to peripheral domains of late endosomal/lysosomal compartments, reproducing the pattern previously observed in MØs (Czibener et al., 2006). Remarkably, during maturation Syt VII was massively translocated to the cell surface simultaneously with MHC-II. In a time course analysis, we found that the kinetics of MHC-II surface translocation in Syt VII-deficient cells is delayed, when compared to wild type DCs. Thus, at least in part, the lysosomal Ca2+ sensor Syt VII appears to be involved in regulating the translocation of MHC-II from late endosomes/lysosomes to the plasma membrane. This delayed MHC-II transport kinetics did not correlate with a defect in antigen presentation, possibly due to the difficulty of detecting small differences with the currently available in vitro assays (results not shown). A smaller reduction in surface translocation was also observed with the co-stimulatory maturation marker CD86. This finding could be related to a more generalized defect in the maturation program of Syt VII-deficient DCs. However, the marked plateau in the levels of MHC-II surface expression observed between 4 and 8 h after LPS stimulation was not observed with CD86, which continued increasing as cells underwent maturation. Thus, the differences in CD86 surface levels observed in the later time points might result from a population of molecules co-localizing/co-trafficking with MHC-II. This is consistent with prior observations (Turley et al., 2000), although that study did not distinguish if the colocalized CD86/MHC-II was biosynthetic or endocytic-recycling in nature. Taken together, our findings add the regulation of exocytosis by Syt VII to the numerous functional differences known to exist between MØs and DCs. In MØs, Syt VII mediates lysosomal delivery to nascent phagocytic cups, while in DCs the function of this Ca2+ sensor appears restricted to later events involved in maturation.
Acknowledgments
This work was supported by grants from the NIH to N.W.A. We thank Amy Chow for help and advice during the early stages of this project.
Abbreviations
- DC
dendritic cell
- MØ
macrophage
- Syt VII
synaptotagmin VII
References
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Andrews NW, Chakrabarti S. There’s more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol. 2005;11:626–631. doi: 10.1016/j.tcb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A, Tucker WC, Chapman ER. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol Biol Cell. 2005;16:4755–4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Fraisier V, Raposo G, Hurbain I, Sibarita JB, Chavrier P, Galli T, Niedergang F. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J. 2004;23:4166–4176. doi: 10.1038/sj.emboj.7600427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- Chow A, Toomre D, Garrett W, Mellman I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- Cox D, Lee DJ, Dale BM, Calafat J, Greenberg S. A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc Natl Acad Sci USA. 2000;97:680–685. doi: 10.1073/pnas.97.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czibener C, Sherer NM, Becker SM, Pypaert M, Hui E, Chapman ER, Mothes W, Andrews NW. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol. 2006;174:997–1007. doi: 10.1083/jcb.200605004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delemarre FG, Kors N, van Rooijen N. The in situ immune response in popliteal lymph nodes of mice after macrophage depletion. Differential effects of macrophages on thymus-dependent and thymus-independent immune responses. Immunobiology. 1990;180:395–404. doi: 10.1016/S0171-2985(11)80301-3. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Sjolin C, Schreiber AD, Trimble WS, Grinstein S. v-SNARE-dependent secretion is required for phagocytosis. Proc Natl Acad Sci USA. 1998;95:11691–11696. doi: 10.1073/pnas.95.20.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holevinsky KO, Nelson DJ. Membrane capacitance changes associated with particle uptake during phagocytosis in macrophages. Biophys J. 1998;75:2577–2586. doi: 10.1016/S0006-3495(98)77703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Ingmundson A, Horner SM, Cicchetti G, Allen PG, Pypaert M, Cunningham JM, Mothes W. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic. 2003;4:785–801. doi: 10.1034/j.1600-0854.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber A, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]