Abstract
A detailed qualitative and quantitative characterization of goat colostrum oligosaccharides (GCO) has been carried out for the first time. Defatted and deproteinized colostrum samples, previously treated by size exclusion chromatography (SEC) to remove lactose, were analyzed by nanoflow liquid chromatography-quadrupole-time of flight mass spectrometry (Nano-LC-Chip-Q-TOF MS). Up to 78 oligosaccharides containing hexose, hexosamine, fucose, N-acetylneuraminic acid or N-glycolylneuraminic acid monomeric units were identified in the samples, some of them detected for the first time in goat colostra. As a second step, a hydrophilic interaction liquid chromatography coupled to mass spectrometry (HILIC-MS) methodology was developed for the separation and quantitation of the main GCO, both acidic and neutral carbohydrates. Among other experimental chromatographic conditions, mobile phase additives and column temperature were evaluated in terms of retention time, resolution, peak width and symmetry of target carbohydrates. Narrow peaks (wh: 0.2–0.6 min) and good symmetry (As: 0.8–1.4) were obtained for GCO using an acetonitrile:water gradient with 0.1% ammonium hydroxide at 40 °C. These conditions were selected to quantify the main oligosaccharides in goat colostrum samples. Values ranging from 140 to 315 mg L−1 for neutral oligosaccharides and from 83 to 251 mg L−1 for acidic oligosaccharides were found. The combination of both techniques resulted to be useful to achieve a comprehensive characterization of GCO.
Keywords: Goat colostrum, Oligosaccharides, Mass spectrometry, Hydrophilic interaction liquid, chromatography, Nanoflow liquid, chromatography-quadrupole-time of flight, mass spectrometry
1. Introduction
Goat milk is a complex mixture of nutritive and bioactive components with reported health benefits such as carbohydrates, lipids and proteins [1]. Although lactose is the main carbohydrate, presence of other oligosaccharides (OS) similar to those found in human milk, has been reported [2]. Among them, some studies indicate the existence of: (i) neutral oligosaccharides, whose structures are mainly based on lactose with the addition of neutral monosaccharides such as glucose or galactose (Hex), N-acetylglucosamine or N-acetylgalactosamine (HexNAc) and fucose or deoxyhexose (Fuc) and (ii) acidic oligosaccharides, containing acidic components such as N-acetylneuraminic (Neu5Ac) or N-glycolylneuraminic acid (Neu5Gc) [3,4]. Some of these oligosaccharides, such as those containing fucosyl- or sialyl-groups have been described to have prebiotic and pathogen binding activities [5–9]. Although much effort has been focused on the composition, structure and bioactiv-ity of OS in human milk, scarce information about both qualitative and quantitative composition of goat milk OS is available. Since it is well known that bioactive properties are directly related to OS chemical structure, the search of novel sensitive and reproducible methods for the analysis of goat milk OS is of special relevance. Moreover, it is expectable that goat colostrum has higher amounts of OS than goat milk in a similar way to bovine or human milk [3,10], representing an interesting source of bioactive OS.
Among the different techniques used for OS analysis, high performance liquid chromatography (LC) is one of the most widespread. Human milk OS have been successfully analyzed by normal phase [11] and reverse phase LC [11–13], although a previous derivatization step is required to improve carbohydrates retention [14]. High performance anion exchange chromatography (HPAEC) provides better separation without a previous derivatization step and it has been widely used for goat milk OS characterization and quantitation [2,15–19]. However, the complex profiles obtained for OS mixtures with different linkage variants and the use of high pH and high salts concentrations in mobile phases make this technique not compatible with mass spectrometry (MS), impairing their complete characterization [20].
Hydrophilic interaction liquid chromatography (HILIC) is a powerful LC operation mode for the analysis of complex OS mixtures (galactooligosaccharides, gentiooligosaccharides, etc.), providing an appropriate resolution and good peak shapes [14,20]. Moreover, mobile phases used in HILIC are compatible with MS and even the use of a high percentage of organic solvents enhances the ionization and increase sensitivity which makes this technique appropriate for structural and glycomic research [21]. However, applications of HILIC to the analysis of mammal milks are scarce. Marino et al. [22] developed a methodology for the analysis of bovine colostrum OS based on their fluorescent labeling, pre-fractionation by weak anionic exchange chromatography and separation by HILIC using an amide based column and a fluorescence detector. Structural assignment of 37 free glycans was carried out by a combination of HILIC analyses, exoglycosidase digestion, desalting and offline MS/MS analyses. HILIC has also been used for the successful determination of six acidic OS in bovine milk, bovine colostrum, and infant formulas [23] in combination with high-resolution selected reaction monitoring mass spectrometry (HILIC-HRSRM-MS). Nevertheless, to the best of our knowledge, HILIC-MS has not been previously used for goat milk OS analysis, being the optimization of the method a requirement for their comprehensive characterization.
In recent years, the use of nano-liquid chip-based technologies mainly coupled to MS or tandem MS (MS/MS) techniques have demonstrated to be extremely helpful for OS identification and it has been applied to milk characterization due to its high sensitivity and capacity for compositional verification [4]. Nano-LC-Chip technology coupled to time of flight (TOF) MS has been successfully used for OS analysis of human milk [24], porcine milk [25] and bovine milk [26,27]. An exhaustive characterization of OS in goat’s milks with and without the genetic ability to synthesize αs1-casein by nano flow liquid chromatography-quadrupole-TOF MS (Nano-LC-Chip-Q-TOF MS) with a porous graphitized carbon column has been recently reported [4]. Twenty nine goat milk OS, 11 of which were detected by the first time, were identified and verified via MS/MS analyses. Moreover, a goat milk oligosaccharide library was also created, which gathered information available in the literature with the new identifications. This methodology has been proven to be an excellent tool for the identification of OS in mammal milks due to its high sensitivity and mass resolution; however, it has not been previously applied to the analysis of goat colostrum samples which could be of interest for further exploitation of goat colostrum oligosaccharides (GCO) as prebiotics.
In this study, goat colostrum samples, previously purified by size exclusion chromatography (SEC) to remove lactose, were firstly submitted to Nano-LC-Chip-Q-TOF MS analysis in order to exhaustively characterize their oligosaccharide fraction. As a second step, a HILIC-MS methodology was developed for the separation and quantitation of the main GCO, both acidic and neutral compounds.
2. Materials and methods
2.1. Chemicals and reagents
All reagents were of analytical grade or better. Acetic acid from Normasolv (Barcelona, Spain), ammonium acetate, ammonium hydroxide from Panreac (Barcelona, Spain) and ethanol of analytical grade were purchased from Lab-Scan (Gliwice, Poland). Acetonitrile (ACN) and formic acid HPLC-MS grade were purchased from Fisher-Scientific (Fair Lawn, NJ, USA). ESI-TOF Low concentration Tuning Mix G1969–85000 was purchased from Agilent Technologies (Santa Clara, CA, USA).
Analytical standards of β-4-galactosyl-lactose, maltotriose and maltotetraose were obtained from Sigma Chemical Co. (St. Louis, MO, USA). 6′-Sialyl-lactose (6′-SL) sodium salt, 3′-sialyl-lactose (3′-SL) sodium salt, 2′-fucosyl-lactose (2′-FL) and 3′-sialyl-N-acetyllactosamine were purchased from Carbosynth (Berkshire, UK). Standard solutions in ACN:water (50:50, v:v) were filtered through nylon FH membranes (0.22 µm; Millipore, Bedford, MA, USA) before injection.
2.2. Colostrum samples
For this study, colostrum samples from four Murciano-Granadina goats (CS1–CS4) were obtained from an experimental farm located at Estación Experimental del Zaidín (Granada, Spain). In addition, colostrum from twelve individual Murciano-Granadina goats reared at Hermanos Archiduque farm (Granada, Spain) were collected and pooled (CS5). Collected samples were immediately frozen at −80°C until further analysis. Animals were cared and handled in accordance with the Spanish guidelines for experimental animal protection (Royal Decree 53/2013 on the protection of animals used for experimentation or other scientific purposes) in line of corresponding European Directive (2010/63/EU). An experimental protocol was approved by the Ethics Committee for Animal Research from the Animal Nutrition Unit.
2.3. Fat and protein removal
Fat and proteins were removed from the samples following the methodology described by Martinez-Ferez et al. [15] with small modifications. Briefly, samples were defatted by centrifugation at 6500 × g for 15min at 5°C, then kept in an ice bath for 30min and filtrated through Whatman No. 1 filter paper to remove the supernatant lipid layer, which was discarded.
The total protein fraction was precipitated by adding two volumes of cold ethanol to the skimmed colostrum samples and shaking for 2 h in an ice bath. The solution was then centrifuged at 6500 × g for 30 min at 5 °C and supernatant was carefully collected. Ethanol was evaporated from the sample in a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) at 37 °C and the remaining aqueous solution containing the carbohydrate fraction was frozen and lyophilized.
2.4. Colostrum oligosaccharides isolation
Considering the high amounts of lactose present in goat colostrum and the interference of this disaccharide in the analysis of minor oligosaccharides, samples were submitted to SEC fractionation to remove mono- and disaccharides, obtaining an enriched oligosaccharide fraction. Briefly, 25 mL of colostrum carbohydrate solution (20% wt:v) was injected into a Bio-Gel P2 (Bio-Rad, Hercules, CA, USA) column (90 cm × 5 cm) using water as the mobile phase at a flow of 1.5mLmin−1 and maintained at 4°C. The degree of polymerization (DP) of collected fractions was determined by electrospray ionization-mass spectrometry (ESI-MS) on an Agilent 1200 series HPLC system (Hewlett-Packard, Palo Alto, CA, USA) coupled to a quadrupole HP-1100 mass detector at positive polarity selecting the corresponding m/z values. Fractions with DP ≥3 were pooled and freeze-dried.
2.5. Chromatographic analyses
2.5.1. Qualitative analysis (Nano-LC-Chip-Q-TOF MS)
Prior to MS analysis, purified and dried OS of CS1–CS5 were reconstituted to a final concentration of 0.1 mg mL−1 with nanopure water. MS analysis was performed with an Agilent 6520 accurate-mass Quadrupole-Time-of-Flight (Q-TOF) LC/MS with a microfluidic nano-electrospray chip (Agilent Technologies, Santa Clara, CA, USA) as previously described [28]. The chip employed contained enrichment and analytical columns, both packed with graphitized carbon. Chromatographic elution was performed with a binary gradient of 3% ACN/0.1% formic acid in water (solvent A), and 90% ACN/0.1% formic acid in water (solvent B). The column was initially equilibrated and eluted with a flow rate of 0.3 µL min−1 for the nano pump and 4 µL min−1 for the capillary pump. The 65-min gradient was programmed as follows: 0–2.5 min, 0% B; 2.5–20 min, 0–16% B; 20–30 min, 16–44% B; 30–35 min, 44–100% B; 35–45 min, 100% B; 45–65 min, 0% B. Data were acquired in the positive ionization mode with a 450–2500 mass/charge (m/z) range. The electrospray capillary voltage was 1600–1700 V. The acquisition rate was 0.63 spectra/s for both MS and MS/MS modes. Automated precursor selection was employed based on abundance, with up to 6 MS/MS per MS. The precursor isolation window was narrow (1.3 m/z). Fragmentation energy was set at 1.8V/100Da with an offset of −2.4 V. Internal calibration was performed using m/z 922.009 and 1221.991 as the reference masses (ESI-TOF Low concentration Tuning Mix G1969–85000, Agilent Technologies).
For OS identification, the Find Compounds by Formula function of Mass Hunter Qualitative Analysis Version B.06.00 (Agilent Technologies) was used to generate a list of deconvoluted masses selected to be in a range of 450–1500 m/z with a >1000 height count and a typical isotopic distribution of small biological molecules. Charge states allowed were 1–2. The function matched the masses of oligosaccharides with the goat milk oligosaccharide databases [4] creating a list of OS compositions with their specific retention time (RT).
Oligosaccharide compositions were confirmed by tandem MS (MS/MS) analysis using the same method previously described recording 6 MS/MS per each MS analysis. Compounds selected for MS/MS analysis were those with a count higher than 1000. Once OS were confirmed by MS/MS and their RT established, the relative abundance of each OS were determined by integration of individual peaks using the Batch Targeted Feature Extractor from MassHunter Profinder Version B.06.00 (Agilent Technologies) and using the MS library created in a previous work [4]. The retention time window allowed for compound – matching was ±0.5 min with the addition of ±0.25% of the RT at each time point.
2.5.2. Quantitative analysis (HILIC-QMS)
GCO analyses were performed on an Agilent 1200 series HPLC system (Hewlett-Packard, Palo Alto, CA, USA) equipped with an oven (Kariba Instruments, UK) and coupled to a quadrupole HP-1100 mass detector (Hewlett-Packard, Palo Alto, CA, USA) provided with an electrospray ionization (ESI) source. Samples (5 µL) were injected using a Rheodyne 7725 valve.
LC experiments were carried out on an ethylene bridge hybrid with trifunctionally-bonded amide phase (BEH X-Bridge column); 150 mm × 4.6 mm; 3.5 µm particle size, 135Å pore size, Waters (Hertfordshire, UK) at a flow rate of 0.4 mL min−1. Different binary gradients consisting of acetonitrile (ACN):water with addition of different additives (0.1% ammonium hydroxide, 0.1% acetic acid or 5 mM ammonium acetate) and column temperatures (30–60 °C) were assayed. Injection volume was 5 µL.
The electrospray ionization source was operated under positive or negative polarity using the following MS parameters: capillary voltage, 4kV; temperature, 300 °C; nitrogen drying gas flow, 12 L min−1; nebulizer (N2, 99.5% purity) pressure, 276kPa; and fragmentor voltage, 80–110 V. Adducts formed under optimal conditions were evaluated. In positive mode, mono-sodiated adducts [M+Na]+ were primarily formed for the different samples and only minor abundances of [M+K]+ and [M+H]+ were observed. Similarly, in negative mode, [M−H]− were detected. Therefore, ions corresponding to [M+Na]+ in positive mode and [M−H]− in negative mode of the oligosaccharides under analysis were monitored in SIM mode using default variable fragmentor voltages. Data were processed using HPChem Station software version 10.02 (Hewlett-Packard, Palo Alto, CA, USA).
Optimization of the method was carried out on the basis of RT, peak width at half height (wh), peak tailing measured by the peak asymmetry factor (As): calculated as the ratio of the back half to front half widths at 10% of the peak height, and resolution (Rs), calculated as 2(tR2 −tR1)/(wb1 +wb2), where 1 and 2 refer to two consecutive eluting carbohydrates and wb is the peak width at base. Rs values should be higher than 1.0 to get an appropriate separation and As close to 1 to get symmetric peaks.
Quantitative analysis was performed in triplicate by the external standard method, using calibration curves within the range 0.25–100 mg L−1 for maltotriose, maltotetraose, 2′-FL, 3′-SL and 6′-SL. Prior to quantitation of OS in all colostrum samples, matrix effect was evaluated by quantifying target analytes in solutions of CS5 before and after SEC treatment diluted in water at different ratios (1:1–1:50, v/v). Reproducibility of the method was estimated on the basis of the intra-day and inter-day precision, calculated as the relative standard deviation (RSD) of retention times and concentrations of oligosaccharide standards obtained in n = 5 independent measurements. Limit of detection (LOD) and limit of quantitation (LOQ) were calculated as three and ten times, respectively, the signal to noise ratio (S/N).
3. Results and discussion
3.1. Qualitative analysis of goat colostrum oligosaccharides
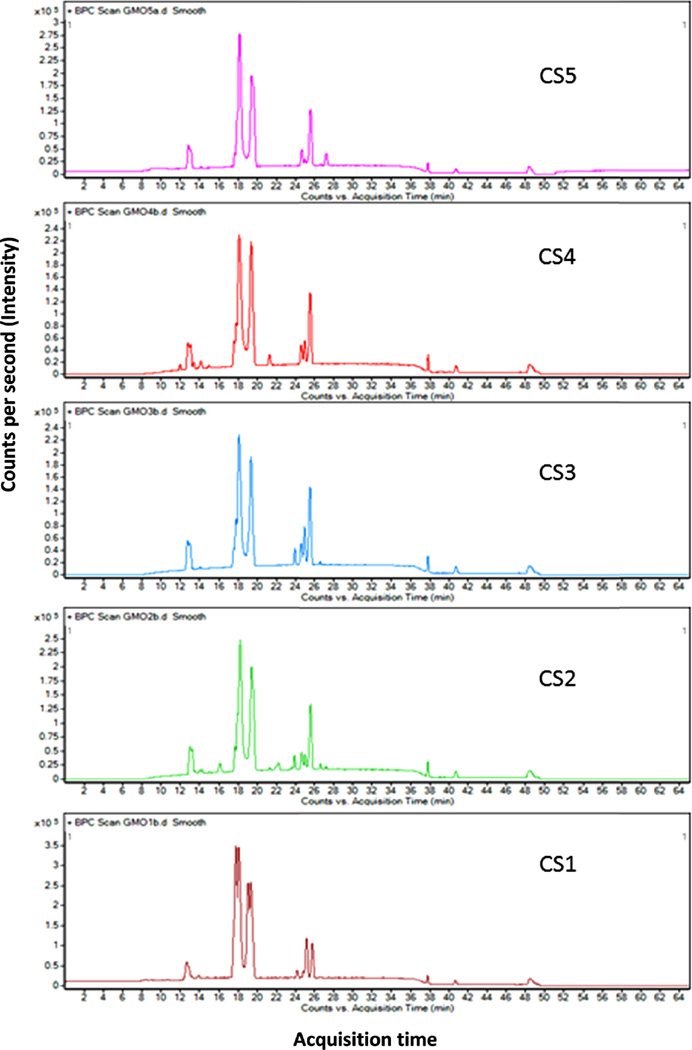
Several studies have pointed out the efficiency of MS related techniques to characterize OS from different biological fluids [24,25,29].The Nano-LC-Chip-Q-TOF MS system is an excellent tool for oligosaccharide characterization in different mammal milks, allowing the identification of over 150 different OS in human milk and 55 in bovine milk [28–30]. In this work, a great variety of GCO structures were identified (based on their RT and accurate masses), showing a different profile than human or bovine milk.
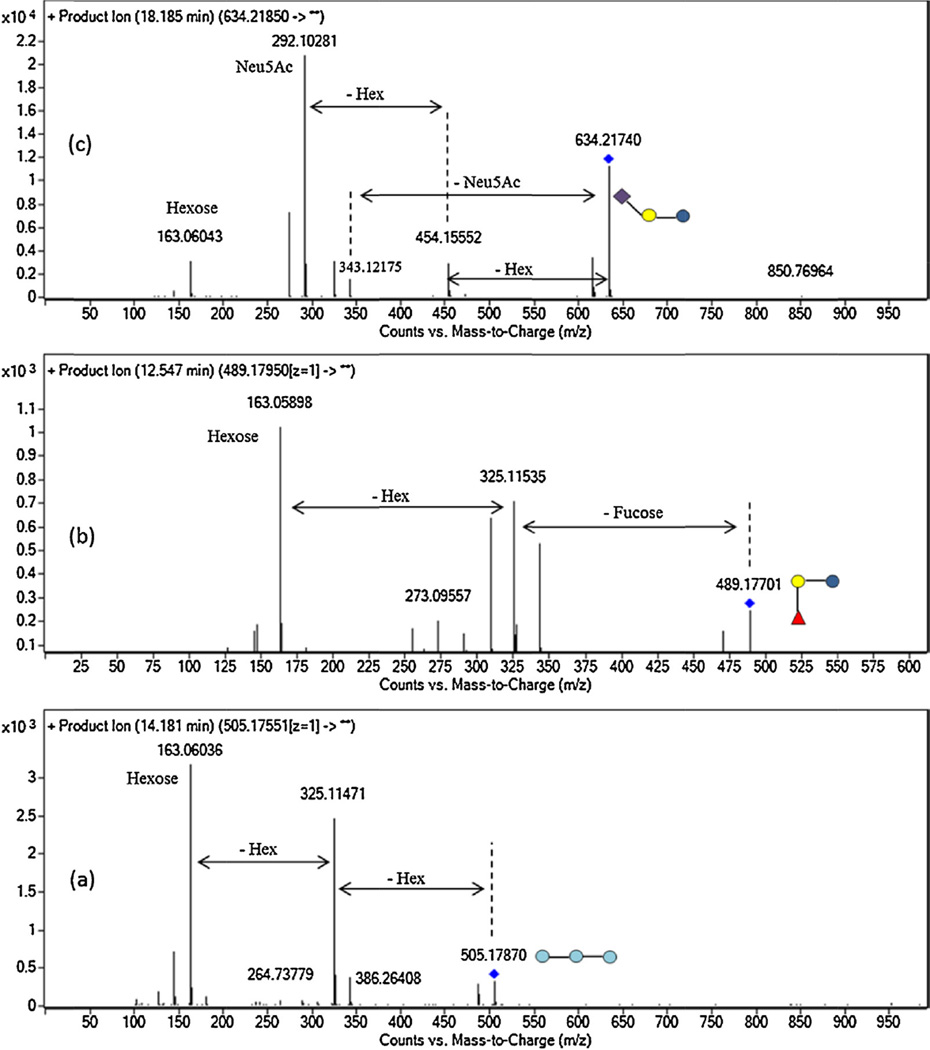
Considering the high content of lactose in goat colostrum, qualitative structural analysis of GCO required a previous purification step based on SEC, leading to a reduction of 99.9% in lactose concentration (Fig. 1S in supplementary material). A partial loss of neutral OS containing three monomeric units was also observed. Table 1 presents the list of OS identified by nano-LC-QTOF MS in the five goat colostrum samples previously purified by SEC. A total of 78 compounds were identified as oligosaccharides, 59 of which have been confirmed by their MS/MS spectrum. These results indicate that GCO show greater complexity compared to those of other domestic animals [3,4,9,15,19,31]. Similar nano-LC profiles were observed among the samples analyzed (see Fig. 1), although the whole set of OS was not identified in all samples tested. Fig. 2 shows MS/MS spectrum of hexosyl-lactose, 2′-FL, and 3′-SL as representative of each OS type (neutral, fucosylated and acidic). The corresponding losses of the different monomeric units of these OS are indicated in the figure. MS/MS spectra of all OS identified in the samples analyzed are also available in Fig. 2S of supplementary material.
Table 1.
List of oligosaccharides found in goat milk colostrum samples. The empirical formula as well as neutral mass and MS/MS verification are reported for oligosaccharides in all samples.
| # | Compositiona | Formula | Neutral mass | Verified by MS/MS | Presence in individual samples and abundanceb |
||||
|---|---|---|---|---|---|---|---|---|---|
| CS1 | CS2 | CS3 | CS4 | CS5 | |||||
| 1 | 1_1_1_0_0 | C20H35N1O15 | 529.201 | X | Y§ | Y§ | Y§ | Y§ | Y§ |
| 2 | 1_1_0_0_1 | C25H42N2O20 | 690.232 | √ | Y§ | N | N | N | N |
| 3 | 1_1_0_0_1 | C25H42N2O20 | 690.233 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 4 | 1_1_0_0_1 | C25H42N2O20 | 690.232 | √ | Y¥ | Y‡ | Y‡ | Y‡ | Y‡ |
| 5 | 1_1_0_1_0 | C25H42N2O19 | 674.238 | √ | Y‡ | Y | Y‡ | Y‡ | Y¥ |
| 6 | 1_1_0_1_0 | C25H42N2O19 | 674.238 | √ | Y¥ | Y¥ | Y¥ | Y¥ | Y¥ |
| 7 | 1_1_0_1_0 | C25H42N2O19 | 674.239 | √ | N | Y§ | Y§ | Y§ | Y§ |
| 8 | 1_1_0_1_0 | C25H42N2O19 | 674.236 | √ | Y‡ | N | N | N | N |
| 9 | 1_1_0_1_0 | C25H42N2O19 | 674.239 | √ | N | Y§ | Y§ | Y§ | Y§ |
| 10 | 1_1_0_1_0 | C25H42N2O19 | 674.238 | √ | N | Y‡ | Y‡ | Y§ | Y‡ |
| 11 | 2_0_0_0_1 | C23H39NO20 | 649.207 | √ | Y‡ | Y§ | Y§ | Y§ | Y§ |
| 12 | 2_0_0_0_1 | C23H39NO20 | 649.206 | √ | Y¥ | Y‡ | Y‡ | Y‡ | Y‡ |
| 13 | 2_0_0_0_1 | C23H39NO20 | 649.207 | √ | Y¥ | Y‡ | Y‡ | Y‡ | Y‡ |
| 14 | 2_0_0_0_1 | C23H39NO20 | 649.206 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 15 | 2_0_0_0_1 | C23H39NO20 | 649.207 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 16 | 2_0_0_0_2 | C34H56N2O29 | 956.296 | √ | Y‡ | Y§ | Y‡ | Y‡ | Y§ |
| 17 | 2_0_0_0_2 | C34H56N2O29 | 956.298 | √ | Y‡ | Y§ | Y§ | Y§ | Y§ |
| 18 | 2_0_0_0_2 | C34H56N2O29 | 956.296 | √ | Y‡ | N | Y§ | N | Y§ |
| 19 | 2_0_0_0_2 | C34H56N2O29 | 956.297 | √ | Y‡ | N | N | N | N |
| 20 | 2_0_0_1_0 | C23H39NO19 | 633.211 | √ | Y¥¥ | Y¥¥ | Y¥¥ | Y¥¥ | Y¥¥ |
| 21 | 2_0_0_1_0 | C23H39NO19 | 633.212 | √ | Y¥ | Y¥ | Y¥ | Y¥ | Y¥ |
| 22 | 2_0_0_1_1 | C34H56N2O28 | 940.303 | X | Y‡ | Y§ | Y‡ | Y‡ | Y‡ |
| 23 | 2_0_0_1_1 | C34H56N2O28 | 940.302 | √ | Y‡ | Y§ | Y§ | Y§ | Y§ |
| 24 | 2_0_0_1_1 | C34H56N2O28 | 940.301 | √ | Y‡ | Y§ | Y§ | Y§ | Y§ |
| 25 | 2_0_0_1_1 | C34H56N2O28 | 940.302 | √ | Y‡ | N | N | N | N |
| 26 | 2_0_0_2_0 | C34H56N2O27 | 924.306 | √ | Y‡ | Y‡ | Y‡ | Y§ | Y‡ |
| 27 | 2_0_0_2_0 | C34H56N2O27 | 924.307 | √ | Y§ | Y‡ | Y‡ | Y‡ | Y‡ |
| 28 | 2_0_0_2_0 | C34H56N2O27 | 924.307 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 29 | 2_0_1_0_0 | C18H32O15 | 488.174 | √ | Y§ | Y§ | Y§ | Y‡ | Y§ |
| 30 | 2_1_0_0_0 | C20H35N16 | 545.195 | √ | Y§ | Y‡ | Y‡ | Y§ | Y‡ |
| 31 | 2_1_0_0_0 | C20H35NO16 | 545.195 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 32 | 2_1_0_0_0 | C20H35NO16 | 545.195 | √ | Y§ | Y‡ | Y‡ | Y§ | Y§ |
| 33 | 2_1_0_0_0 | C20H35NO16 | 545.195 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 34 | 2_1_0_0_0 | C20H35NO16 | 545.195 | √ | Y‡ | Y‡ | Y‡ | Y§ | Y§ |
| 35 | 2_1_0_1_0 | C31H52N2O24 | 836.293 | √ | Y§ | Y§ | Y§ | Y§ | Y§ |
| 36 | 2_2_0_0_0 | C28H48N2O21 | 748.276 | X | Y§ | N | N | N | Y§ |
| 37 | 2_2_0_0_0 | C28H48N2O21 | 748.275 | √ | Y§ | Y§ | Y§ | Y§ | Y§ |
| 38 | 2_2_0_0_0 | C28H48N2O21 | 748.275 | X | Y‡ | N | Y§ | Y‡ | Y§ |
| 39 | 3_0_0_0_0 | C18H32O16 | 504.169 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y§ |
| 40 | 3_0_0_0_0 | C18H32O16 | 504.168 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 41 | 3_0_0_0_0 | C18H32O16 | 504.169 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 42 | 3_0_0_0_0 | C18H32O16 | 504.169 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 43 | 3_0_0_0_0 | C18H32O16 | 504.169 | √ | Y§ | Y§ | Y‡ | Y‡ | Y§ |
| 44 | 3_0_0_0_0 | C18H32O16 | 504.169 | √ | Y‡ | Y‡ | Y‡ | Y‡ | N |
| 45 | 3_0_0_0_0 | C18H32O16 | 504.169 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 46 | 3_0_0_0_1 | C29H49NO25 | 811.260 | √ | Y‡ | Y§ | Y‡ | Y‡ | Y§ |
| 47 | 3_0_0_1_0 | C29H49NO24 | 795.266 | √ | Y§ | Y§ | Y§ | Y§ | Y§ |
| 48 | 3_0_0_1_0 | C29H49NO24 | 795.264 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 49 | 3_0_0_1_0 | C29H49NO24 | 795.265 | √ | Y§ | Y‡ | Y‡ | Y‡ | Y‡ |
| 50 | 3_0_0_2_0 | C40H66N2O32 | 1086.360 | X | Y§ | Y§ | Y§ | N | Y§ |
| 51 | 3_1_0_0_0 | C26H45NO21 | 707.248 | √ | Y§ | Y§ | Y§ | Y§ | Y§ |
| 52 | 3_1_0_0_0 | C26H45NO21 | 707.249 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y§ |
| 53 | 3_1_0_0_0 | C26H45NO21 | 707.248 | √ | Y‡ | Y§ | Y‡ | Y‡ | Y§ |
| 54 | 3_1_1_0_0 | C32H55NO25 | 853.305 | X | Y§ | Y§ | Y§ | Y§ | Y§ |
| 55 | 3_3_0_0_0 | C42H7lN3O31 | 1113.411 | X | Y§ | Y§ | Y§ | Y§ | Y§ |
| 56 | 4_0_0_0_0 | C24H42O21 | 666.222 | √ | Y§ | Y§ | Y§ | Y§ | Y§ |
| 57 | 4_0_0_0_0 | C24H42O21 | 666.222 | √ | Y§ | Y§ | Y‡ | Y§ | Y§ |
| 58 | 4_0_0_0_0 | C24H42O21 | 666.221 | √ | Y§ | Y§ | Y‡ | Y§ | Y§ |
| 59 | 4_0_0_0_0 | C24H42O21 | 666.222 | √ | Y§ | Y§ | Y§ | Y‡ | Y‡ |
| 60 | 4_0_0_0_0 | C24H42O21 | 666.222 | √ | Y§ | Y§ | Y§ | Y‡ | Y‡ |
| 61 | 4_0_0_0_0 | C24H42O21 | 666.220 | √ | Y§ | Y§ | Y§ | Y‡ | Y§ |
| 62 | 4_0_0_0_0 | C24H42O21 | 666.220 | √ | Y§ | Y§ | Y§ | Y§ | Y§ |
| 63 | 4_0_0_0_0 | C24H42O21 | 666.222 | √ | Y§ | Y§ | Y§ | Y‡ | Y§ |
| 64 | 4_0_0_0_0 | C24H42O21 | 666.222 | √ | Y§ | Y§ | Y§ | Y‡ | Y§ |
| 65 | 4_1_0_0_0 | C32H55NO26 | 869.301 | √ | Y‡ | Y‡ | Y‡ | Y‡ | Y‡ |
| 66 | 4_1_0_0_0 | C32H55NO26 | 869.302 | √ | Y‡ | Y§ | Y‡ | Y‡ | Y‡ |
| 67 | 4_2_0_0_0 | C40H68N2O31 | 1072.385 | X | Y§ | Y§ | Y‡ | Y§ | Y‡ |
| 68 | 4_2_0_1_0 | C51H85N3O39 | 1363.486 | X | N | N | Y§ | N | Y§ |
| 69 | 4_2_0_1_0 | C51H85N3O39 | 1363.481 | X | Y§ | Y§ | Y‡ | N | Y§ |
| 70 | 5_0_0_0_0 | C30H52O26 | 828.277 | X | Y§ | N | Y§ | Y§ | Y§ |
| 71 | 5_0_0_0_0 | C30H52O26 | 828.276 | X | Y§ | Y§ | Y§ | Y§ | Y§ |
| 72 | 5_0_0_0_0 | C30H52O26 | 828.272 | X | Y§ | N | N | Y§ | Y§ |
| 73 | 5_0_0_0_0 | C30H52O26 | 828.276 | X | N | N | Y§ | Y‡ | Y§ |
| 74 | 5_0_0_0_0 | C30H52O26 | 828.278 | X | Y§ | Y§ | Y§ | Y§ | Y§ |
| 75 | 6_0_0_0_0 | C36H62O31 | 990.327 | X | Y§ | Y§ | Y§ | Y§ | Y§ |
| 76 | 6_0_0_0_0 | C36H62O31 | 990.330 | X | Y§ | N | Y§ | Y§ | N |
| 77 | 6_0_0_0_0 | C36H62O31 | 990.331 | X | Y§ | Y§ | N | Y§ | Y§ |
| 78 | 6_0_0_0_0 | C36H62O31 | 990.331 | X | N | N | N | Y§ | N |
Composition (in order): Hexose_HexNAc_Fucose_NeuAc_NeuGc.
Relative abundance
low abundance (<0.2%)
medium abundance (0.2–5%)
high abundance(5–20%)
very high abundance (>20%)
compound abundance was too low to achieve good MS/MS spectra.
Fig. 1.
Nano-LC-Chip-Q-TOF MS profiles of goat colostrum oligosaccharides: CS1, CS2, CS3, CS4, and CS5.
Fig. 2.
MS/MS spectra of: (a) hexosyl-lactose 3_0_0_0_0 (m/z 505.176), (b) 2′-FL (m/z 489.181), and (c) 3′-SL (634.218). Light blue circle: hexose; dark blue circle: glucose; yellow circle: galactose; red triangle: fucose; violet diamond: Neu5Ac. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
From the 78 oligosaccharides identified, 40 (51.3%) are neutral non-fucosylated, 3 (3.8%) neutral fucosylated and 35 (44.9%) corresponded to sialylated (Ne5Ac/Neu5Gc) oligosaccharides. The predominant OS found in these colostrum fractions analyzed were sialyl-lactoses (neutral mass 633.211) followed by Hex-HexNAc-Neu5Ac (neutral mass 674.238) and Hex-HexNAc-Neu5Gc (neutral mass 690.232) residues.
Regarding neutral OS, 7 isomers of the neutral oligosaccharide galactosyl-lactose (neutral mass 504.169), 9 isomers of digalactosyl-lactose (neutral mass 666.222), 5 isomers of tri-galactosyl-lactose (neutral mass 828.277) and 4 isomers of tetragalactosyl-lactose (neutral mass 990.331) were also detected. Different isomers of N-acetylglucosaminyl-lactose (neutral mass 545.195) and N-acetylglucosaminyl-hexosyl-lactose (neutral mass 707.249) and N-acetylglucosaminyl-dihexosyl-lactose (neutral mass 869.301) were also found in all the colostra. These results are in good agreement with those found by Meyrand et al. [4] in goat milks, although a higher number of isomers of each oligosaccharide have been detected in the present work. Additionally, three oligosaccharides containing fucose were found in these samples (fucosyl-lactosamine, 2′-fucosyl-lactose and lacto-N-fuco-pentaose); some of these OS were detected in low abundance which hindered our ability to achieve good tandem spectra as further confirmation.
A total of 35 acidic OS, containing N-acetylneuraminic monomers (18), N-glycolylneuraminic monomers (13) and 4 containing both (isomers of sialyl-N-glycolyl-neuraminyl-lactose), were detected in goat colostrum (Table 1). To the best of our knowledge, these results show the greatest number of acidic OS found in goat milk, also including a higher Neu5Gc presence than that reported in previous studies (54.8% vs. 29.4%) [4,19,31].
Conventionally, a step of reduction of carbohydrate aldehydes into their alditols form is performed using sodium borohydride. However, due to the low abundance of some fucosylated OS, the reduction was not performed to avoid unwanted sample losses associated with intense washing of residual borates (incompatible with the subsequent mass spectrometry analysis). Therefore, in some cases, the oligosaccharide isomers separated by nano-LC may include α and β anomers.
3.2. Quantitative analysis of goat colostrum oligosaccharides
3.2.1. Optimization of HILIC-Q MS conditions
The pool goat colostrum CS5 sample after SEC treatment was chosen as a representative sample for the optimization of the HILIC-Q MS method. Molecular ion adducts for fucosyl-lactose, galactosyl-lactoses, sialyl-lactoses, digalactosyl-lactoses, sialyl-lactosamines and glycolyl-neuraminyl-lactosamines were selected for the optimization of HILIC-QMS conditions at both positive and negative mode (Table 2). According to results shown in Table 1, several peaks were detected for selected m/z ions depending on the different conditions; however, only the main ones were considered for the optimization of the method. Chromatographic peaks corresponding to [M+Na]+ 656 and 511 m/z ions under positive polarity and [M−H]− 632 and 487 m/z ions under negative polarity were assigned to 6′-SL and 3′-SL and 2′-FL, respectively, by comparison of their retention times and MS data with those of commercial standards.
Table 2.
Molecular ion adducts registered for colostrum goat milk oligosaccharides ([M+Na]+ in positive ionization mode and [M−H]− in negative ionization mode). Symbol code: neutral (N); acidic (A).
| Peak no | Oligosaccharides | [M+Na]+ | [M−H]− | |
|---|---|---|---|---|
| 1 | α-2′Fucosyl-lactose | 511 | 487 | N |
| 2 | Galactosyl-lactoses | 527 | 503 | N |
| 3 | 3′Sialyl-lactose and 6′sialyl-lactose | 656 | 632 | A |
| 4 | Di-hexosyl-lactoses | 689 | 665 | N |
| 5 | Sialyl-lactosamine | 697 | 673 | A |
| 6 | Glycolyl-neuraminyl-lactosamine | 713 | 689 | A |
The use of BEH-amide stationary phase was evaluated for the analysis of GCO. Different gradients of acetonitrile:water using 0.1% ammonium hydroxide as additive were assayed for the analysis of CS5 oligosaccharides. First of all, initial gradient conditions were evaluated, using different percentages of aqueous phase (10, 15 and 20%); 15% was selected for following experiments considering the appropriate retention times (14–16 min) of the first eluting compounds (acidic OS). Percentage of the aqueous phase was also increased up to 50% and 80% in 50 min; 50% was enough for the elution of target carbohydrates. Finally, gradient rate was also evaluated: aqueous phase was modified from 15% to 50% in 30, 40 and 50 min; whereas 30 and 40 min were too fast for the appropriate elution of all the compounds, 50 min provided the best conditions. These elution results were slightly improved reducing final time to 46 min, when all target carbohydrates had eluted. In all cases, 10 min were required at 50% aqueous phase to clean de column. Then, initial conditions were recovered in 1 min and finally equilibrated for 15 min. These conditions were also applied to both 0.1% acetic acid and 5 mM ammonium acetate additives.
Table 3 shows the chromatographic parameters (RT, wh, As and Rs) considered for the selected carbohydrates under positive polarity. In all cases, acidic OS eluted before the neutral ones; this effect was more notable working under basic conditions (0.1% ammonium acetate as additive) where two eluting zones were clearly distinguished in CS5 profiles: (i) acidic oligosaccharides (14.6–16.5 min) and (ii) neutral oligosaccharides (32.6–42.5 min). The main separation mechanism in HILIC seems to be based on the partitioning between a water-enriched layer on the surface of the polar stationary phase and the relatively hydrophobic eluent [32] which mainly affect neutral carbohydrates. However, although BEH is considered a neutral stationary phase, ionization of residual surface silanol groups in this stationary phase at pH above 4 could impart negative charges to the column [32]. Under working conditions, negatively charged acidic oligosaccharides (pKa of sialic acid = 2.6) would be electrostatically repelled by the stationary phase and elute at shorter retention times.
Table 3.
Chromatographic parameters obtained using different additive of aqueous phase. Acetonitrile:waterelution program: 15–50% aqueous phase in 46min, 50% aqueous phase for 10min at 30°C.
| 0.1% ammonium hydroxide ()) |
0.1% acetic acid () |
5 mM ammonium acetate () |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [M+Na]+ | RT(min) | As | Wh (min) | Rs | [M+Na]+ | RT (min) | As | Wh (min) | Rs | [M+Na]+ | RT(min) | As | Wh (min) | Rs |
| 656 | 14.6 | 1.3 | 0.3 | 0.2 | 656 | 22.0 | 2.8 | 0.5 | 0.4 | 713 | 26.4 | 1.1 | 0.3 | 0.1 |
| 697 | 14.7 | 1.3 | 0.4 | 0.0 | 713 | 22.3 | 1.8 | 0.5 | 0.1 | 697 | 26.4 | 0.6 | 0.2 | 0.2 |
| 713 | 14.7 | 1.0 | 0.5 | 2.5 | 697 | 22.4 | 0.8 | 0.6 | 2.2 | 656 | 26.5 | 1.0 | 0.2 | 4.1 |
| 656 | 16.4 | 1.4 | 0.3 | 0.1 | 656 | 24.4 | 1.2 | 0.5 | 6.4 | 713 | 28.1 | 1.1 | 0.3 | 0.3 |
| 713 | 16.5 | 1.4 | 0.3 | 18.5 | 511 | 28.3 | 0.8 | 0.3 | 6.5 | 656 | 28.2 | 0.9 | 0.3 | 0.7 |
| 511 | 32.6 | 1.3 | 0.4 | 3.3 | 527 | 31.1 | 0.7 | 0.6 | 0.6 | 713 | 28.6 | 0.7 | 0.4 | 4.3 |
| 527 | 35.6 | 1.2 | 0.6 | 1.9 | 527 | 31.4 | 1.3 | 0.3 | 1.0 | 511 | 30.8 | 1.1 | 0.2 | 5.8 |
| 527 | 36.5 | 1.0 | 0.2 | 1.8 | 527 | 32.1 | 1.1 | 0.4 | 3.6 | 527 | 33.8 | 0.6 | 0.4 | 1.4 |
| 689 | 42.5 | 0.7 | 3.6 | 689 | 41.3 | 1.6 | 2.8 | 527 | 34.5 | 1.1 | 0.2 | 12.2 | ||
| 689 | 40.0 | 0.8 | 2.0 | |||||||||||
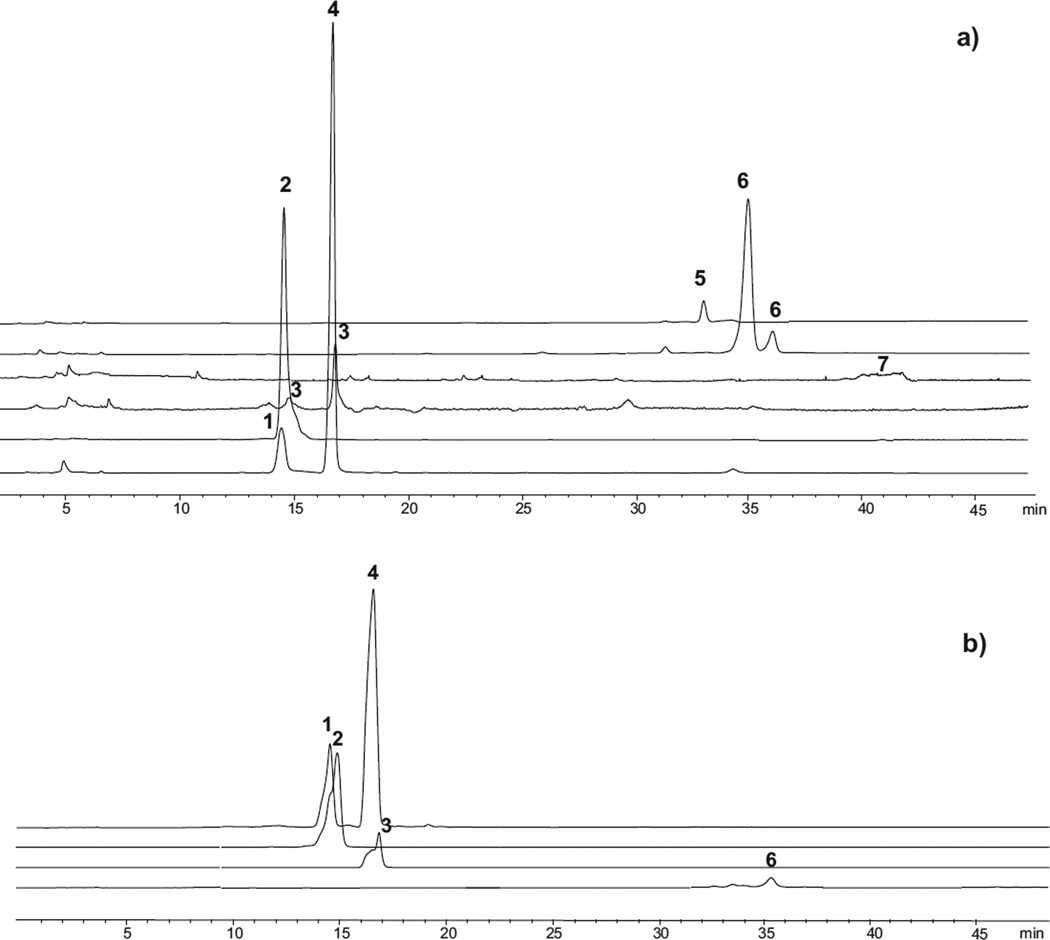
No noticeable differences in the resolution values for the different conditions assayed were observed. Although, as previously mentioned, a better separation between acidic and neutral oligosaccharides was achieved using 0.1% ammonium hydroxide as additive, coelutions between 3′-SL, sialyl-lactosamine and a glycolyl-neuraminyl-lactosamine and 6′-SL and a glycolyl-neuraminyl-lactosamine were observed. This behavior was also detected using 5 mM ammonium acetate as additive. A better separation was observed under acidic conditions except for 3′-SL and a glycolyl-neuraminyl-lactosamine. Different isomers of [M+Na]+ 527 and 689 m/z coeluted at the same retention time under all conditions, resulting in broad peaks mainly for digalactosyl-lactoses (wh: 2.0–3.6 min). Regarding the other oligosaccharides, in general, good peak width and symmetry values were obtained under both 0.1% ammonium hydroxide and 5 mM ammonium acetate (wh: 0.2–0.6 min and As: 1.0–1.4 and wh: 0.2–0.4min and As: 0.6–1.1, respectively), whereas slightly broader peaks with poor symmetry were observed using 0.1% acetic acid as additive (wh: 0.3–0.6 min and As: 0.7–2.8). Fig. 3a shows the HILIC-MS profile of registered CS5 oligosaccharides eluted under basic conditions and positive polarity.
Fig. 3.
Extracted ion chromatographic profile of CS5 oligosaccharides obtained using BEH amide column and 0.1% ammonium hydroxide as mobile phase additive. (a) ESI interface working under positive polarity and (b) under negative polarity. (1) 3′-Sialyl-lactose; (2) sialyl-lactosamine; (3) glycolyl-neuraminyl-lactosamine; (4) 6í-sialyl-lactose; (5) 2′-fucosyl-lactose; (6) galactosyl-lactoses; (7) di-hexosyl-lactoses.
Under negative polarity, [M−H]− ions (632, 673 and 689 m/z) corresponding to acidic carbohydrates (6′-SL and 3′-SL, sialyl-lactosamine and glycolyl-neuraminyl-lactosamine, respectively) of CS5 were clearly detected using acetonitrile:water with 0.1% acetic acid, 0.1% ammonium hydroxide and 5 mM ammonium acetate as additives. However, in all cases neutral carbohydrates could not be determined or were only slightly detected under these conditions (Fig. 3b). Therefore, further works were carried out under positive polarity.
The effect of temperature was also evaluated using BEH amide column with acetonitrile:water and 0.1% ammonium hydroxide as mobile phase. Three different temperatures (30, 40, 60°C) were assayed (Table 3 and Table 4). As expected, as temperature increased a higher decrease in RT of all the carbohydrates was observed (e.g. differences in RT values of 3.6 min for 3′-SL and 2.3 min for 2′-FL between 30 and 60 °C were observed). In general, narrower peaks with good symmetry were obtained at 40 and 60 °C (e.g. wh = 0.2–0.4 min; AS =0.7–1.3), compared to elutions at 30 °C (e.g. wh =0.2–0.6 min; AS = 0.7–1.4), whereas resolution was only slightly affected by temperature. Considering these results and in order to avoid high temperatures which could affect the stability of the stationary phase, 40 °C was selected for the analysis of the oligosaccharides under study.
Table 4.
Chromatographic parameters obtained using different oventemperatures. Acetonitrile:water + 0.1% ammonium hydroxide elution program: 15–50% aqueous phase in 46 min, 50% aqueous phase for 10 min.
| 40 °C |
60 °C |
|||||||
|---|---|---|---|---|---|---|---|---|
| [M+Na]+ | RT (min) | As | Wh (min) | Rs | RT(min) | As | Wh (min) | Rs |
| 656 | 13.9 | 0.9 | 0.3 | 0.2 | 11.0 | 0.7 | 0.4 | 0.4 |
| 713 | 14.0 | 1.1 | 0.4 | 0.0 | 11.4 | 1.3 | 0.5 | 0.4 |
| 697 | 14.0 | 1.2 | 0.4 | 3.5 | 11.8 | 0.7 | 0.4 | 2.4 |
| 656 | 16.1 | 0.7 | 0.2 | 0.1 | 13.6 | 0.7 | 0.4 | 0.2 |
| 713 | 16.1 | 1.1 | 0.3 | 35.6 | 13.7 | 1.1 | 0.2 | 40.0 |
| 511 | 31.9 | 1.1 | 0.2 | 6.5 | 30.3 | 1.0 | 0.3 | 7.4 |
| 527 | 35.1 | 0.7 | 0.3 | 1.7 | 33.9 | 0.7 | 0.3 | 1.8 |
| 527 | 36.0 | 1.1 | 0.2 | 2.2 | 34.8 | 1.1 | 0.2 | 2.2 |
| 689 | 42.0 | 0.8 | 3.0 | 40.7 | 0.7 | 3.0 | ||
3.2.2. Analytical parameters
Once the chromatographic conditions were selected, different analytical parameters were considered for the validation of the method before quantitative analysis.
External standard method was used for the quantitative analysis using calibration curves within the range 100–0.25 mg L−1 for 2′-FL, maltotriose, maltotetraose, 3′- and 6′-SL. These compounds were selected as representative of neutral fucosylated trisaccharides, neutral non-fucosylated trisaccharides, neutral tetrasaccharides and acidic OS, respectively. The obtained correlation coefficients from these calibration curves ranged from 0.92 to 0.99.
Considering that GCO are present at low levels, limit of detection (LOD), limit of quantitation (LOQ) and precision (RSD, %) data for a standard mixture including 6′-SL, 2′-FL and maltotriose, as representative standards of target compounds, were calculated. As shown in Table 5, the lowest LOD and LOQ values were obtained for 6′-SL(3.28 and 10.94ngmL−1, respectively), whereas the highest were found for 2′-FL (160.62 and 535.41 ngmL−1, respectively). Good precision values were obtained for all standards analyzed (RSD ranging 6.0–8.1%).
Table 5.
Limits of detection (LOD) and of quantitation (LOQ) and precision (relative standard deviation, % RSD) for a standard mixture analyzed by HILIC-Q MS using acetoni-trile:water with 0.1% ammonium hydroxide as mobile phase and 40°C as oven temperature.
| Compound | LOD (ng mL−1) |
LOQ (ng mL−1) |
Precision (RSD, %) |
|---|---|---|---|
| 2′-Fucosyl-lactose | 160.62 | 535.41 | 6.9 |
| 6′-Sialyl-lactose | 3.28 | 10.94 | 8.1 |
| Maltotriose | 19.76 | 65.87 | 6.0 |
| Maltotetraose | 114.21 | 380.69 | 7.2 |
The potential effect on the quantitative determination of GCO of the sample matrix was also considered by analyzing different dilutions (1:1–1:50, v/v) of CS5 before and after SEC treatment. No differences in carbohydrates concentrations associated to a possible matrix effect were found for the different dilutions.
3.2.3. Quantitation of oligosaccharides in colostrum samples
Table 6 shows the quantitative data (mg L−1 goat colostrum) of the most abundant GCO for the five samples. Considering the potential loss of OS with three monomer units during SEC treatment, oligosaccharides marked with an asterisk in the table (2′-FL, 3′-SL, 6′-SL, sialyl-lactosamine, galactosyl-lactoses, fucosyl-lactosamine, glycolyl-neuraminyl-lactosamine and glycolyl-neuraminyl-lactose) were quantified in the original samples only after fat and protein removal (before performing SEC).
Table 6.
Content (mg L−1) of oligosaccharides in colostrum samples analyzed by HILIC-Q MS using acetonitrile:water with 0.1% ammonium hydroxide as mobile phase and 40 °C as oven temperature.
| Compositiona | [M+Na]+ | RT(min) | CS1 | CS2 | CS3 | CS4 | CS5 | |
|---|---|---|---|---|---|---|---|---|
| 2′-Fucosyl-lactosec | 20100 | 511 | 32.6 | 2.2 (0.4)b | 2.9 (0.1) | 7.7 (0.1) | 31.6 (0.3) | 16 (1) |
| Galactosyl-lactosec | 30000 | 527 | 35.1 | 145 (10) | 128 (13) | 125 (11) | 233 (11) | 266 (21) |
| Galactosyl-lactosec | 30000 | 527 | 36.0 | 3.6 (0.5) | 2.0 (0.3) | 2.7 (0.7) | 5 (1) | 8.3 (2.2) |
| Sialyl-lactosaminec | 11010 | 697 | 14.0 | 37.80 (0.01) | 31.31 (0.01) | 33 (4) | 65 (6) | 5.7 (0.8) |
| Sialyl-lactosaminec | 11010 | 697 | 14.3 | 8.0 (0.6) | 5.2 (0.4) | 6 (2) | 8.6 (0.5) | 4 (1) |
| 3′-Sialyl-lactosec | 20010 | 656 | 13.9 | 3.05 (0.01) | 5.73 (0.02) | 12 (1) | 12(0.7) | 10 (1) |
| 6′-Sialyl-lactosec | 20010 | 656 | 16.2 | 53 (5) | 50 (2) | 70 (1) | 124(2) | 29 (4) |
| Glycolyl-neuraminyl-lactosaminec | 11001 | 713 | 12.4 | 2.26 (0.02) | 1.48 (0.01) | 0.7 (0.5) | 5.5 (0.5) | 1.5 (0.8) |
| Glycolyl-neuraminyl-lactosaminec | 11001 | 713 | 14.0 | 0.6 (0.1) | 0.24 (0.03) | 1.41 (0.02) | 0.98 (0.01) | 2.24 (0.03) |
| Glycolyl-neuraminyl-lactosaminec | 11001 | 713 | 16.1 | 13.6 (0.5) | 2.7 (0.1) | 2.8 (0.4) | 8 (1) | 5.1 (0.1) |
| Glycolyl-neuraminyl-lactosaminec | 11001 | 713 | 16.2 | 10.6 (0.6) | 2.6 (0.1) | 4.5 (0.7) | 5 (1) | 5.1 (0.1) |
| Glycolyl-neuraminyl-lactosec | 20001 | 672 | 13.7 | 0.2 (0.1) | 0.61 (0.04) | 1.30(0.01) | 3.0 (0.3) | 0.3 (0.5) |
| Glycolyl-neuraminyl-lactosec | 20001 | 672 | 16.3 | 5.14 (0.03) | 3.93 (0.02) | 7 (1) | 19(2) | 5 (1) |
| Glycolyl-neuraminyl-lactosec | 20001 | 672 | 17.8 | 19.8 (0.3) | 5.5 (0.1) | 12 (1) | 30(2) | 5 (2) |
| Fucosyl-lactosaminec | 11100 | 552 | 30.6 | 3.1 (0.1) | 4.1 (0.1) | 6.00 (0.05) | 6.15 (0.05) | 5.9 (0.7) |
| Sialyl-hexosyl-lactose | 30010 | 818 | 16.2 | 0.05 (0.01) | 0.93 (0.02) | 0.1 (0.1) | 0.10 (0.01) | 1.3 (0.4) |
| Sialyl-hexosyl-lactose | 30010 | 818 | 17.4 | 0.05 (0.01) | -d | - | 0.03 (0.01) | 1 (1) |
| Sialyl-hexosyl-lactose | 30010 | 818 | 17.9 | 0.07 (0.01) | 0.08 (0.01) | - | 0.05 (0.01) | 1.2 (0.2) |
| Sialyl-glycolyl-neuraminyl-lactose | 20011 | 963 | 9.5 | - | - | - | - | 1.1 (0.2) |
| Sialyl-glycolyl-neuraminyl-lactose | 20011 | 963 | 12.1 | 0.21 (0.02) | 0.27 (0.03) | 0.16(0.03) | - | 1.1 (0.2) |
| Acetyl-glucosaminyl-hexosyl-lactose | 31000 | 730 | 26.7 | - | - | - | - | 1.4 (0.4) |
| Acetyl-glucosaminyl-hexosyl-lactose | 31000 | 730 | 27.6 | - | 0.75 (0.01) | 0.4 (0.3) | 1.19 (0.07) | 10 (2) |
| Acetyl-glucosaminyl-lactose | 21000 | 568 | 31.7 | 0.33 (0.03) | 0.3 (0.1) | 0.3 (0.1) | 0.63 (0.01) | 1.1 (0.1) |
| Acetyl-glucosaminyl-lactose | 21000 | 568 | 32.5 | 0.46 (0.02) | 0.26 (0.02) | 0.20(0.01) | 0.7 (0.1) | 3 (1) |
| Acetyl-glucosaminyl-lactose | 21000 | 568 | 32.8 | 1.4 (0.2) | 0.6 (0.2) | 0.60(0.01) | 3.9 (0.1) | 1.5 (0.6) |
| Acetyl-glucosaminyl-lactose | 21000 | 568 | 33.9 | 0.11 (0.01) | 0.06 (0.01) | 0.29(0.01) | 0.21 (0.01) | 0.7 (0.2) |
| Dihexosyl-lactose | 40000 | 689 | 39.5 | 0.22 (0.01) | 0.22 (0.02) | - | 1.31 (0.05) | 1.6 (0.1) |
| Dihexosyl-lactose | 40000 | 689 | 40.6 | - | - | - | 1.98 (0.03) | 2.0 (0.1) |
| Dihexosyl-lactose | 40000 | 689 | 41.9 | 1.2 (0.1) | 0.58 (0.01) | - | 4.65 (0.05) | 6.9 (0.1) |
| Total oligosaccharides | 311.84 | 251.22 | 293.16 | 572.24 | 404.11 |
Composition (in order): Hex_HexNAc_Fucose_NeuAc_NeuGc.
Standard deviation in brackets.
Oligosaccharides analyzed before SEC treatment.
Non detected OS.
A high variability in quantified OS concentrations was observed among the different colostrum samples. CS4 showed the highest concentrations of OS (572.24 mg L−1) whereas CS2 and CS3 showed the lowest values (251.22 and 293.16 mg L−1, respectively). Values ranging from 140 to 315 mg L−1 for neutral oligosaccharides and from 83 to 251 mg L−1 for acidic oligosaccharides were found. The most abundant OS were galactosyl-lactoses (separation of all the isomers was not possible and these compounds were quantified together: 124.92–265.77 mg L−1). Concentration of these neutral carbohydrates in colostrum samples are higher than those reported in the literature for goat milks [4]. Regarding fucosyl-oligosaccharides, 2′-FL showed higher concentrations (2.21–31.59 mg L−1) than fucosyl-lactosamine (3.08–6.15 mg L−1). As indicated before, concentration of 6′-SL (28.85–123.76 mg L−1) was higher than that of 3′-SL (3.05–11.99 mg L−1) in all the colostrum samples. These results could suggest an OS profile closer to human milk than bovine milk, where 3′-SL is the predominant form in the latter. Relatively high amounts of sialyl-lactosamine isomers were also found in colostrum samples (5.17–8.56 and 5.66–65.12 mg L−1), whereas lower concentrations of other acidic OS were observed (Table 6). Although some of these oligosaccharides have been detected in previous works in mature goat milks [4,10,15], to the best of our knowledge, this is the first time that such a large number of oligosaccharides have been quantified in goat colostrum.
4. Conclusions
A high number of both neutral and acidic OS has been detected for the first time in different goat colostrum samples by Nano-LC-Chip-Q-TOF MS. Moreover, a HILIC-QMS method has successfully been developed for the first time for the quantitative analysis of these OS. Regarding mobile phase, acetonitrile:water with 0.1% ammonium hydroxide as additive has proven to be the most appropriate eluent to achieve good results in terms of peak width, peak symmetry and resolution. This method has proven to be successful for the quantitation of several OS in colostrum samples. Up to 0.57 g L−1 of total OS could be estimated, galactosyl-lactoses being the predominant carbohydrates followed by sialyl- and fucosyl-oligosaccharides, respectively. Overall, findings contained in this work strengthen the potential of goat colostrum as an efficient source of naturally-occurring bioactive OS.
Supplementary Material
Acknowledgments
This work has been funded by Junta de Andalucía (project AGR2011-7626), CSIC (project i-link0827), Comunidad Autónoma de Madrid (Spain) and European funding from FEDER program (AVANSECAL-CM S2013/ABI-3028) and Fundación Ramón Areces. This work was also supported by the UC Davis RISE program and the National Institutes of Health awards R21AT006180, R01AT007079, R01AT008759-02. Authors also thank I. Calvillo for her technical assistance.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.chroma.2015.09.060.
References
- 1.Casado B, Affolter M, Kussmann M. OMICS-rooted studies of milk proteins oligosaccharides and lipids. J. Proteomics. 2009;73:196–208. doi: 10.1016/j.jprot.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Ferez A, Zapata JE, Guadixa A, Almecija MC, Gomez M, Guadix EM. Obtention ofgoat milk permeates enriched in lactose-derived oligosaccharides. Desalination. 2009;245:730–736. [Google Scholar]
- 3.Urashima T, Saito T, Nakamura T, Messer M. Oligosaccharides of milk and colostrums in non-human mammals. Glycoconj. J. 2001;18:357–371. doi: 10.1023/a:1014881913541. [DOI] [PubMed] [Google Scholar]
- 4.Meyrand M, Dallas DC, Caillat H, Bouvier F, Martin P, Barile D. Comparison of milk oligosaccharides between goats with and without the genetic ability to synthesize αs1 -casein. Small Rumin. Res. 2013;113:411–420. doi: 10.1016/j.smallrumres.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Brand-Miller J, McVeagh P, Petocz P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001;74:510–515. doi: 10.1093/ajcn/74.4.510. [DOI] [PubMed] [Google Scholar]
- 6.Park YW. Bioactive components in goat milk. In: Park YW, editor. Bioactive Components in Milk and Dairy Products. UK: Wiley-Blackwell; 2009. pp. 43–82. [Google Scholar]
- 7.Kunz C, Rudloff S. Health promoting aspects of milk oligosaccharides. Int. Dairy J. 2006;16:1341–1346. [Google Scholar]
- 8.Yu Z-T, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, Heidtman M, Newburg DS. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology. 2013;23:169–177. doi: 10.1093/glycob/cws138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viverge D, Grimmonprez L, Solere M. Chemical characterization of sialyl oligosaccharides isolated from goat (Capra hircus) milk. Biochim. Biophys. Acta. 1997;1336:157–164. doi: 10.1016/s0304-4165(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 10.Mehra R, Kelly P. Milk oligosaccharides: structural and technological aspects. Int. Dairy J. 2006;16:1334–1340. [Google Scholar]
- 11.Charlwood J, Tolson D, Dwek M, Camilleri P. A detailed analysis of neutral and acidic carbohydrates in human milk. Anal. Biochem. 1999;273:261–277. doi: 10.1006/abio.1999.4232. [DOI] [PubMed] [Google Scholar]
- 12.Warren C, Chaturvedi P, Newburg A, Oftedal O, Tilden C, Newburg D. Comparison of oligosaccharides in milk specimens from humans and twelve other species. Adv. Exp. Med. Biol. 2001;501:325–332. doi: 10.1007/978-1-4615-1371-1_40. [DOI] [PubMed] [Google Scholar]
- 13.Asakuma S, Akahori M, Kimura K, Watanabe Y, Nakamura T, Tsunemi M, Arai I, Sanai Y, Urashima T. Sialyl oligosaccharides of human colostrum: changes in concentration during the first three days of lactation. Biosci. Biotechnol. Biochem. 2007;71:1447–1451. doi: 10.1271/bbb.60529. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Hernández O, Calvillo I, Lebrón-Aguilar R, Moreno FJ, L Sanz M. Hydrophilic interaction liquid chromatography coupled to mass spectrometry for the characterization of prebiotic galactooligosaccharides. J. Chromatogr. 2012;A1220:57–67. doi: 10.1016/j.chroma.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Ferez A, Rudloff S, Guadix A, Henkel CA, Pohlentz G, Boza JJ, Guadix EM, Kunz C. Goats’ milk as a natural source of lactose-derived oligosaccharides: isolation by membrane technology. Int. Dairy J. 2006;16:173–181. [Google Scholar]
- 16.Martinez-Ferez A, Guadix A, Zapata-Montoya JE, Guadix EM. Influence of transmembrane pressure on the separation of caprine milk oligosaccharides from protein by cross-flow ultrafiltration. Int. J. Dairy Technol. 2008;61:333–339. [Google Scholar]
- 17.Lara-Villoslada F, Debras E, Nieto A, Concha A, Galvez J, Lopez-Huertas E, Boza J, Obled C, Xaus J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin. Nutr. 2006;25:477–488. doi: 10.1016/j.clnu.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira DL, Wilbey RA, Grandison AS, Duarte LC, Roseiro LB, Separation of oligosaccharides from caprine milk whey. prior to prebiotic evaluation. Int. Dairy J. 2012;24:102–106. [Google Scholar]
- 19.Claps S, Di Napoli MA, Sepe L, Caputo AR, Rufrano D, Di Trana A, Annic-chiarico G, Fedele V. Sialyloligosaccharides content in colostrum and milk of two goat breeds. Small Rumin. Res. 2014;121:116–119. [Google Scholar]
- 20.Brokl M, Hernández-Hernández O, CSoria A, L Sanz M. Evaluation of different operation modes of high performance liquid chromatography for the analysis of complex mixtures of neutral oligosaccharides. J. Chromatogr. 2011;A1218:7697–7703. doi: 10.1016/j.chroma.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Wuhrer M, de Boer AR, Deelder AM. Structural glycomics using hydrophilic interaction liquid chromatography (HILIC) with mass spectrometry. Mass Spec-trom. Rev. 2009;28:192–206. doi: 10.1002/mas.20195. [DOI] [PubMed] [Google Scholar]
- 22.Marino K, Lane JA, Abrahams JL, Struwe WB, Harvey DJ, Marotta M, Hickey RM, Rudd PM. Method for milk oligosaccharide profiling by 2-aminobenzamide labeling and hydrophilic interaction chromatography. Glycobiology. 2011;21:1317–1330. doi: 10.1093/glycob/cwr067. [DOI] [PubMed] [Google Scholar]
- 23.Fong B, Ma K, McJarrow P. Quantification of bovine milk oligosaccharides using liquid chromatography-selected reaction monitoring-mass spectrometry. J. Agric. Food Chem. 2011;59:9788–9795. doi: 10.1021/jf202035m. [DOI] [PubMed] [Google Scholar]
- 24.Ninonuevo MR, Perkins PD, Francis J, Lamotte LM, LoCascio RG, Freeman SL, Mills DA, German JB, Grimm R, Lebrilla CB. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J. Agric. Food Chem. 2008;56:618–626. doi: 10.1021/jf071972u. [DOI] [PubMed] [Google Scholar]
- 25.Tao N, Ochonicky KL, German JB, Donovan SM, Lebrilla CB. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food Chem. 2010;58:4653–4659. doi: 10.1021/jf100398u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao N, DePeters EJ, Freeman S, German JB, Grimm R. Bovine milkglycome. J. Dairy Sci. 2008;91:3768–3778. doi: 10.3168/jds.2008-1305. [DOI] [PubMed] [Google Scholar]
- 27.Barile D, Marotta M, Chu C, Mehra R, Grimm R, Lebrilla CB, German JB. Neutral and acidic oligosaccharides in Holstein-Friesian colostrum during the first 3 days of lactation measured by high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. J. Dairy Sci. 2010;93:3940–3949. doi: 10.3168/jds.2010-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis ofsialylated human milk oligosaccharides. J. Proteome Res. 2011;10:856–868. doi: 10.1021/pr101006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB, Barile D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology. 2013;23:664–676. doi: 10.1093/glycob/cwt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J. Proteome Res. 2010;9:4138–4151. doi: 10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albretch S, Lane JA, Marino K, Al-Bushada KA, Carrington SD, Hickey RM, Rudd PM. A comparative study of free oligosaccharides in the milk of domestic animals. Br.J. Nutr. 2014;111:1313–1328. doi: 10.1017/S0007114513003772. [DOI] [PubMed] [Google Scholar]
- 32.Buszewski B, Noga S. Hydrophilic interaction liquid chromatography (HILIC) - a powerful separation technique. Anal. Bioanal. Chem. 2012;402:231–247. doi: 10.1007/s00216-011-5308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.