Cell polarity, growth and signaling require organelle transport by cytoskeletal motor proteins that are precisely regulated in time and space. Probing these complex, dynamic processes requires experimental techniques with comparable temporal and spatial precision. Inducible dimerization offers the ability to recruit motor proteins to organelles in living cells. Approaches include rapamycin-induced dimerization of motors and cargo-bound binding partners [1] or the recent application of the TULIP light-inducible dimerization system [2,3]. In the latter system, motor recruitment is activated by blue light, and relaxes to an OFF state in the dark within seconds. While rapid relaxation is desirable for some applications, many experiments require sustained motor recruitment. Here, we use a photocaged chemical dimerizer to achieve sustained, spatially-defined motor recruitment to individual organelles with a single pulse of light. We demonstrate the general applicability of the system by recruiting microtubule plus end-directed kinesin-1 and minus end-directed dynein motors to peroxisomes and mitochondria in HeLa cells and primary neurons, leading to alterations in organelle transport on timescales from <10 seconds to >10 minutes after photoactivation.
We recently developed a photoactivatable chemical dimerizer, cTMP–Htag, a synthetic small molecule comprising a Halotag ligand linked to photocaged trimethoprim (TMP). This molecule is designed to heterodimerize Halotag (Halo) and Escherichia coli DHFR (eDHFR) fusion proteins [4]. Here we use light to recruit eDHFR-tagged molecular motors or motor effectors to specific organelles. cTMP–Htag is cell permeable and covalently binds the Halotag protein, which we localized to the cytosolic surface of either peroxisomes or mitochondria [1,4]. While photocaged, TMP does not bind eDHFR. Uncaging with a pulse of ~400 nm light recruits eDHFR-fusions to the organelle surface (Figure 1A). Photoactivation is spatially restricted to the illuminated organelle since uncaged TMP remains covalently tethered to the Halotag anchor. TMP–eDHFR binding is noncovalent, so individual motor–eDHFR proteins may bind and release, but at steady state the interaction sustains robust motor recruitment. Dimerization can be reversed within minutes by addition of free TMP [4].
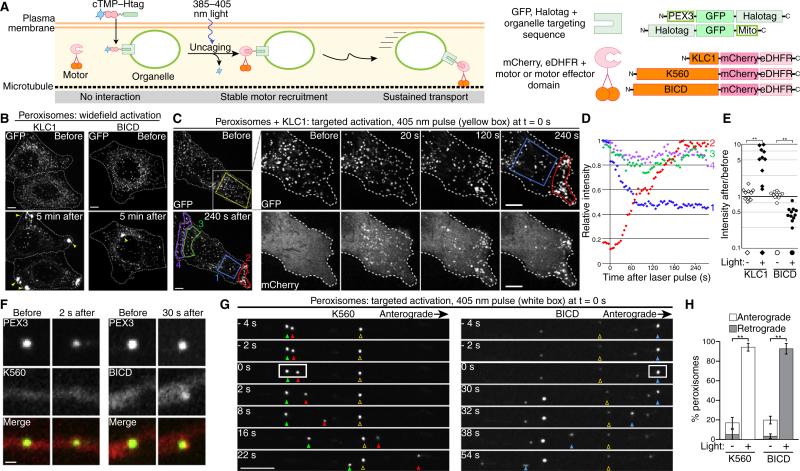
Figure 1. Optogenetic control over organelle transport.
(A) Schematic of experimental approach and protein constructs. (B–E) HeLa cells and (F–H) primary rat hippocampal neurons expressing PEX3–GFP–Halo and BICD–mCherry–eDHFR, KLC1–mCherry–eDHFR or K560–mCherry–eDHFR as indicated were incubated with 10 μM cTMP–Htag prior to imaging. (B) GFP images show peroxisomes before and after widefield motor recruitment; dashed lines show cell outlines. Peroxisomes accumulated (arrowheads) in the periphery (KLC1), or center (BICD) in 100% of activated cells (n > 15 cells for each, 2 independent experiments). (C,D) KLC1 was recruited to peroxisomes in a defined region (yellow box) at t = 0. Whole-cell images (left) show GFP; insets show area in white square in GFP and mCherry. (D) GFP quantification of regions (1–4) marked in (C) shows peroxisome depletion from the interior of the photoactivated region (1, blue) and accumulation at the nearest edge of the cell (2, red), while unilluminated regions (3, 4, green and purple) are unaffected. (E) Following targeted KLC1 or BICD recruitment to peroxisomes (e.g. panel C or Figure S1F), the fold change in average GFP intensity (as a proxy for peroxisome density) was calculated for a photoactivated region (filled symbols) and a comparable unactivated region (open symbols) in each cell (n ≥ 10 cells each, similar results from 2 independent experiments). (F) Representative images of K560 and BICD recruitment to peroxisomes in neurons before photoactivation and immediately prior to motility. (G) Peroxisome movement in axons after photoactivation in a defined region (white box) at t = 0. Filled and open arrowheads mark photoactivated and unactivated peroxisomes, respectively. (H) Quantification of the percentage of peroxisomes exhibiting anterograde or retrograde movement (mean ± SEM, n = 10 neurons from 3 independent experiments). **p < 0.002, Student's t-test. Scale bar in (F) is 500 nm, all others 5 μm.
We tested three constructs: the constitutively active motor domain of kinesin-1 (amino acids 1–560, K560); an amino-terminal fragment of kinesin light chain 1 (KLC1), which binds and recruits kinesin heavy chain; and an amino-terminal fragment of Bicaudal D (BICD), a motor effector that binds and recruits dynein. To localize Halotag protein, we used the peroxisome-targeting sequence from human PEX3 or the mitochondrial outer membrane targeting sequence (Mito) from Listeria monocytogenes ActA (Figure 1A).
HeLa cells expressing PEX3–GFP–Halo, together with either KLC1–mCherry–eDHFR or BICD-–mCherry–eDHFR, were treated with cTMP–Htag. Before uncaging, peroxisomes localized uniformly (Figure 1B), with motor or effector constructs diffuse throughout the cytosol. In response to a 500 ms widefield pulse of 387 ± 5 nm light, the motor and effector constructs relocalized to peroxisomes within 30 seconds (Figure S1A,B) and transported them to the periphery or to the center of the cell, respectively, as predicted for kinesin- or dynein-driven motility (Figure 1B, Movie S1). Recruiting K560 or BICD to mitochondria induced transport as well as a striking increase in elongated mitochondria within 5–20 seconds (Figure S1C,D). KLC1 recruitment relocalized mitochondria more slowly, over ~10 minutes, without pronounced morphological changes (Figure S1E). These observations highlight the organelle-specific and motor/effector-specific regulation of intracellular transport [5].
The power of optogenetics is its potential for localized control on subcellular length scales. Using 405 nm light, we photoactivated defined regions within HeLa cells (Figures 1C–E and S1F,G), or individual organelles within axons of hippocampal neurons, in which microtubules are uniformly organized with minus ends oriented toward the cell body (Figures 1F,G and S2, Movies S2 and S3). Motor or effector recruitment in both cell types led to transport of peroxisomes and mitochondria in the predicted directions, while unilluminated organelles in the same cells were unaffected (Figures 1E,H and S2C). In neurons, for example, within 5 min of photoactivation, >90% of illuminated peroxisomes moved >5 μm toward microtubule plus ends for K560 (anterograde) or toward microtubule minus ends for BICD (retrograde), whereas unilluminated peroxisomes exhibited low-frequency, mixed motility (Figure 1H). In neurons, K560 recruitment induced peroxisome motility 10 ± 2 s (mean ± SEM) after photoactivation, before a detectable increase in mCherry fluorescence. In contrast, BICD recruitment induced motility 32 ± 6 s after illumination, following a clear increase in mCherry fluorescence (Figure 1F). These observations are consistent with previous findings that intracellular cargo transport requires fewer kinesin than dynein motors, which function in larger teams [6,7].
Our results demonstrate the utility of cTMP–Htag for manipulating organelle transport within living cells with spatial and temporal control. Motor recruitment after uncaging is stable, leading to sustained transport of individual organelles over several minutes after a single pulse of light. Because continuous illumination is not required, we can observe phenomena such as activated organelles bypassing unactivated organelles (Figures 1G and S2B). Moreover, cTMP–Htag is insensitive to 488 nm light, allowing GFP imaging without inducing uncaging, and Halotag protein is compatible with both amino- and carboxy-terminal fusion partners. In contrast, the TULIP system, also used for optogenetic control of organelle transport, requires repeated illumination for sustained transport and is sensitive to 488 nm light [2,3]. These complementary systems offer the choice of transient [3] or sustained (this study) motor recruitment.
In cells, endogenous motors are tightly regulated by mechanisms including auto-inhibition, effector binding and scaffolding proteins [5,8]. To better understand intracellular dynamics, multiple approaches must be employed. The use of optogenetics to recruit motors to organelles with temporal and spatial specificity is an exciting addition to the toolkit to dissect motor function within the cell, and to test downstream effects of localized perturbations of organelle transport on cellular physiology.
Supplementary Material
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes experimental procedures, two figures and three movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2015.03.056.
REFERENCES
- 1.Kapitein LC, Schlager MA, van der Zwan WA, Wulf PS, Keijzer N, Hoogenraad CC. Probing intracellular motor protein activity using an inducible cargo trafficking assay. Biophys. J. 2010;99:2143–2152. doi: 10.1016/j.bpj.2010.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, Sosnick TR, Weiss EL, Glotzer M. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods. 2012;9:379–384. doi: 10.1038/nmeth.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Bergeijk P, Adrian M, Hoogenraad CC, Kapitein LC. Optogenetic control of organelle transport and positioning. Nature. 2015;518:111–114. doi: 10.1038/nature14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballister ER, Aonbangkhen C, Mayo AM, Lampson MA, Chenoweth DM. Localized light-induced protein dimerization in living cells using a photocaged dimerizer. Nat. Commun. 2014;5:5475. doi: 10.1038/ncomms6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu MM, Holzbaur ELF. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 2014;10:564–574. doi: 10.1016/j.tcb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricks AG, Holzbaur ELF, Goldman YE. Force measurements on cargoes in living cells reveal collective dynamics of microtubule motors. Proc. Natl. Acad. Sci. USA. 2012;109:18447–18452. doi: 10.1073/pnas.1215462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai AK, Rai A, Ramaiya AJ, Jha R, Mallik R. Molecular adaptations allow dynein to generate large collective forces inside cells. Cell. 2013;152:172–182. doi: 10.1016/j.cell.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Hancock WO. Bidirectional cargo transport: moving beyond tug of war. Nat. Rev. Mol. Cell Biol. 2014;9:615–628. doi: 10.1038/nrm3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.