Abstract
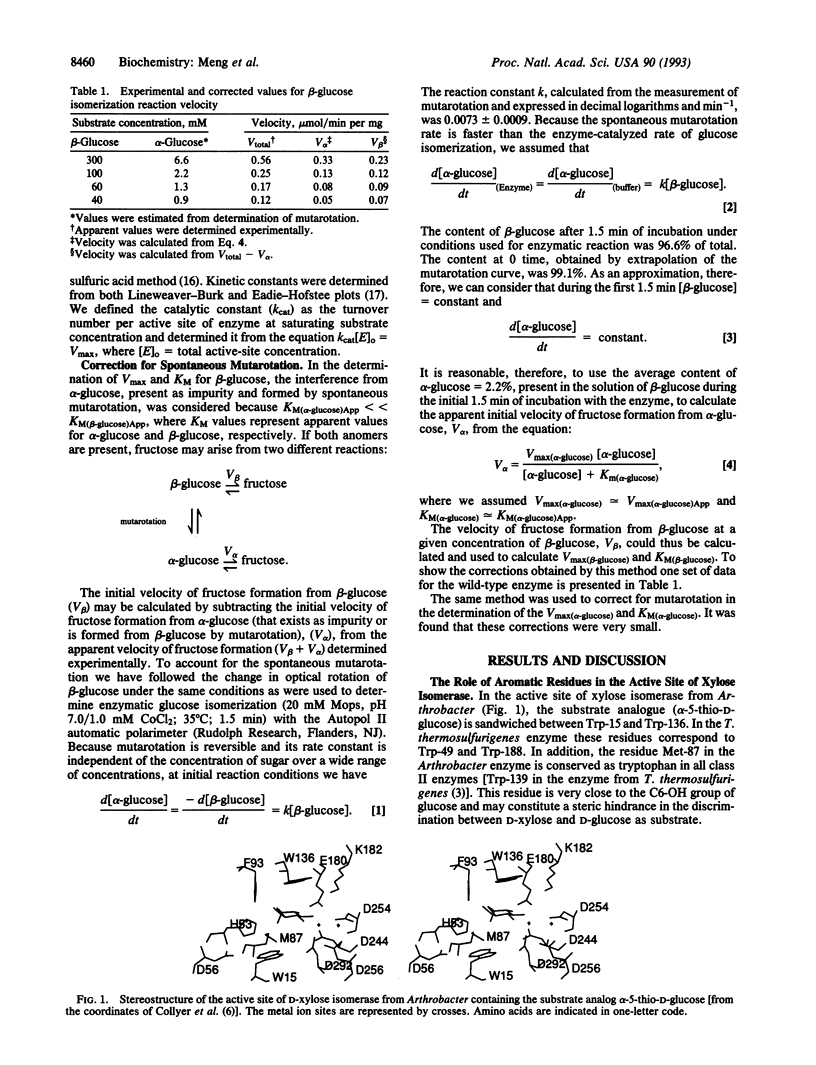
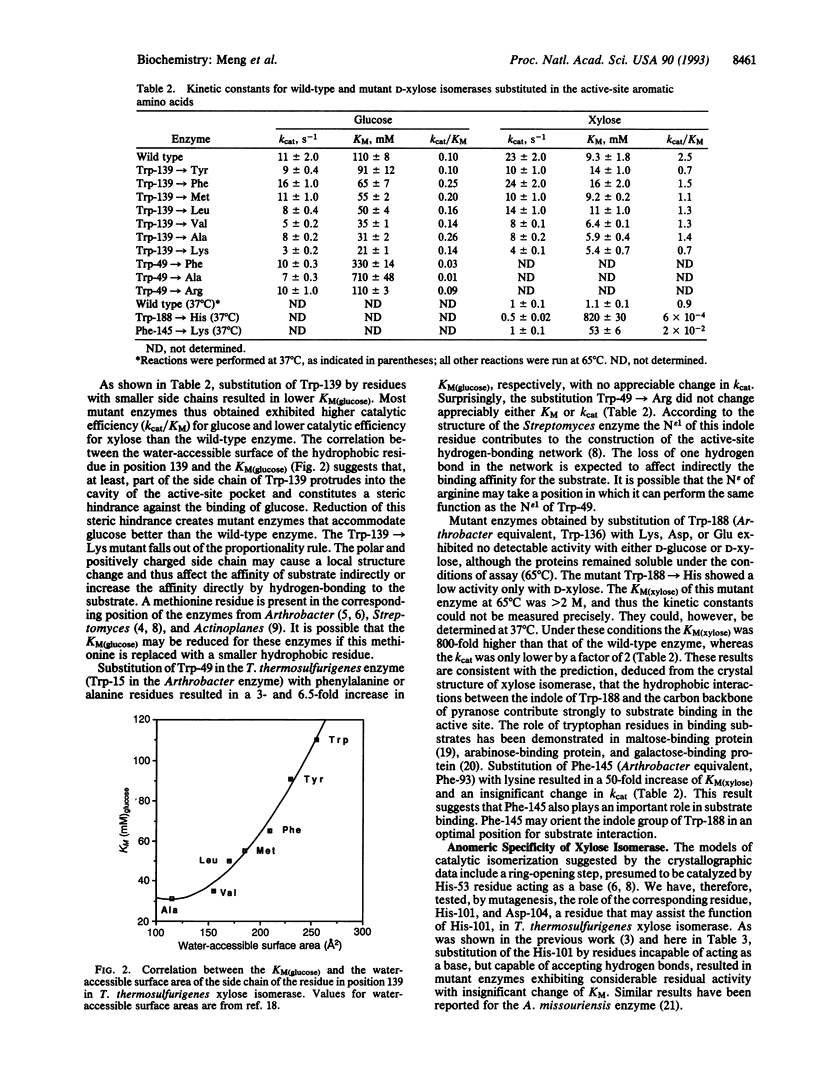
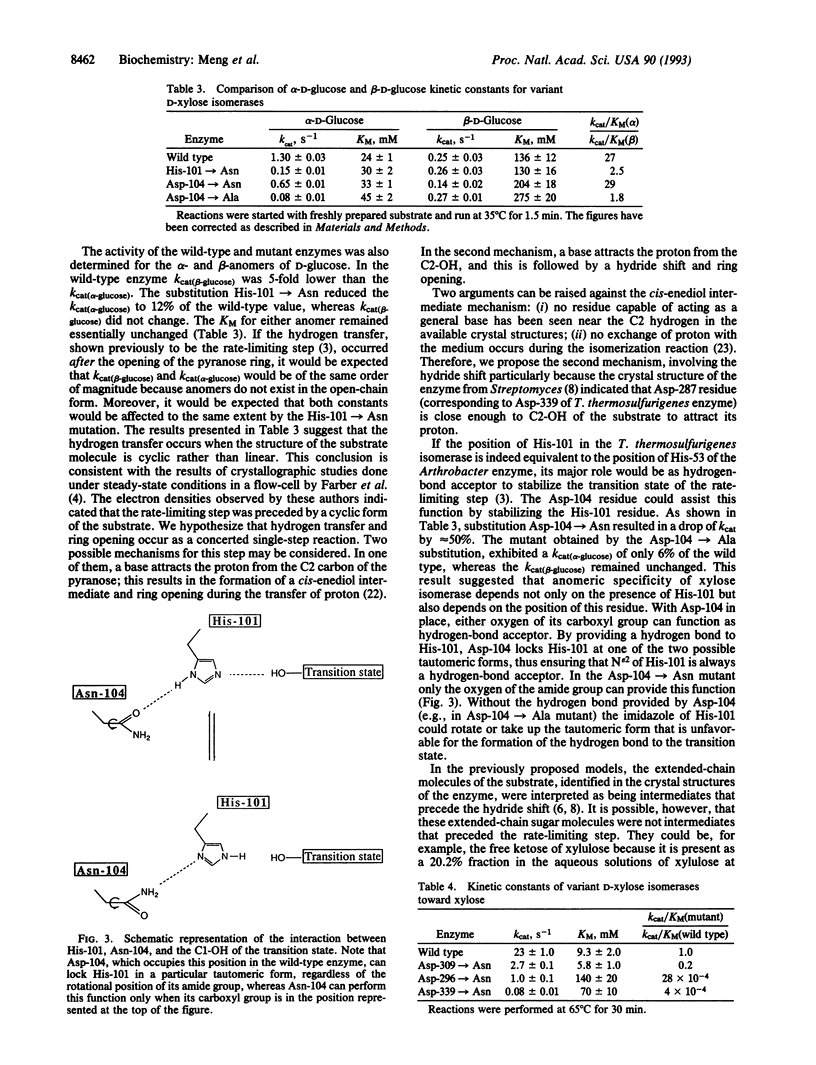
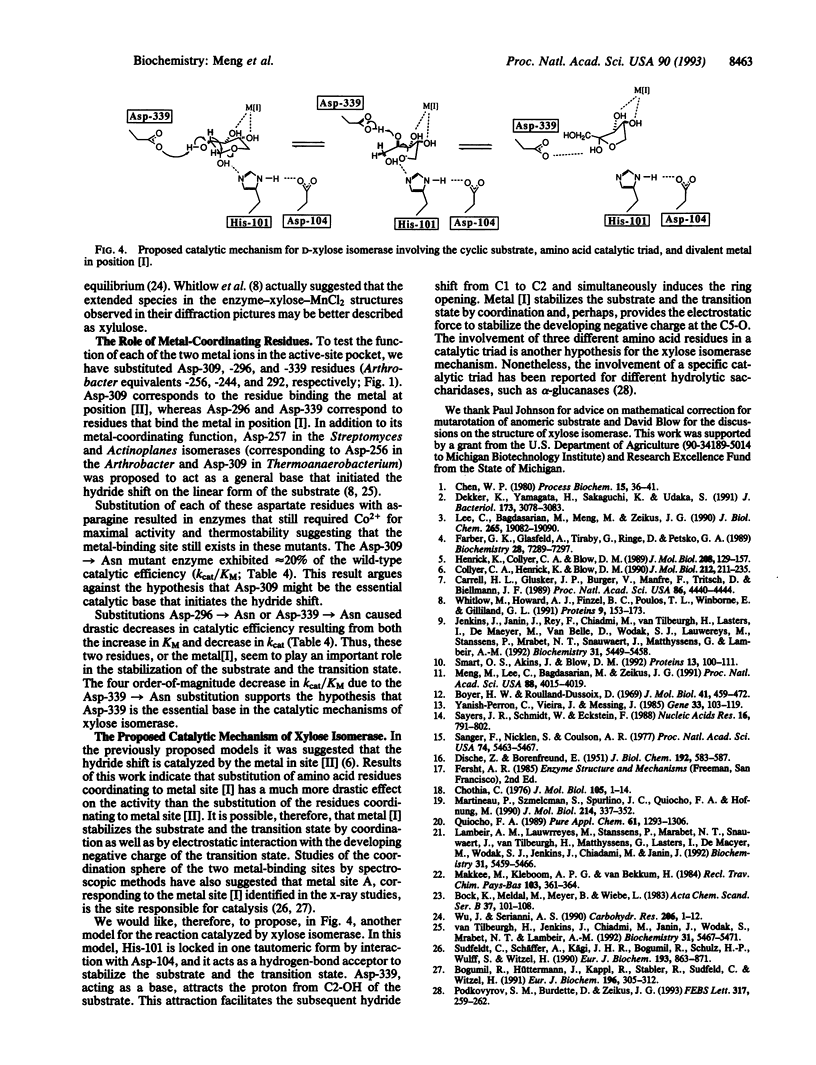
The functions of individual amino acid residues in the active site of Thermoanaerobacterium thermosulfurigenes D-xylose ketol-isomerase (EC 5.3.1.5) were studied by site-directed substitution. The role of aromatic residues in the active-site pocket was not limited to the creation of a hydrophobic environment. For example, Trp-188 provided for substrate binding and Trp-139 allowed for the discrimination between D-xylose and D-glucose. Substrate discrimination was accomplished by steric hindrance caused by the side chain of Trp-139 toward the larger glucose molecule. Preference of the enzyme for the alpha-anomer of glucose depended on the His-101/Asp-104 pair. Wide differences observed in the catalytic constant (kcat) for alpha- versus beta-glucose in the wild-type enzyme and the fact that only the kcat for alpha-glucose was changed in the His-101-->Asn mutants strongly suggest that the substrate molecule entering the hydride-shift step is still in the cyclic form. On the basis of these results a revised hypothesis for the catalytic mechanism of D-xylose isomerase has been proposed that involves His-101, Asp-104, and Asp-339 functioning as a catalytic triad.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock K., Meldal M., Meyer B., Wiebe L. Isomerization of D-glucose with glucose-isomerase. A mechanistic study. Acta Chem Scand B. 1983;37(2):101–108. doi: 10.3891/acta.chem.scand.37b-0101. [DOI] [PubMed] [Google Scholar]
- Bogumil R., Hüttermann J., Kappl R., Stabler R., Sudfeldt C., Witzel H. Visible, EPR and electron nuclear double-resonance spectroscopic studies on the two metal-binding sites of oxovanadium (IV)-substituted D-xylose isomerase. Eur J Biochem. 1991 Mar 14;196(2):305–312. doi: 10.1111/j.1432-1033.1991.tb15818.x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Carrell H. L., Glusker J. P., Burger V., Manfre F., Tritsch D., Biellmann J. F. X-ray analysis of D-xylose isomerase at 1.9 A: native enzyme in complex with substrate and with a mechanism-designed inactivator. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4440–4444. doi: 10.1073/pnas.86.12.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- Dekker K., Yamagata H., Sakaguchi K., Udaka S. Xylose (glucose) isomerase gene from the thermophile Thermus thermophilus: cloning, sequencing, and comparison with other thermostable xylose isomerases. J Bacteriol. 1991 May;173(10):3078–3083. doi: 10.1128/jb.173.10.3078-3083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber G. K., Glasfeld A., Tiraby G., Ringe D., Petsko G. A. Crystallographic studies of the mechanism of xylose isomerase. Biochemistry. 1989 Sep 5;28(18):7289–7297. doi: 10.1021/bi00444a022. [DOI] [PubMed] [Google Scholar]
- Jenkins J., Janin J., Rey F., Chiadmi M., van Tilbeurgh H., Lasters I., De Maeyer M., Van Belle D., Wodak S. J., Lauwereys M. Protein engineering of xylose (glucose) isomerase from Actinoplanes missouriensis. 1. Crystallography and site-directed mutagenesis of metal binding sites. Biochemistry. 1992 Jun 23;31(24):5449–5458. doi: 10.1021/bi00139a005. [DOI] [PubMed] [Google Scholar]
- Lambeir A. M., Lauwereys M., Stanssens P., Mrabet N. T., Snauwaert J., van Tilbeurgh H., Matthyssens G., Lasters I., De Maeyer M., Wodak S. J. Protein engineering of xylose (glucose) isomerase from Actinoplanes missouriensis. 2. Site-directed mutagenesis of the xylose binding site. Biochemistry. 1992 Jun 23;31(24):5459–5466. doi: 10.1021/bi00139a006. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Bagdasarian M., Meng M. H., Zeikus J. G. Catalytic mechanism of xylose (glucose) isomerase from Clostridium thermosulfurogenes. Characterization of the structural gene and function of active site histidine. J Biol Chem. 1990 Nov 5;265(31):19082–19090. [PubMed] [Google Scholar]
- Martineau P., Szmelcman S., Spurlino J. C., Quiocho F. A., Hofnung M. Genetic approach to the role of tryptophan residues in the activities and fluorescence of a bacterial periplasmic maltose-binding protein. J Mol Biol. 1990 Jul 5;214(1):337–352. doi: 10.1016/0022-2836(90)90165-I. [DOI] [PubMed] [Google Scholar]
- Meng M., Lee C., Bagdasarian M., Zeikus J. G. Switching substrate preference of thermophilic xylose isomerase from D-xylose to D-glucose by redesigning the substrate binding pocket. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4015–4019. doi: 10.1073/pnas.88.9.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkovyrov S. M., Burdette D., Zeikus J. G. Analysis of the catalytic center of cyclomaltodextrinase from Thermoanaerobacter ethanolicus 39E. FEBS Lett. 1993 Feb 15;317(3):259–262. doi: 10.1016/0014-5793(93)81288-b. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Eckstein F. 5'-3' exonucleases in phosphorothioate-based oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Feb 11;16(3):791–802. doi: 10.1093/nar/16.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart O. S., Akins J., Blow D. M. Molecular mechanics simulations of a conformational rearrangement of D-xylose in the active site of D-xylose isomerase. Proteins. 1992 Apr;13(2):100–111. doi: 10.1002/prot.340130203. [DOI] [PubMed] [Google Scholar]
- Sudfeldt C., Schäffer A., Kägi J. H., Bogumil R., Schulz H. P., Wulff S., Witzel H. Spectroscopic studies on the metal-ion-binding sites of Co2(+)-substituted D-xylose isomerase from Streptomyces rubiginosus. Eur J Biochem. 1990 Nov 13;193(3):863–871. doi: 10.1111/j.1432-1033.1990.tb19410.x. [DOI] [PubMed] [Google Scholar]
- Whitlow M., Howard A. J., Finzel B. C., Poulos T. L., Winborne E., Gilliland G. L. A metal-mediated hydride shift mechanism for xylose isomerase based on the 1.6 A Streptomyces rubiginosus structures with xylitol and D-xylose. Proteins. 1991;9(3):153–173. doi: 10.1002/prot.340090302. [DOI] [PubMed] [Google Scholar]
- Wu J., Serianni A. S., Vuorinen T. Furanose ring anomerization: kinetic and thermodynamic studies of the D-2-pentuloses by 13C-n.m.r. spectroscopy. Carbohydr Res. 1990 Sep 30;206(1):1–12. doi: 10.1016/0008-6215(90)84001-b. [DOI] [PubMed] [Google Scholar]
- van Tilbeurgh H., Jenkins J., Chiadmi M., Janin J., Wodak S. J., Mrabet N. T., Lambeir A. M. Protein engineering of xylose (glucose) isomerase from Actinoplanes missouriensis. 3. Changing metal specificity and the pH profile by site-directed mutagenesis. Biochemistry. 1992 Jun 23;31(24):5467–5471. doi: 10.1021/bi00139a007. [DOI] [PubMed] [Google Scholar]



