Abstract
The right temporoparietal junction (rTPJ) is engaged by tasks that manipulate biological motion processing, Theory of Mind attributions, and attention reorienting. The proximity of activations elicited by these tasks raises the question of whether these tasks share common cognitive component processes that are subserved by common neural substrates. Here, we used high-resolution whole-brain functional magnetic resonance imaging in a within-subjects design to determine whether these tasks activate common regions of the rTPJ. Each participant was presented with the 3 tasks in the same imaging session. In a whole-brain analysis, we found that only the right and left TPJs were activated by all 3 tasks. Multivoxel pattern analysis revealed that the regions of overlap could still discriminate the 3 tasks. Notably, we found significant cross-task classification in the right TPJ, which suggests a shared neural process between the 3 tasks. Taken together, these results support prior studies that have indicated functional heterogeneity within the rTPJ but also suggest a convergence of function within a region of overlap. These results also call for further investigation into the nature of the function subserved in this overlap region.
Keywords: attention reorienting, biological motion, fMRI, overlap, Theory of Mind
Introduction
The right temporoparietal junction (rTPJ) lies at the intersection of the temporal and parietal cortices. Its boundaries are anatomically indistinct, but researchers have included within its designation the angular gyrus, the posterior superior temporal sulcus (pSTS) and its horizontal and ascending branches, the posterior extent of the supramarginal gyrus, and the dorsal-anterior extent of the lateral occipital cortex (Decety and Lamm 2007; Carter and Huettel 2013). Recent meta-analytical and review articles have highlighted the functional heterogeneity of the rTPJ, which has been associated with such disparate cognitive processes such as attention, memory, language, and social cognition (Cabeza et al. 2012; Carter and Huettel 2013).
The heterogeneity of tasks that engage the rTPJ has raised the question of whether the TPJ is composed of cortical regions that perform many different functions or whether the TPJ has a common function that supports the various tasks that engage it. Recently, researchers have sought to provide integrative accounts of this region's function that emphasize global abstract functions. For example, some have proposed that this area serves as a convergence zone of sensory and contextual information to enable abstract representations of perceptual experience for semantic memory (Binder and Desai 2011), or to bind episodic features for episodic memory (Shimamura 2011). Others have suggested that the TPJ is the site of convergence for social, attention, memory, and language processing streams, which are then integrated to create a social context for decision-making involving other social agents (Carter and Huettel 2013). The TPJ is also thought to update one's internal model of the current environment (i.e., contextual updating) and adjusting expectations based on incoming sensory information (Geng and Vossel 2013). Additionally, a recent study has proposed that the TPJ serves to attribute sensory awareness to oneself and to others (Kelly et al. 2014). The superior temporal sulcus (STS), the posterior end of which is usually included within the designation of rTPJ, has also been ascribed multifunctionality, and it has been suggested that the function served by the STS varies according to the nature of its coactivations with different brain networks (Hein and Knight 2008).
What is the nature of the evidence supporting these differing perspectives? For neuroimaging data, evidence for whether the rTPJ supports common or different functions is based, often implicitly, upon spatial (i.e., anatomical) dissociations in activation. Roughly speaking, if 2 tasks activate spatially different areas, they are usually thought to rely on different functions, whereas an overlap of activation implies a common function. This logic is similar to the double-dissociation concept in brain lesion studies but is complicated by the myriad statistical issues in determining whether a brain region is activated above baseline, and by technical and judgment issues about how to set statistical thresholds. Overlapping fMRI activations are also not conclusive evidence for a common neural function, as a common activation might be artifactual—for example, it might reflect a confluence of venous drainage from adjacent brain regions. Nevertheless, task activation overlap is at least suggestive of a common neural function, whereas the absence of overlap renders such an interpretation moot.
As we review later, the evidence concerning task activation overlap in the TPJ is inconclusive. This is largely because, with a few exceptions discussed later, most prior studies have not directly compared different tasks in the same participants. Here, we address this issue by comparing the activation patterns of 3 tasks that are frequently used to activate the rTPJ in studies of biological motion processing, Theory of Mind, and attention reorienting.
Our choice of these tasks stemmed from our laboratory's history of studying the pSTS' response to biological motion (i.e., the movement associated with living entities) than to non-biological motion (Allison et al. 2000), and the modulation of pSTS activity to biological motion by contextual information (Pelphrey et al. 2003; Pelphrey et al. 2004). We, and others, have also found that this region is engaged when observers consider the intentions and goal-directedness of motion, even when they are made by non-biological entities, such as moving geometric shapes or machines (Castelli et al. 2002; Schultz et al. 2003; Tavares et al. 2008; Gao et al. 2012; Shultz and McCarthy 2012; Lee et al. 2014). These findings suggest that the pSTS is involved in intention processing, which bears some similarities to Theory of Mind, that is, when one considers the thoughts and beliefs of other people, which also consistently recruits the rTPJ (Saxe 2006).
A subset of the work that implicates the pSTS in intention processing has used experimental tasks where the observer's expectation of a social agent's action is violated (Pelphrey et al. 2003; Pelphrey et al. 2004; Saxe et al. 2004; Brass et al. 2007; Vander Wyk et al. 2009). This observation has brought to light the similarities between activations to biological motion and to attention-reorienting tasks, such as the Posner-cueing task (Posner 1980) where participants are occasionally incorrectly cued about the spatial location of a target, which also engage the TPJ (Corbetta et al. 2008). Given the proximity of activations elicited by Theory of Mind and attention-reorienting tasks, there is also not surprisingly a considerable literature discussing the similarities between Theory of Mind and attention reorienting (Decety and Lamm 2007; Corbetta et al. 2008). All together, these 3 tasks seem to be interrelated, hence their inclusion in this study.
The spatial distributions of activation elicited by these different tasks have been discussed in recent meta-analyses. Biological motion activations have been reported to activate ventral portions of the rTPJ, Theory of Mind tasks have been reported to activate superior and posterior regions of the rTPJ, whereas attention-reorienting activations have been reported to activate superior and anterior regions of the rTPJ (Decety and Lamm 2007; Carter and Huettel 2013). However, as meta-analyses compare activations across different subject groups and studies, the question of whether the similarity or differences in activation loci associated with the different tasks could be due to interstudy and intersubject variability inevitably remains. For this reason, a few recent studies have compared a subset of these tasks in the same individuals.
Gobbini et al. (2007) presented participants with Theory of Mind stories, social animations of geometric shapes (Heider and Simmel 1944), and biological motion. They reported overlapping regions of activation in the pSTS to biological motion and to social animations, but did not find activations in this overlap region to Theory of Mind stories. Saxe et al. (2009) also did not find any overlap between Theory of Mind and biological motion activations in the pSTS when both these tasks were run in the same children. Bahnemann et al. (2010), however, did find overlapping activation to Theory of Mind and biological motion in the pSTS, possibly because they used the same stimuli to investigate both tasks whereas the previous 2 studies used different stimuli for each task. Interestingly, Deen and McCarthy (2010) also found that the same pSTS region that responded to biological motion was activated more strongly when participants read Theory of Mind stories with strong biological motion imagery compared with those without such imagery.
Studies have also directly compared activations with Theory of Mind and attention reorienting in the same participants. Mitchell (2008) presented participants with Theory of Mind stories and an attention-reorienting (i.e., Posner cueing) task and found that both tasks engaged a common region in the TPJ. However, Scholz et al. (2009), using higher resolution functional imaging, found minimal overlap that lay at the periphery of activations to each task. No study to our knowledge has directly contrasted biological motion and attention-reorienting tasks, although as previously mentioned, many studies have shown that actions that violate the observer's expectations often result in greater activity in the TPJ.
Based on the literature reviewed, it remains unclear whether biological motion, Theory of Mind, and attention-reorienting tasks engage common regions in rTPJ. Moreover, no study to our knowledge has compared all 3 tasks in the same participants. In the current study, we compared activations with all 3 tasks in the same participants to assess the extent to which the tasks engaged common neural activity in the TPJ. We used a multiplexed imaging sequence (Feinberg et al. 2010) to acquire high-resolution whole-brain fMRI data with 2-mm isotropic voxels to improve our ability to spatially discriminate among tasks. Furthermore, recognizing that overlapping activations can still contain task-specific information (Peelen et al. 2006; Downing et al. 2007), we also used multivoxel pattern analysis (MVPA) to determine whether the pattern of activity in any region of overlap could still discriminate the 3 tasks.
Materials and Methods
Participants
Twenty right-handed, healthy adults (11 male, mean age 24.2 ± 3.68 years) participated in the study. All had normal or corrected-to-normal vision and no history of neurological or psychiatric illnesses. The protocol was approved by the Yale Human Investigation Committee, and informed consent was obtained for all participants. Due to a technical error, the stimulus timing files were corrupted for 1 participant and so this report is based on data from 19 subjects.
Experimental Design and Stimuli
Three tasks were compared: biological motion, Theory of Mind, and attention reorienting (described later). Stimuli were presented using Psychtoolbox 3.0.8 (Brainard 1997; Pelli 1997) in MATLAB 7.8 (The Mathworks, Inc.).
Biological Motion
Stimuli were from Engell and McCarthy (2013) and consisted of dynamic point-light displays of human figures (Johansson 1973) and their scrambled counterparts. Stimuli were presented in alternating 12-s blocks of point-display figures (“Bio”) and 12-s blocks of scrambled point-lights (“Scrambled”). Each stimulus block consisted of six 2-s presentations of different point-light displays. To ensure that participants paid attention to the stimuli, 2-s presentations of static frames of the upright point-light figures (for Bio blocks) or their inverted counterparts (for Scrambled blocks) were randomly interspersed throughout the run. Participants were simply asked to count how many times static frames appeared. Task blocks were separated by 12-s fixation intervals. The task consisted of a total of 18 Bio blocks and 18 Scrambled blocks, which were grouped into 3 runs, each with 6 Bio blocks and 6 Scrambled blocks.
Theory of Mind
Stimuli were obtained from the website of Dr Rebecca Saxe's lab (http://saxelab.mit.edu/superloc.php, last accessed November 24, 2014; credit David Dodell-Feder, Nicholas Dufour, and Rebecca Saxe) and consisted of 30 short stories—15 false belief and 15 false “photo” stories (examples below). These stimuli were originally developed based on an item analysis of stories that best activated the rTPJ in Dodell-Feder et al. (2011). As the downloaded stimulus set only consisted of 20 stories, 10 additional stories were obtained from Dodell-Feder et al. (2011). Specifically, the 5 belief stories that elicited the next highest TPJ activations (after the stories chosen for the initial set of 10) were chosen, along with the 5 corresponding photo stories. Each story was presented for 10 s, following which a True/False statement about the story was presented for 4 s. Participants responded “True” or “False” to the statement using left and right button presses.
False belief stories (“Belief”) described situations in which a protagonist's initial correct belief about a situation became incorrect (i.e., false) because of a change in the situation that occurred without the protagonist's knowledge. For example, “The morning of the high school dance Sarah placed her high heel shoes under her dress and then went shopping. That afternoon, her sister borrowed the shoes and later put them under Sarah's bed.” The True/False statement that followed read, “Sarah gets ready assuming her shoes are under the dress.” False photo stories (“Photo”) similarly described situations in which a documentation (such as a photo, painting and piece of writing) of a physical description became outdated because of changes that occurred over time. For example, “A long time ago, an explorer mapped a small island. Since then, the water levels rose and only a tiny part of the island is now left above water.” The True/False statement that followed read, “On the explorer's maps, the island appears to be mostly submerged.” The crucial difference between Belief and Photo stories is that the outdated content was another's mental representation in the Belief stories but a physical representation in the Photo stories. Therefore, performing the task required participants to consider either the mental state of another person or the physical state of the documentation.
Each story and its corresponding True/False statement made up one 14-s trial. Trials were separated by 12-s fixation intervals. The order of Belief and Photo conditions was pseudo-randomized with the restriction that no more than 2 trials of the same condition were presented consecutively. Trials were grouped into 3 runs, each run consisting of 5 Belief trials and 5 Photo trials.
Analysis of the behavioral results for the Theory of Mind task revealed that there was no difference in response times to belief stories (M = 2629 ms) and photo stories (M = 2607 ms), t(18) = 0.44, P = 0.667.
Attention Reorienting
A Posner attention cueing task was used, in which participants had to respond to a visual target that appeared on the screen at 1 of 2 locations. We followed closely the design used in Mitchell (2008). A fixation cross and 2 square frames on the left and right of the cross were displayed for the entire duration of the task. Participants were instructed to fixate on the central cross throughout the task, but eye movements were not monitored. At the start of each 4-s trial, the fixation cross turned green for 700 ms, after which an arrow centered on the cross appeared for 800 ms cuing the participant to the left or right frame. After a jittered interval of 500–2000 ms, a target (i.e., a circle) appeared in either the left or right frame for 100 ms and participants were instructed to press the left or right button corresponding to the side that the target appeared as quickly as possible. In Valid trials, the target appeared where the arrow cued (“Valid”). Crucially, however, in Invalid trials, the target appeared on the opposite side (“Invalid”), requiring participants to reorient their attention from the cued location. After the target disappeared, the fixation cross and 2 square-frames remained on the screen for the remainder of the trial, as well as during the jittered inter-trial interval of 1–7 s.
The task consisted of a total of 180 Valid and 60 Invalid trials. Trials were grouped into 3 runs, each with 60 Valid trials and 20 Invalid trials. The program “optseq2” (http://surfer.nmr.mgh.harvard.edu/optseq, last accessed November 24, 2014) was used to generate the optimal sequence and separation of trials for maximal statistical efficiency of rapid-presentation event-related hemodynamic response estimation for each run (Dale 1999).
Participants took longer to respond to Invalid trials (M = 470 ms) than to Valid trials (M = 448 ms), t(18) = 4.54, P < 0.001, confirming that participants' attention was cued by the arrow and had to be re-oriented during Invalid trials.
Participants first performed 2 runs of the Biological Motion task, then 2 runs of Theory of Mind task, then 2 runs of the Attention-Reorienting tasks, after which they performed a third run of each of the 3 tasks. The last run of each task was performed at the end to ensure that participants would have at least 2 runs of each task if the imaging session was shortened due to technical problems. However, all participants completed 3 runs of each task.
Image Acquisition and Preprocessing
Data were acquired using a 3T Siemens TIM Trio scanner with a 32-channel head coil. Functional images were acquired using a multiband echo-planar pulse sequence (TR = 2000 ms, TE = 32 ms, flip angle = 62°, FOV = 210 × 202 mm, matrix = 104 × 100, slice thickness = 2 mm, 60 slices, voxel size = 2 mm3). Two structural images were acquired for registration: T1 coplanar images were acquired using a T1 Flash sequence (TR = 335 ms, TE = 2.61 ms, flip angle = 70°, FOV = 210 × 210 mm, matrix = 192 × 192, slice thickness = 2 mm, 60 slices), and high-resolution images were acquired using a 3D MP-RAGE sequence (TR = 2530 ms, TE = 2.77 ms, flip angle = 7°, FOV = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm, 176 slices).
Image preprocessing was performed using the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl, last accessed November 24, 2014). Structural and functional images were skull-stripped using the Brain Extraction Tool. The first 3 volumes (6 s) of each functional data set were discarded to allow for MR equilibration. Functional images then underwent motion correction (using the MCFLIRT linear realignment), spatial smoothing using a 3-mm FWHM Gaussian kernel, and high-pass filtering with a 0.01 Hz cut-off to remove low-frequency drift. Finally, the functional images were registered to the coplanar images, which were in turn registered to the high-resolution structural images, using non-linear registration, and then normalized to the Montreal Neurological Institute's MNI152 template.
fMRI Data Analysis
Whole-brain voxel-wise regression analyses were performed using FSL FEAT. Each task was analyzed separately. The linear model for each task consisted of 2 explanatory variables, one for the condition of interest and one for the control condition (i.e., variables for the 12-s “Bio” and “Scrambled” blocks for the Biological Motion task, the 14-s “Belief” and “Photo” trials for the Theory of Mind task, and the 4-s “Valid” and “Invalid” trials for the Attention-Reorienting task). All variables were modeled as boxcar functions, where a value of 1 was assigned to time-points associated with the event and a value of 0 to time-points not associated with the event, and then convolved with a gamma function. Contrasts comparing parameter estimates obtained from the regression analyses were defined at the subject level to identify brain regions that showed condition-specific effects (i.e., Bio > Scrambled for the Biological Motion task, Belief > Photo for the Theory of Mind task, and Invalid > Valid for the Attention-Reorienting task). Subject-level analyses combining multiple runs were conducted using a fixed-effects model.
Group-level analyses were performed on the parameter estimates obtained from the contrasts calculated at the subject level using a mixed-effects model, where the mixed-effects variance comprised both the fixed (within-subject) and random effects (between-subject) variance. The random-effects component of variance was estimated using FSL's FLAME 1 + 2 procedure.
Identifying Individual Task Activations
To identify regions that were activated to each task, voxels were first thresholded at a level of Z > 3.1. Cluster-correction using Gaussian Random Field theory was then applied to the thresholded voxels to correct for multiple comparisons (Worsley et al. 1996). Clusters, defined as contiguous sets of voxels with Z > 3.1, that survived the correction at a cluster probability of P < 0.05 were considered significant activations.
Identifying Overlapping Activations
To identify regions that were activated in all 3 tasks, the activation map for each task was first thresholded at a voxel-wise level of Z > 1.65 and cluster probability of P < 0.05. The overlap of all 3 tasks was then defined as the intersection of voxels that survived this threshold in all 3 task contrasts, resulting in a voxel-wise conjoint probability of P < 0.001 for the overlapping regions.
We also identified the spatial overlap of activation for pairs of tasks; that is, Biological Motion and Theory of Mind, Biological Motion and Attention Reorienting, and Theory of Mind and Attention Reorienting. Here, the activation map for each task was first thresholded at a voxel-wise level of Z > 2.3 and a cluster probability of P < 0.05. The overlap was then defined as the intersection of voxels that survived this threshold in both task contrasts, resulting in a voxel-wise conjoint probability of P < 0.001 for the overlapping regions. Only resulting clusters of at least 20 voxels are reported to exclude voxels that only overlapped at the extreme edges of task-specific clusters. The activation overlap between pairs of tasks does not exclude voxels that also overlap with the third task.
Multivoxel Pattern Analysis
Multivoxel pattern analysis was performed to ascertain if regions commonly activated by all 3 tasks nevertheless responded to each task in a discriminable manner, suggestive of a task-specific spatial pattern of activity. We used a combination of AFNI (Cox 1996), PyMVPA (Hanke et al. 2009), and MATLAB 7.14 (The MathWorks, Inc.) for these analyses. A linear support vector machine (LIBSVM; Chang and Lin 2011) was used as the classifier, with automatically scaling of the cost parameter C to the norm of the data. MVPA was performed both within-participant to assess the discriminability of tasks within each participant and across participants to assess the spatial consistency of task activations between participants.
Within-participant Classification Analysis
For each participant, beta estimates for each block (for Biological Motion task) or trial (for Theory of Mind and Attention-Reorienting tasks) were obtained for each condition in each task using AFNI's 3dDeconvolve and 3dREMLfit functions. One of the 19 participants was excluded from this analysis because the timing file for 1 Theory of Mind run was corrupted for this participant. All within-participant classification analyses were performed using the voxels in each region of the overlap of all 3 tasks as features.
To assess the discriminability of the 3 tasks, the mean of the beta estimates for the control conditions in each task were subtracted from the beta estimates from the condition of interest to control for effects of no interest, such as visual differences between the tasks. Specifically, we subtracted the mean of Scrambled estimates from each Bio estimate, the mean of Photo estimates from each Belief estimate, and the mean of Valid estimates from each Invalid estimate. A three-way classification analysis was then performed using the control-subtracted beta estimates as the data samples for the training and testing data sets. The samples were not weighted. The voxels in each sample were mean-normalized (by z-scoring) to remove amplitude differences between tasks. This ensured that any task discrimination observed was driven by differences in spatial pattern of activity, and not by differences in mean activation differences between tasks (Coutanche 2013). This is particularly important in this study because of the different nature of the tasks and controls. Multiclass classification was implemented using a one-against-one scheme. A leave-one-run-out cross-validation scheme was used, and the number of samples used for each task was balanced in each cross-validation fold using PyMVPA's Balancer option. Group-level classification accuracies were obtained by averaging the accuracies from all participants.
Group-level significance was assessed using permutation testing at the participant-level and bootstrap sampling at the group level (Stelzer et al. 2013). That is, the data labels for each participant were permuted (within each run) 100 times using a data set-wise permutation scheme of both testing and training labels (Etzel and Braver 2013). The classification analysis was performed using each permuted label set to yield 100 chance accuracies for each participant. We then randomly drew 1 of the chance accuracies from each participant and averaged these accuracies to obtain a chance group-level accuracy. This was repeated 105 times to create a group-level null distribution. The true group-level classification accuracy was compared with the null distribution to obtain the P-value associated with the accuracy.
To further assess whether the 3 tasks possibly share a common neural process within the regions of overlap, we also performed a cross-task classification analysis where the classifier was trained on the whole data set from 1 task and tested on the whole data set from each of the other 2 tasks (no cross-validation was performed). For example, the classifier was first trained to discriminate Bio and Scrambled samples in the Biological Motion task and then tested to assess whether this trained classifier could discriminate Belief and Photo samples in the Theory of Mind task and Invalid and Valid samples in the Attention-Reorienting task. Again, the voxels in each sample were first z-scored before the classification analysis was conducted. Group-level significance testing followed the same approach as the three-way classification analysis, except that only the cross-task test labels were permuted for each participant.
Cross-participant Classification Analysis
To assess the spatial consistency of the activations elicited by each task across participants, we performed a cross-participant three-way classification analysis to assess whether the regions of overlap could discriminate the 3 tasks across participants. Here, training and testing data consisted of the Z-score summary statistic for each of the task contrasts (i.e., Bio > Scrambled, Belief > Photo, Invalid > Valid) from each participant. The voxels in each sample were mean-normalized (by z-scoring) to remove amplitude differences between tasks. A leave-one-subject-out cross-validation scheme was used. To assess the significance of classification accuracies, an empirical null distribution was also obtained by performing the classification analysis on 1000 permutations of the data labels.
Results
Regions Activated by Each Task
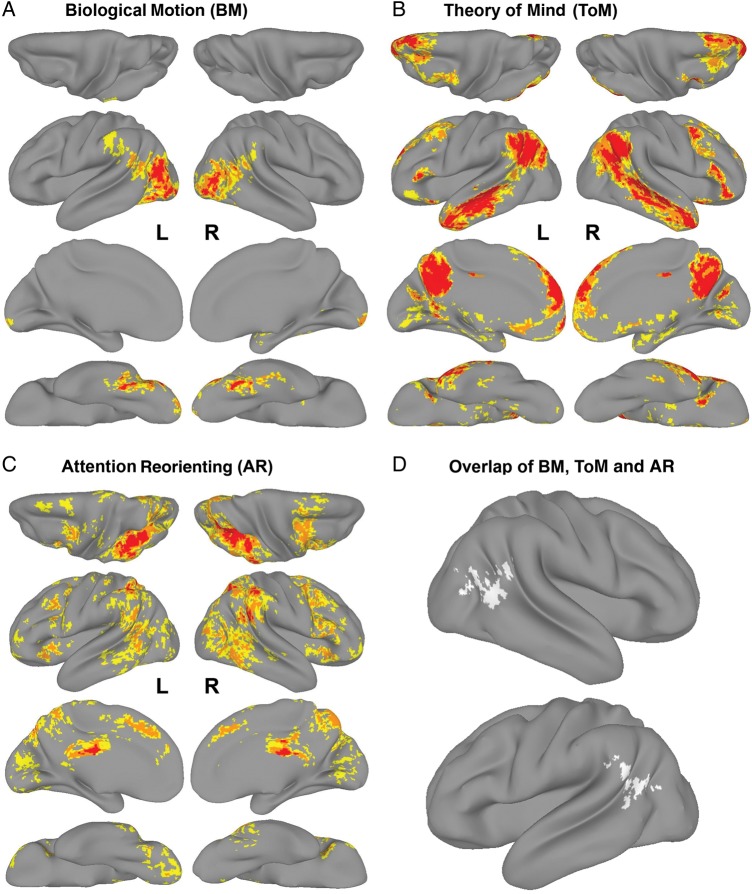
The Bio > Scrambled contrast from the Biological Motion task evoked clusters of activation in right and left lateral occipital cortex extending into occipital pole, as well as right and left fusiform gyrus (Fig. 1A, in red). The Belief > Photo contrast from the Theory of Mind task evoked clusters of activation that included right and left temporoparietal cortex extending inferiorly to anterior temporal gyrus, medial prefrontal cortex, precuneus, posterior cingulate cortex, right and left inferior and middle frontal gyri (Fig. 1B, in red), and the cerebellum (not shown). The Invalid > Valid contrast from the Attention-Reorienting task evoked clusters of activation that included right and left intraparietal sulcus, right temporoparietal cortex, posterior cingulate cortex, precuneus, and left superior lateral occipital cortex (Fig. 1C, in red). Coordinates of cluster maxima for individual task activations are reported in Supplementary Table 1.
Figure 1.
Activation maps from each task (cluster-corrected at P < 0.05) are displayed on a surface-rendered brain using AFNI′s SUMA tool (http://afni.nimh.nih.gov/afni/suma, last accessed October 20, 2014): (A) Bio > Scrambled contrast from the Biological Motion task, (B) Belief > Photo contrast from the Theory of Mind task, (C) Invalid > Valid contrast from the Attention-Reorienting task. For each activation map, the dorsal (top row), lateral (second row), medial (third row), and ventral (fourth row) surfaces are displayed. Activation maps are colored according to an activation gradient: red = Z > 3.1, orange = Z > 2.3, yellow = Z > 1.65. Only activations in red are considered significant activations. The regions in orange and yellow reflect regions that were considered in the two-way (Fig. 2, in red) and in the three-way (Fig. 1D) conjunctions, respectively. (D) The regions of overlap that were activated by all 3 tasks, that is, right TPJ/LOC and left TPJ. The regions of overlap in each hemisphere are from 1 cluster in volume space and only appear separated on the surface.
Regions Commonly Activated by all Three Tasks
Two regions were commonly activated by all 3 tasks: the right TPJ, extending posteriorly into lateral occipital cortex, and the left TPJ, also extending posteriorly into lateral occipital cortex and anteriorly into the supramarginal gyrus (Fig. 1D, in white).
Regions Commonly Activated by Pairs of Tasks
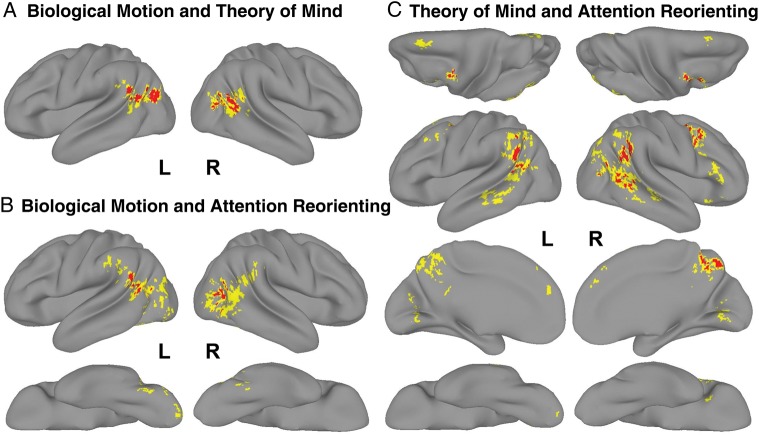
Regions activated by both the Biological Motion and Theory of Mind tasks included bilateral TPJ extending posteriorly into the lateral occipital cortex (Fig. 2A, in red). Regions activated by both the Biological Motion and Attention-Reorienting tasks included left TPJ and right lateral occipital cortex (Fig. 2B, in red). Regions activated by both the Theory of Mind and Attention-Reorienting tasks included left TPJ, right TPJ extending to lateral occipital cortex and middle temporal gyrus, precuneus, and bilateral middle frontal gyrus/precentral sulcus (Fig. 2C, in red) and left cerebellum (not shown).
Figure 2.
Surface rendering of regions that were commonly activated by pairs of tasks: (A) Biological Motion and Theory of Mind (lateral surface), (B) Biological Motion and Attention Reorienting (lateral and ventral surfaces), and (C) Theory of Mind and Attention Reorienting (dorsal, lateral, medial, and ventral surfaces). Activation maps are colored according to an activation gradient: red = conjoint P < 0.001, yellow = conjoint P < 0.0025. Only activations in red are considered significant conjunctions. Regions in yellow reflect regions that were considered in the three-way conjunction (Fig. 1D). Surfaces views that had no activations are not shown. Only clusters of at least 20 voxels are shown.
Within-participant Classification Analysis
The three-way classification of tasks in each participant yielded significant above-chance accuracies for the region of overlap in right TPJ (M = 56.2%, P < 0.001), as well as for the region of overlap in left TPJ (M = 50.5%, P < 0.001). Inspection of the classification confusion matrix revealed that all tasks were successfully classified (Supplementary Table 2).
The cross-task classification analyses revealed that for the right TPJ, a classifier trained on the Theory of Mind task was able to discriminate Invalid from Valid samples in the Attention-Reorienting task (M = 51.3%, P = 0.003), and a classifier trained on the Attention-Reorienting task was able to discriminate Bio from Scrambled samples in the Biological Motion task (M = 52.0%, P = 0.045) and Belief from Photo samples in the Theory of Mind task (M = 53.7%, P = 0.029). For the left TPJ, a classifier trained on the Biological Motion task was able to discriminate Invalid from Valid samples in the Attention-Reorienting task (M = 51.0%, P = 0.024). All classification accuracies are reported in Supplementary Table 3.
Cross-participant Classification Analysis
The cross-participant classification analysis revealed that the region of overlap in the right TPJ successfully discriminated between the 3 tasks (403 voxels, M = 50.9%, P = 0.005). Inspection of the classification confusion matrix revealed that all tasks were successfully classified (Supplementary Table 4). The region of overlap in left TPJ, however, did not successfully discriminate between the 3 tasks (322 voxels, M = 36.8%, P = 0.328).
Discussion
Biological motion processing, Theory of Mind, and attention-reorienting tasks have been reliably shown to activate the rTPJ, which raises the question of whether these 3 seemingly disparate tasks engage common neural substrates. To help answer this question, we presented participants with these 3 tasks in a within-subjects design to investigate whether these tasks activate overlapping regions in rTPJ. We found that even though each task engaged different brain regions (Fig. 1A–C), they nevertheless all activated common regions in right and left TPJ (Fig. 1D). These results suggest the possibility that these 3 tasks are supported by a common function performed in the TPJ.
Notably, the cross-task classification analysis revealed that a classifier trained on the Attention-Reorienting task also performed successful discrimination on the Biological Motion and Theory of Mind tasks, which provides stronger support for the presence of a shared neural process, since overlapping activations could merely be attributed to downstream venous drainage or smoothing. However, the cross-task classification was not necessarily symmetric. That is, a classifier trained on the Biological Motion task did not successful discriminate the Attention-Reorienting conditions. This suggests that whereas the most discriminative feature of attention reorienting may also play a role in biological motion processing (and Theory of Mind), the most discriminative feature of biological motion processing may not necessarily play a role in attention reorienting. We also found that a classifier trained on the Theory of Mind task performed successful discrimination on the Attention-Reorienting task, providing further support for shared neural processes between these 2 tasks, as has been proposed in the literature. Evidence for common neural signatures between tasks was largely absent in the left TPJ.
Interestingly, a within-participant three-way pattern classification analysis revealed that overall, the 3 tasks could be discriminated from the spatial pattern of activity in the region of overlap in the right and left TPJ, suggesting that there was still task-specific information within these areas of overlap. Results of the cross-participant classification analyses suggest that the spatial pattern of activation by each task is spatially consistent across participants in the right TPJ, but not so in the left TPJ, which had a bias toward classifying tasks as the Biological Motion task.
Altogether, results from the three-way discrimination and the cross-task classification in the right TPJ suggest common, but also task-specific, patterns of activity among the 3 tasks. How might these 2 results be reconciled? The common pattern of activity could reflect a neural process that is particularly discriminative within the Attention-Reorienting task that is also shared between the other 2 tasks. Contextual updating, as proposed by Geng and Vossel (2013), could be one such process. The application of contextual updating to attention reorienting and Theory of Mind was proposed in their review. Specifically, attention reorienting involves violation of expectations and thus requires stimulus response mappings and expectations to be updated. In Theory of Mind tasks, observers have to update contextual representations reflecting their own perspective of the current situation to that reflecting the other person's perspective. Although not included in Geng and Vossel's discussion, we propose that contextual updating can also be applied to biological motion processing. Point-light displays of human figures are coherent and therefore provide an internal model of expected movements and actions. Every frame in the dynamic display can therefore be used to update the model. Scrambled displays, by virtue of being random, arguably offer the observer no internal model for which to update, and therefore, the process of updating is less engaged.
Even if the region of overlap performs a common function such as contextual updating, the pattern of the input to or output from this region could be different depending on the task being performed (Cabeza et al. 2011; Cabeza et al. 2012; Geng and Vossel 2013). The task-specific pattern of activity could be reflecting this difference. For example, the Biological Motion task also engaged regions associated with motion, body form, and face processing (Engell and McCarthy 2013), the Theory of Mind task also engaged regions in the default mode network (Buckner et al. 2008) and the Attention-Reorienting task also engaged regions in the central executive and salience networks (Seeley et al. 2007). It is possible, therefore, that the region of overlap in the TPJ was functionally connected with regions in these different networks during the different tasks. This view is similar to the suggestion by Hein and Knight (2008) that the STS supports different cognitive operations depending on its connections with different networks, except that here, we consider that the rTPJ may be performing 1 common function. Indeed, Cabeza et al. (2011; 2012) have also proposed that the ventral parietal cortex could have an overarching function, but it has subregions that, through different functional connectivity, apply the function to different types of information according to different goals. Although our study was not designed to compare connectivity between tasks, we conducted an exploratory analysis on our data to test this possibility. We found preliminary evidence that the regions of overlap indeed show task-unique patterns of connectivity. These analyses and results are reported in the Supplementary Data.
Our finding of overlapping activations in the TPJ stands in contrast to some previous studies that did not find overlapping activations when comparing Biological Motion and Theory of Mind tasks (Gobbini et al. 2007; Saxe et al. 2009), or Theory of Mind and Attention-Reorienting tasks (Scholz et al. 2009). However, the differences in results could be due to the different statistical thresholds used across the studies. In the studies that did not find any overlap, each task activation map was thresholded at a conservative voxel-wise level of P < 0.001, which may have precluded some task-related activity from being further considered in the conjunction analysis. Indeed, in Gobbini et al. (2007), rTPJ activations to false belief stories were only present at a lower threshold of P < 0.01. Moreover, a voxel would have had to survive a conjoint probability of P < 10−6 to be considered a voxel of overlap from 2 activation maps, which may be an overly stringent criterion. In this study, we used a voxel-wise threshold of P < 0.05 for each task to allow as much task-related activity as statistically reasonable into the conjunction analysis. The conjoint probability of voxels surviving the conjunction analysis of 3 activation maps was nevertheless P < 0.001. Strikingly, no other regions of overlap were found, which demonstrates the specificity of the overlap in the TPJ and highlights the unique role of the TPJ in all 3 tasks.
The tasks contrasted in this study are only a subset of tasks that reportedly engage the rTPJ. The pSTS has also been suggested as a site for multisensory integration and has been shown to respond to stimuli of different modalities (Beauchamp 2005; Beauchamp et al. 2008). Likewise, the angular gyrus subsection of the TPJ is also implicated in semantic and episodic memory processes (Binder and Desai 2011; Shimamura 2011). Expanding the battery of tasks may reveal even greater heterogeneity within the TPJ and may also uncover a more specific region of overlap. For example, the region of overlap extending posteriorly into the lateral occipital cortex could reflect the visual nature of all 3 of the tasks used in this study and could cease to be commonly activated if an auditory task is included. The expansion of tasks may require a correspondingly more inclusive model of the rTPJ's common function, if one is to be proposed.
In conclusion, we identified a region in the TPJ that was commonly activated by 3 separate tasks—biological motion processing, Theory of Mind, and attention reorienting. The pattern of activity in this area of overlap, however, not only successfully discriminated the 3 tasks, demonstrating task-specific information in this “common” region, but also yielded some successful cross-task classification, suggesting a shared neural process between the 3 tasks. The results from this study support the view of the TPJ as a convergence zone as suggested by previous reviews (Binder and Desai 2011; Shimamura 2011; Carter and Huettel 2013), and calls for further investigation into the underlying cognitive and neural processes performed by this common yet distinct area of the TPJ.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Health (MH005286 to G.M.) and the Yale University Faculty of Arts and Sciences (FAS) Imaging Fund.
Supplementary Material
Notes
We thank Marcia Johnson and Rebecca van den Honert for providing helpful feedback on drafts of this manuscript. We also thank the anonymous reviewers for providing critical insights that strengthened the quality of this work. Conflict of Interest: None declared.
References
- Allison T, Puce A, McCarthy G. 2000. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 4:267–278. [DOI] [PubMed] [Google Scholar]
- Bahnemann M, Dziobek I, Prehn K, Wolf I, Heekeren HR. 2010. Sociotopy in the temporoparietal cortex: common versus distinct processes. Soc Cogn Affect Neurosci. 5:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS. 2005. See me, hear me, touch me: multisensory integration in lateral occipital-temporal cortex. Curr Opin Neurobiol. 15:145–153. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. 2008. Touch, sound and vision in human superior temporal sulcus. Neuroimage. 41:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. 2011. The neurobiology of semantic memory. Trends Cogn Sci. 15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. 1997. The Psychophysics Toolbox. Spat Vis. 10:433–436. [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergely G. 2007. Investigating action understanding: inferential processes versus action simulation. Curr Biol. 17:2117–2121. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. 2012. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 16:338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Mazuz YS, Stokes J, Kragel JE, Woldorff MG, Ciaramelli E, Olson IR, Moscovitch M. 2011. Overlapping parietal activity in memory and perception: evidence for the attention to memory model. J Cogn Neurosci. 23:3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Huettel SA. 2013. A nexus model of the temporal–parietal junction. Trends Cogn Sci. 17:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. 2002. Autism, asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 125:1839–1849. [DOI] [PubMed] [Google Scholar]
- Chang C, Lin C. 2011. LIBSVM. ACM Trans Intell Syst Technol. 2:1–27. [Google Scholar]
- Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron. 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutanche MN. 2013. Distinguishing multi-voxel patterns and mean activation: why, how, and what does it tell us? Cogn Affect Behav Neurosci. 13:667–673. [DOI] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Dale AM. 1999. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. 2007. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neurosci. 13:580–593. [DOI] [PubMed] [Google Scholar]
- Deen B, McCarthy G. 2010. Reading about the actions of others: Biological motion imagery and action congruency influence brain activity. Neuropsychologia. 48:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. 2011. fMRI item analysis in a theory of mind task. Neuroimage. 55:705–712. [DOI] [PubMed] [Google Scholar]
- Downing PE, Wiggett AJ, Peelen MV. 2007. Functional magnetic resonance imaging investigation of overlapping lateral occipitotemporal activations using multi-voxel pattern analysis. J Neurosci. 27:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, McCarthy G. 2013. Probabilistic atlases for face and biological motion perception: an analysis of their reliability and overlap. Neuroimage. 74:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel JA, Braver TS. 2013. MVPA permutation schemes: permutation testing in the land of cross-validation. 2013 Int. Work. Pattern Recognit Neuroimaging, Philadelphia, PA; 140–143. [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. 2010. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 5:e15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Scholl BJ, McCarthy G. 2012. Dissociating the detection of intentionality from animacy in the right posterior superior temporal sulcus. J Neurosci. 32:14276–14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Vossel S. 2013. Re-evaluating the role of TPJ in attentional control: contextual updating? Neurosci. Biobehav Rev. 37:2608–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. 2007. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci. 19:1803–1814. [DOI] [PubMed] [Google Scholar]
- Hanke M, Halchenko YO, Sederberg PB, Hanson SJ, Haxby JV, Pollmann S. 2009. PyMVPA: a python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics. 7:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider F, Simmel M. 1944. An experimental study of apparent behavior. Am J Psychol. 57:243–259. [Google Scholar]
- Hein G, Knight RT. 2008. Superior temporal sulcus--It's my area: or is it? J Cogn Neurosci. 20:2125–2136. [DOI] [PubMed] [Google Scholar]
- Johansson G. 1973. Visual perception of biological motion and a model for its analysis. Percept Psychophys. 14:201–211. [Google Scholar]
- Kelly YT, Webb TW, Meier JD, Arcaro MJ, Graziano MSA. 2014. Attributing awareness to oneself and to others. Proc Natl Acad Sci USA. 111:5012–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Gao T, McCarthy G. 2014. Attributing intentions to random motion engages the posterior superior temporal sulcus. Soc Cogn Affect Neurosci. 9:81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. 2008. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 18:262–271. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Wiggett AJ, Downing PE. 2006. Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron. 49:815–822. [DOI] [PubMed] [Google Scholar]
- Pelli DG. 1997. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 10:437–442. [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. 2004. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 16:1706–1716. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. 2003. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 41:156–170. [DOI] [PubMed] [Google Scholar]
- Posner MI. 1980. Orienting of attention. Q J Exp Psychol. 32:3–25. [DOI] [PubMed] [Google Scholar]
- Saxe R. 2006. Uniquely human social cognition. Curr Opin Neurobiol. 16:235–239. [DOI] [PubMed] [Google Scholar]
- Saxe R, Whitfield-Gabrieli S, Scholz J, Pelphrey KA. 2009. Brain regions for perceiving and reasoning about other people in school-aged children. Child Dev. 80:1197–1209. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N. 2004. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 42:1435–1446. [DOI] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. 2009. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 4:e4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P. 2003. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 358:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. 2011. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 11:277–291. [DOI] [PubMed] [Google Scholar]
- Shultz S, McCarthy G. 2012. Goal-directed actions activate the face-sensitive posterior superior temporal sulcus and fusiform gyrus in the absence of human-like perceptual cues. Cereb Cortex. 22:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J, Chen Y, Turner R. 2013. Statistical inference and multiple testing correction in classification-based multi-voxel pattern analysis (MVPA): random permutations and cluster size control. Neuroimage. 65:69–82. [DOI] [PubMed] [Google Scholar]
- Tavares P, Lawrence AD, Barnard PJ. 2008. Paying attention to social meaning: an FMRI study. Cereb Cortex. 18:1876–1885. [DOI] [PubMed] [Google Scholar]
- Vander Wyk BC, Hudac CM, Carter EJ, Sobel DM, Pelphrey KA. 2009. Action understanding in the superior temporal sulcus region. Psychol Sci. 20:771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. 1996. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 4:58–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.