Abstract
The ability to integrate information from different senses, and thereby facilitate detecting and localizing events, normally develops gradually in cat superior colliculus (SC) neurons as experience with cross-modal events is acquired. Here, we demonstrate that the portal for this experience-based change is association cortex. Unilaterally deactivating this cortex whenever visual–auditory events were present resulted in the failure of ipsilateral SC neurons to develop the ability to integrate those cross-modal inputs, even though they retained the ability to respond to them. In contrast, their counterparts in the opposite SC developed this capacity normally. The deficits were eliminated by providing cross-modal experience when cortex was active. These observations underscore the collaborative developmental processes that take place among different levels of the neuraxis to adapt the brain's multisensory (and sensorimotor) circuits to the environment in which they will be used.
Keywords: auditory, cat, multisensory integration, superior colliculus, visual
Introduction
The capability of superior colliculus (SC) neurons to integrate information from multiple senses enhances their sensitivity to external events and their role in detection and orientation behavior (Stein and Stanford 2008). Given its obvious survival value and the vulnerability of the neonate, the protracted postnatal time course for the development of multisensory integration capabilities (Wallace and Stein 1997) seems surprising. It appears, however, to be necessitated by the extensive experience needed to learn to synthesize information from systems with fundamentally different operational parameters (Stein 2012). In the absence of early cross-modal experience, such as occurs when animals are reared with visual or auditory restriction, cat SC neurons fail to develop their ability to integrate these converging sensory inputs to generate enhanced responses (Wallace et al. 2004; Yu et al. 2010; Xu, Yu, Rowland et al. 2012) yet retain the ability to do so in adulthood if sufficient cross-modal experience is obtained (Yu et al. 2010).
There is a second critical factor for the expression of multisensory integration: the presence of influences from association cortex. The SC receives its sensory information from a host of cortical and subcortical sources (Stein and Meredith 1993). Of these, inputs from ipsilateral association cortex (the anterior ectosylvian sulcus, AES; and the rostral lateral suprasylvian sulcus, rLS) are particularly important. If they are deactivated or ablated at any stage of life, SC neurons are rendered incapable of integrating cross-modal cues (Wallace and Stein 2000; Jiang et al. 2001; Jiang et al. 2002; Jiang et al. 2006; Alvarado et al. 2007; Jiang et al. 2007; Alvarado et al. 2009). These observations underscore the necessity of a functionally active association cortex for the expression of SC multisensory integration but provide little insight into what role it plays during development.
That the development of SC multisensory integration is dependent both on multisensory experience and influences from association cortex is unlikely to be coincidental. Indeed, recent computational models (Cuppini et al. 2011; 2012) suggest that this cortex, which is highly sensitive to sensory experience (Rauschecker 1995; Carriere et al. 2007), plays an essential role in incorporating cross-modal experience into the SC circuit, thereby crafting a multisensory integrative capacity that best suits the environment in which it will be used. This theoretical model was favored in explaining the results of a recent study in which chronic (4–8 weeks) pharmacological deactivation of association cortex during early life was shown to disrupt the maturation of multisensory (i.e., visual–auditory) integration in the SC for 1.5–4 years (Rowland et al. 2014). This interpretation was based on the assumption that pharmacological deactivation rendered association cortex insensitive to cross-modal experience, thereby delaying its acquisition of the experience required to facilitate multisensory integration in SC neurons as opposed to producing a general disruption of activity-dependent maturational mechanisms (Catalano and Shatz 1998; Yuste and Sur 1999; Khazipov and Luhmann 2006; Blankenship and Feller 2010).
The present experiments were designed to test this assumption directly by isolating the factor of cross-modal experience from other activity-dependent factors in cortical maturation. This was accomplished using dark-reared animals that had not yet developed visual–auditory integration capability. Association cortex was deactivated (unilaterally) only during brief periods, once per week, and only when visual–auditory experience was possible. The multisensory capabilities of ipsilateral and contralateral SC neurons were then assessed after the accumulation of sufficient experience for the development of visual–auditory integration in similarly reared animals (Yu et al. 2010).
Materials and Methods
All protocols used here were in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition (NRC 2011) and were approved by the institutional Animal Care and Use Committee of Wake Forest Medical School, an AAALAC-accredited institution. Two adult male cats reared in the dark from birth were used for these experiments. The results from these animals were indistinguishable and were collapsed for purposes of analysis.
Surgical Preparation
Aseptic surgical techniques for implanting deactivation coils were similar to those previously described (Jiang et al. 2001; Alvarado et al. 2007). Animals were pretreated with dexamethasone sodium phosphate (1 mg/kg, i.m.) 12 h prior to the surgery. On the day of surgery, the animal was sedated with ketamine hydrochloride (20 mg/kg, i.m.) and acepromazine maleate (0.1 mg/kg, i.m.) in the dark room, masked, and transported to the surgical suite in a light-tight carrier. It was then intubated and placed in the stereotaxic head-holder. Anesthesia for surgery was maintained with isoflurane (1.5–3%), the animal was artificially respired, and body temperature maintained at 37–38°C using a heating pad. Blood pressure, heart rate, CO2 level, and core temperature were monitored continuously (Digital Vital Signs Monitor, VetSpecs VSM7).
Craniotomy-Exposed AES/rLS Unilaterally
The overlying dura was reflected, and the sulcal walls in AES and rLS were gently opened to allow the insertion of cryogenic deactivation coils that were prefabricated from 21-gauge steel stainless tubing (inner diameter, 0.025 inch; outer diameter, 0.032 inch) and shaped to fit the sulci (Lomber et al. 1999). The area was covered with moist gelfoam, and the stems of the coils were housed within a protective stainless steel well with a removable cap. The stems and the well were secured to the skull with bone screws and cement. A second stainless steel recording well was secured to the skull over a craniotomy that gave access to both the ipsilateral and contralateral SC. Postsurgical analgesics were administrated as needed.
Exposure Procedure
The exposure procedure followed that previously found to be highly effective in developing multisensory integration capability in SC neurons of dark-reared animals (Yu et al. 2010). Animals were anesthetized with ketamine hydrochloride (20–30 mg/kg, i.m.) and acepromazine maleate (0.1 mg/kg, i.m.) in the dark room, masked, and transported to the experimental room in a light-attenuating carrier. The animal was intubated through the mouth, a holding plate was attached to the recording well, and the intubation tube was connected to the respirator for artificial respiration during paralysis (pancuronium bromide; 0.1 mg/kg, i.v.) induced to prevent eye and ear movement. During experimentation, anesthesia, paralysis, and hydration were maintained via continuous intravenous infusion of ketamine hydrochloride (6–8 mg/kg/h) and pancuronium bromide (0.05 mg/kg/h) in 5% dextrose Ringer's solution (3–6 mL/h) through the saphenous vein. Blood pressure, heart rate, SpO2, and respiratory CO2 level as well as core body temperature were monitored continuously (Digital Vital Signs Monitor, VetSpecs VSM7). End tidal CO2 was maintained at 4–5%. SpO2 was maintained at >90%. Body temperature was kept at 37–38°C using a heating pad. Pupils were dilated with ophthalmic atropine sulfate (1%), and the eyes were fitted with contact lens to prevent corneal drying and to focus the eyes.
Exposure sessions were conducted once per week, and each session consisted of multiple periods of cross-modal exposure. Within a session, the cross-modal exposure periods involved presenting a spatiotemporally congruent visual–auditory stimulus at 2-s intervals randomly at 1 of 5 locations (see Fig. 1), beginning 5 min after deactivation onset (see below). The visual stimulus component was a bar of light (25.4 cd/m2) moving upward for 200 ms. This was back-projected (LC 4445 Philips projector) onto a tangent screen 45 cm in front of the animal, with a uniform background (0.62 cd/m2). The auditory stimulus component was a broadband noise burst of 200 ms at 75 dB SPL against an ambient background of 48.4–52.7 dB SPL. There were a total of 750–1000 stimulus presentations (∼150–200 per location) per exposure period.
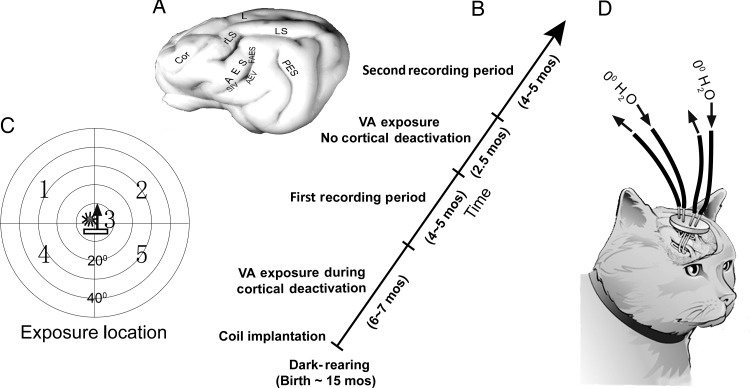
Figure 1.
Experiment design. (A) A schematic of the brain with AES (and rLS) identified and its visual (AEV), auditory (FAES), and somatosensory (SIV) subdivisions. (B) The diagonal timeline shows the periods in which sequential exposure and recording sessions took place. (C) The cross-modal (visual–auditory) exposure stimulus was presented randomly at 5 spatial locations shown on a diagram of visual–auditory (each circle = 10°). The icons at Position 3 depict the cross-modal component stimuli: a bar of light moved in the direction of the arrow, and a stationary noise burst. (D) Removable tubes at the ends of the deactivation coils were used to circulate refrigerated water to deactivate adjacent tissue during the initial cross-modal exposure period.
Cryogenic cortical deactivation was the method of choice here because the spatial and temporal pattern of its effectiveness in cat association cortex has been examined in detail, and its speed of action and reversibility, reliability, restricted extent, and impact on SC multisensory integration are ideally suited for these experiments (Jiang et al. 2001). Unilateral cryogenic cortical block of AES and rLS is induced within several minutes of circulating refrigerated (0°C) water through the implanted deactivation coils until the temperature of the adjacent tissue (within 2 mm) stabilizes at a level below that required for AES and rLS function. Robust cortical activity can be returned within minutes of active or passive rewarming (Jiang et al. 2001; Alvarado et al. 2007; Yu et al. 2013). Furthermore, its effectiveness in this context is such that even incomplete deactivation of the targeted area is sufficient to eliminate multisensory integration in SC neurons (Alvarado et al. 2009).
Each deactivation period was 40 min or less to avoid possible tissue damage. After each period, the deactivation coils were emptied and the cortex passively re-warmed for 10 min until reaching 37–38°. Soon thereafter, another deactivation period began. This was repeated 5–7 times/experiment to achieve the scheduled number of 1000 exposures/location/session. Upon completion of the exposure session the animal recovered and was masked and transported back to its home cage in the dark as described earlier.
Electrophysiological recordings were initiated after ∼26 cross-modal exposure sessions, when each neuron had reached the criterion of ∼26 000 exposure trials (some neurons had receptive fields encompassing more than 1 exposure site and thus had greater numbers of exposures, see Fig. 1, and Results). After completing this first phase of the study, a second phase of cross-modal exposures and recording sessions was initiated with the same animals in which there was no cortical deactivation during the exposure trials. Only 2 exposure sites contralateral to the previously deactivated cortex were engaged (Fig. 1).
Recording
Weekly recording sessions involved methods previously used for examining SC multisensory integration capabilities in normal and dark-reared animals (Yu et al. 2009; 2010). Anesthesia, paralysis, and vital signs monitoring were as above. The eye ipsilateral to the SC being examined was occluded with an opaque contact lens. Glass-coated parylene-insulated tungsten electrodes (impedance: 1–3 MΩ at 1 KHz) were lowered to the surface of the SC manually and then advanced via a hydraulic microdrive. Neural signals were recorded, amplified, and routed to an oscilloscope and audio monitor for online assessment and to a computer for data recording. They were evaluated by amplitude and wave shape to identify isolated individual neurons. At the end of an experiment, the animal recovered and was returned to its home cage in the dark (using the above transport methods) when stable respiration and coordinated locomotion were achieved.
Test Stimuli
Visual stimuli were moving light bars (size: 6° × 2° or 15° × 3°; intensity: 4.8–36.5 cd/m2) and flashing stationary spots (size: 4°; duration: 50 ms; intensity: 4.8–13.1 cd/m2). Auditory stimuli were 100-ms broadband noise bursts of 60–75 dB SPL, delivered by speakers mounted on a 25-cm hoop and separated by 15°. The hoop could be rotated around the head.
For each neuron, visual, and auditory receptive fields were mapped with the visual and auditory test stimuli and a test location was selected within a highly responsive area of its overlapping receptive fields in order to provide the highest probability of exposing multisensory integration (Kadunce et al. 2001). Then, visual and auditory test stimuli were presented individually and in spatiotemporally concordant combinations in pseudo-random order. To further minimize the possibility of erroneously concluding that a neuron was incapable of multisensory integration as a consequence of cortical deactivation, each neuron that failed to exhibit statistically significant response enhancement to the standard multisensory test battery was subjected to additional tests. These involved stimuli at multiple stimulus onset asynchronies and multiple locations within the area of receptive field overlap, and 3 different stimulus parameters (intensity, movement direction, bar lengths, or stationary flashed spots).
Data Analysis
Only multisensory neurons responding to visual and auditory stimuli individually were examined. Multisensory enhancement (ME) was quantified by percent difference between the response to the cross-modal stimulus and the response to the most effective modality-specific component stimulus. Statistical tests included the t-test and Mann–Whitney U, ANOVA, and chi-square tests as appropriate (alpha = 0.05). Summary data were expressed as mean ± standard error.
Results
Effect of Cortical Deactivation on the Development of Multisensory Integration
A total of 133 of 215 visual–auditory SC neurons met the study criteria (see Materials and Methods). Each had both its overlapping receptive fields encompassing at least 1 exposure site so that it received the minimum number of cross-modal exposure trials (n = 26 000) during unilateral deactivation of AES and rLS (Fig. 1). Eighty-six of these neurons were located in the SC ipsilateral and 47 in homotopic locations of the SC contralateral to the deactivated cortex. No differences were noted in the laminar location of these samples nor in their receptive field sizes (t-test, P = 0.81), or the vigor of their responses as measured by the mean number of impulses/trial to the visual (ipsilateral: 7.26 ± 5.53 vs. contralateral: 6.06 ± 5.59) and auditory (ipsilateral: 3.5 ± 3.62 vs. contralateral: 4.08 ± 3.15) test stimuli presented.
The minimum number of exposures provided to these neurons far exceeded that required for initiating the development of SC multisensory integration capabilities in similarly reared, albeit intact, animals and approached that needed to reach its normal adult-like incidence (Yu et al. 2010). Approximately half the neurons studied had receptive fields encompassing 2 exposure sites and thus received double the minimum number of exposures (ipsilateral: n = 44; contralateral, n = 22). Approximately one quarter of the neurons studied had receptive fields encompassing 3 exposure sites, thereby receiving triple the minimum number of exposures (ipsilateral, n = 22; contralateral, n = 12).
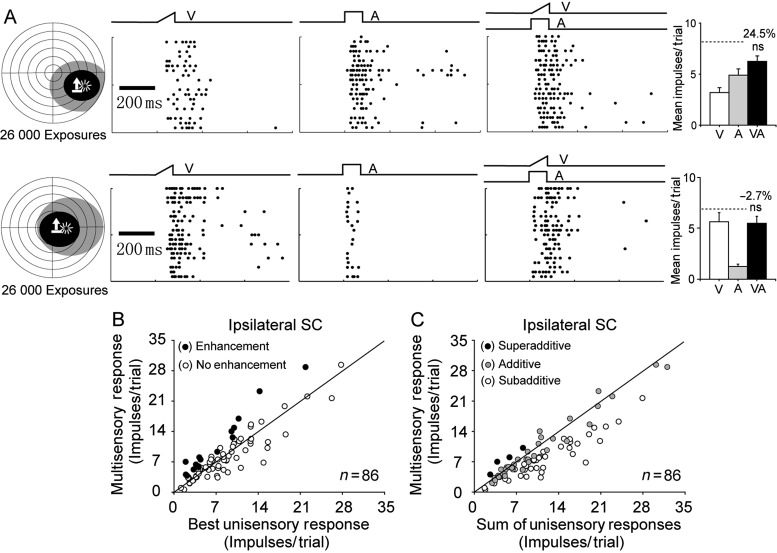
Despite the large number of cross-modal exposure trials during cortical deactivation, only 17% (15 of 86) of ipsilateral SC multisensory neurons exhibited multisensory integration capabilities; an incidence far below that expected based on data from the contralateral SC of these animals (see below) and on data from the SC of normal animals (Meredith and Stein 1986; Perrault et al. 2005; Alvarado et al. 2007; Pluta et al. 2011). There were no obvious differences between these integrating and non-integrating neurons. Their receptive field sizes overlapped one another, and they did not differ significantly in the robustness of their unisensory responses to either the visual or the auditory stimuli presented (non-integrating vs. integrating visual responses: visual: 7.51 ± 5.63 vs. 6.07 ± 5.04 impulses/trial, t-test, P = 0.36; auditory: 3.41 ± 3.60 vs. 3.93 ± 3.80 impulses/trial, t-test, P = 0.61). In addition, most (13 of 15) neurons showing multisensory integration capabilities were not encountered until at least 4 recording sessions had taken place, raising the possibility that they had been influenced by these previous testing procedures (Wallace et al. 2004; Yu et al. 2010) and/or reflected the limitations of the cryogenic blockade technique (Jiang et al. 2001). However, the efficacy of the integrative capabilities of these neurons, as measured by ME, was not significantly different from that of neurons in the contralateral SC (t-test, P = 0.36, also see below).They were, nevertheless, the exception to the rule: The vast majority (83%) of ipsilateral SC neurons responded no better to the combination of visual and auditory stimuli than to the most effective of them alone, and representative examples are provided in Figure 2A.
Figure 2.
Cortical deactivation blocked the experience-induced development of multisensory integration in ipsilateral SC neurons. (A) Two exemplar neurons after 26 000 cross-modal exposure trials at each of 5 sites during AES/rLS deactivation. Left: The visual (black) and auditory (gray) receptive fields of these neurons are shown, with icons identifying stimulus locations as described in Figure 1. Middle: Raster plots show each neuron's responses to these stimuli individually and in combination, with trials ordered from bottom to top. The ramps and square waves above rasters represent traces of the visual (V), auditory (A), and cross-modal (VA) stimuli. Right: Bar graphs summarize response (impulses) magnitudes (mean ± SEM) evoked by these stimuli. Percentages represent the ME, and the dashed line the sum of mean unisensory responses. Note that neither neuron's multisensory response was significantly greater than its most effective unisensory response (ns = not statistically significant). (B,C) Population comparisons in which each neuron's response to the cross-modal stimulus was plotted against its response to the most effective component stimulus in (B), and against the sum of visual and auditory responses in (C). Filled (open) circles represent neurons whose multisensory response was (was not) significantly enhanced above its highest unisensory response (B) or greater than the sum of visual and auditory responses (C). Note that the vast majority of neurons failed to exhibit multisensory integration.
Data for the entire ipsilateral population are summarized in Figure 2B,C. In these figures, each neuron's multisensory response is compared with the (unisensory) response elicited by the most effective component stimulus (Fig. 2B) and to the sum of the 2 unisensory responses (Fig. 2C). Each data point represents the mean response based on the most effective combination of test stimuli at the most effective receptive field location. Note that most points in Figure 2B fell near the line of equality, indicating that no multisensory integration was obtained. Thus, the average ME for the entire population was very low (8.2 ± 4.6%). These results indicated that the development of multisensory integration capabilities was severely compromised in SC neurons ipsilateral to the deactivated cortex.
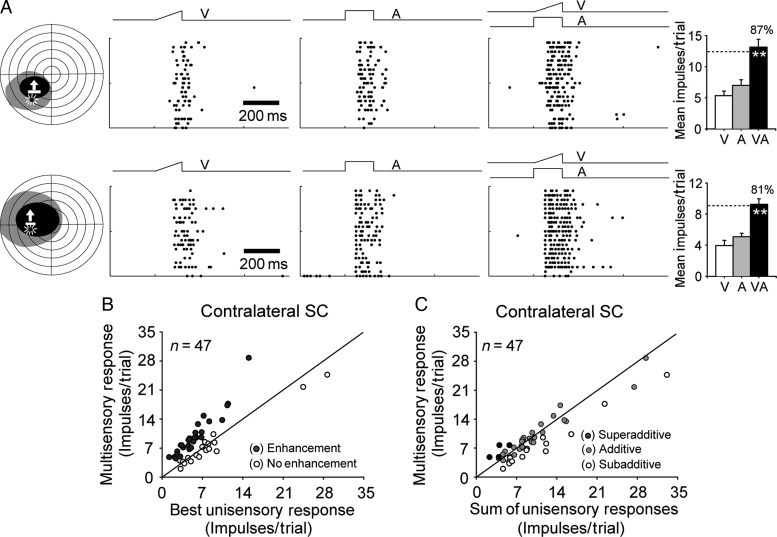
In contrast, sampling the contralateral SC within the same time frame revealed that it developed a substantial complement (60%, 28 of 47) of neurons capable of multisensory integration. Their incidence (chi-square tests, X2 = 22.77, P < 0.0001) and ME (47.9 ± 8.7% versus 8.2 ± 4.6%; t-test, P < 0.001) was significantly higher than it was in ipsilateral neurons and similar to that found in other dark-reared animals that were later provided with cross-modal exposure during adulthood (Yu et al. 2010). Two representative examples are illustrated in Figure 3A, one in which the overlapping receptive fields encompassed 1 exposure site (top) and one in which they encompassed 2 exposure sites (bottom). Both neurons had multisensory responses to the cross-modal stimulus that were significantly greater than those to the most effective of the modality-specific component stimuli, thereby meeting the criterion for multisensory integration. The population data are shown in Figure 3B,C. Apparently, deactivation of the contralateral cortex had little, if any, effect on the ability of SC neurons to develop the ability to integrate their cross-modal inputs.
Figure 3.
Cortical deactivation did not block the experience-induced development of multisensory integration in contralateral SC neurons. (A) Shown are raster plots and bar graphs summarizing the responses of 2 representative neurons having significantly enhanced responses to the cross-modal stimulus (multisensory integration). (B) Population comparisons of responses to the cross-modal and most effective component stimuli reveal that the majority of neurons developed multisensory integration (filled circles). (C) At these levels of effectiveness, most of the multisensory responses were additive, clustering around the line of equality representing the sum of their unisensory counterparts. **P < 0.001. Conventions are from Figure 2.
Effects of Subsequent Cross-modal Exposure on Ipsilateral SC Neurons
In the second experimental series, ipsilateral SC neurons were provided cross-modal exposure while the cortex was active. The cross-modal exposure in this case was provided at 2 selected sites. A total of 66 of 86 visual–auditory SC neurons met the criterion of having both receptive fields encompassing at least 1 of these exposure sites and 45 of them had receptive fields that encompassed both exposure sites.
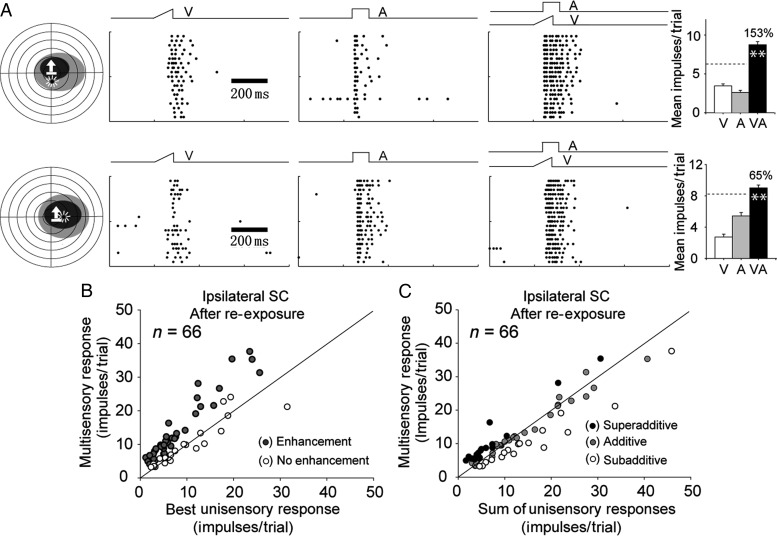
Now, a near-normal complement of neurons (65%, 43 of 66) exhibited the ability to integrate their cross-modal inputs. This finding was consistent with observations detailing the effectiveness of cross-modal exposure in dark-reared animals without concurrent cortical deactivation (Yu et al. 2010). Two representative examples are illustrated in Figure 4A. The one at the top had its receptive fields encompassing 1 exposure site, and the one at the bottom encompassed both exposure sites. Both neurons showed significantly enhanced multisensory responses. The incidence of multisensory integration and ME was similar, albeit slightly higher, in this sample than that observed in the contralateral SC after the initial exposure period (incidence: 65% vs. 60%; ME: 66.3 ± 8.5% vs. 47.9 ± 8.7%, see Fig. 4B). However, neither difference was statistically significant.
Figure 4.
Ipsilateral SC neurons developed multisensory integration capability after re-exposure to cross-modal stimuli when cortex was not deactivated. (A) Shown are the enhanced multisensory responses of 2 representative neurons. (B,C) Population comparisons in which each neuron's multisensory response magnitude was plotted against its corresponding best unisensory response and against the sum of its unisensory responses. Conventions are the same as in Figure 2 and Figure 3.
Discussion
The present study demonstrates that ipsilateral association cortex (i.e., AES and rLS) must be active during cross-modal experience for the development of SC multisensory integration. The training sessions in which visual–auditory experience was provided to dark-reared animals during cortical deactivation took place once per week and did not prohibit ipsilateral SC neurons from developing robust responses to the individual visual and auditory exposure stimuli. However, deactivation of association cortex did selectively disrupt the ability of these multisensory neurons to use such experience to craft the computational means required to synthesize these cross-modal inputs. The neural responses to the cross-modal combination after exposure were no greater than that generated by the most effective component stimulus individually. In contrast, neurons in the opposite SC, which could obtain the same cross-modal experience but whose inputs from their ipsilateral cortex were not deactivated, developed normal visual–auditory multisensory integration within this same time frame. It was not until ipsilateral association cortex was provided with cross-modal experience in the absence of deactivation that their SC target neurons developed multisensory integration capability. Apparently, association cortex serves as a portal through which cross-modal experience enhances the functional capability of this SC multisensory circuit and simultaneously adapts it to the environment in which it will be used (see also Xu, Yu, Rowland et al. 2012). Whether this is due to changes in the constituent AES-SC neurons themselves or in the local circuitry that they affect in the SC remains to be determined.
Yet, the importance of AES for mediating the effect of experience in the multisensory circuit is consistent with the predictions of recent models, in which the ability of SC neurons to integrate cross-modal cues is also dependent on specific, experience-based conformational changes in the AES-SC projection (Cuppini et al. 2011; 2012). In the model, cross-modal cues produce correlated activity in multisensory SC neurons and their unisensory afferents from cortex. Simple Hebbian learning rules operate on this correlated activity to change the synaptic weights of the cortical projections onto both the multisensory SC neurons and local inhibitory populations, which alters the functional circuit to increase the access of these descending inputs to their common multisensory SC target neuron. The findings also reveal that the deficits observed with long-term functional deactivation of cortex in early life noted by Rowland et al. (2014) could have resulted from blocking the cortex's access to relevant cross-modal relationships. Methodological differences between the present study (i.e., controlled cross-modal exposure) and the study by Rowland et al. (2014) (normal housing) may have contributed to the differing time courses for the acquisition of multisensory integration capability (weeks versus years). Alternatively, the impact of disrupting activity-dependent maturation during early life may explain the long time course in the study by Rowland et al. (2014) even though animals were being reared in a normal housing environment, rich in cross-modal stimuli.
In the present study, visual–auditory multisensory integration capability was acquired within a month of having only weekly exposure to visual–auditory stimuli, a time frame similar to that noted previously with similar training procedures after dark-rearing by Yu et al. (2010) and after noise-rearing by Xu, Yu, Stanford et al. (2012). This suggests another, albeit counterintuitive, possibility for the long-duration effect noted by Rowland et al. (2014): That the normal environment is a far less effective one for instantiating the multisensory integration capability explored here than is an impoverished laboratory environment in which access to cross-modal stimuli is limited to one such configuration. Whether this difference in acquisition speed extends to features of multisensory integration not explored here cannot be assessed. However, it appears reasonable to posit that the precise spatiotemporal concordance of the visual and auditory cues in all previous laboratory exposure studies, and the absence of any competing stimuli during those periods, may be more efficacious in this regard than a normal environment precisely because of the lack of stimulus ambiguity. In the normal environment, similar external cross-modal events often appear under very different conditions so that there is substantial variation in the relative intensities of the cross-modal cues, as well as their relative timing and spatial alignment. They also appear in the presence of many other competing stimuli. All of these factors are likely to increase the time required to learn cross-modal associations. If so, one would expect that the neonate would more rapidly develop multisensory integration capabilities if presented with invariant cross-modal stimuli in a controlled environment, than it does under normal rearing conditions. These possibilities remain to be explored.
It should be noted, however, that the present observations, which were made in mature animals reared in darkness to obviate early development of this process, are assumed to reflect the same mechanisms that are active during early maturation. Strong support for the assumption that influences from association cortex would play a primary role in the normal development of this SC capability is provided by the strong correlation between their time courses (Wallace and Stein 2000). But, it is also important to recognize that despite apparent equivalencies in their “naivete” with respect to visual-nonvisual events, the brain of the mature dark-reared animal is not equivalent to that of the neonate. Many developmental processes are altered by this sort of early sensory deprivation, and a good deal of opportunism occurs among the sensory afferents that compete for synaptic space during the period of rapid maturational change that characterizes early postnatal life (Rauschecker and Harris 1983; Rauschecker 1995; Finney et al. 2001; Lomber et al. 2010; Merabet and Pascual-Leone 2010).
Yet, these factors do not preclude development of the anatomical substrate for this process (convergent cortico-collicular afferents from the visual and auditory subdivisions of association cortex) in dark-reared animals. In the absence of cross-modal experience, this input to the SC enhances all sensory responses and does so nonselectively rather than selectively enhancing responses to cross-modal stimuli (Yu et al. 2013). That it retains the capability to achieve this selectivity well into adulthood reflects an inherent flexibility that appears to characterize many higher-order cortical circuits and in this case was able to do so despite whatever opportunistic changes had already taken place. The present results, coupled with prior observations (Bolognini et al. 2005; Yu et al. 2010; Xu, Yu, Rowland et al. 2012), suggest that such alterations can be induced by appropriate cross-modal experience throughout life even if there is some age-related decline that could not be assessed with the present tests.
Funding
This research was supported by NIH Grants EY016716 and NS036916 as well as funds from the Wallace Foundation, the Tab Williams Family Foundation, and the National Natural Science Foundation of China (Grant No. 31300910).
Notes
We thank Nancy London for technical assistance. Conflict of Interest: None declared.
References
- Alvarado JC, Stanford TR, Rowland BA, Vaughan JW, Stein BE. 2009. Multisensory integration in the superior colliculus requires synergy among corticocollicular inputs. J Neurosci. 29:6580–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JC, Stanford TR, Vaughan JW, Stein BE. 2007. Cortex mediates multisensory but not unisensory integration in superior colliculus. J Neurosci. 27:12775–12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. 2010. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 11:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Rasi F, Coccia M, Ladavas E. 2005. Visual search improvement in hemianopic patients after audio-visual stimulation. Brain. 128:2830–2842. [DOI] [PubMed] [Google Scholar]
- Carriere BN, Royal DW, Perrault TJ, Morrison SP, Vaughan JW, Stein BE, Wallace MT. 2007. Visual deprivation alters the development of cortical multisensory integration. J Neurophysiol. 98:2858–2867. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Shatz CJ. 1998. Activity-dependent cortical target selection by thalamic axons. Science. 281:559–562. [DOI] [PubMed] [Google Scholar]
- Cuppini C, Magosso E, Rowland BA, Stein BE, Ursino M. 2012. Hebbian mechanisms help explain development of multisensory integration in the superior colliculus: a neural network model. BiolCybern. 6:691–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppini C, Stein BE, Rowland BA, Magosso E, Ursino M. 2011. A computational study of multisensory maturation in the superior colliculus (SC). Exp Brain Res. 213:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney EM, Fine I, Dobkins KR. 2001. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 4:1171–1173. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Rowland BA, Stein BE. 2007. Multisensory orientation behavior is disrupted by neonatal cortical ablation. J Neurophysiol. 97:557–562. [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. 2006. Neonatal cortical ablation disrupts multisensory development in superior colliculus. J Neurophysiol. 95:1380–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Jiang H, Stein BE. 2002. Two corticotectal areas facilitate multisensory orientation behavior. J Cogn Neurosci. 14:1240–1255. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wallace MT, Jiang H, Vaughan JW, Stein BE. 2001. Two cortical areas mediate multisensory integration in superior colliculus neurons. J Neurophysiol. 85:506–522. [DOI] [PubMed] [Google Scholar]
- Kadunce DC, Vaughan JW, Wallace MT, Stein BE. 2001. The influence of visual and auditory receptive field organization on multisensory integration in the superior colliculus. Exp Brain Res. 139:303–310. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. 2006. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 29:414–418. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Meredith MA, Kral A. 2010. Cross-modal plasticity in specific auditory cortices underlies visual compensations in the deaf. Nat Neurosci. 13:1421–1427. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Horel JA. 1999. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods. 86:179–194. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. 2010. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 11:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. 1986. Spatial factors determine the activity of multisensory neurons in cat superior colliculus. Brain Res. 365:350–354. [DOI] [PubMed] [Google Scholar]
- Perrault TJ, Jr, Vaughan JW, Stein BE, Wallace MT. 2005. Superior colliculus neurons use distinct operational modes in the integration of multisensory stimuli. J Neurophysiol. 93:2575–2586. [DOI] [PubMed] [Google Scholar]
- Pluta SR, Rowland BA, Stanford TR, Stein BE. 2011. Alterations to multisensory and unisensory integration by stimulus competition. J Neurophysiol. 106:3091–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. 1995. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 18:36–43. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Harris LR. 1983. Auditory compensation of the effects of visual deprivation in the cat's superior colliculus. Exp Brain Res. 50:69–83. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Jiang W, Stein BE. 2014. Brief cortical deactivation early in life has long-lasting effects on multisensory behavior. J Neurosci. 34:7198–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE. 2012. The New Handbook of Multisensory Processing. Cambridge: (MA): MIT Press. [Google Scholar]
- Stein BE, Meredith MA. 1993. The Merging of the Senses. Cambridge: (MA): MIT Press. [Google Scholar]
- Stein BE, Stanford TR. 2008. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 9:255–266. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Perrault TJ, Jr, Hairston WD, Stein BE. 2004. Visual experience is necessary for the development of multisensory integration. J Neurosci. 24:9580–9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. 1997. Development of multisensory neurons and multisensory integration in cat superior colliculus. J Neurosci. 17:2429–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Stein BE. 2000. Onset of cross-modal synthesis in the neonatal superior colliculus is gated by the development of cortical influences. J Neurophysiol. 83:3578–3582. [DOI] [PubMed] [Google Scholar]
- Xu J, Yu L, Rowland BA, Stanford TR, Stein BE. 2012. Incorporating cross-modal statistics in the development and maintenance of multisensory integration. J Neurosci. 32:2287–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yu L, Stanford TR, Rowland BA, Stein BE. 2012. Independent, but otherwise normal, experiences with auditory and visual information is not sufficient for the maturation of multisensory integration capabilities. In: 2012 Abstract Viewer/Itinerary Planner, Program No 36921/KK8 New Orleans, LA Society for Neuroscience. [Google Scholar]
- Yu L, Rowland BA, Stein BE. 2010. Initiating the development of multisensory integration by manipulating sensory experience. J Neurosci. 30:4904–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Stein BE, Rowland BA. 2009. Adult plasticity in multisensory neurons: short-term experience-dependent changes in the superior colliculus. J Neurosci. 29:15910–15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Xu J, Rowland BA, Stein BE. 2013. Development of cortical influences on superior colliculus multisensory neurons: effects of dark-rearing. Eur J Neurosci. 37:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Sur M. 1999. Development and plasticity of the cerebral cortex: from molecules to maps. J Neurobiol. 41:1–6. [DOI] [PubMed] [Google Scholar]