Abstract
Episodic memories are established and maintained by close interplay between hippocampus and other cortical regions, but degradation of a fronto-striatal network has been suggested to be a driving force of memory decline in aging. We wanted to directly address how changes in hippocampal-cortical versus striatal-cortical networks over time impact episodic memory with age. We followed 119 healthy participants (20–83 years) for 3.5 years with repeated tests of episodic verbal memory and magnetic resonance imaging for quantification of functional and structural connectivity and regional brain atrophy. While hippocampal-cortical functional connectivity predicted memory change in young, changes in cortico-striatal functional connectivity were related to change in recall in older adults. Within each age group, effects of functional and structural connectivity were anatomically closely aligned. Interestingly, the relationship between functional connectivity and memory was strongest in the age ranges where the rate of reduction of the relevant brain structure was lowest, implying selective impacts of the different brain events on memory. Together, these findings suggest a partly sequential and partly simultaneous model of brain events underlying cognitive changes in aging, where different functional and structural events are more or less important in various time windows, dismissing a simple uni-factorial view on neurocognitive aging.
Keywords: aging, longitudinal, memory, resting-state functional connectivity, structural connectivity
Introduction
After the discovery of the critical role of the hippocampus for episodic memory of >50 years ago (Scoville and Milner 1957), it has been clear that episodic memories are established and maintained by repeated replays through a hippocampal-cortical dialog (Buzsaki 1996; Moscovitch et al. 2006; Vilberg and Davachi 2013). Hence, lesions and atrophy to the hippocampus, as seen in, for example, age-related neurodegenerative conditions such as Alzheimer's disease (AD), cause dramatic reductions in memory performance. Episodic memory function tends to decline also in normal aging, related to volumetric reductions of the hippocampus and other medial temporal lobe structures (Rodrigue and Raz 2004; Murphy et al. 2010; Nyberg et al. 2012; Persson et al. 2012; Fjell et al. 2014). However, due to substantial atrophy also in prefrontal cortex in healthy aging (Raz et al. 2005; Fjell, Walhovd et al. 2009), with accompanying reductions of specific cognitive functions (Rabbitt et al. 2001), it has been suggested that decline in efficiency of fronto-striatal networks may be responsible for memory decline in aging (Buckner 2004; Head et al. 2005). The striatum and hippocampus are conventionally viewed as complementary learning and memory systems (DeCoteau et al. 2007), with extensive evidence indicating an important role for especially the dorsal or “associative” striatum (Parent and Hazrati 1995), for example, caudate and putamen, for learning and memory (Packard and Knowlton 2002; Helie et al. 2015). Impact on memory from striatum may be indirect, through is critical involvement in executive functioning (Ward et al. 2013; Leunissen et al. 2014; Niemann et al. 2014; Rae et al. 2015), or by affection of memory processes directly. Contributions from hippocampal and striatal networks to memory function have been partly disentangled, with fronto-striatal functional connectivity (FC), especially with the caudate, playing an important role during learning of different kinds of information, from acquisition of single associations to generalized, categorical representations (Helie et al. 2010; Antzoulatos and Miller 2014), and hippocampal-cortical FC being critical for memory consolidation (Vilberg and Davachi 2013). Hence, we wanted to directly address how changes in functional and structural connectivity of hippocampal-cortical versus striatal-cortical networks over time impact episodic learning and memory in aging.
FC is a promising metric for investigating memory decline in aging, as lower FC of large-scale brain systems in older adults has been related to declining cognitive function, including memory ([Andrews-Hanna et al. 2007; Wang et al. 2010; He et al. 2012; Onoda et al. 2012; Mevel et al. 2013; Geerligs et al. 2014], but see [Ystad et al. 2010; Adamczuk et al. 2016] for reports of inverse relationships). In accordance with the classical dedifferentiation theory of aging (Lindenberger and Baltes 1994), it has also been suggested that higher intranetwork connections, for example, efficiency of communications within networks, and lower internetwork connections, reflecting specificity and selectivity of the network, may be positively related to cognitive function in aging (Salami et al. 2012; Spreng and Schacter 2012; Antonenko and Floel 2014). However, higher functional segregation has also been negatively related to memory in aging (Sala-Llonch et al. 2014), and a recent study found elevated FC between left and right hippocampus to be related to declining memory over 20 years (Salami et al. 2014). So far, most studies of FC–memory relationships have been cross-sectional. A longitudinal design allows investigating whether individual changes in FC over time can be responsible for the commonly observed decline in episodic memory from ∼60 years (Nyberg et al. 2012). This is especially interesting in a multimodal perspective, taking into account changes both in functional and structural connectivity, as well as brain atrophy. In line with this, 1 rare longitudinal study found that resting-state FC change over 6 years within the default mode network (DMN) correlated with memory change in middle-aged and older adults, independently of gray matter atrophy (Persson et al. 2014).
Targeting FC change in hippocampal-cortical and fronto-striatal networks, we aimed to test to what extent FC could explain change in episodic memory function measured longitudinally, and the relationship to structural connectivity changes and atrophy. Specifically, we tested the following main hypotheses:
H1: Fronto-striatal FC change will be more strongly related to memory change in older adults than in young, in accordance with the theory of a partly frontal basis for episodic memory decline in aging (Buckner 2004; Head et al. 2005).
H2: Hippocampal-based functional networks will be more strongly related to long-term recall than to learning, with the opposite pattern for striatal-based networks.
H3: Structural and FC changes will be related to memory change partly independently of ongoing atrophy, as previous cross-sectional studies have shown FC to be at least partly independent of brain volumetric measures (Damoiseaux et al. 2008; Mowinckel et al. 2012; Onoda et al. 2012).
Materials and Methods
Sample
The longitudinal sample was drawn from the ongoing project “Cognition and Plasticity through the Lifespan” (Storsve et al. 2014; Walhovd et al. 2014) at the Research Group for Lifespan Changes in Brain and Cognition (LCBC), Department of Psychology, University of Oslo. All procedures were approved by the Regional Ethical Committee of Southern Norway (REK-Sør), and written consent was obtained from all participants. For the first wave of data collection, participants were recruited through newspaper ads. Recruitment for the second wave was by written invitation to the original participants. At both time points, participants were screened with a health interview. Participants were required to be right handed, fluent Norwegian speakers, and have normal or corrected to normal vision and hearing. At both time points, exclusion criteria were history of injury or disease known to affect central nervous system (CNS) function, including neurological or psychiatric illness or serious head trauma, being under psychiatric treatment, use of psychoactive drugs known to affect CNS functioning, and MRI contraindications. Moreover, participants were required to score ≥26 on the Mini Mental State Examination (MMSE) (Folstein et al. 1975), have a Beck Depression Inventory (BDI; Beck and Steer 1987) score of ≤16, and obtain a normal IQ or above (IQ ≥ 85) on the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1999). At both time points, all scans were evaluated by a neuroradiologist and were required to be deemed free of significant injuries or conditions. At follow-up, an additional set of inclusion criteria was employed: MMSE change from time point 1 to time point 2 <10%; California Verbal Learning Test II—Alternative Version (CVLT II; Delis et al. 2000) immediate and long delay T-score >30; CVLT II immediate and long delay change from time point 1 to time point 2 <60%.
Two hundred and eighty-one participants completed Tp1 assessment. For the follow-up study, 42 opted out, 18 could not be located, 3 did not participate due to health reasons (the nature of these were not disclosed), and 3 had MRI contraindications, yielding a total of 66 dropouts (35 females, mean [SD] age = 47.3 [20.0] years). Independent samples t-tests revealed that dropouts had significantly lower FSIQ (t = −3.92, P < 0.001) and BDI (t = −2.02, P = 0.046) scores but comparable CVLT and MMSE scores (P > 0.05). More detailed dropout characteristics are published elsewhere (Storsve et al. 2014). Of the 215 participants that completed MRI and neuropsychological testing at both time points, 8 failed to meet 1 or more of the additional inclusion criteria for the follow-up study described earlier, 4 did not have adequately processed diffusion MRI data, and 2 were outliers (4 or more tracts showing change values of >6 SD from mean). This resulted in a follow-up sample of 201 participants (118 females) aged 20–84 years at Tp1 (Storsve et al. 2014; Walhovd et al. 2014). Of these, rsBOLD was not acquired for the first 81, yielding a complete sample of 120 with quality-checked cognitive and functional and anatomical MRI data for both time points. Tp1 rsBOLD data were lacking for all participants between 52 and 63 years, and we therefore formed 2 age groups: a young group of 23–52 years and a group of older adults of 63–86 years. One participant lacked complete memory testing at Tp2 due to technical problems, and only 117 had usable DTI scans. Further, in rare cases, Tracula would fail in identifying a tract for a given person. This was the case for one participant where right SLFP and for one where right Uncinate was not identified by Tracula. Since mean value across hemispheres were used for statistical analyses, left hemisphere values were used for these to avoid excluding them from the sample. Sample descriptives are provided in Table 1.
Table 1.
Sample descriptives
| Young | Older adults | Significance | |
|---|---|---|---|
| n | 64 | 56a | |
| Age | 32.9 (23–52) | 71.6 (63–86) | * |
| Sex (females/males) | 40/24 | 29/27 | |
| Education | 15.9 (12–23) | 16.5 (8–26) | |
| IQ | 119 (101–133) | 120 (90–146) | |
| MMSE | 29.6 (27–30) | 29.0 (26–30) | * |
| Follow-up interval | 3.4 (2.7–4.0) | 3.1 (2.8–3.8) | * |
Age, IQ, and MMSE values from Tp2, education from Tp1. Mean (range) values are provided.
aOne participant lacked valid memory scores.
*Difference between age groups is significant (P < 0.05).
Memory Testing
CVLT II was used to assess memory at Tp1 and CVLT II—Alternative Version (Delis et al. 2000) at Tp2. A list of 16 concrete words was read 5 times consecutively, and each time, the participants were immediately instructed to recall as many of the items as possible. After these 5 trials, another 16-item list was read, with instructions of immediate recall, whereupon the participants were asked to recall the first list again (immediate, 5 min, recall). This was followed by a cued recall test. After a 30-min delay, the participants were asked, without having been forewarned, to recall this list again. Variables of interest were learning (sum of correctly recalled words from trial 1 to 5), immediate (5 min) recall, and delayed recall (30 min). As these measures are derived from the same test, correlations between them were expected. A more powerful approach could be to include multiple tests of the same underlying psychological construct, but longitudinal data from similar tests besides CVLT were unfortunately not available.
MRI Acquisition
Imaging data were collected using a 12-channel head coil on a 1.5T Siemens Avanto scanner (Siemens Medical Solutions) at Rikshospitalet, Oslo University Hospital. The same scanner and sequences were used at both time points.
For morphometry
The pulse sequence used for morphometric analyses included 2 repetitions of a 160 slices, sagittal T1-weighted magnetization prepared rapid gradient echo sequences with the following parameters: repetition time(TR)/echo time(TE)/time to inversion(TI)/flip angle(FA) = 2400 ms/3.61 ms/1000 ms/8°, matrix = 192 × 192, field of view (FOV) = 240, voxel size = 1.25 × 1.25 × 1.20 mm, scan time 4 min 42 s.
For FC
The resting-state BOLD sequence included 28 transversally oriented slices (no gap), measured using a BOLD-sensitive T2*-weighted EPI sequence (TR = 3000 ms, TE = 70 ms, FA = 90°, voxel size = 3.44 × 3.44 × 4 mm, FOV = 220, descending acquisition, GRAPPA acceleration factor = 2), producing 100 volumes and lasting for ∼5 min. Three dummy volumes were collected at the start to avoid T1 saturation effects.
For structural connectivity
Diffusion-weighted MRI (dMRI) was performed using a single-shot twice-refocused spin-echo echo planar imaging pulse sequence optimized to minimize eddy current-induced distortions (Reese et al. 2003) (primary slice direction, axial; phase encoding direction, columns; repetition time, 8200 ms; echo time, 82 ms; voxel size, 2.0 mm isotropic; number of slices, 64; FOV, 256; matric size, 128 × 128 × 64; b-value, 700 s/mm2; number of diffusion encoding gradients directions, 30; number of b = 0 images, 10; number of acquisitions, 2). Acquisition time was 11 min 21 s.
MRI Analysis
Image processing and analyses of FC and atrophy were performed at the Neuroimaging Analysis Laboratory, Research Group for LCBC, Department of Psychology, University of Oslo, whereas DTI analyses were run at the Martinos Center for Biomedical Imaging, Harvard Medical School, Boston.
Morphometry
Morphometric analyses were performed by the use of FreeSurfer v. 5.1 (http://surfer.nmr.mgh.harvard.edu/) (Dale et al. 1999; Fischl et al. 1999, 2002; Fischl and Dale 2000), please see a detailed account elsewhere (Storsve et al. 2014; Walhovd et al. 2014). In short, hippocampus, caudate, and putamen were segmented through automatically assigning a neuroanatomical label to each voxel in an MRI volume based on probabilistic information automatically estimated from a manually labeled training set (Fischl et al. 2002). The training set included both healthy persons in the age range of 18–87 years and a group of Alzheimer's disease patients in the age range 60–87 years, and the classification technique employs a registration procedure that is robust to anatomical variability, including the ventricular enlargement typically associated with aging. The technique has previously been shown to be comparable in accuracy to manual labeling (Fischl et al. 2002; Fischl, Salat et al. 2004). An atlas-based normalization procedure was also used, which has been shown to increase the robustness and accuracy of the segmentations across scanner platforms (Smith et al. 2007). All volumes were inspected for accuracy, and minor manual edits were performed when needed by a trained operator on the baseline images, usually restricted to removal of nonbrain tissue included within the cortical boundary. The cross-sectionally processed images were subsequently run through the longitudinal stream in FreeSurfer (Reuter et al. 2012), with high sensitivity and robustness as well as inverse consistency (Reuter et al. 2010; Reuter and Fischl 2011). In addition, probabilistic methods (temporal fusion) were applied to further reduce the variability across time points. This yielded estimates of atrophy between time points for the hippocampus, caudate, and putamen.
Functional Connectivity
Resting-state functional imaging data were preprocessed following LCBC's custom analysis stream. Images were motion corrected, slice timing corrected, and smoothed (5-mm FWHM) in volume space using FSL's FMRI Expert Analysis Tool (FEAT; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Then, FSL's Multivariate Exploratory Linear Optimized Decomposition into Independent Components was used in combination with FMRIB's ICA-based Xnoiseifier (FIX) to auto-classify independent components into signal and noise components and remove the noise components from the 4D fMRI data (Salimi-Khorshidi et al. 2014). A prerequisite for FIX classification is a hand-labeled training set of typical signal and noise components. After manually inspecting and validating that it fitted to our data, we used the default classification template provided with the FIX-toolbox. Freesurfer-defined individually estimated anatomical masks of cerebral white matter (WM) and cerebrospinal fluid/lateral ventricles (CSF) were resampled to each individual's functional space. All anatomical voxels that “constituted” a functional voxel had to be labeled as WM or CSF for that functional voxel to be considered a functional representation of noncortical tissue. Average time series were then extracted from functional WM- and CSF-voxels and were regressed out of the FIX-cleaned 4D volume together with a set of estimated motion parameters (rotation/translation) and their derivatives. Following recent recommendations about noise removal from resting-state data (Hallquist et al. 2013), we also band-pass filtered the data (0.009–0.08 Hz) after regression of confound variables. In-scanner head motion may substantially impact measures of FC (Satterthwaite et al. 2012; Van Dijk et al. 2012), with the risk of causing spurious correlations, especially when comparing groups of participants where differences in head movement may exist. Thus, in addition to regressing out estimated motion parameters from the time series before they were entered into further analyses, and band-pass filtering the data according to current recommendations, motion was also included as a covariate in all statistical analyses (see Statistical analyses).
FC between hippocampus, caudate, and the putamen was calculated as the correlation between the average time series of all voxels within each structure and every voxel in the ipsilateral cerebral hemispheres, each correlation being variance-stabilized using the Fisher z-transformation (Silver and Dunlap 1987), yielding 3 FC maps for each participant for each hemisphere. FC analyses were done ipsilaterally, so that FC from right hippocampus and right striatum was calculated for the right hemisphere only, and vice versa for the left hemisphere. Contralateral rsFC was also calculated, and presented in Supplemental material.
Structural connectivity
For dMRI analyses, TRActs Constrained by UnderLying Anatomy (TRACULA), part of FreeSurfer v.5.3 were to delineate major WM tracts of interest (Yendiki et al. 2011). This is a novel algorithm for automated global probabilistic tractography that estimates the posterior probability of each pathway given the diffusion-weighted MRI data. The posterior probability is decomposed into a data likelihood term, which uses the “ball-and-stick” model of diffusion (Behrens et al. 2007), and a pathway prior term, which incorporates prior anatomical knowledge on the pathways from an independent set of 33 training subjects included in the tract atlas that is used by TRACULA, described in Yendiki et al. (2011). The segmentation labels required by TRACULA were obtained by processing the T1-weighted images of the study subjects with the automated cortical parcellation and subcortical segmentation tools in FreeSurfer (Fischl et al. 2002; Fischl, Salat et al. 2004; Fischl, van der Kouwe et al. 2004). The pathways reconstructed for the present study were selected due to their possible importance for memory processing, for example, the uncinate fasciculus (UNC), cingulum–cingulate gyrus bundle, cingulum–angular bundle, and the superior longitudinal fasciculus-temporal terminations, but we believe the literature so far is too scarce to make specific hypothesis about possible differential roles for these tracts in memory. A note of caution should be made: Although we use the term structural connectivity as an umbrella concept to describe the different diffusion properties, we observe in the tracts, a caveat that is always present in diffusion MRI studies is that structural connectivity measures derived from water diffusion are indirect measurements of axonal bundles. Thus, we would like to remind the reader that this is a simplification.
We also used a novel approach to estimate motion during the dMRI scans. Since the contrast in dMRI arises from microscopic random motion of water, it is particularly sensitive to macroscopic head motion during acquisition, which can lead to observation of spurious group differences if proper care is not taken (Yendiki et al. 2013). To quantify head motion in each dMRI scan, we derived volume-by-volume translation and rotation from the affine registration between volumes that are commonly used to reduce misalignment between images due to head motion and eddy currents. In addition, we quantified slice-by-slice signal drop-out specific to dMRI (Benner et al. 2011). This way, we estimated slower, between-volume motion captured by the registration-based measures, as well as more rapid, within-volume motion, captured by the intensity-based measures (see Yendiki et al. (2013) for details). A sum variable expressing total motion during dMRI was included as covariate in all analyses (see below).
Statistical Analyses
The same analyses were done for both time points, and longitudinal changes in brain measures derived from the 3 modalities (FC, morphometry, and dMRI parameters) were quantified as the difference of each measure between time points. For all analyses, sex, movement at both time points, and interval between scans were included as covariates of no interest. For analyses of FC, motion parameters during BOLD scanning were included, summarized as the mean relative displacement estimated by FSL's MCFLIRT, were included for both time points. For analyses of structural connectivity, motion parameters during dMRI scanning were additionally included, calculated as described earlier. In analyses where age group was not a variable of interest, age was also included as covariate. Since sex can affect change in episodic memory function (Josefsson et al. 2012) and profoundly impact brain volume (Fjell, Westlye et al. 2009), sex was covaried out from all analyses.
Statistical analyses on the cortical surface were run with general linear models (GLMs) implemented in FreeSurfer. All results were tested against an empirical null distribution of maximum cluster size across 10 000 iterations using Z Monte Carlo simulations as implemented in FreeSurfer (Hayasaka and Nichols 2003; Hagler et al. 2006) synthesized with a cluster-forming threshold of P < 0.05 (two-sided), yielding clusters corrected for multiple comparisons across the surface. Effects of age on the relationship between FC change and memory change were tested by comparing the slopes for the FC–memory relationships between age groups. These analyses were conducted to address Hypothesis 1—that fronto-striatal FC change would be more strongly related to memory change in older adults than in young—and Hypothesis 2—that hippocampal-based functional networks would be more strongly related to long-term recall than to learning, with the opposite pattern for striatal-based networks.
The relationship between rsFC change and dMRI change was tested by partial correlations, and the critical P-value corrected for number of comparisons with Sidak's correction by a factor of 16 (4 [tract-of-interest {TOI}], 2 dMRI metrics [FA and MD], analyses performed in 2 age groups [young and elderly]), adjusted for the correlations between the dependent variables (http://www.quantitativeskills.com/ available from: URLsisa/calculations/bonfer.htm.). For multimodal analyses, multiple regression analyses or partial correlation analyses were run. For multimodal analyses, stepwise regressions were run to decide whether each modality yielded unique contributions in explaining memory change. Memory change was the dependent variable, and in the first step, FC was entered along with all nuisance variables. In the next step, SC change from a TOI was added, and the change in coefficients inspected. In a third step, atrophy was added as a third variable of interest, and the coefficients again inspected. The rational for these analyses was to test whether the relationships between FC and memory change could be explained by concurrent change in diffusion characteristics or atrophy. In accordance with Hypothesis 3, we expected that SC and FC would be related to memory change partly independently of ongoing atrophy. Thus, FC was calculated between regions that already showed significant relationships to memory change in the initial analyses. The FC–memory relationships tested in these regression analyses must not be regarded as independent of the initial analyses, and the results must be interpreted as tests of whether rsFC–memory relationships can occur independently of structural degeneration.
Age effects on brain change—memory change relationships were tested by introducing an age × brain measure interaction term as an additional covariate. Memory change was calculated as Tp2 score/Tp1 score.
Results
Age Effects on Memory
There were strong cross-sectional relationships between age and memory scores at each time point (Tp) (partial r, controlling for sex: learning −0.63/ −0.57 [Tp1/Tp2]; 5-min recall −0.61/ −0.49; 30-min recall −0.60/−0.44, all P < 0.001). A quadratic age term (age × age) was not significant, and the linear model was therefore not rejected. Accordingly, age at Tp1 did not correlate significantly with change in any of the 3 memory scores, but a tendency for relatively worse performance over time with increasing age was seen for 30-min recall (r = −0.17, P = 0.062, co-varying out sex and follow-up interval). Change in learning was more closely related to change in 5-min recall (r = 0.70) than to change in 30-min recall (r = 0.54, difference between correlations Z = 3.00, P = 0.003), whereas change in 5-min recall was not significantly more related to either (correlation between change in 5 and change in 30-min recall r = 0.67). We also tested whether memory performance changed from baseline to follow up by one-sample t-tests. In the young group, significant increases were found for all measures: learning 4.2%, 5-min recall 9.1%, and 30-min recall 5.2% (all P < 0.05). In the older group, no significant change was found.
Cortico-subcortical Functional Connectivity Patterns
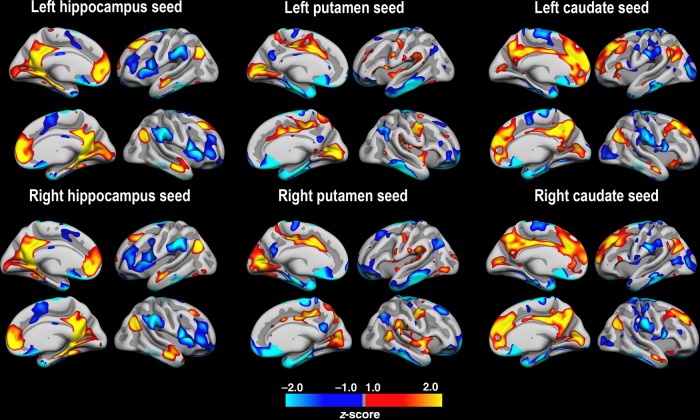
Functional connectivity between each of the subcortical structures and the cerebral cortex is shown in Figure 1, averaged across time points. As massive significantly positive relationships existed across almost the entire brain surface, the statistical maps were z-transformed and thresholded at 1 < z < −1. Effects were largely corresponding across hemispheres. To highlight degree of overlap between cortical connectivity profiles of the 3 structures, a right hemisphere conjunction map of all 3 seed regions was created (Fig. 2). Please note that no formal statistical testing was done for these analyses. Hippocampal connectivity, but not caudate or putamen connectivity, was seen in the entorhinal and the parahippocampal cortex, as well as in the inferior temporal lobe and the fusiform gyrus. Caudate connectivity, but not hippocampal or putamen connectivity, was seen in regions of the superior frontal cortex. Putamen connectivity, but not hippocampal or caudate connectivity, was seen mainly in superior temporal gyrus, the posterior-most part of the middle temporal gyrus and the insula. Caudate and the hippocampus showed several regions of overlapping connectivity, and these coincided with the DMN, for example, medially in posterior cingulate, medial orbitofrontal cortex extending into the anterior cingulate and the superior frontal cortex, and laterally in the temporo-parietal junction and the anterior part of the middle temporal cortex. Putamen showed very limited overlap in connectivity patterns with the hippocampus and the caudate. Thus, each subcortical seed region showed a distinct pattern of cortical FC, with seemingly more overlap between hippocampus and the caudate than between putamen and any of the 2 others.
Figure 1.
Cortico-subcortical FC patterns. Resting-state FC (FC) between the 3 seed structures and the cerebral cortex. FC was computed as the mean of the 2 time points. Massive positive relationships were observed, so the maps were z-transformed to allow inspections of regions of relatively higher (red-yellow) versus lower (blue-cyan) FC.
Figure 2.
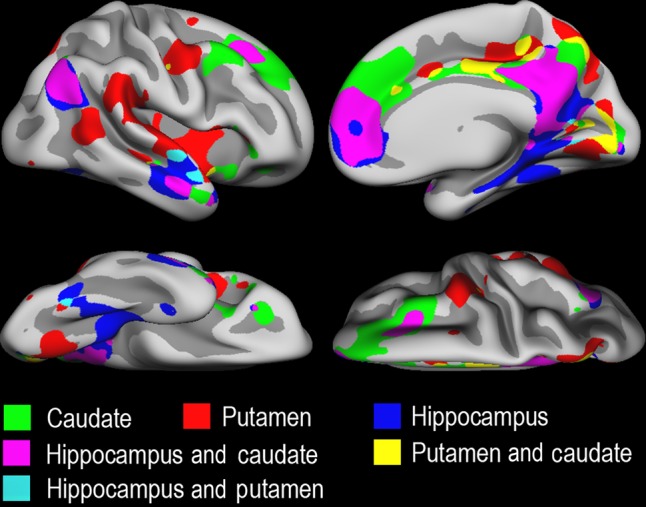
Overlapping cortico-subcortical FC patterns. A conjunction map was created to illustrate degree of overlapping versus unique FC patterns for each seed structure, for each seed structure from Figure 1 thresholded at z ≥ 1.
Effects of Age on Change on Cortico-subcortical Functional Connectivity
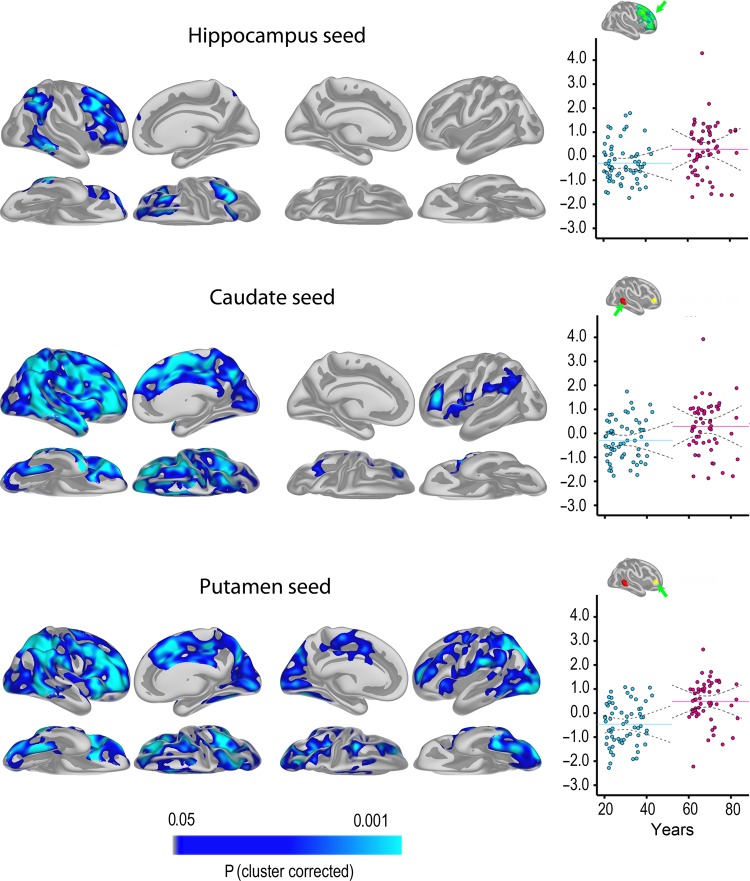
GLMs were run to test whether change in FC varied as a function of age. Results are shown in Figure 3 (see Supplemental information for contralateral results).
Figure 3.
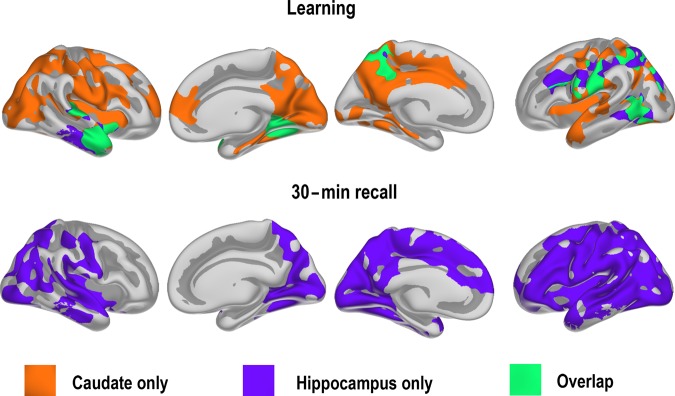
Age–change interactions .The maps show effects of age group on FC change. Blue-cyan indicates more negative change in the group of younger versus older participants, corrected for multiple comparisons by Monte Carlo simulations. The scatterplots illustrate mean difference in FC (z-transformed correlations) for each participant from selected regions shown in green (hippocampus), red (caudate), or yellow (putamen). The regions were selected to be representative of the effects shown in the surface maps. Mean and 95% confidence interval of the mean are shown as solid and dotted lines, respectively.
Across the cortico-subcortical networks, the young group showed reduced FC, whereas the older group showed an increase. For hippocampus, FC change, for example, difference in FC between time points, was significantly different between the 2 age groups in the right hemisphere, especially on the lateral surface, encompassing posterior parts of the temporal lobe, the temporo-parietal junction, and a large portion of the prefrontal cortex, including both inferior, middle, and superior frontal gyri and regions of in the inferior and superior parietal cortex. Somewhat weaker effects of age on FC change were seen in the left hemisphere (P < 0.05), but these did not survive corrections (see Supplemental Information). The differences in FC change from Tp1 to Tp2 between the 2 age groups in striatal-cortical connectivity were considerably more widespread, covering most of the lateral surface and a major part of the medial in the right hemisphere, with more limited effects in the left, but no formal statistical tests were performed to decide whether these hemispheric differences were statistically significant. One interesting difference between the caudate and the putamen effect was that change in FC between putamen and the anterior part of the lateral temporal cortex did not vary with age, in contrast to what was observed for the caudate.
As can be seen from the scatterplots for selected ROIs, there is 1 outlier in the older group. Thus, contrasts between age groups in FC change were re-run excluding values exceeding z = 3. Significant differences between groups in FC change were confirmed for all ROIs by independent samples t-tests.
Due to the strongly age-dependent change rates in FC, all subsequent analyses were run for each group separately.
FC and Memory Function
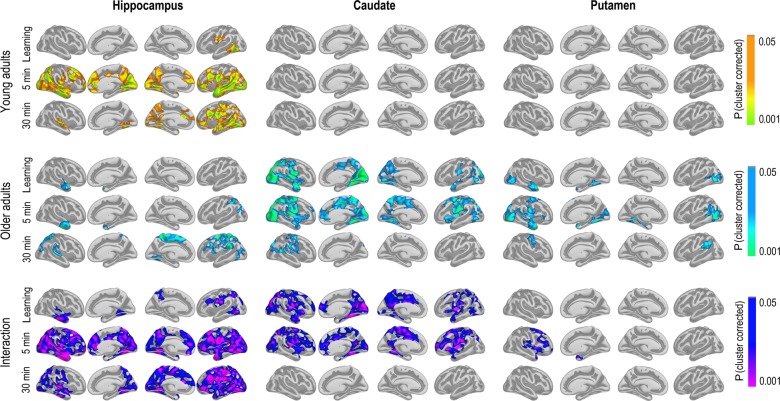
GLMs were run to test the relationship between change in learning, 5-min recall and 30-min recall and change in cortico-subcortical FC. We first tested for age interactions in FC—memory relationships, for example, regions where change in FC was differently related to memory change across age groups. The results are shown in Figure 4 (bottom panel). We found widespread interactions, caused by reduced FC being associated with relatively better memory performance in the young adults (top panel), and some more limited relationships between increased FC associated with relatively better memory performance in the older adults (middle panel). For the hippocampus, this pattern of effects was seen in large areas of the cortex for recall after 5 min and 30 min, both on the lateral and the medial surface. For 5-min recall, effects were overlapping with, but not restricted to, hub regions of the DMN both laterally and medially. For 30-min recall, the effects were most widespread in the left hemisphere, with effects in right being most pronounced in lateral temporal as well as posterior cortical regions. Effects were also found for learning, including in the lateral temporal cortex in both hemispheres, and around the precuneus and lateral parietal and frontal cortex in the left, but these were substantially less extensive. Scatterplots are shown in Figure 5 (see Supplemental information for contralateral results).
Figure 4.
Maps of the significant relationships between FC change and memory change. The 3 main columns represent the hippocampus, caudate, and putamen as seeds, respectively. The 3 main rows represent the young group, the older group, and the age interactions, for example, the effect of age on the relationship between FC change and memory change. Within each main row, each line represents learning, 5-min recall, and 30-min recall, respectively. The results were corrected for multiple comparisons by Monte Carlo simulations.
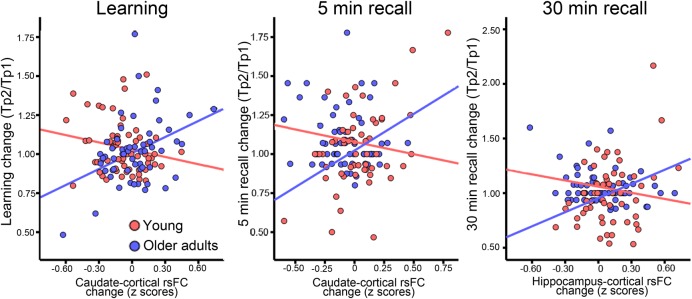
Figure 5.
rsFC–memory relationships. Scatterplots illustrating the relationship between change in memory scores between time points and change in rsFC. The rsFC data are extracted from the significant age-interaction analyses for the right hemisphere showed in Figure 4. Left panel: caudate-cortical rsFC change versus learning change. Middle panel: caudate-cortical rsFC change versus 5-min recall. Right panel: hippocampal-cortical rsFC change versus 30-min recall change.
For caudate, age interactions were found for learning and 5-min recall exclusively. These effects covered the MTL extending superiorly toward the retrosplenial cortex/posterior cingulate in both hemispheres, but also parts of the middle or frontal cingulum and posterior sections of the superior frontal gyrus. Widespread effects were also seen on the lateral surface, both in the temporal, frontal, parietal, and occipital cortex. These age interactions were caused by significant FC–memory relationships found exclusively in the group of older adults.
For putamen, age effects were found only in the right hemisphere 5-min recall condition, most widely distributed on the lateral surface. These were caused by significant effects in the older group only.
To highlight the differential contributions from hippocampus and caudate FC change, a conjunction figure was created based on the age-interaction maps for the right hemisphere in Figure 4. Figure 6 shows that age affected the effect of caudate FC change on learning across large regions of the cortex, overlapping with effects of hippocampal FC change in minor regions only. In contrast, for 30-min recall, age affected the hippocampal FC effect on recall only. Thus, caudate FC change and hippocampal FC change seemed to impact different aspects of memory function across age groups.
Figure 6.
Unique and common patterns of FC–memory change relationships. Conjunction maps of the significant right hemisphere age interactions in Figure 4. The figure shows that age affected the effect of caudate FC change on learning across large regions of the cortex, overlapping with effects of hippocampal FC change in minor regions only. In contrast, for 30-min recall, age affected the hippocampal FC effect on recall only. Thus, caudate FC change and hippocampal FC change impacted different aspects of memory function across age groups.
Multimodal Analyses
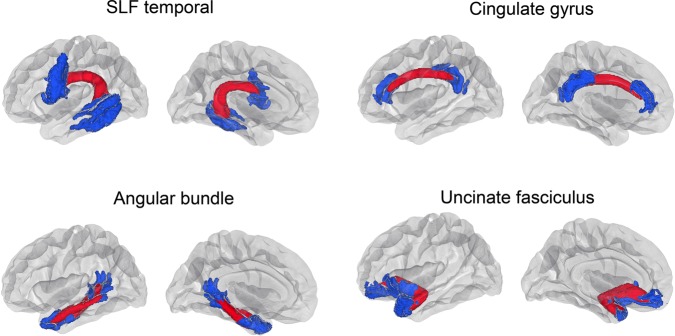
Having established that changes in FC are associated with changes in memory function, we conducted a set of post hoc multimodal analyses to test effects of atrophy and structural connectivity change on the above-identified FC–memory relationship. These analyses focused on the hippocampus and caudate, since these showed the most extensive relationship to memory. For the multimodal analyses, change in hippocampal—cortical FC and caudate—cortical FC was extracted for all vertices that showed a significant age effect on 5-min recall in the right hemisphere. This was chosen as the basis for extracting data due to the robust relationship observed in the initial analyses, and it would thus yield a strong test if atrophy or structural connectivity change could explain this relationship. Atrophy was calculated for hippocampus and caudate (mean of right and left hemisphere), since the effects of age on the FC—memory change relationships were strongest for these structures. In addition, change in structural connectivity in selected tracts was calculated. The cingulate angular bundle, superior longitudinal fasciculus temporal part, and the UNC were chosen as tracts of interests (TOIs) because they connect the medial temporal lobe to other cortical regions, thus being potentially important for memory, and the cingulum–cingulate bundle because it connects the precuneus and posterior cingulate to the prefrontal cortex, which constitute hub regions critical for different aspects of memory (Buckner and Carroll 2007; Andrews-Hanna et al. 2010) (see Fig. 6 for delineation of the tracts). Fornix would have been another possible TOI but is not segmented in Tracula. Other tools for tractography exist that could be used to segment the fornix. In this study, however, we preferred to use the longitudinal stream of TRACULA, since this is specifically designed to reconstruct tracts from longitudinal diffusion data by taking advantage of a subject's data from all time points jointly. This is a rather unique approach, designed to ensure point-to-point correspondence between the tracts reconstructed at different time points, while eliminating any bias toward any single time point. Changes in the fractional anisotropy (FA) and the mean diffusivity (MD) between time points, averaged across right and left hemisphere tracts, were used in the analyses (Figure 7).
Figure 7.
Structural connectivity tracts of interest. Four relevant white matter tracts were delineated by the use of TRACULA: superior longitudinal fasciculus temporal part (SLF temporal), cingulate gyrus, angular bundle, and uncinated fasciculus. Tract endings were projected into the cortex (blue) for visualization purposes, whereas the delinated tracts in the white matter are shown in red.
First, FC was correlated with MD and FA change in the 4 TOIs by partial correlations. The critical α-level after corrections for number of comparisons by Sidak's correction was set to 0.0094, similar to P < 0.05 uncorrected and a median correlation between dependent variables of r = 0.39. In the young group, a relationship between hippocampal—cortical FC change and FA change in the uncinate was identified (r = −0.37, P = 0.007). This means that more FA decrease was associated with less FC decrease. In the older adult group, a relationship was observed between caudate FC change and MD change in the cingulate bundle (r = 0.38, P = 0.009), meaning that more increase in MD was associated with more increase in FC. Uncinate FA was therefore included in hippocampal analyses, whereas cingulate MD was included in caudate analyses.
Similar analyses were done for hippocampal and caudate atrophy, defined as difference in volume between time points. No relationships between atrophy and FC approached significance in either age group. Larger reductions of hippocampal volume from Tp1 to Tp2 were observed in the older adults compared with the younger, and the opposite pattern was seen for the caudate (both P < 0.05). This means that the effect of FC on memory seems to be strongest in the time periods when volumetric reductions are the lowest, as illustrated in Figure 8. In essence, changes in caudate-cortical FC are related to changes in memory in elderly and caudate volume change relatively little among elderly. Similarly, changes in hippocampal-cortical FC are related to changes in memory in young and hippocampal volume change relatively little among young.
Figure 8.
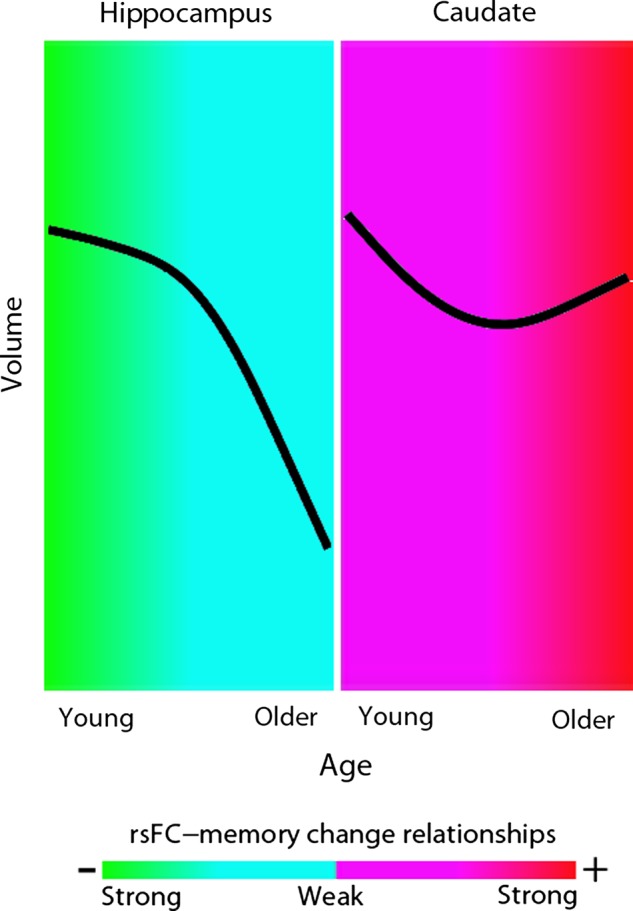
Timing of influence of FC on memory change. The figure illustrates that the relationship between FC change and memory is strongest in the time periods where rate of change of a given structure typically is the lowest. Imposed on the color scale depicting the strength of the relationship between FC change and memory change, the black lines show the typical age trajectory of the volume of the hippocampus (left) and the caudate (right). The curves were obtained from a nonparametric smoothing spline fit based on cross-sectional MR scans from 1100 healthy participants, published in Fjell et al. (2013). This general pattern of change was confirmed in the present longitudinal data.
To test whether the observed relationships between change in memory and change in FC could be explained by changes in tract integrity, multiple regressions were run with 5-min recall as dependent variable and FC change as predictor. These analyses were followed up by similar analyses where changes in MD and/or FA in significant TOIs were included as additional covariates. 5-min recall was chosen as the dependent variable because the most extensive age effects on the FC–memory relationship were seen in this condition. The analyses were first run for hippocampal-cortical FC change. In the first model, hippocampal FC change was significantly related to 5-min recall (young: standardized partial β = −0.40, P = 0.002/older adults: standardized partial β = 0.31, P = 0.024), which was already given since the ROIs were based on the previous surface analyses. Adding FA change in the uncinate as an additional covariate had negligible effects on the contributions from FC change (young: standardized partial β = −0.40, P = 0.005/older adults: standardized partial β = 0.32, P = 0.029), whereas uncinate FA did not contribute significantly in either age group. Adding hippocampal atrophy and both FA and MD change in all 4 TOIs as additional covariates in a further expanded model still yielded significant contributions from FC change (young: standardized partial β = −0.37, P = 0.009/older adults: standardized partial β = 0.41, P = 0.019). For the young group, MD change in the uncinate also contributed to explain 5-min recall (standardized partial β = −0.36, P = 0.04), meaning that relatively less decrease in MD was associated with more favorable recall outcome. No other variable of interest explained unique variance. To test whether the inclusion of the structural connectivity and atrophy variables could explain the effects of age on the relationship between FC change and memory change, an interaction term of the Z-transformed values of age × FC change was added as an additional covariate. This term was highly significant (standardized partial β = −0.37, P < 0.001), showing unique age effects on the relationship between FC and memory, independently of ongoing atrophy and structural connectivity changes.
A similar multiple regression strategy was used for change in caudate-cortical FC. The first model confirmed the contribution from FC change to 5-min recall change for the older group from the surface analyses (standardized partial β = 0.50, P < 0.0005), with only a trend for the young group (standardized partial β = −0.23, P = 0.084). In the next model, MD change in the cingulate bundle was added as an additional covariate. In the young group, neither FC nor MD change contributed uniquely, whereas in the older group, both did (FC: standardized partial β = 0.59, P < 0.0001/MD: standardized partial β = −0.30, P = 0.026), meaning that relative increase in FC and relative reduction of MD was associated with better recall outcome. Adding caudate atrophy and both FA and MD change in all 4 TOIs as additional covariates in the further expanded model rendered the cingulum MD change no longer significant, whereas FC still contributed uniquely (standardized partial β = 0.60, P < 0.0005). Similar to the hippocampus-cortical connectivity analyses, an interaction term of the Z-transformed values of age × FC change was added and was found to be highly significant (standardized partial β = 0.32, P < 0.001).
Finally, a stepwise multiple regression analysis with 5-min recall change as dependent variable was run. In the first block, all the covariates used in all analyses were entered (age, movement at both time points during BOLD and dMRI scanning, interval, and sex). In the next block, FC changes for both hippocampus cortex and caudate cortex were entered, along with FA and MD change in the 4 TOIs and atrophy of hippocampus and caudate, in a stepwise pattern (P < 0.05 for inclusion, P ≥ 0.10 for removal). For the young group, hippocampal-cortical FC (standardized partial β = −0.42, P < 0.001) and MD change in the angular bundle explained unique variance in memory (standardized partial β = −0.28, P < 0.05). In the group of older adults, caudate-cortical FC (standardized partial β = 0.64, P < 0.00005) and MD change in the cingulate explained unique variance in memory (standardized partial β = −0.33, P < 0.05).
Discussion
The present study demonstrated that cortico-subcortical FC change was related to change in memory function, with effects of SC anatomically closely aligned. While hippocampal-cortical FC changes impacted recall performance for both younger and older adults, striatal-cortical FC affected memory in the group of older only. This is in line with a view of episodic memory deficits in normal aging as caused partly by disturbance of a striatal-prefrontal network. Multimodal analyses further showed that caudate functional and cingulum SC changes were related to recall performance among the older adults, whereas hippocampal functional and angular SC were predictive in the younger. The FC–memory relationships were in opposite directions across age groups, but SC–memory relationships were in the same and expected directions regardless of age—less increase in MD was associated with more favorable memory outcome. Interestingly, FC–memory relationships were strongest when the specific subcortical structure showed the least volumetric reductions, suggesting a complex and age-varying pattern of influences on memory function throughout adult life from connectivity and atrophy. This can be interpreted within a partly sequential and partly simultaneous model of brain events underlying cognitive changes in aging, with influences from different functional and structural events varying in strength over time. According to a sequential model, brain events can be ordered in time, with some being initiated before others, such as in the popular dynamic biomarker model of AD (Jack et al. 2010, 2013). Although not necessarily in conflict with the sequential model, a simultaneous model will put more focus on brain events ongoing in parallel (Fjell et al. 2013; Jagust 2013).
Discussions of the Main Hypotheses
Changes in FC and memory were related in an age-dependent way. In one longitudinal study of DMN FC in middle-aged and older adults, no net change was observed between time points, but increased FC still predicted better memory outcome (Persson et al. 2014). The direction of this relationship was similar to the present findings in the older group. Several cross-sectional studies have also indicated a positive relationship between FC of large-scale brain systems and cognitive function, including memory ([Andrews-Hanna et al. 2007; Wang et al. 2010; He et al. 2012; Onoda et al. 2012; Mevel et al. 2013; Geerligs et al. 2014; Ward et al. 2015], but see [Ystad et al. 2010]), but the direction of effects has still been shown to differ as a function of network and network properties (Salami et al. 2012; Spreng and Schacter 2012; Antonenko and Floel 2014; Sala-Llonch et al. 2014).
We hypothesized that fronto-striatal FC change would be more important for memory change in older adults than in young and that hippocampal-based functional networks would be more related to long-term recall than to learning, with the opposite pattern for striatal-based networks. Interestingly, there were distinct anatomical patterns of connectivity–memory change relationships for young versus elderly. As exemplified in Figure 6, age interactions were prominent for caudate connectivity in the learning condition and for hippocampus connectivity in the 30-min recall condition. This was caused by the strongest relationships being observed between hippocampus FC and recall (5 and 30 min) for the young, and between caudate FC and learning and 5-min recall for the older. Supported by the correlations between the different memory scores, a rough partial distinction can be made between cognitive processes important for learning and processes important for long-term storage. The 5-min recall condition depends equally on both types, while learning and 30-min recall to a lesser degree depend on the same processes.
The impact of caudate FC on learning scores in the older adults can be interpreted within a view of episodic memory decline in aging as partly caused by decline in fronto-striatal circuits critical for executive functions (Alexander et al. 1990; Buckner 2004; Head et al. 2005). We and others have previously demonstrated the relevance of MTL atrophy for decline of long-term memory in normal aging (Rodrigue and Raz 2004; Murphy et al. 2010; Nyberg et al. 2012; Persson et al. 2012; Fjell et al. 2014). The present findings suggest that changes in striatal-cortical connectivity may be more important in the initial phases of learning and short-term consolidation, with change in hippocampal-cortical connectivity being more relevant for long-term consolidation. This is interesting, as previous studies have shown increased FC between prefrontal cortex and striatum during category learning (Antzoulatos and Miller 2014) and perirhinal-hippocampal connectivity as a marker for long-term memory consolidation (Vilberg and Davachi 2013). It seems that these phenomena apply to changes over time in specific memory functions. The importance of hippocampal-cortical FC change for somewhat longer-term consolidation was even more evident in the young group than the older, where hippocampal FC was related to recall whereas striatal FC was not related to any aspect of memory function. Previous volumetric studies have suggested that hippocampus is relatively preserved well into middle age, before decline accelerates (Walhovd et al. 2005; Fjell et al. 2013). In contrast, caudate has been found to decline the most in the first part of adult life, with only minor volume reductions or even increases seen after 60 years (Walhovd et al. 2005; Fjell et al. 2013). This pattern was confirmed in the present data, with timing of volume decrease versus FC–memory relationships illustrated in Figure 8. In light of this, it may seem like the influence of FC from each of these structures is largest in the time periods where volume is the most stable.
Multimodal Results
FC changes impacted cognitive function independently of atrophy and reduced structural connectivity, in line with our hypothesis and some previous cross-sectional studies that corrected for gray matter volume (Damoiseaux et al. 2008; Mowinckel et al. 2012; Onoda et al. 2012). This result could also be expected based on the depicted patterns in Figure 8, where effects of FC on memory function were highest when rates of change were the lowest. Interestingly, structural and FC both contributed uniquely to explain change in memory function, yielding a more elaborated picture of brain events underlying memory change. For the young group, hippocampal-cortical FC change along with MD change in the angular bundle explained unique variance in memory. For the older adults, caudate-cortical FC and MD change in the cingulum explained unique variance. This pattern of effects aligned nicely with the basic connectivity profiles of hippocampus and caudate in Figures 1 and 2 and the age-dependent effects of FC on memory. The caudate showed high FC along almost the complete cingulum in the older group. Although hippocampus also showed FC with posterior and anterior cingulum regions, strong unique hippocampal FC was seen along the parahippocampal and entorhinal cortex, aligning closely with the angular bundle running through the MTL and connecting its posterior and anterior parts. Thus, the structural and functional effects on memory yielded anatomically high correspondence within each age group but still were mostly independent in explaining memory change. Thus, there was unique information in structural connectivity measures that was not captured by FC. In line with this, Salami et al. (2014) found elevated rsFC in aging to be related to memory decline and partly accounted for by age-related decline in WM diffusion properties. In the present study, the direct correlation between changes in each measure was harder to interpret, as positive FA change was associated with negative FC change in the young group and the opposite pattern was found for MD in the old group. In general, structural connectivity is sparser than FC, and 2 regions that do not have a direct structural connection may be connected functionally through a third region. Thus, as a specific structural connection deteriorates, more regions could be recruited to perform a functional task as a means of compensation. Therefore, one cannot always expect a straightforward relationship between structural and FC.
Relationships between FC Change and Age
Changes in FC varied as a function of age, evident across extended regions of the cortex. No apparent differences between regions belonging to “task positive” versus “task negative” networks or intra- versus inter-network effects were observed. We observed a tendency for FC to increase over time in the older group. Highly variable results have been reported in previous literature, with for instance one very large study finding positive age correlations in somatosensory and subcortical networks and negative age correlations with FC density in other networks (Tomasi and Volkow 2012). Other studies have found higher FC in selected networks, including DMN, in elderly with subjective memory complaints compared with age-matched controls (Hafkemeijer et al. 2013), genetic risk for sporadic AD (Filippini et al. 2009; Westlye et al. 2011), Aβ accumulation (Lim et al. 2014; Adamczuk et al. 2016), and AD diagnosis (Zhang et al. 2010; Agosta et al. 2012), although reduced FC is a more common finding in elderly at risk for neurodegenerative conditions, as reviewed in Sheline and Raichle (2013).
In several studies finding higher FC in the risk groups, FC is still positively related to cognitive function (Agosta et al. 2012; Lim et al. 2014; Adamczuk et al. 2016). Increased FC could thus be interpreted as a compensatory response to reduced brain integrity, for example, due to the accelerated reductions in WM integrity (Sexton et al. 2014) or hippocampal volume (Walhovd et al. 2005; Fjell, Walhovd et al. 2009), which are typically seen from ∼60 years of age. However, the compensation account is challenging, since episodic memory function typically declines after 60 years (Josefsson et al. 2012; Nyberg et al. 2012), which is difficult to reconcile with the pattern of increased FC in this age range, which at the same time is associated with better memory outcome. The positive relationship between FC and memory also renders the alternative account of disinhibition or dedifferentiation in aging less likely. In a previous simulation study, we found that increased FC in elderly could be observed in a substantial proportion of the simulations, even with no assumption of increased FC included in the model and replication of age-dependent FC–memory relationships (Fjell et al. unpublished data). With the conflicting results from cross-sectional studies and the almost complete lack of longitudinal investigations, it is pivotal that more efforts are devoted to test how FC changes over time in individuals at different ages and in different populations. Despite this, the observation of a positive relationship between FC and preservation of memory within this critical age period is reasonable and in coherence with another longitudinal FC aging study (Persson et al. 2014). Importantly, this observation is not dependent on the direction of the net change in group mean connectivity among the older adults. The FC–memory relationship can be interpreted within the frames of the brain maintenance theory (Nyberg et al. 2012) without making any assumptions about compensatory processes. The finding of reduced FC in the young group likely has complex causes but could conceivably in part be caused by ongoing maturation, at least among the youngest participants. WM development is ongoing until 30 years or more (Westlye et al. 2010; Walhovd et al. 2011; Grydeland et al. 2013; Sexton et al. 2014), with the FC changes reflecting such maturational processes.
Limitations
The present follow-up interval was a little more than 3 years. Due to the paucity of longitudinal rsFC studies, it is not known whether this is enough to measure reliable changes in FC, especially in young and healthy participants. Previous studies have shown that structural connectivity (Sexton et al. 2014) and atrophy (Storsve et al. 2014) seem to be reliably tracked over this time interval, and even shorter in elderly participants (Fjell, Walhovd et al. 2009). However, more longitudinal studies are needed to establish the optimal follow-up time for rsFC. A related issue is the practice effect commonly observed in longitudinal memory studies. In the present study, the young participants showed increased memory scores over time whereas the older adults did not show significant change in any direction. It is likely that the actual score at follow-up reflects initial ability, and time-related reduction and a practice effect. Thus, no change could indicate a reduction in memory function. Also, there are individual differences in change in rsFC and memory between time points which likely have significance, even though not seen as net change in memory at a group level. This is the same reasoning used by Persson et al. (2014), where FC did not show longitudinal change at a group level, but increased FC still predicted better memory outcome. In any case, the lack of reduction in memory function on a group level complicates the interpretation of the relationships between memory change and connectivity in the present data, and it is possible that a longer follow-up interval would yield a less complex picture.
In the present study, we were interested in testing specific hypotheses about changes in medial temporal versus striatal networks and their implications for memory function since these have been hypothesized to be fundamental in major theories of cognitive aging. Thus, we chose to examine these networks by a direct seed-based approach, targeting the structures of interest specifically. The rsFC profile of the hippocampus in the present study overlapped with the DMN, including medial prefrontal and posterior parietal cortex, temporo-parietal junction/angular gyrus and anterior lateral temporal cortex, although regions also outside DMN were included. The other networks did not correspond closely to any of the previously described “canonical” networks. An alternative approach would thus be to extract previously established “canonical” networks where strong FC has been demonstrated previously (Smith et al. 2009; Yeo et al. 2011), and test to what degree changes in these would have implications for memory function.
Summary and Conclusions
In summary, this longitudinal study demonstrated that changes in functional and structural connectivity are both related to memory outcome over 3 years and that each class of measure explains unique variance. The finding that FC has the most explanatory power in the time periods when the rate of volumetric reductions is lowest for the specific seed structure suggests a sequential model of brain events underlying cognitive changes, where different events explain more or less variance in various time windows. While hippocampal-cortical FC as hypothesized was important for long-term recall and especially for the young adult, the results also supported the fronto-striatal hypothesis of aging, especially related to learning and short-term recall. The increased FC over time in the older adults was surprising and, on the backdrop of a general lack of longitudinal studies combined with conflicting results from cross-sectional studies, demonstrated that more efforts are needed to understand how FC changes over time at different ages and in different populations. The current results suggest interesting venues for further research, and much work remains before firm conclusions can be reached about the combined relationship between structural and function connectivity and atrophy in explaining age-related deficits in episodic memory.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org.
Funding
This work was supported by the Department of Psychology, University of Oslo (A.B.S., K.B.W. and A.M.F.), the Norwegian Research Council (to K.B.W. and A.M.F.), the European Research Council's Starting Grant scheme (ERC grant agreement 313440 to K.B.W. and 283634 to A.M.F.), the US-Norway Fulbright Foundation (to A.B.S.), the National Institute for Biomedical Imaging and Bioengineering (R00-EB008129, R01-EB006758) (to A.Y.), and the National Institutes of Health Blueprint for Neuroscience Research (5U01-MH093765) (to A.Y.), part of the multi-institutional Human Connectome Project. Additional resources were provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health (to A.Y.). This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grants S10RR023401, S10RR019307, S10RR019254, S10RR023043.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Adamczuk K, De Weer A-S, Nelissen N, Dupont P, Sunaert S, Bettens K, Sleegers K, Van Broeckhoven C, Van Laere K, Vandenberghe R. 2016. Functional changes in the language network in response to increased amyloid β deposition in cognitively intact older adults. Cereb Cortex. 26:358–373. [DOI] [PubMed] [Google Scholar]
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. 2012. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging. 33:1564–1578. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. 1990. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 85:119–146. [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. 2010. Functional-anatomic fractionation of the brain's default network. Neuron. 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. 2007. Disruption of large-scale brain systems in advanced aging. Neuron. 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D, Floel A. 2014. Healthy aging by staying selectively connected: a mini-review. Gerontology. 60:3–9. [DOI] [PubMed] [Google Scholar]
- Antzoulatos EG, Miller EK. 2014. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 83:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer R. 1987. Beck Depression Inventory Scoring Manual. New York: The Psychological Corporation. [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. 2007. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner T, van der Kouwe AJ, Sorensen AG. 2011. Diffusion imaging with prospective motion correction and reacquisition. Magn Reson Med. 66:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. 2004. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 44:195–208. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. 2007. Self-projection and the brain. Trends Cogn Sci. 11:49–57. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. 1996. The hippocampo-neocortical dialogue. Cereb Cortex. 6:81–92. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. 2008. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. 2007. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci USA. 104:5644–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. 2000. California Verbal Learning Test - Second Edition (CVLT - II). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. 2004. Sequence-independent segmentation of magnetic resonance images. NeuroImage 23 Suppl. 1:S69–S84. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. 2004. Automatically parcellating the human cerebral cortex. Cereb Cortex. 14:11–22. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. 2009. One-year brain atrophy evident in healthy aging. J Neurosci. 29:15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. 2009. Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer's disease. J Neurosci. 29:8774–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB, for the Alzheimer Disease Neuroimaging I. 2014. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex. 24:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB, et al. 2013. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging. 34:2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. ÄúMini-mental state, Äù: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 12:189–198. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, Lorist MM. 2014. Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp. 35:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. 2013. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci. 33:18618–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, Altmann-Schneider I, Oleksik AM, van de Wiel L, Middelkoop HA, van Buchem MA, van der Grond J, Rombouts SA. 2013. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect. 3:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr., Saygin AP, Sereno MI. 2006. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage. 33:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. 2013. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. 2003. Validating cluster size inference: random field and permutation methods. NeuroImage. 20:2343–2356. [DOI] [PubMed] [Google Scholar]
- He J, Carmichael O, Fletcher E, Singh B, Iosif AM, Martinez O, Reed B, Yonelinas A, Decarli C. 2012. Influence of functional connectivity and structural MRI measures on episodic memory. Neurobiol Aging. 33:2612–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. 2005. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cereb Cortex. 15:732–739. [DOI] [PubMed] [Google Scholar]
- Helie S, Ell SW, Ashby FG. 2015. Learning robust cortico-cortical associations with the basal ganglia: an integrative review. Cortex A J Devot Study Nervous Syst Behav 64C:123–135. [DOI] [PubMed] [Google Scholar]
- Helie S, Roeder JL, Ashby FG. 2010. Evidence for cortical automaticity in rule-based categorization. J Neurosci. 30:14225–14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, et al. 2013. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. 2010. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. 2013. Vulnerable neural systems and the borderland of brain aging and neurodegeneration. Neuron. 77:219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson M, de Luna X, Pudas S, Nilsson LG, Nyberg L. 2012. Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. J Am Geriatrics Soci. 60:2308–2312. [DOI] [PubMed] [Google Scholar]
- Leunissen I, Coxon JP, Caeyenberghs K, Michiels K, Sunaert S, Swinnen SP. 2014. Subcortical volume analysis in traumatic brain injury: the importance of the fronto-striato-thalamic circuit in task switching. Cortex J Devot Study Nervous Syst Behav. 51:67–81. [DOI] [PubMed] [Google Scholar]
- Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, et al. 2014. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease. Brain. 137:221–231. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. 1994. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 9:339–355. [DOI] [PubMed] [Google Scholar]
- Mevel K, Landeau B, Fouquet M, La Joie R, Villain N, Mezenge F, Perrotin A, Eustache F, Desgranges B, Chetelat G. 2013. Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiol Aging. 34:1292–1301. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. 2006. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 16:179–190. [DOI] [PubMed] [Google Scholar]
- Mowinckel AM, Espeseth T, Westlye LT. 2012. Network-specific effects of age and in-scanner subject motion: a resting-state fMRI study of 238 healthy adults. NeuroImage. 63:1364–1373. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Holland D, Donohue M, McEvoy LK, Hagler DJ, Jr., Dale AM, Brewer JB. 2010. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. NeuroImage. 53:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C, Godde B, Staudinger UM, Voelcker-Rehage C. 2014. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience 281C:147–163. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lovden M, Riklund K, Lindenberger U, Backman L. 2012. Memory aging and brain maintenance. Trends Cogn Sci. 16:292–305. [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S. 2012. Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci. 24:2186–2198. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. 2002. Learning and memory functions of the Basal Ganglia. Ann Rev Neurosci. 25:563–593. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. 1995. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 20:91–127. [DOI] [PubMed] [Google Scholar]
- Persson J, Pudas S, Lind J, Kauppi K, Nilsson LG, Nyberg L. 2012. Longitudinal structure-function correlates in elderly reveal MTL dysfunction with cognitive decline. Cereb Cortex. 22:2297–2304. [DOI] [PubMed] [Google Scholar]
- Persson J, Pudas S, Nilsson L-G, Nyberg L. 2014. Longitudinal assessment of default-mode brain function in aging. Neurobiol Aging. 35:2107–2117. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lowe C, Shilling V. 2001. Frontal tests and models for cognitive aging. Eur J Cogn Psychol. 13:5–28. [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB. 2015. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci. 35:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. 2003. Reduction of eddy-current distortion in diffusion MRI using a twice-refocused spin echo. Magnet Reson Med. 49:177–182. [DOI] [PubMed] [Google Scholar]
- Reuter M, Fischl B. 2011. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B. 2010. Highly accurate inverse consistent registration: A robust approach. NeuroImage. 53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. 2012. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. 2004. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci. 24:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Junque C, Arenaza-Urquijo EM, Vidal-Pineiro D, Valls-Pedret C, Palacios EM, Domenech S, Salva A, Bargallo N, Bartres-Faz D. 2014. Changes in whole-brain functional networks and memory performance in aging. Neurobiol Aging. 35:2193–2202. [DOI] [PubMed] [Google Scholar]
- Salami A, Eriksson J, Nyberg L. 2012. Opposing effects of aging on large-scale brain systems for memory encoding and cognitive control. J Neurosci. 32:10749–10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Pudas S, Nyberg L. 2014. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proc Natl Acad Sci US A. 9:17654–17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 90:449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. 2012. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, Fjell AM. 2014. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 34:15425–15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME. 2013. Resting state functional connectivity in preclinical Alzheimer's disease. Biol Psychiatry. 74:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver N, Dunlap W. 1987. Averaging correlation coefficients: Should Fisher's z-transformation be used? J Appl Psychol. 72:1979–1981. [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc Natil Acad Sci USA. 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, et al. 2007. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2:499–503. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. 2012. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 22:2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB. 2014. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2012. Aging and functional brain networks. Mol Psychiatry. 17, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Davachi L. 2013. Perirhinal-hippocampal connectivity during reactivation is a marker for object-based memory consolidation. Neuron. 79:1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. 2005. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 26:1261–1270; discussion 1275–1268. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Storsve AB, Westlye LT, Drevon CA, Fjell AM. 2014. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging. 35:1055–1064. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, et al. 2011. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 32:916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O'Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, Sperling RA. 2010. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. NeuroImage. 51:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Mormino EC, Huijbers W, Schultz AP, Hedden T, Sperling RA. 2015. Relationships between default-mode network connectivity, medial temporal lobe structure, and age-related memory deficits. Neurobiol Aging. 36:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P, Seri, Cavanna AE. 2013. Functional neuroanatomy and behavioural correlates of the basal ganglia: evidence from lesion studies. Behav Neurol. 26:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT. 2011. Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE epsilon4 carriers: relationships with memory performance. J Neurosci. 31:7775–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. 2010. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 20:2055–2068. [DOI] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. 2013. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage 88C:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, et al. 2011. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinformatics. 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, Lundervold A. 2010. Subcortical functional connectivity and verbal episodic memory in healthy elderly--a resting state fMRI study. NeuroImage. 52:379–388. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, Teng GJ. 2010. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 256:598–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.