Abstract
Learning mechanisms are based on synaptic plasticity processes. Numerous studies on synaptic plasticity suggest that the regulation of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) plays a central role maintaining the delicate balance of inhibition and excitation. However, in humans, a link between learning outcome and GABA levels has not been shown so far. Using magnetic resonance spectroscopy of GABA prior to and after repetitive tactile stimulation, we show here that baseline GABA+ levels predict changes in perceptual outcome. Although no net changes in GABA+ are observed, the GABA+ concentration prior to intervention explains almost 60% of the variance in learning outcome. Our data suggest that behavioral effects can be predicted by baseline GABA+ levels, which provide new insights into the role of inhibitory mechanisms during perceptual learning.
Keywords: GABA, magnetic resonance spectroscopy, plasticity, tactile learning
Introduction
Cellular studies suggest that changes in synaptic transmission are characterized by a complex balance between inhibition and excitation (Abbott and Nelson 2000; Dorrn et al. 2010; Carcea and Froemke 2013). In particular, the inhibitory neurotransmitter γ-aminobutyric acid (GABA) is implicated in numerous mechanisms stabilizing and shaping neural excitation (Lewis et al. 2009; Cybulska-Klosowicz et al. 2013). Animal models provided evidence that during learning, GABAergic circuits are involved in homeostatic plasticity mechanisms that maintain a precise balance between excitation and inhibition (Benali et al. 2008), promoting network stability [see Turrigiano and Nelson (2000) and Feldman (2009) for a review].
Recent advances in magnetic resonance spectroscopy (MRS) have enabled the reliable assessment of cortical GABA levels in humans (Rothman et al. 1993; Puts et al. 2011; Puts and Edden 2012), offering a unique opportunity to explore the role of GABA in learning processes in humans in vivo. In the case of motor learning, recent studies in humans have investigated the role of GABA in plastic changes induced by central stimulation protocols (Jung and Ziemann 2009; Kim et al. 2014). For example, anodal transcranial direct current stimulation (tDCS) applied over primary motor cortex in human subjects increased the magnitude of motor-evoked potentials, which was assumed to be due to a reduction in central GABA levels (Kim et al. 2014).
Perceptual improvements as a consequence of “tactile” learning can be reliably induced not only by training and practice, but also by brief, training-independent sensory learning through repetitive somatosensory stimulation (rSS; Seitz and Watanabe 2005; Seitz and Dinse 2007; Beste and Dinse 2013). This high-frequency intermittent tactile stimulation can be regarded as a long-term potentiation (LTP)-like protocol that enforces Hebbian learning, thereby linking cellular plasticity mechanisms to human perceptual learning (Godde et al. 2000; Dinse et al. 2003; Pleger et al. 2003). Several studies have shown that rSS applied to the index finger improves tactile spatial two-point discrimination (2ptD) of that finger, presumably via functional reorganization within the somatosensory cortices (Pleger et al. 2001, 2003; Dinse et al. 2003; Freyer et al. 2012). Notably, the contralateral hand is not stimulated, and indeed remains perceptually and cortically unaffected, offering a unique internal control condition (Godde et al. 2000; Pleger et al. 2001; Dinse et al. 2003). We used this approach to investigate the link between tactile learning and GABAergic inhibition, assuming a relationship between rSS-induced behavioral improvements and GABA+ levels in the sensorimotor cortices. Furthermore, we considered baseline GABA+ level to be of importance to investigate the predictive power of central inhibition in the somatosensory system for upcoming tactile learning success.
Materials and Methods
Subjects
In total, 24 subjects (all right-handed, 14 male) with no previous history of psychological disorders or any known hand or head injuries were enrolled in the study. Subjects gave their written informed consent and received monetary compensation at the end of the experiment. The experimental protocol had been approved by the local ethics committee of the Ruhr-University Bochum and was performed in accordance with the Declaration of Helsinki. Questionnaire scores concerning depression (Beck's Depression Inventory, BDI) and anxiety trait (State-Trait Anxiety Inventory) were surveyed in addition to visual analog scales on tiredness, arousal, and demand (the latter prior and post to each MRS assessment). Handedness was confirmed using the Edinburgh Handedness Inventory. Data from 6 subjects had to be excluded due to one of the following reasons: Medication with central modes of action (1), BDI scores higher than 18 (1), hand injuries (1), poor-quality water reference data, that is, water scans with >2 repeats rejected due to poor fitting (1), and movement artifacts larger than 3 mm translation or 3° rotation during MRS sessions, as estimated from peaks and steps in the time course of water spectra (2), thus leaving data sets from 18 subjects for final analyses (10 male, mean age 23.8 ± 3.5 years).
Assessment of 2ptD Thresholds
2ptD thresholds were assessed on the tip of the index finger (D2) of both hands by using the method of constant stimuli (Godde et al. 2000; Pleger et al. 2001, 2003; Dinse et al. 2003; Ragert et al. 2003, 2008). All subjects underwent one training session in order to familiarize themselves with the testing procedure (TEST). A second session prior to rSS served as a baseline (BASE). A third assessment (POST) was performed 45–90 min after rSS intervention (67.5 ± 2.81 min). Changes of interest were between the BASE and the POST sessions.
A custom-made device was used to assess the 2ptD thresholds at a fixed location on the skin of the fingertips by rapidly switching between stimuli. The stimuli consisted of 7 pairs of brass needles with individual spacing ranging from 0.7 to 2.5 mm in increments of 0.3 mm and a single needle as zero distance (control condition). Brass pins were 0.7 mm thick with blunt tips of approximately 200 μm in diameter. Tactile stimuli were applied for approximately 1 s with application forces ranging between 150 and 200 mN. The subjects were instructed to place their finger on the support and to maintain this initial position of the finger throughout the experiment. Probes were presented 8 times in a randomized order resulting in 64 trials per session. Subjects were not informed about the ratio of paired to single needles being 7 : 1. The participants had to decide immediately after stimulus contact if they had the sensation of 1 or 2 needles being applied by reporting the percept of a single needle, or any ambiguous stimulus, as “1” and the distinct percept of 2 needle tips as “2.” The tip spacing was plotted against the percentage of double-tip responses given and fitted by a binary logistic regression, resulting in a psychometric function where chance level of the sigmoid fit marked the individual 2ptD threshold. The behavioral gain was calculated according to the following equation:
| (1) |
with a positive gain indicating an improvement in tactile perception, that is, lower 2ptD thresholds after rSS. To provide evidence that a change in discrimination sensitivity was not due to a change in response criterion, we calculated the discrimination index d-prime (d′). The d′ value equals the difference between the z-transform of the hit rate (z(H)) and the z-transform of the false alarm rate (z(F)) [d′ = z(H) − z(F)]. The hit rate describes the probability of discriminating 2 tips whenever 2 tips are presented, whereas the false alarm rate describes the probability of detecting 2 tips when only one is present. To carry out the numerical calculation in case of zero false alarm rates, the false alarm rate was set to 0.0165 (1/2N, with N = 8 being the number of control trials).
Electrical Repetitive Sensory Stimulation Protocol
rSS was applied for 45 min to the dominant right hand. The rSS sequence was applied to the fingertips of all digits and consisted of stimulus trains of 2 s [including 2 × 0.5 s ramps, single-pulse duration: 0.2 ms (square), frequency: 20 Hz] and intertrain intervals of 5 s, played back from a digital storage that triggered a standard TENS device (Pierenkemper, Germany). Electrical pulses were transmitted by adhesive surface electrodes (4 cm2, Pierenkemper) fixed to the first and third segment of each finger (cathode placed proximal) and current intensity was adjusted individually for each subject (mean intensity 9.48 ± 3.37 mA) to maintain a stable percept of stimulating the fingertips across participants.
Magnetic Resonance Imaging Specifications
All subjects were scanned on a 3-T scanner (PHILIPS Achieva X-series, Best, the Netherlands) using the body coil as a transmitter and a 32-channel head coil as a receiver. All MRI acquisitions were performed identically in both BASE and POST sessions. First, T1-weighted images were acquired using an MPRAGE sequence [repetition time (TR) 8.5 ms, echo time (TE) 3.9 ms, flip angle 8°, voxel size (1 mm)3, field of view 256 × 256 × 220 mm], in order to enable anatomically guided MRS voxel placement and tissue segmentation.
The MRS voxel localization within the sensorimotor cortex was determined in the axial plane as being centered on the “hand knob” area of the precentral gyrus (Yousry et al. 1997), and rotated in both the sagittal and coronal planes so that the outermost face of the voxel was aligned to the cortical surface (Puts et al. 2011; Puts and Edden 2012; blue- and orange-shaded areas in Fig. 1B).
Figure 1.
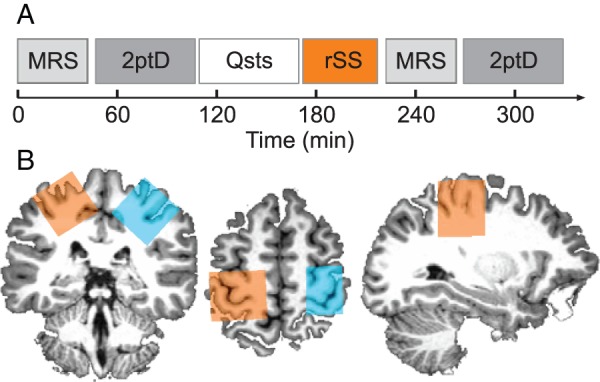
Experimental schedule and GABA-edited MRS voxel location. (A) For each subject (n = 18), we measured the local GABA+ concentration in a sensorimotor voxel of each brain hemisphere by means of GABA-edited MRS, followed by the assessment of 2ptD thresholds of the index fingers of both the left and right hand. After baseline assessments, subjects filled out questionnaires concerning personality traits (Qsts). During the subsequent rSS intervention, one hand received 45 min of intermittent high-frequency stimulation, whereas the other hand served as a control condition. The time between the end of rSS and the start of the second MRS session ranged between 5 and 40 min (20.0 ± 1.98, mean ± SEM). (B) A single subject example of MRS voxel positions at baseline in the stimulated (orange) and control (blue) sensorimotor cortices on its individual T1-weighted image. The single GABA-edited spectra around 3 ppm obtained within were fitted with a Gaussian + baseline model to result in GABA+ concentrations in institutional units, which were afterwards CSF-corrected for that particular voxel.
The Mescher-Garwood-Point Resolved Spectroscopy (MEGA-PRESS, Mescher et al. 1998) sequence was used to obtain GABA+-edited spectra from single-voxel acquisitions over the right and left sensorimotor cortices using the following parameters: voxel size 3 cm3, TR 2000 ms, TE 68 ms, 14 ms sinc-Gaussian editing pulses applied at 7.46 and 1.9 ppm, 320 acquisitions in total with 20 averages of OFF and ON scans interleaved every 16 scans, spectral bandwidth of 2 kHz with a sampling rate of 2048 points. Regional saturation technique slabs were applied to suppress fat signals from the skull, whereas variable power radio-frequency pulses with optimized relaxation delays were used for water suppression. A separate nonwater-suppressed scan followed the acquisition. Macromolecules were not suppressed and therefore those at the 1.72-ppm resonance were also partially inverted by the 1.9-ppm editing pulse. Since this signal is coupled with the 3.00-ppm resonance (Behar et al. 1994), those macromolecules would also have been affected by the editing pulse and therefore contribute to the difference spectra. Thus, GABA+ in this study refers to GABA including macromolecules. MRS sessions were scheduled so as to avoid effects of frequency drift on GABA+-edited MRS (Dydak and Schär 2006; Harris et al. 2014).
Postprocessing
GABA+ concentration was calculated using the GABA Analysis Toolkit “Gannet,” which uses a Gaussian + baseline model to fit the edited GABA+ signal and a Lorentz-Gaussian lineshape to fit the unsuppressed water signal (Edden et al. 2014).
Brain volumes within each sensorimotor voxel (matching the MRS voxels) were segmented into gray matter (GM), white matter (WM), as well as cerebrospinal fluid (CSF) fractions using the segmentation routine implemented in the VBM8 toolbox (http://www.neuro.uni-jena.de/vbm/, last accessed November 23, 2015) as part of SPM8.
Institutional units for GABA+/H2O were corrected post hoc for voxel tissue fraction by calculating the ratio of GABA+ units and the sum of GM and WM fractions according to the following equation:
| (2) |
and are stated as CSF-corrected individual GABA+ values. The gain of GABA+ concentration was calculated according to the equation:
| (3) |
with a positive gain indicating an increase in central GABA+ concentration following rSS.
Statistics
All results are quoted as mean ± SEM, unless stated otherwise. Correlations between GABA+ concentration and 2ptD threshold were tested using Pearson correlation coefficients (R). Other statistical tests comprised paired t-tests as well as repeated-measures analysis of variance (ANOVA). An interactive stepwise regression (initial model containing no terms; entrance tolerance P = 0.05, exit tolerance P = 0.10) was performed on post-intervention 2ptD threshold as a dependent variable and the following set of independent variables: baseline GABA+ concentration of the stimulated sensorimotor voxel and baseline 2ptD threshold of the stimulated hand. Between group comparisons of stimulation versus control, data were performed by means of Fisher-R-to-Z transformations (comparing correlations) and Student's t-statistics (comparing slopes and intercepts; Statistics toolbox and in-house scripts; MATLAB, R2009a, The MathWorks, Inc., USA).
Results
Improvements in Tactile Discrimination
After 45 min of intermittent high-frequency stimulation of all fingers of the right hand, tactile discrimination performance of the stimulated index finger improved by 12% on average, while performance of the nonstimulated left hand index finger (serving as a control) remained unaltered (Fig. 2B), confirming previously reported improvements after rSS (Ragert et al. 2008; repeated-measures ANOVA with factor site F1,17 = 0.46, P = 0.506, factor time F1,17 = 5.30, P = 0.034, and interaction site × time F1,17 = 18.51, P < 0.001). With respect to the discrimination index (d′), we found an increase in d′ after rSS [1.51 ± 0.21 (pre) vs. 1.75 ± 0.35 (post)] on the stimulated hand, whereas the d′ of the control hand decreased slightly [1.63 ± 0.31 (pre) vs. 1.54 ± 0.38 (post)]. A two-factor repeated-measures ANOVA showed a significant stimulation site × time interaction on tactile sensitivity (F1,17 = 28.39, P < 0.001), such that d′ of the stimulated hand increased significantly more than on the nonstimulated hand. Linear regression analyses revealed no significant correlation between baseline 2ptD threshold and 2ptD gain (stimulated hand: Pearson's R = 0.06, P = 0.808; control hand: Pearson's R = 0.20, P = 0.427). Baseline 2ptD thresholds of the stimulated hand correlated marginally with post-interventional 2ptD thresholds (Pearson's R = 0.46, P = 0.056), yet we found a significant correlation between pre- and post-interventional 2ptD thresholds of the control hand (Pearson's R = 0.55, P = 0.018).
Figure 2.
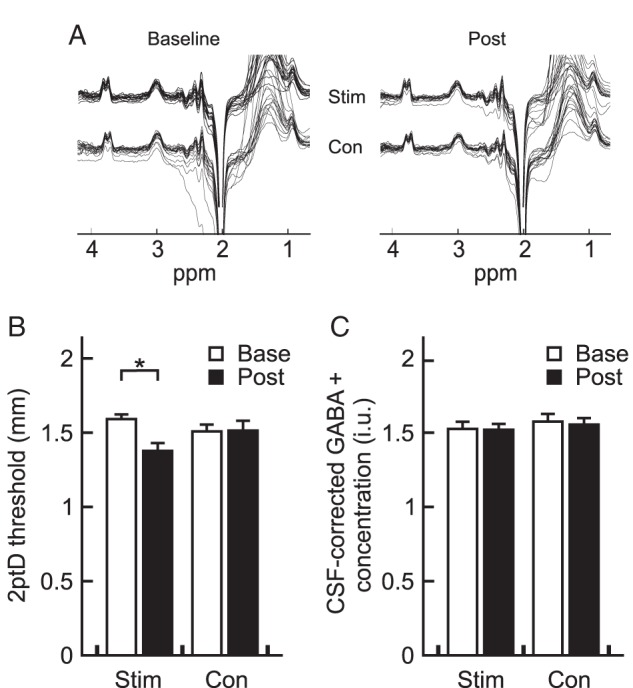
Changes in central GABA+ concentration and behavioral performance. (A) Representative MEGA-PRESS difference spectra from an individual subject depicted as single repeats during baseline and post sessions, for both hemispheres. (B) Average tactile 2ptD performance of the index fingers of both the stimulated (Stim) and control (Con) hand using the method of constant stimuli. White bars give the mean of the baseline assessments, whereas black bars indicate the means across participants after the rSS intervention. Here, asterisk indicates the rSS-related significant difference between 2ptD thresholds of the stimulated hand (P < 0.05, paired t-test). Error bars show SEM. (C) Averages of CSF-corrected local GABA+ concentrations of both stimulated (Stim) and non-stimulated (Con) sensorimotor cortices. Conventions are identical to (B).
Intervention-Related Changes in GABA+ Concentration
Local GABA concentration was measured before and after rSS, bilaterally in 3 cm3 voxels centered on the hand knob area of the precentral gyrus (Fig. 1B). The delay between end of rSS and start of POST MRS acquisition (Δtime) ranged between 5 and 40 min (20.0 ± 1.98 min). In contrast to the clear improvement of perceptual performance across all participants, we observed no net changes in GABA+ concentration after rSS in the stimulated hemisphere (Fig. 2C), as tested with a repeated-measures two-factor ANOVA with factor site F1,17 = 1.84, P = 0.192; factor time F1,17 = 0.21, P = 0.655; and interaction site × time F1,17 = 0.04, P = 0.846.
For each of the 4 conditions (pre-/post-stimulation, stimulated/control site), the coefficient of variation (CV) between subjects of GABA+ measurements was approximately 13% (mean 12.6%, range 10.9–14.1%), in line with previously published data from the same acquisition region and protocol (Evans et al. 2010). No correlation between GABA+ concentration and fit error was observed (stimulated hemisphere: baseline Pearson's R = 0.19, P = 0.53; post: Pearson's R = −0.33, P = 0.185; control hemisphere: baseline Pearson's R = 0.12, P = 0.625; post: Pearson's R = −0.16; P = 0.528).
Also, we found no correlation between GABA+ concentration and GM tissue content of the MRS voxels surviving a Bonferroni-corrected P = 0.0125 (stimulated hemisphere: baseline Pearson's R = 0.44, P = 0.071; post Pearson's R = −0.06, P = 0.825; control hemisphere: baseline Pearson's R = 0.03, P = 0.894; post Pearson's R = 0.01, P = 0.962).
To check whether Δtime takes influence on the GABA+ concentrations, we looked up Δtime from our records and performed correlation analyses. We found no correlation between Δtime and post-interventional GABA+ (stimulated hemisphere: Pearson's R = −0.366, P = 0.14; control hemisphere: Pearson's R = −0.018, P = 0.94), or Δtime and gain in GABA+ (stimulated hemisphere: Pearson's R = −0.148, P = 0.56; control hemisphere: Pearson's R = 0.153, P = 0.55).
Relationship Between GABA+ Levels and Tactile Performance
First, we tested whether GABA+ was related to performance, either at baseline or after the intervention. We did not find any significant correlations, either for the stimulated, or for the control site (baseline − stimulated hand: Pearson's R = −0.20, P = 0.422; baseline − nonstimulated hand: Pearson's R = 0.03, P = 0.913; post-intervention − stimulated hand: Pearson's R = −0.40, P = 0.103; post-intervention − nonstimulated hand: Pearson's R = −0.33, P = 0.179).
Secondly, to obtain further insights into the underlying mechanisms mediating the perceptual gain, we analyzed the changes in perceptual performance and baseline GABA+. Correlation analysis revealed a strong positive relation between the improvement of tactile performance after rSS in the stimulated hemisphere and GABA+ concentration at baseline (Pearson's R = 0.74, P < 0.001, Fig. 3A, left panel), which was absent in the nonstimulated hemisphere serving as a control (Pearson's R = 0.28, P = 0.262, Fig. 3A, right panel). Between-group comparisons proved both correlation coefficients to be significantly different (P = 0.036). Furthermore, only for the stimulated hand, baseline GABA+ concentration itself predicted learning outcome (performance after intervention), that is, absolute 2ptD threshold after rSS, explaining 57% of the variance (Pearson's R = −0.76, P < 0.001, Fig. 3B). There was a significant group difference between the correlations between baseline GABA+ and post-interventional 2ptD thresholds with respect to correlation coefficients (P = 0.011). To exclude a regression to the mean effect for the perceptual improvement, we performed a multiple regression with baseline GABA+ as a predictor and post-interventional performance as a dependent variable; baseline performance was entered into the model as a nuisance variable. Even when controlling for baseline performance, there was a highly significant association between baseline GABA+ levels and post-interventional performance: There was a trend for a positive association between baseline and post-interventional 2ptD threshold (R2 = 0.21, F = 4.23, P = 0.06), however, adding baseline GABA+ as a predictive term significantly improved the model (R2 = 0.67, F = 15.02, P < 0.001).
Figure 3.
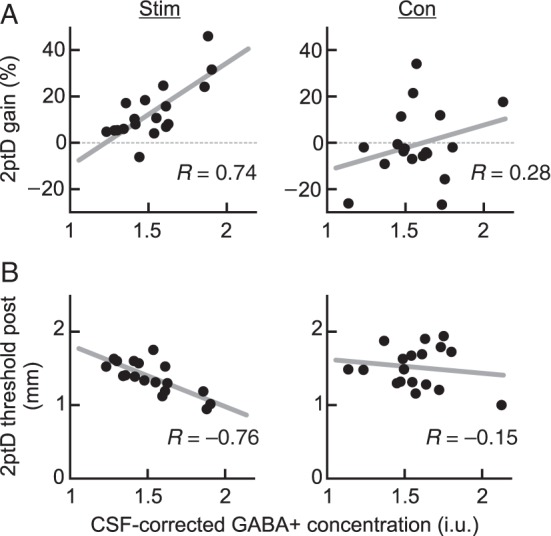
Relating changes in tactile improvement and behavioral performance to CSF-corrected baseline GABA+ concentration. (A) Correlation between baseline GABA+ level and gain in 2ptD performance of the corresponding hand observed for each subject (n = 18) and hemisphere. A positive 2ptD gain indicates increased tactile acuity, whereas negative values represent worsened performance following either unilateral rSS intervention of that hand, or none intervention at all. Only the linear regression between the hand/voxel pairing of the stimulated sites reaches statistical significance (P < 0.001). (B) Correlation between baseline GABA+ level and absolute 2ptD thresholds of the corresponding hand observed for each subject (n = 18) and hemisphere after rSS intervention. The lower the 2ptD threshold, the better the tactile acuity following either unilateral rSS intervention of that hand, or none intervention at all. Only the linear regression between baseline GABA+ and 2ptD threshold of the stimulated sites reaches significance (Pearson's R = −0.76, P < 0.001).
One concern with our results is the number of statistical test that have been performed. On each of 2 sides, it might be reasonable to test for correlations between baseline GABA and 2ptD performance prestimulation, poststimulation, and change, and between GABA post-stimulation and 2ptD performance change and post-stimulation. A Bonferroni correction for this total of 10 statistical tests would reduce the threshold of significance from 0.05 to 0.005, a stringent multiple-comparisons correction. The correlations between baseline GABA and change in 2ptD performance and between baseline GABA and performance poststimulation both remain significant after this correction.
Gender Effects on Neurochemistry and Behavior
In total, data from 8 female and 10 male participants were subject to final analyses. We observed no gender-related effects in our data: The groups did not differ with respect to age (P = 0.277), GABA+ concentration of the stimulated or control hemisphere [baseline GABA+ concentration (P = 0.336 and 0.729)], post-interventional GABA+ concentration (P = 0.686 and 0.268), or 2ptD thresholds of the stimulated or control hand [baseline 2ptD thresholds (P = 0.548 and 0.271)], post-interventional 2ptD thresholds (P = 0.319 and P = 0.891), and 2ptD gain (P = 0.128 and P = 0.519). Furthermore, we found no significant gender-related differences concerning the correlation coefficients, slopes, or intercepts of the correlations as depicted in Figure 3.
Discussion
We used GABA-MRS to investigate the relationship between local GABA+ concentrations in sensorimotor regions and individual improvement of tactile perception induced by rSS to the tip of the index finger. To this end, we applied intermittent high-frequency tactile stimulation, which, in respect to timing, resembles LTP-like protocols used in synaptic plasticity research (Beste and Dinse 2013). The particular advantage of this approach is that changes in perceptual performance paralleled by cortical reorganization can be brought about within less than an hour.
We show that an rSS intervention of 45 min is sufficient to drive significant improvement of tactile discrimination in the stimulated hand selectively, with baseline GABA+ concentrations in the contralateral hemisphere predicting tactile performance, suggesting that baseline GABA levels play a crucial role in perceptual learning. As such, we found that almost 60% of the interindividual variability of tactile learning success could be explained by baseline GABA+ levels in the corresponding sensorimotor area. On a group level, there was no consistent up- or down-regulation of GABA, as average GABA+ concentration was maintained at a surprisingly constant level. Substantial variation in learning outcome is a typical observation not only in real life, but also under laboratory conditions (Fahle and Henke-Fahle 1996; Schmitt et al. 2002). Both neurophysiological conditions and cognitive factors such as attention are known to play a critical role in learning success (Freyer et al. 2013). In this regard, rSS is of particular interest, because attention can be excluded as a contributing factor (Godde et al. 2000). Therefore, other factors must explain the observed variability. For example, it has been shown that genetic predisposition interferes with learning, as is the case in BDNF polymorphisms (Kleim et al. 2006; Cheeran et al. 2008). Also, recently published results suggest that the cortical thickness in primary sensorimotor cortex explained about 50% of variance in learning (Conde et al. 2012). Furthermore, the baseline alpha power (mu-rhythm) recorded in somatosensory cortices, and the event-related alpha desynchronization predicted about two-thirds of the learning outcome (Freyer et al. 2013). Our study now provides evidence that local baseline GABA+ concentration also determines the success of training-independent learning.
The notion of a critical role of GABA in plasticity processes is in line with previous findings, according to which the administration of the GABAA-agonist lorazepam prevented the typical rSS-induced improvement of discrimination performance (Dinse et al. 2003). Other studies have shown that a tDCS-induced reduction in GABAergic inhibition correlated significantly with the learning rate during a force-field adaptation/deadaptation task (Kim et al. 2014), and also with a speed-up in reaction time during a motor sequence task (Stagg et al. 2011).
There are ways to obtain indirect markers of intracortical inhibition which are assumed to involve GABAergic mechanisms, for example, paired-pulse suppression protocols. These methods provide evidence that changes in behavior and perception following central stimulation, such as TMS or tDCS, or peripheral stimulation such as rSS, are associated with a net increase in cortical excitability due to a strengthening of excitatory and a suppression of inhibitory circuits [see Ziemann et al. (1996); Butefisch et al. (2000); Di Lazzaro et al. (2006); Höffken et al. (2007)]. However, paired-pulse behavior is far from being fully understood, and conceivably many other transmitter systems other than GABA may contribute to changes in paired-pulse behavior.
MRS has been shown to be a reliable tool for GABA detection of appropriate resolution and accuracy in physiological concentrations with test–retest reliability on the order of 10% (Gorman et al. 2011; Evans et al. 2012), given consistent voxel positioning between single MRS sessions, and minimized subject motion. Specifically, Evans et al. have shown between-subject CVs for the same sensorimotor voxel location and a similar 10-min MEGA-PRESS GABA acquisition to be 12% (and in the same study, within-subject CVs of 9%). Our own data among a similar recruitment cohort show equivalent between-subject reproducibility of 12.6%. Here, individual voxels were positioned by a single experimenter according to defined anatomical features, and screenshots of this initial position helped to preserve a similar voxel position during the post-interventional MRS sessions. Furthermore, most of our subjects were familiar with the MR environment and managed to avoid excessive head motion.
The MR signal interpreted as local GABA concentration is the sum of metabolic GABA located within the synaptic cleft or coupled with GABA receptors (active states), and in the cell body or at extrasynaptic sites (inactive states; Rae 2014). Using MRS, our data revealing the predictive power of local GABA+ concentration in perceptual learning cannot distinguish between these physiological states. However, in the absence of a net change in regional GABA+ concentration, our results are likely to reflect a fast switch in physiological states, possibly mediated by cellular GABA turnover which has been shown to be an imminent step in GABAergic regulation (Levy et al. 2002). Fast up- and down-regulations of inhibitory neurotransmitters have been reported for rat somatosensory cortex following intracortical microstimulation, confirming that GABA regulation can occur within short periods of time (Benali et al. 2008). Furthermore, fast GABA down-regulation in the human somatosensory and motor systems has also been reported following altered afferent input (Levy et al. 2002), or during motor training (Floyer-Lea et al. 2006). With respect to the findings of Floyer-Lea and colleagues, who, despite partial recovery, found a reduction in GABA concentration lasting 20–40 min after cessation of a motor learning task, any changes in GABA+ concentration in our paradigm would have accordingly been detected by our POST measurement. By starting the post-interventional GABA-MRS with variable delay after the end of rSS, we might have increased the variance in the observed post-interventional GABA+ concentrations. However, as reported above, we found no correlation, not even a trend, between Δtime and GABA+ concentrations after rSS, or between Δtime and GABA+ gain.
More work is needed to disentangle inhibitory and excitatory mechanisms within the somatosensory system that contribute to task performance and improvement thereof. Furthermore, an interesting prospect for future control studies would be to apply the rSS protocol to the nondominant hand, or use a within-subject design to stimulate dominant and nondominant hands in succession and see whether the same regulatory mechanism can be obtained for both dominant and nondominant hemispheres.
In summary, our study combining a tactile learning paradigm with GABA-MRS shows that baseline GABA+ level predicts both improvement in a tactile perception task following repetitive sensory stimulation, and poststimulation performance.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB874-A1 to M.T. and M.L., SFB874-A5 to H.R.D., and SFB874-A8 to T.S.-W.; DFG Research Unit 1581 to B.G. and M.T.); the Ruhr-University Bochum (BoNeuroMed to L.H.; FORUM- F767-12 to T.K.); the Mercator Research Center Ruhr (MERCURE; Pr-2010-0017 to M.T., H.R.D., and M.L.); and the National Institutes of Health (P41 EB015909, R01 EB016089, and R21 MH098228 to R.A.E.E. and N.A.J.P.). Funding to pay the Open Access publication charges for this article was provided by the Deutsche Forschungsgemeinschaft (grants SFB874-A1, SFB874-A5 and SFB874-A8).
Notes
We appreciate the continuous scientific support of PHILIPS Germany. Conflict of Interest: None declared.
References
- Abbott LF, Nelson SB. 2000. Synaptic plasticity: taming the beast. Nat Neurosci. 3(Suppl):1178–1183. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Spencer DD, Petroff OAC. 1994. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magn Reson Med. 32:294–302. [DOI] [PubMed] [Google Scholar]
- Benali A, Weiler E, Benali Y, Dinse HR, Eysel UT. 2008. Excitation and inhibition jointly regulate cortical reorganization in adult rats. J Neurosci. 28:12284–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Dinse HR. 2013. Learning without training. Curr Biol. 23:R489–R499. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. 2000. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA. 97:3661–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcea I, Froemke RC. 2013. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Prog Brain Res. 207:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. 2008. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 586:5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde V, Vollmann H, Sehm B, Taubert M, Villringer A, Ragert P. 2012. Cortical thickness in primary sensorimotor cortex influences the effectiveness of paired associative stimulation. Neuroimage. 60:864–870. [DOI] [PubMed] [Google Scholar]
- Cybulska-Klosowicz A, Posluszny A, Nowak K, Siucinska E, Kossut M, Liguz-Lecznar M. 2013. Interneurons containing somatostatin are affected by learning-induced cortical plasticity. Neuroscience. 254:18–25. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. 2006. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 575:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. 2003. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 301:91–94. [DOI] [PubMed] [Google Scholar]
- Dorrn A, Yuan K, Barker A. 2010. Developmental sensory experience balances cortical excitation and inhibition. Nature. 465:932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dydak U, Schär M. 2006. MR spectroscopy and spectroscopic imaging: comparing 3.0T versus 1.5T. Neuroimaging Clin N Am. 16:269–283. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. 2014. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 40:1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, McGonigle DJ, Edden RAE. 2010. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 31:204–209. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Puts NAJ, Robson SE, Boy F, McGonigle DJ, Sumner P, Singh KD, Edden RAE. 2012. Subtraction artifacts and frequency (Mis-)alignment in J-difference GABA editing. J Magn Reson Imaging. 38(4):970–975. [DOI] [PubMed] [Google Scholar]
- Fahle M, Henke-Fahle S. 1996. Interobserver variance in perceptual performance and learning. Invest Ophthalmol Vis Sci. 37:869–877. [PubMed] [Google Scholar]
- Feldman DE. 2009. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 32:33–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. 2006. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 95:1639–1644. [DOI] [PubMed] [Google Scholar]
- Freyer F, Becker R, Dinse HR, Ritter P. 2013. State-dependent perceptual learning. J Neurosci. 33:2900–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer F, Reinacher M, Nolte G, Dinse HR, Ritter P. 2012. Repetitive tactile stimulation changes resting-state functional connectivity—implications for treatment of sensorimotor decline. Front Hum Neurosci. 6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B, Stauffenberg B, Spengler F, Dinse HR. 2000. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci. 20:1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman RLO, Michels L, Edden RA, James B, Martin E. 2011. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging. 33:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Glaubitz B, Near J, Evans JC, Puts NAJ, Schmidt-Wilcke T, Tegenthoff M, Barker PB, Edden RAE. 2014. The impact of frequency drift on GABA-edited MR spectroscopy. Magn Reson Med. 72(4):941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffken O, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M. 2007. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J Physiol. 584:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung P, Ziemann U. 2009. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci. 29:5597–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Stephenson MC, Morris PG, Jackson SR. 2014. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7T magnetic resonance spectroscopy study. Neuroimage. 99C:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. 2006. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 9:735–737. [DOI] [PubMed] [Google Scholar]
- Levy LM, Ziemann U, Chen R, Cohen LG. 2002. Rapid modulation of GABA in sensorimotor cortex induced by acute deafferentation. Ann Neurol. 52:755–761. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. 2009. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci. 106:17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11:266–272. [DOI] [PubMed] [Google Scholar]
- Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M. 2001. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci USA. 98:12255–12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Foerster A, Ragert P, Dinse HR, Schwenkreis P, Malin J, Nicolas V, Tegenthoff M. 2003. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron. 40:643–653. [DOI] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE. 2012. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 60:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE, Evans JC, McGlone F, McGonigle D. 2011. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 31:16556–16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CD. 2014. A guide to the metabolic pathways and function of metabolites observed in human brain (1)h magnetic resonance spectra. Neurochem Res. 39:1–36. [DOI] [PubMed] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, Tegenthoff M. 2003. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci Lett. 348:105–108. [DOI] [PubMed] [Google Scholar]
- Ragert P, Kalisch T, Bliem B, Franzkowiak S, Dinse HR. 2008. Differential effects of tactile high- and low-frequency stimulation on tactile discrimination in human subjects. BMC Neurosci. 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. 1993. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 90:5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C, Kromeier M, Bach M, Kommerell G. 2002. Interindividual variability of learning in stereoacuity. Graefes Arch Clin Exp Ophthalmol. 240:704–709. [DOI] [PubMed] [Google Scholar]
- Seitz A, Watanabe T. 2005. A unified model for perceptual learning. Trends Cogn Sci. 9:329–334. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Dinse HR. 2007. A common framework for perceptual learning. Curr Opin Neurobiol. 17:148–153. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. 2011. The role of GABA in human motor learning. Curr Biol. 21:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. 2000. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 10:358–364. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. 1997. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark. Brain. 120(1):141–157. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. 1996. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 109:127–135. [DOI] [PubMed] [Google Scholar]


