Abstract
Chronic kidney disease (CKD), impairment of kidney function, is a serious public health problem, and the assessment of genetic factors influencing kidney function has substantial clinical relevance. Here, we report a meta-analysis of genome-wide association studies for kidney function–related traits, including 71,149 east Asian individuals from 18 studies in 11 population-, hospital- or family-based cohorts, conducted as part of the Asian Genetic Epidemiology Network (AGEN). Our meta-analysis identified 17 loci newly associated with kidney function–related traits, including the concentrations of blood urea nitrogen, uric acid and serum creatinine and estimated glomerular filtration rate based on serum creatinine levels (eGFRcrea) (P < 5.0 × 10−8). We further examined these loci with in silico replication in individuals of European ancestry from the KidneyGen, CKDGen and GUGC consortia, including a combined total of ~110,347 individuals. We identify pleiotropic associations among these loci with kidney function–related traits and risk of CKD. These findings provide new insights into the genetics of kidney function.
Chronic kidney disease—the impairment of kidney function—constitutes a serious public health burden on society worldwide, with increased risks of mortality and morbidity1, 2. Biochemical measures of kidney function that are commonly used in clinical practice include the concentrations of blood urea nitrogen, serum creatinine and uric acid and glomerular filtration rate (GFR). Heritability estimates have shown that genetic factors contribute significantly to interindividual variance in kidney function3, and recent developments in genome-wide association studies (GWAS) have identified a number of genetic loci associated with measurements of kidney function4, 5, 6, 7, 8, 9, 10, 11, 12, 13. However, most of these studies were conducted in populations of European ancestry4, 6, 7, 8, 10, 11, 12, 13, and the extension of GWAS approaches to non-European populations would provide an opportunity to discover additional loci. We report a large-scale meta-analysis of GWAS and a replication study of kidney function–related traits involving 71,149 east Asian subjects performed by AGEN9, 14, 15, 16 in which 11 cohorts participated (BBJ, SP2, SiMES, SINDI, SCES, KARE, HEXA, TWSC, TWT2D, GenSalt and CAGE; Online Methods and Supplementary Note).
In this study, we evaluated four kidney function–related traits (Supplementary Tables 1 and 2): the concentrations of blood urea nitrogen (n = 57,178), uric acid (n = 33,074) and serum creatinine (n= 61.919) and eGFRcrea (n = 62,087). Blood urea nitrogen concentration reflects the amount of nitrogen in the blood and is related to protein metabolism, including excretion by the kidneys17. Uric acid is the end product of purine metabolism, and impaired renal excretion of uric acid leads to hyperuricemia. Epidemiological studies suggest that uric acid is a risk factor for various diseases, including gout and myocardial infarction13. Serum creatinine levels and eGFRcrea are the most common kidney function measures used for the definition of CKD1, 2, for which extensive genetic studies in European populations have been conducted4, 6, 7, 8.
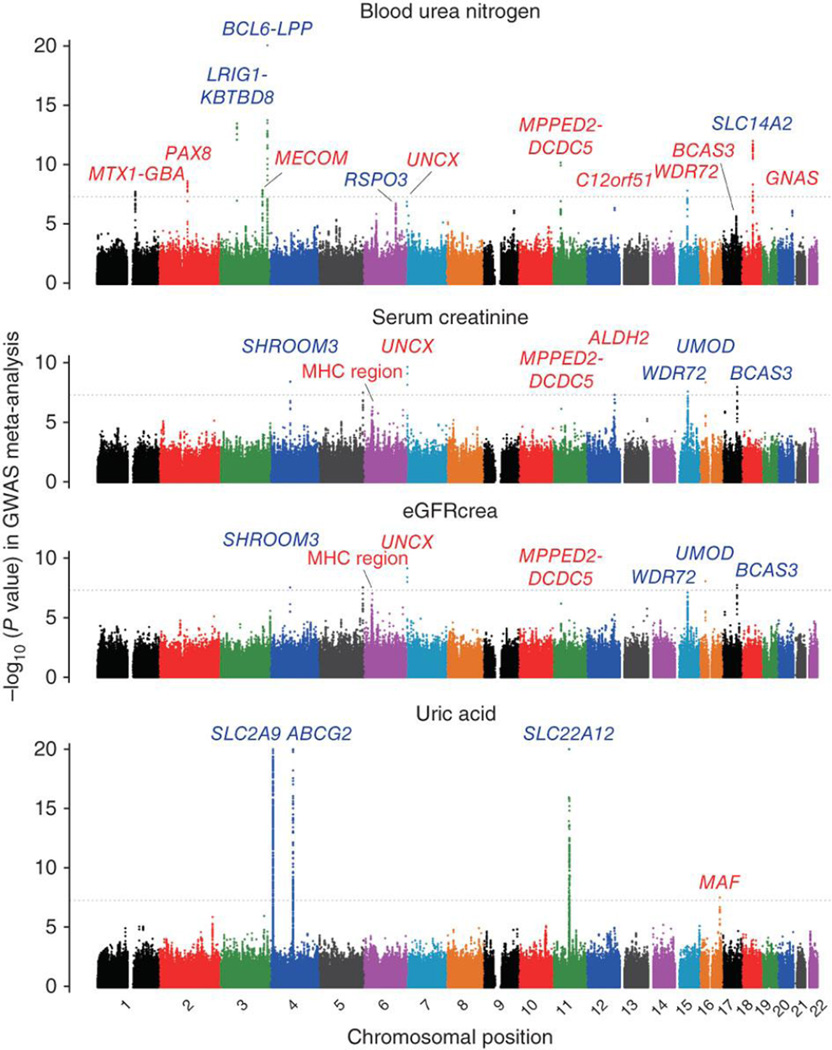
The GWAS meta-analysis included 51,327 east Asian individuals and evaluated approximately 2.4 million autosomal SNPs with a minor allele frequency (MAF) of ≥0.01. These SNPs were obtained by imputation of genotypes on the basis of HapMap Phase 2 panels (Supplementary Tables 3 and 4). The inflation factors of the test statistics were modest (λGC = 1.060, 1.072, 1.079 and 1.031 for blood urea nitrogen, serum creatinine, eGFRcrea and uric acid, respectively), which suggested that population structures did not have a substantial impact on the results of the meta-analysis. Quantile-quantile plots of the P values indicated notable discrepancies in their tails from those anticipated under the null hypothesis of no association, indicating the presence of significant associations in the meta-analysis (Supplementary Fig. 1). We identified 25 associations that satisfied the genome-wide significance threshold of P < 5.0 × 10−8. Of these, eight, seven, six and four genetic loci were found to associated with blood urea nitrogen, serum creatinine, eGFRcrea and uric acid, respectively (Supplementary Table 5).
We then performed an in silico replication study using data from an additional 19,822 east Asians for the loci that associated at P < 5.0 × 10−6 in the GWAS meta-analysis. Through the combined study of the GWAS meta-analysis and replication, we identified 32 significant associations at P < 5.0 × 10−8 (13, 8, 7 and 4 loci for blood urea nitrogen, serum creatinine, eGFRcrea and uric acid, respectively; Fig. 1 and Supplementary Table 5). We found that seven of these newly associated loci were associated with both serum creatinine concentration and eGFRcrea, with the same landmark SNPs involved at each locus, which reflects the close relationship between these two phenotypes (R2 = 0.76 for common log-transformed values; Supplementary Table 6)1, 2. Among the loci identified in the combined analysis, associations at 15 loci were previously reported4, 5, 6, 7, 8, 9, 10, 11, 12, 13: LRIG1-KBTBD8, BCL6-LPP, RSPO3 and SLC14A2 for blood urea nitrogen concentration (smallest P = 8.8 × 10−30 at BCL6-LPP); SHROOM3, WDR72, UMOD and BCAS3 for serum creatinine concentration (smallest P = 1.2 × 10−13 at WDR72); SHROOM3, WDR72, UMOD and BCAS3 for eGFRcrea (smallest P = 6.0 × 10−13 at WDR72); and SLC2A9, ABCG2 and SLC22A12 for uric acid concentration (smallest P = 1.6 × 10−65 at SLC2A9). At the UMOD locus, the rs12917707 variant associated with eGFRcrea in Europeans4, 7 had a low MAF (<0.01) and was not evaluated in our GWAS meta-analysis. However, we identified another variant that showed a significant association with eGFRcrea (P = 3.6 × 10−10 at rs11864909; MAF = 0.19; r2 = 0.02 with rs12917707).
Figure 1. Manhattan plots of the GWAS meta-analysis for kidney function–related traits.
Shown are the −log10 (P values) of the SNPs for the concentrations of blood urea nitrogen, serum creatinine and uric acid, and for eGFRcrea. The genetic loci that satisfied the genome-wide significance threshold of P < 5.0 × 10−8 (gray horizontal dotted line) in the combined study of the GWAS meta-analysis and replication are labeled for each of the traits. The newly identified loci are colored red, and the previously known loci are colored blue. The SNPs for which the P value was smaller than 1.0 × 10−20 are indicated at the upper limit of each plot.
In addition, we identified 17 loci newly associated with kidney function–related traits (Table 1 and Supplementary Fig. 2). Namely, we identified associations at nine loci for blood urea nitrogen concentration (MTX1-GBA, PAX8, MECOM,UNCX, MPPED2-DCDC5, C12orf51, WDR72, BCAS3 and GNAS at 1q22, 2q13, 3q26, 7p22, 11p14, 12q24.13, 15q21, 17q23 and 20q13, respectively; smallest P = 4.5 × 10−16 at rs10767873 in MPPED2-DCDC5), four loci for serum creatinine concentration (the major histocompatibility (MHC) region, UNCX, MPPED2-DCDC5 and ALDH2 at 6p21, 7p22, 11p14 and 12q24.2, respectively; smallest P = 4.6 × 10−11 at rs10277115 in UNCX), three loci for eGFRcrea (the MHC region, UNCX and MPPED2-DCDC5 at 6p21, 7p22 and 11p14, respectively; smallest P = 1.0 × 10−10 at rs10277115 in UNCX) and one locus for uric acid concentration (MAF at 16q23, P = 1.1 × 10−9 at rs889472). Combinations of these identified loci explained 1.3%, 0.54%, 0.55% and 2.3% of interindividual variance in blood urea nitrogen, serum creatinine, eGFRcrea and uric acid, respectively.
Table 1.
Loci newly associated with kidney function-related traits
| rsIDa | Chr. | Position (bp) | Band | Gene | A1/A2b | Freq.c | GWAS meta-analysis |
Replication study |
Combined study |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE)d | P | β (SE)d | P | β (SE)d | P | |||||||
| Blood urea nitrogen (n = 39,717 for GWAS meta-analysis, n = 17,461 for replication) | ||||||||||||
| rs2049805 | 1 | 153461604 | 1q22 | MTX1-GBA | T/C | 0.17 | 0.0072 (0.0013) |
1.9 × 10−8 | 0.0072 (0.0017) |
2.3 × 10−5 | 0.0072 (0.0010) |
1.8 × 10−12 |
| rs11123170 | 2 | 113695411 | 2q13 | PAX8 | G/G | 0.35 | 0.0059 (0.0010) |
2.4 × 10−9 | 0.0035 (0.0014) |
0.014 | 0.0051 (0.0008) |
3.3 × 10−10 |
| rs16853722 | 3 | 170633326 | 3q26 | MECOM | C/T | 0.29 | 0.0059 (0.0010) |
1.3 × 10−8 | 0.0072 (0.0014) |
2.7 × 10−7 | 0.0064 (0.0008) |
2.7 × 10−14 |
| rs10275044 | 7 | 1240371 | 7p22 | UNCX | T/A | 0.34 | 0.0079 (0.0015) |
1.3 × 10−7 | 0.0053 (0.0019) |
0.0056 | 0.0069 (0.0012) |
4.3 × 10−9 |
| rs10767873 | 11 | 30725254 | 11p14 |
MPPED2- DCDC5 |
C/T | 0.69 | 0.0068 (0.0010) |
7.0 × 10−11 | 0.0068 (0.0014) |
1.4 × 10−6 | 0.0068 (0.0008) |
4.5 × 10−16 |
| rs2074356 | 12 | 111129784 | 12q24.13 | C12orf51 | A/G | 0.23 | 0.0064 (0.0013) |
4.4 × 10−7 | 0.0063 (0.0020) |
0.0012 | 0.0064 (0.0011) |
1.8 × 10−9 |
| rs17730281 | 15 | 51695240 | 15q21 | WDR72 | G/A | 0.58 | 0.0054 (0.0010) |
1.5 × 10−8 | 0.0046 (0.0013) |
4.5 × 10−4 | 0.0051 (0.0008) |
3.0 × 10−11 |
| rs11868441 | 17 | 56594003 | 17q23 | BCAS3 | G/A | 0.75 | 0.0062 (0.0013) |
2.1 × 10−6 | 0.0055 (0.0015) |
2.2 × 10−4 | 0.0059 (0.0010) |
2.1 × 10−9 |
| rs6026584 | 20 | 56902468 | 20q13 | GNAS | T/C | 0.32 | 0.0055 (0.0011) |
7.1 × 10−7 | 0.0046 (0.0016) |
0.0033 | 0.0052 (0.0009) |
8.8 × 10−9 |
| Serum creatinine (n = 42,257 for GWAS meta-analysis, n = 19,662 for replication) | ||||||||||||
| rs3828890 | 6 | 31548648 | 6p21 | MHC region | G/C | 0.11 | 0.0074 (0.0015) |
5.3 × 10−7 | 0.0060 (0.0018) |
0.0011 | 0.0069 (0.0012) |
2.6 × 10−9 |
| rs10277115 | 7 | 1251721 | 7p22 | UNCX | T/A | 0.35 | 0.0060 (0.0009) |
2.2 × 10−10 | 0.0034 (0.0015) |
0.022 | 0.0052 (0.0008) |
4.6 × 10−11 |
| rs963837 | 11 | 30705666 | 11p14 |
MPPED2- DCDC5 |
T/C | 0.64 | 0.0036 (0.0007) |
7.5 × 10−7 | 0.0040 (0.0012) |
0.0013 | 0.0037 (0.0006) |
3.4 × 10−9 |
| rs671 | 12 | 110726149 | 12q24.2 | ALDH2 | A/G | 0.27 | 0.0047 (0.0009) |
5.0 × 10−8 | 0.0040 (0.0013) |
0.0015 | 0.0045 (0.0007) |
2.8 × 10−10 |
| eGFRcrea (n = 42,451 for GWAS meta-analysis, n = 19,636 for replication) | ||||||||||||
| rs3828890 | 6 | 31548648 | 6p21 | MHC region | G/C | 0.11 | −0.0091 (0.0017) |
9.8 × 10−8 | −0.0062 (0.0020) |
0.0018 | −0.0079 (0.0013) |
1.2 × 10−9 |
| rs10277115 | 7 | 1251721 | 7p22 | UNCX | T/A | 0.35 | −0.0066 (0.0011) |
7.3 × 10−10 | −0.0039 (0.0016) |
0.014 | −0.0058 (0.0009) |
1.0 × 10−10 |
| rs963837 | 11 | 30705666 | 11p14 |
MPPED2- DCDC5 |
T/C | 0.64 | −0.0041 (0.0008) |
6.3 × 10−7 | −0.0048 (0.0013) |
3.8 × 10−4 | −0.0043 (0.0007) |
1.1 × 10−9 |
| Uric acid (n = 21,417 for GWAS meta-analysis, n = 11,657 for replication) | ||||||||||||
| rs889472 | 16 | 78203490 | 16q23 | MAF | C/A | 0.57 | 0.0758 (0.0136) |
2.8 × 10−8 | 0.0584 (0.0227) |
0.010 | 0.0711 (0.0117) |
1.1 × 10−9 |
Chr., chromosome; SE, standard error, Freq., frequency.
SNPs in the newly identified loci associated with kidney function-related traits.
The allele that increased blood urea nitrogen, serum creatinine or uric acid concentration or that decreased eGFRcrea was defined as allele 1 (Al) and is indicated on the basis of the forward strand of NCBI Build 36.
Frequency of allele 1 in the GWAS meta-analysis.
Effect size of allele 1 on common log-transformed values of blood urea nitrogen or serum creatinine concentration, eGFRcrea or non-transformed values of uric acid concentration.
To determine whether the associations that we observed were relevant to populations of European ancestry, we evaluated the newly associated loci (at P < 5.0 × 10−8 in our combined meta-analysis) in Europeans by using the results of studies by the KidneyGen (n = 23,812 for serum creatinine concentration)6, CKDGen (n = 67,093 for eGFRcrea)7 and GUGC (n = 110,347 for uric acid concentration; A. Köttgen et al., personal communication) consortia. Nine of the 15 loci that reached P < 5.0 × 10−8 for serum creatinine, eGFRcrea and uric acid measures in our study also showed significant associations in the European study (P < 0.05/15 = 0.0033, Bonferroni correction for the number of loci with available results), including the MPPED2-DCDC5 locus for eGFRcrea (P= 5.3 × 10−8 at rs963837; Supplementary Table 5).
We also evaluated the loci previously reported to be associated with kidney function measures, after excluding the 14 loci that had already been identified in our study (Supplementary Table 7)4, 5, 6, 7, 8, 9, 10, 11, 12, 13. Of the 31 loci evaluated, we replicated associations at 8 loci in our study (P< 0.05/31 = 0.0016, Bonferroni correction for the number of loci), including in CPS1, RGS14, STC1, RNASEH2C-OVOL1 and SLC6A13 for eGFRcrea and GCKR, LRP2 and LRRC16A-SLC17A1 for uric acid concentration.
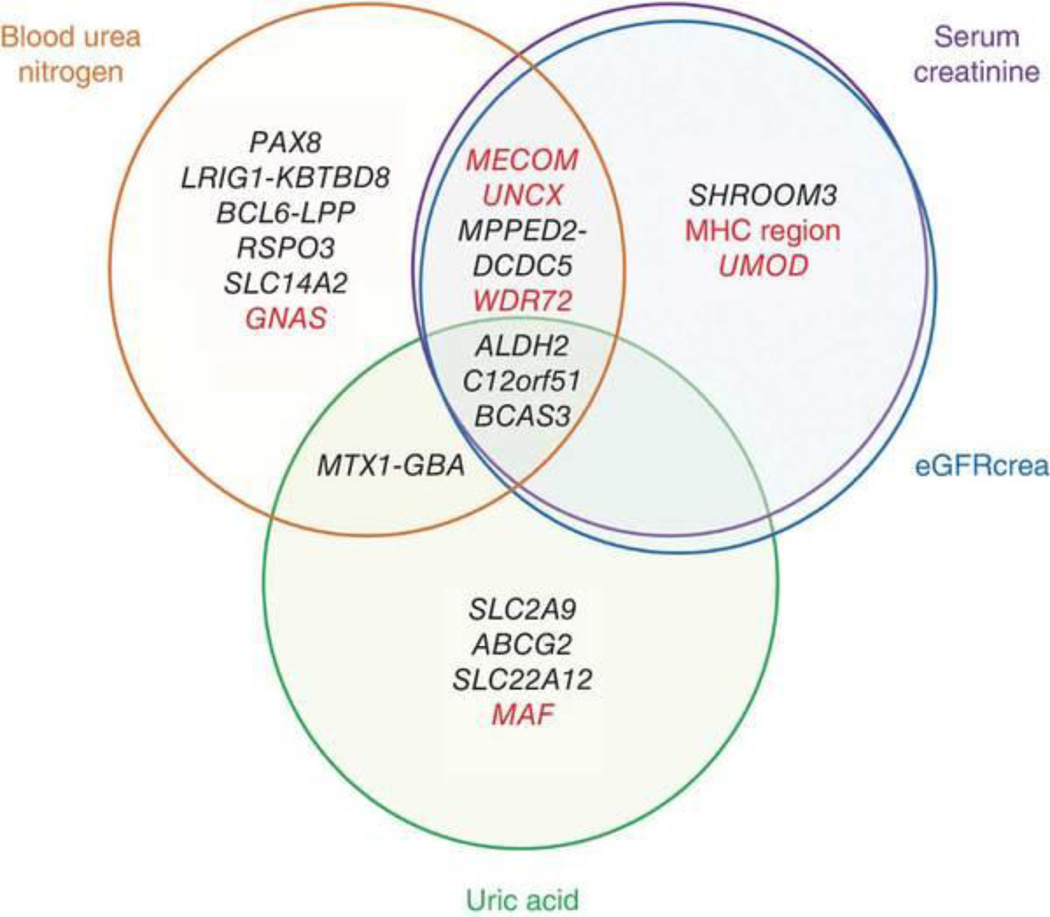
As the evaluated phenotypes reflect both common and unique biological aspects of kidney status, it is of interest to understand whether the loci associated with kidney function traits show pleiotropic patterns of associations18. We evaluated the associations of the identified loci within the evaluated kidney function–related traits and risk of stage 3+ CKD (defined as eGFRcrea of <60 ml/min/1.73 m2; Fig. 2, Table 2 and Supplementary Table 8)1, 2. Of 21 unique loci, 9 yielded significant associations with three or more phenotypes (P < 0.05/21 = 0.0024, Bonferroni correction for the number of loci). In particular, the ALDH2, C12orf51 and BCAS3 loci had significant associations with all of the evaluated kidney function–related traits. We also observed significant risk for CKD at several loci, including in MECOM, the MHC region, UNCX, WDR72, UMOD, MAF and GNAS. Because of the definition of CKD1, 2, previous studies assessed CKD risk primarily at the loci associated with serum creatinine concentration and eGFRcrea4, 6, 7, 8. However, our results suggest that genetic risk for CKD would also be contributed to by other kidney function–related loci, such as MAF and GNAS. Recent studies suggested the superiority of eGFR based on serum cystatin C concentration (eGFRcys) relative to eGFRcrea, especially for predicting GFR in subjects with normal or mildly reduced GFR, and assessment of the genetic factors underlying eGFRcys in east Asians would thus be warranted.
Figure 2. Venn diagram of pleiotropic associations of the identified loci.
Genetic loci identified in the study are classified on the basis of the results of the pleiotropic association study of kidney function–related traits (Table 2 and Supplementary Table 8). Genes that showed significant associations with risk for stage 3+ CKD are colored red.
Table 2.
Pleiotropic associations of the identified loci with kidney function–related traits and CKD risk
| Blood urea nitrogen (n = 57,178) |
Serum creatinine (n = 61,919) |
eGFRcrea (n = 62,087) |
Uric acid (n = 33,074) |
CKD (8,805 cases and 35,259 controls) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Pb | Pb | Pb | Pb | |||||
| rs2049805 | 1 | 153461604 | 1q22 | MTX1-GBA | 1.8 × 10−12 | 0.027 | 0.046 | 0.0022 | 0.75 |
| rs11123170 | 2 | 113695411 | 2q13 | PAX8 | 3.3 × 10−10 | 0.0059 | 0.0082 | 0.079 | 0.0058 |
| rs13069000 | 3 | 66881640 | 3p14 | LRIG1-KBTBD8 | 1.4 × 10−19 | 0.24 | 0.42 | 0.0093 | 0.94 |
| rs16853722 | 3 | 170633326 | 3q26 | MECOM | 2.7 × 10−14 | 9.4 × 10−4 | 8.3 × 10−4 | 0.76 | 9.9 × 10−4 |
| rs10937329 | 3 | 189196412 | 3q27 | BCL6-LPP | 8.8 × 10−30 | 0.90 | 0.93 | 0.36 | 0.40 |
| rs3775948 | 4 | 9604280 | 4p16 | SLC2A9 | 0.061 | 0.40 | 0.36 | 1.6 × 10−65 | 0.13 |
| rs13146355 | 4 | 77631164 | 4q21 | SHROOM3 | 0.043 | 9.4 × 10−12 | 6.6 × 10−11 | 0.16 | 0.090 |
| rs2725220 | 4 | 89178946 | 4q22 | ABCG2 | 0.20 | 0.18 | 0.23 | 4.2 × 10−30 | 0.44 |
| rs3828890 | 6 | 31548648 | 6p21 | MHC region | 0.14 | 2.6 × 10−9 | 1.2 × 10−9 | 0.051 | 0.0016 |
| rs1936800 | 6 | 127477757 | 6q22 | RSPO3 | 1.2 × 10−11 | 0.64 | 0.57 | 0.78 | 0.72 |
| rs10277115 | 7 | 1251721 | 7p22 | UNCX | 1.9 × 10−9 | 4.6 × 10−11 | 1.0 × 10−10 | 0.014 | 1.7 × 10−6 |
| rs10767873 | 11 | 30725254 | 11p14 | MPPED2-DCDC5 | 4.5 × 10−16 | 4.3 × 10−7 | 1.8 × 10−7 | 0.012 | 0.0055 |
| rs504915 | 11 | 64220661 | 11q13 | SLC22A12 | 0.68 | 0.32 | 0.39 | 3.3 × 10−63 | 0.74 |
| rs671 | 12 | 110726149 | 12q24.2 | ALDH2 | 1.3 × 10−5 | 2.8 × 10−10 | 7.8 × 10−8 | 1.6 × 10−6 | 0.16 |
| rs2074356 | 12 | 111129784 | 12q24.13 | C12orf51 | 1.8 × 10−9 | 1.9 × 10−9 | 6.5 × 10−8 | 1.6 × 10−5 | 0.14 |
| rs17730281 | 15 | 51695240 | 15q21 | WDR72 | 3.0 × 10−11 | 3.6 × 10−14 | 1.3 × 10−13 | 0.29 | 1.3 × 10−8 |
| rs11864909 | 16 | 20308340 | 16p12 | UMOD | 0.0058 | 1.1 × 10−10 | 3.6 × 10−10 | 0.87 | 7.0 × 10−4 |
| rs889472 | 16 | 78203490 | 16q23 | MAF | 0.30 | 0.29 | 0.30 | 1.1 × 10−9 | 0.0012 |
| rs11868441 | 17 | 56594003 | 17q23 | BCAS3 | 2.1 × 10−9 | 0.010 | 0.0098 | 0.0089 | 0.062 |
| rs9895661 | 17 | 56811371 | 17q23 | BCAS3 | 0.65 | 7.4 × 10−11 | 4.8 × 10−11 | 9.3 × 10−4 | 0.0060 |
| rs7227483 | 18 | 41441128 | 18q12 | SLC14A2 | 6.7 × 10−18 | 0.32 | 0.32 | 0.033 | 0.11 |
| rs6026584 | 20 | 56902468 | 20q13 | GNAS | 8.8 × 10−9 | 0.19 | 0.10 | 0.0041 | 0.0022 |
Detailed results of the analysis are provided in Supplementary Table 8.
Indicated on the basis of the forward strand of NCBI Build 36.
P values that satisfied the Bonferroni correction based on the number of loci (α = 0.05, n = 21; P < 0.0024) are shown in bold.
In this study, we identified new associations at MTX1-GBA, PAX8, MECOM, the MHC region, UNCX, MPPED2-DCDC5, ALDH2, C12orf51, WDR72, MAF, BCAS3 and GNAS with kidney function–related traits. MTX1 has an essential role in embryonic development, and GBA encodes glucocerebrosidase, an enzyme mediating glycolipid metabolism19. Both are known as causal genes in Gaucher disease19, a lysosomal storage disease, although kidney function decline has not been implicated in pathogenesis. PAX8 is a member of the PAX gene family and is widely expressed in renal tissues20. MECOM (also known as EVI1) encodes a transcriptional regulator involved in hematopoiesis21. The MHC region contains a large number of genes related to the immune system, including human leukocyte antigen (HLA) genes. The SNP that was found to be associated with serum creatinine concentration and eGFRcrea (rs3828890) was located in the MHC class I region22 and was in moderate linkage disequilibrium with the HLA-DRB1*1302 and HLA-DQB1*0604 alleles (D′ > 0.65 and r2 > 0.40 for both alleles)23. UNCX encodes a paired-type homeobox transcription factor that has essential roles in skeleton formation and kidney development24. The function of MPPED2 is as yet unknown, and DCDC5 encodes a protein with two doublecortin domains, which serve as protein-interaction platforms25. It is noteworthy that the MTX1-GBA, MECOM and MPPED2-DCDC5 loci have been reported to influence serum magnesium levels26, which are maintained by renal regulation of magnesium reabsorption. The loci in ALDH2, WDR72 and BCAS3 have been reported to be associated with some kidney function measures5, 7, although the biological roles of these genes in renal homeostasis have not been substantially explored. Although the function of the protein encoded by C12orf51 has not been examined, this locus was reported to be associated with serum lipid and liver enzyme concentrations in east Asians9. MAF encodes a leucine zipper transcription factor and has been implicated in the pathogenesis of minimal-change nephrotic syndrome (MCNS)27. Defects in MAF cause juvenile-onset pulverulent cataract as well as congenital cerulean cataract (CCA4)28. GNAS encodes the heterotrimeric G protein Gsα, and the associated locus in this gene is also associated with multiple metabolic traits, including blood pressure, in Europeans29. Nevertheless, other genes near each of the loci could also be candidates, and further functional assessment is desirable.
In conclusion, in this large-scale meta-analysis in east Asian populations, we identified multiple loci newly associated with kidney function–related traits and pleiotropic associations. Our study should make an important contribution to the enhanced understanding of the genetic architecture of kidney function.
URLs
International HapMap Project, http://hapmap.ncbi.nlm.nih.gov/; MACH and mach2qtl software, http://www.sph.umich.edu/csg/abecasis/MACH/index.html; IMPUTE and SNPTEST software, http://www.stats.ox.ac.uk/~marchini/software/gwas/gwas.html; BEAGLE software, http://faculty.washington.edu/browning/beagle/beagle.html; PLINK software, http://pngu.mgh.harvard.edu/~purcell/plink/; R statistical software, http://cran.r-project.org/; SNAP software, http://www.broadinstitute.org/mpg/snap/index.php.
Subjects
The 71,149 subjects included in the GWAS meta-analysis for kidney function–related traits (n = 57,178, 61,919, 62,087 and 33,074 for blood urea nitrogen, eGFRcrea and uric acid, respectively) were obtained from 18 studies conducted in the following 11 population-, hospital- or family-based cohorts of east Asian populations through the collaborations of AGEN9, 14, 15, 16: the BioBank Japan Project (BBJ), the Singapore Prospective Study Program (SP2), the Singapore Malay Eye Study (SiMES), the Singapore Indian Study (SINDI), the Singapore Chinese Eye Study (SCES), the Korea Association Resource project (KARE), the Health Examinee shared control study (HEXA), the Taiwan Super Control Study (TWSC), the Taiwan Type 2 Diabetes Consortium (TWT2D), the Genetic Epidemiology Network of Salt Sensitivity (GenSalt) and Cardio-metabolic Genome Epidemiology (CAGE). Of these, 51,327 subjects were enrolled in the GWAS meta-analysis, and 19,822 subjects were enrolled in the in silico replication study. Some of the subjects were included in previous studies of east Asian populations9, 14, 15, 16. All participants in each cohort provided written informed consent for participation in the study, as approved by the ethical committees of each of the institutional review boards. Each study established a consensus on subject participation and phenotype definition and analytical protocol for the project. Detailed descriptions of the participating cohorts and the characteristics of the subjects are provided in Supplementary Tables 1 and 2 and in the Supplementary Note. Details of the European studies enrolled by the KidneyGen (n = 23,812 for serum creatinine concentration), CKDGen (n = 67,093 for eGFRcrea) and GUGC (n= 110,347 for uric acid concentration) consortia, including subject details and the study designs, have been described at length elsewhere (refs 6, 7 and A. Köttgen et al., personal communication).
Genotyping and quality control
Genotyping platforms and quality control criteria, including exclusion of closely related subjects and outliers in terms of ancestry and cutoff values for sample call rate, SNP call rate, MAF and Hardy-Weinberg equilibrium P value are provided for each study (Supplementary Table 3 and Supplementary Note). Genotype imputation was performed on the basis of the HapMap Phase 2 panels (Japanese in Tokyo, Japan (JPT) and Han Chinese in Beijing, China (CHB) populations, except for SiMES and SINDI, for which JPT, CHB, Yoruba in Ibadan, Nigeria (YRI) and Utah residents of Northern and Western European ancestry (CEU) populations were adopted) by using MACH, IMPUTE or BEAGLE software (see URLs). After imputation, we excluded SNPs with MAF of <0.01 or imputation quality score of R2 of <0.5 from each study.
Phenotype modeling
Clinical information on the subjects, including age, gender and mean ± s.d. values for the kidney function–related traits, are provided (Supplementary Table 2). Collection methods for the clinical information in each of the cohorts are described (Supplementary Note). In this study, eGFRcrea was estimated on the basis of serum creatinine levels, using the Japanese coefficient-modified CKD Epidemiology Collaboration (CKD-EPI) equation2. We excluded subjects who were <18 or >85 years old, those who had eGFRcrea of <15 ml/min/1.73 m2 and those who had undergone renal replacement therapy. Subjects with gastrointestinal bleeding, systemic infection or hepatic failure and subjects who had undergone uric acid–lowering therapy (alloprinol, benzbromarone or probenecid) were also excluded from the analyses for blood urea nitrogen and uric acid concentration, respectively.
Genome-wide association study
Associations of SNPs with common log-transformed values of blood urea nitrogen (mg/dl), serum creatinine (mg/dl), eGFRcrea (ml/min/1.73 m2) or non-transformed values of uric acid concentration (mg/dl) were assessed by linear regression models assuming additive effects of the allele dosages of the SNPs using mach2qtl, SNPTEST, PLINK or R statistical software (see URLs). For the subjects in the family-based cohort, generalized linear mixed models accounting for the family structure were applied. In the regression model, gender, age, drinking status (current drinker or not), smoking status (previous or current smoker or not), body mass index and other cohort-specific variables were incorporated as covariates (Supplementary Note).
GWAS meta-analysis
In the GWAS meta-analysis, we included autosomal SNPs that satisfied quality control criteria in three or more GWAS for each of the traits, which yielded between 2.2 and 2.4 million SNPs (Supplementary Table 4). Information about the SNPs, including the coded alleles, was oriented to the forward strand of the NCBI Build 36 reference sequence. GWAS meta-analysis was performed using an inverse variance–weighted method, assuming a fixed-effects model for study-specific effect estimates (β) and standard errors (SE) of the coded alleles of the SNPs, using a Java source code implemented by the authors30, 31. Genomic control corrections were carried out on test statistics from each of the GWAS using study-specific inflation factors (λGC) and were applied again to the results of the GWAS meta-analysis (Supplementary Fig. 1)32.
In silico replication study
The in silico replication study was conducted using additional independent east Asian subjects (Supplementary Tables 1 and 2) for the loci that satisfied P < 5.0 × 10−6 in the GWAS meta-analysis for each of the traits (17, 14, 14 and 6 loci for blood urea nitrogen, serum creatinine, eGFRcrea and uric acid, respectively; Supplementary Table 5). For each of the loci, the SNP that showed the most significant association was selected. The associations of the SNPs were assessed in the same manner as in the GWAS. The combined study of the GWAS meta-analysis and replication was conducted using an inverse variance method, assuming a fixed-effects model30, 31. The SNPs that satisfied P < 5.0 × 10−8 in the combined study were considered to be significantly associated with the relevant kidney function–related trait, and the associations of these SNPs were further evaluated using data in European populations from the KidneyGen, CKDGen and GUGC consortia (refs 6, 7 and A. Köttgen et al., personal communication).
Estimation of explained variance
The interindividual variance in kidney function–related traits explained by the combination of the identified loci (P < 5.0 × 10−8 for each phenotype) was estimated using a genetic risk score model. We calculated the scores of the subjects enrolled in the in silico replication study by the BioBank Japan Project33 (BBJ_5 and BBJ_6; Supplementary Table 2) by summing the dosages of the effect alleles carried by the subjects, which were weighted by the effect sizes of the SNPs obtained from the GWAS meta-analysis. The explained variance was estimated from linear regression models on the covariate-adjusted phenotypes by the scores.
Pleiotropic association analysis for kidney function–related phenotypes
For the genetic loci that showed associations at P < 5.0 × 10−8 in the combined study, pleiotropic associations with the kidney function–related traits and with risk for stage 3+ CKD (defined as eGFRcrea of <60 ml/min/1.73m2)1, 2 were assessed. Associations with CKD risk were assessed using logistic regression models, incorporating the covariates using the subjects obtained from the BioBank Japan Project33 (BBJ_1– BBJ_6; Supplementary Table 2).
Supplementary Material
Acknowledgments
The authors acknowledge the essential roles of AGEN in developing the study. BBJ was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT). SP2 was funded by grants from the Biomedical Research Council of Singapore (BMRC 05/1/36/19/413 and 03/1/27/18/216) and the National Medical Research Council of Singapore (NMRC/1174/2008). SiMES was funded by the National Medical Research Council of Singapore (NMRC 0796/2003, IRG07nov013 and NMRC/STaR/0003/2008) and the Biomedical Research Council of Singapore (BMRC 09/1/35/19/616). SINDI and SCES were funded by grants from the Biomedical Research Council of Singapore (BMRC 09/1/35/19/616 and BMRC 08/1/35/19/550) and the National Medical Research Council of Singapore (NMRC/STaR/0003/2008). Y.-Y.T. acknowledges support from the Singapore National Research Foundation (NRF-RF-2010-05). E.-S.T. receives support from the National Medical Research Council of Singapore through a Clinician Scientist Award. We thank the Singapore BioBank and the Genome Institute of Singapore, Agency for Science, Technology and Research, Singapore, for providing services for tissue archiving and genotyping, respectively. KARE was supported by grants from the Korea Centers for Disease Control and Prevention (4845-301, 4851-302 and 4851-307) and an intramural grant from the Korea National Institute of Health (2010-N73002-00). Y.S.C. acknowledges support from a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2012R1A2A1A03006155). TWSC and TWT2D were supported by the Academia Sinica Genomic Medicine Multicenter Study (40-05-GMM). We acknowledge the National Center for Genome Medicine (NSC100-2319-B-001-001), the National Core Facility Program for Biotechnology of the National Science Council, Taiwan, for technical help in sample management and genotyping. GenSalt was supported by grants (U01HL072507, R01HL087263 and R01HL090682) from the National Heart, Lung, and Blood Institute, the US National Institutes of Health. CAGE was supported by grants for Core Research for Evolutional Science and Technology (CREST) from the Japan Science Technology Agency; the Program for Promotion of Fundamental Studies in Health Sciences, the National Institute of Biomedical Innovation Organization (NIBIO) and the grant of National Center for Global Health and Medicine (NCGM). We thank all the people who supported the Hospital-based Cohort Study at NCGM and the Amagasaki Study. We thank A. Taniguchi, H. Rakugi, K. Sugimoto, K. Kamide and C. Makibayashi for supporting the study.
Footnotes
-
The KidneyGen ConsortiumA full list of contributing members and affiliations is provided in the Supplementary Note.
-
The CKDGen ConsortiumA full list of contributing members and affiliations is provided in the Supplementary Note.
-
The GUGC consortiumA full list of contributing members and affiliations is provided in the Supplementary Note.
Contributions
Y.O. and T. Tanaka designed the overall study. Y.O., X.S., M.J.G., C.-H.C., D.G., F.T. and P.C. analyzed GWAS data. Y.O. performed meta-analysis and other statistical analysis. Y.O., A.T., S.M., T. Tsunoda, K.Y., M.K., Y.N., N. Kamatani and T. Tanaka managed GWAS data of BBJ. X.S., P.C., S.-C.L., T.-Y.W., J.L., T.L.Y., T.A., M.S., Y.-Y.T. and E.-S.T. managed the GWAS data from SP2, SiMES, SINDI and SCES. M.J.G., Y.J.K., J.-Y.L., B.-G.H., D.K. and Y.S.C. managed the GWAS data from KARE and HEXA. C.-H.C., F.-J.T., L.-C.C., S.-J.C.F., Y.-T.C. and J.-Y.W. managed the GWAS data from TWSC and TWT2D. D.G., H.M., D.C.R., J.E.H., S.C. and J.H. managed the GWAS data from GenSalt. F.T., T.K., M.I., T.O. and N. Kato managed the GWAS data from CAGE. J.C.C., W.Z. and J.S.K. managed the data from the KidneyGen Consortium. E.A. managed the data from the GUGC consortium. Y.O., T. Tanaka, E.-S.T., Y.S.C., J.-Y.W., J.H. and N. Kato directed the study and wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
Contributor Information
Yukinori Okada, Laboratory for Statistical Analysis, Center for Genomic Medicine (CGM), RIKEN, Yokohama, Japan; Department of Allergy and Rheumatology, Graduate School of Medicine, University of Tokyo, Tokyo, Japan.
Xueling Sim, Centre for Molecular Epidemiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Center for Statistical Genetics, University of Michigan, Ann Arbor, Michigan, USA.
Min Jin Go, Center for Genome Science, National Institute of Health, Osong Health Technology Administration Complex, Chungcheongbuk-do, Republic of Korea.
Jer-Yuarn Wu, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan; School of Chinese Medicine, China Medical University, Taichung, Taiwan.
Dongfeng Gu, State Key Laboratory of Cardiovascular Diseases, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Fumihiko Takeuchi, Department of Gene Diagnostics and Therapeutics, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan.
Atsushi Takahashi, Laboratory for Statistical Analysis, Center for Genomic Medicine (CGM), RIKEN, Yokohama, Japan.
Shiro Maeda, Laboratory for Endocrinology and Metabolism, CGM, RIKEN, Yokohama, Japan.
Tatsuhiko Tsunoda, Laboratory for Medical Informatics, CGM, RIKEN, Yokohama, Japan.
Peng Chen, Saw Swee Hock School of Public Health, National University of Singapore, Singapore.
Su-Chi Lim, Saw Swee Hock School of Public Health, National University of Singapore, Singapore; Diabetes Centre, Khoo Teck Puat Hospital, Singapore; Department of Medicine, Khoo Teck Puat Hospital, Singapore.
Tien-Yin Wong, Singapore Eye Research Institute, Singapore National Eye Centre, Singapore; Department of Ophthalmology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Centre for Eye Research Australia, University of Melbourne, East Melbourne, Victoria, Australia.
Jianjun Liu, Genome Institute of Singapore, Agency for Science, Technology and Research, Singapore.
Terri L Young, Center for Human Genetics, Duke University Medical Center, Durham, North Carolina, USA.
Tin Aung, Singapore Eye Research Institute, Singapore National Eye Centre, Singapore; Department of Ophthalmology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Mark Seielstad, Institute of Human Genetics, University of California, San Francisco, California, USA.
Yik-Ying Teo, Centre for Molecular Epidemiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Saw Swee Hock School of Public Health, National University of Singapore, Singapore; Genome Institute of Singapore, Agency for Science, Technology and Research, Singapore; Department of Statistics and Applied Probability, National University of Singapore, Singapore; National University of Singapore Graduate School for Integrative Science and Engineering, National University of Singapore, Singapore.
Young Jin Kim, Center for Genome Science, National Institute of Health, Osong Health Technology Administration Complex, Chungcheongbuk-do, Republic of Korea.
Jong-Young Lee, Center for Genome Science, National Institute of Health, Osong Health Technology Administration Complex, Chungcheongbuk-do, Republic of Korea.
Bok-Ghee Han, Center for Genome Science, National Institute of Health, Osong Health Technology Administration Complex, Chungcheongbuk-do, Republic of Korea.
Chien-Hsiun Chen, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan; School of Chinese Medicine, China Medical University, Taichung, Taiwan.
Daehee Kang, Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea.
Fuu-Jen Tsai, School of Chinese Medicine, China Medical University, Taichung, Taiwan.
Li-Ching Chang, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan.
S-J Cathy Fann, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan.
Hao Mei, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, USA.
Dabeeru C Rao, Division of Biostatistics, Washington University School of Medicine, St. Louis, Missouri, USA.
James E Hixson, Human Genetics Center, University of Texas School of Public Health, Houston, Texas, USA.
Shufeng Chen, State Key Laboratory of Cardiovascular Diseases, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Tomohiro Katsuya, Department of Clinical Gene Therapy, Osaka University Graduate School of Medicine, Suita, Japan; Department of Geriatric Medicine and Nephrology, Osaka University Graduate School of Medicine, Suita, Japan.
Masato Isono, Department of Gene Diagnostics and Therapeutics, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan.
Toshio Ogihara, Department of Geriatric Medicine and Nephrology, Osaka University Graduate School of Medicine, Suita, Japan; Morinomiya University of Medical Sciences, Osaka, Japan.
John C Chambers, Department of Epidemiology and Biostatistics, Imperial College London, London, UK.
Weihua Zhang, Department of Epidemiology and Biostatistics, Imperial College London, London, UK.
Jaspal S Kooner, National Heart and Lung Institute, Imperial College London, London, UK.
Eva Albrecht, Institute of Genetic Epidemiology, Helmholtz Zentrum München–German Research Center for Environmental Health, Neuherberg, Germany.
Kazuhiko Yamamoto, Department of Allergy and Rheumatology, Graduate School of Medicine, University of Tokyo, Tokyo, Japan.
Michiaki Kubo, Laboratory for Genotyping Development, CGM, RIKEN, Yokohama, Japan.
Yusuke Nakamura, Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, University of Tokyo, Tokyo, Japan.
Naoyuki Kamatani, Laboratory for International Alliance, CGM, RIKEN, Yokohama, Japan.
Norihiro Kato, Department of Gene Diagnostics and Therapeutics, Research Institute, National Center for Global Health and Medicine, Tokyo, Japan.
Jiang He, Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, Louisiana, USA.
Yuan-Tsong Chen, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan.
Yoon Shin Cho, Center for Genome Science, National Institute of Health, Osong Health Technology Administration Complex, Chungcheongbuk-do, Republic of Korea; Department of Biomedical Science, Hallym University, Gangwon-do, Republic of Korea.
E-Shyong Tai, Saw Swee Hock School of Public Health, National University of Singapore, Singapore; Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Duke–National University of Singapore Graduate Medical School, Singapore.
Toshihiro Tanaka, Laboratory for Cardiovascular Diseases, CGM, RIKEN, Yokohama, Japan.
References
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 2.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am. J. Kidney Dis. 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, et al. Genome-wide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J. Am. Soc. Nephrol. 2004;15:2457–2461. doi: 10.1097/01.ASN.0000135972.13396.6F. [DOI] [PubMed] [Google Scholar]
- 4.Köttgen A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat. Genet. 2009;41:712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamatani Y, et al. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat. Genet. 2010;42:210–215. doi: 10.1038/ng.531. [DOI] [PubMed] [Google Scholar]
- 6.Chambers JC, et al. Genetic loci influencing kidney function and chronic kidney disease. Nat. Genet. 2010;42:373–375. doi: 10.1038/ng.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köttgen A, et al. New loci associated with kidney function and chronic kidney disease. Nat. Genet. 2010;42:376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudbjartsson DF, et al. Association of variants at UMOD with chronic kidney disease and kidney stones—role of age and comorbid diseases. PLoS Genet. 2010;6:e1001039. doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YJ, et al. Large-scale genome-wide association studies in east Asians identify new genetic loci influencing metabolic traits. Nat. Genet. 2011;43:990–995. doi: 10.1038/ng.939. [DOI] [PubMed] [Google Scholar]
- 10.Döring A, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 2008;40:430–436. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- 11.Vitart V, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008;40:437–442. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 12.Kolz M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Q, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ. Cardiovasc. Genet. 2010;3:523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato N, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat. Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada Y, et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat. Genet. 2012;44:302–306. doi: 10.1038/ng.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen W, et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat. Genet. 2012;44:307–311. doi: 10.1038/ng.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyman JL. Blood urea nitrogen and creatinine. Emerg. Med. Clin. North Am. 1986;4:223–233. [PubMed] [Google Scholar]
- 18.Okada Y, et al. Genome-wide association study for C-reactive protein levels identified pleiotropic associations in the IL6 locus. Hum. Mol. Genet. 2011;20:1224–1231. doi: 10.1093/hmg/ddq551. [DOI] [PubMed] [Google Scholar]
- 19.LaMarca ME, et al. A novel alteration in metaxin 1, F202L, is associated with N370S in Gaucher disease. J. Hum. Genet. 2004;49:220–222. doi: 10.1007/s10038-004-0134-7. [DOI] [PubMed] [Google Scholar]
- 20.Tong GX, et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod. Pathol. 2009;22:1218–1227. doi: 10.1038/modpathol.2009.88. [DOI] [PubMed] [Google Scholar]
- 21.Kataoka K, et al. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. J. Exp. Med. 2011;208:2403–2416. doi: 10.1084/jem.20110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MHC Sequencing Consortium. Complete sequence and gene map of a human major histocompatibility complex. Nature. 1999;401:921–923. doi: 10.1038/44853. [DOI] [PubMed] [Google Scholar]
- 23.de Bakker PI, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat. Genet. 2006;38:1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansouri A, et al. Paired-related murine homeobox gene expressed in the developing sclerotome, kidney, and nervous system. Dev. Dyn. 1997;210:53–65. doi: 10.1002/(SICI)1097-0177(199709)210:1<53::AID-AJA6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Reiner O, et al. The evolving doublecortin (DCX) superfamily. BMC Genomics. 2006;7:188. doi: 10.1186/1471-2164-7-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer TE, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. 2010;6:e1001045. doi: 10.1371/journal.pgen.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahali D, et al. A novel approach to investigation of the pathogenesis of active minimal-change nephrotic syndrome using subtracted cDNA library screening. J. Am. Soc. Nephrol. 2002;13:1238–1247. doi: 10.1681/ASN.V1351238. [DOI] [PubMed] [Google Scholar]
- 28.Vanita V, Singh D, Robinson PN, Sperling K, Singh JR. A novel mutation in the DNA-binding domain of MAF at 16q23.1 associated with autosomal dominant “cerulean cataract” in an Indian family. Am. J. Med. Genet. A. 2006;140:558–566. doi: 10.1002/ajmg.a.31126. [DOI] [PubMed] [Google Scholar]
- 29.Ehret GB, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada Y, et al. Identification of nine novel loci associated with white blood cell subtypes in a Japanese population. PLoS Genet. 2011;7:e1002067. doi: 10.1371/journal.pgen.1002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada Y, et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat. Genet. 2012;44:511–516. doi: 10.1038/ng.2231. [DOI] [PubMed] [Google Scholar]
- 32.de Bakker PI, et al. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum. Mol. Genet. 2008;17:R122–R128. doi: 10.1093/hmg/ddn288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura Y. The BioBank Japan Project. Clin. Adv. Hematol. Oncol. 2007;5:696–697. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.