Abstract
Chronic glomerulonephritis (GN), which includes focal segmental glomerulosclerosis and proliferative forms of GN such as IgA nephropathy, increases the risk of hypertension. Hypertension in chronic GN is primarily volume dependent, and this increase in blood volume is not related to the deterioration of renal function. Patients with chronic GN become salt sensitive as renal damage including arteriolosclerosis progresses and the consequent renal ischemia causes the stimulation of the intrarenal renin-angiotensin-aldosterone system(RAAS). Overactivity of the sympathetic nervous system also contributes to hypertension in chronic GN. According to the KDIGO guideline, the available evidence indicates that the target BP should be ≤140mmHg systolic and ≤90mmHg diastolic in chronic kidney disease patients without albuminuria. In most patients with an albumin excretion rate of ≥30mg/24 h (i.e., those with both micro-and macroalbuminuria), a lower target of ≤130mmHg systolic and ≤80mmHg diastolic is suggested. The use of agents that block the RAAS system is recommended or suggested in all patients with an albumin excretion rate of ≥30mg/ 24 h. The combination of a RAAS blockade with a calcium channel blocker and a diuretic may be effective in attaining the target BP, and in reducing the amount of urinary protein excretion in patients with chronic GN.
Keywords: Chronic glomerulonephritis, Volume dependent, RAAS blockade
Prevalence of hypertension in chronic glomerulonephritis
Hypertension is a frequent finding in chronic kidney diseases. Almost all patients develop hypertension when the glomerular filtration rate (GFR) declines. Renal parenchymal hypertension develops in the setting of acute glomerulonephritis (GN), chronic GN, diabetic nephropathy, polycystic kidney disease, hypertensive nephrosclerosis, and renal microvascular disorders. Mild to moderate hypertension occurs in more than 75% of patients with acute forms of GN, such as poststreptococcal GN1). Patients with acute GN have hypertension primarily due to sodium retention leading to fluid overload, as evidenced by suppression of the renin-angiotensin-aldosterone (RAAS) system.
Causes of chronic GN with hypertension include IgA nephropathy (IgAN), membranous nephropathy, membranoproliferative GN, and focal segmental glomerulosclerosis1). According to one report, hypertension was frequently found in focal segmental sclerosis, membranous nephropathy, IgAN, and membranoproliferative GN2). Hypertension is a presenting feature in one third of patients with focal segmental sclerosis. Five years after renal biopsy, 92% of normotensive and 47% of hypertensive patients remained with normal renal function. These findings suggest that the high prevalence of hypertension in chronic GN is related to declining renal function. In IgAN, 9-53% and 7-15% of patients have hypertension and malignant hypertension, respectively. It was also reported that non-dipper hypertension was observed in 93% of the hypertensive IgAN patients3). Hypertension and the lack of a circadian rhythm can accelerate the progression of chronic GN which can, in turn, be slowed by the treatment of hypertension.
Pathophysiology of hypertension in chronic GN
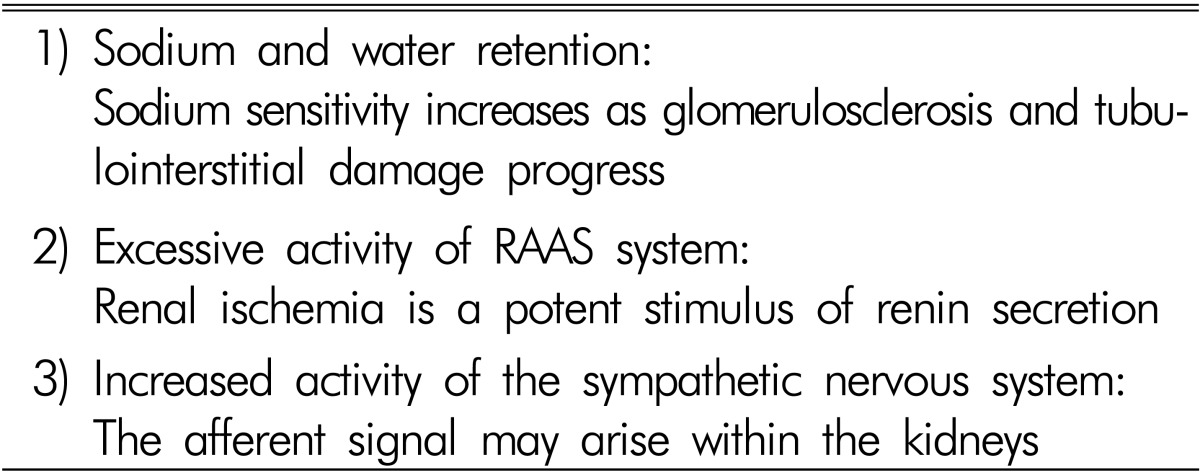
There are three main factors contributing to the development of hypertension in patients with chronic GN, which are similar to those in essential hypertension, but more accentuated (Table 1). Sodium retention is of primary importance. Increased RAAS activity is responsible for the hypertension. Renal ischemia induced by microvascular damage is a potent stimulus of RAAS. Hypertension also results from overactivity of the sympathetic nervous system. Much evidence indicates increased sympathetic nervous activity in renal disease. Renal ischemia is probably a primary event leading to increased sympathetic nervous activity4,5).
Table 1. Pathogenesis of hypertension in chronic glomerulonephritis.
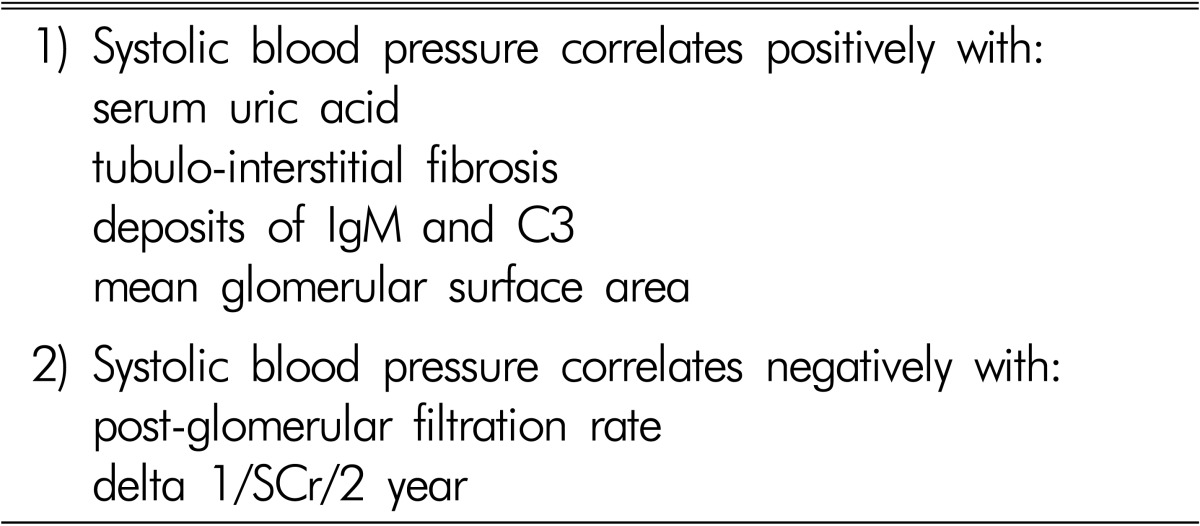
It was earlier reported that the blood volume was high in patients with IgAN, and that mean arterial pressure was correlated with blood volume, but not with plasma renin activity and GFR. Therefore, it has been suggested that hypertension in IgAN is primarily volume dependent, and that this increase in blood volume is not related to the deterioration of renal function6). Among IgAN patients with mild proteinuria, hypertension was associated with glomerular sclerosis, interstitial fibrosis/tubular atrophy, interstitial infiltration, and arteriosclerosis, but was not associated with the mesangial score. Arteriosclerosis was positive in 38.6% of hypertensives compared with 3.2% of normotensives. Hypertension affected the prognosis of mild proteinuric IgAN nephropathy through vascular lesions7). It was reported that in IgAN and membranous nephropathy, the patients with arteriolar hyalinosis and hypertension are characterized by higher values of glomerular sclerosis and tubulointerstitial damage, which are both responsible for reduced capillary bed perfusion pressure leading to a reduction of interstitial blood flow and consequent hypoxia in the interstitial compartment8). Furthermore, the sodium sensitivity index and scores for glomerular sclerosis and tubulointerstitial damage were higher in IgAN patients with normal to high-normal blood pressure (BP) or hypertension than in those with optimal BP9). The mean pressure-natriuresis curve was steeper in the optimal group than that in the normal to high-normal or hypertensive IgAN groups. The sodium sensitivity index was significantly correlated with glomerular sclerosis and tubulointerstitial damage. The increased sodium sensitivity appeared before hypertension and sodium restriction lowered BP to the optimal range and decreased proteinuria. We reported in 234 patients with IgAN that 121 patients (52%) had systolic BP ≥130mmHg and 74 (32%) ≥140mmHg. Systolic BP was positively correlated with serum uric acid concentrations and with pathological findings, including glomerulomegaly and the degree of tubulointerstitial fibrosis and deposits of IgM and C3, and negatively with post-glomerular filtration rate and the slope of change in 1/serum creatinine for 2 years (Table 2). Patients with systolic BP ≥130mmHg compared with those <130mmHg were older and showed more severe clinico-pathological findings such as pre- and post-serum creatinine, amount of proteinuria, glomerulomegaly, tubulointerstitial fibrosis, and deposits of IgM and C310,11,12).
Table 2. Relationship between blood pressure and clinicopathological findings in patients with IgA nephropathy.
It was observed in patients with IgAN that urinary angiotensinogen levels, renal tissue angiotensinogen expression and angiotensin II immunoreactivity were significantly higher, and that urinary angiotensinogen reflects the activity of the intrarenal RAAS system13). Even though the IgAN patients did not show massive renal damage, immunoreactivity of hemeoxygenase-1 and angiotensinogen were increased in these patients at this time point. These data suggest that intrarenal reactive oxygen species and RAAS activation play a pivotal role in the development of IgAN during the early stages and provide a supportive foundation for the effectiveness of RAAS blockade in IgAN14). We observed that urine angiotensinogen levels correlate with the urine protein-to-creatinine ratio and serum creatinine concentrations in patients with IgAN11). Finally, increased urinary angiotensinogen levels induced by salt and associated renal damage leads to the development of salt-sensitive hypertension in patients with IgAN15).
Therefore, it can be speculated that patients with chronic GN become salt sensitive as renal damage progresses, and the consequent reduction of interstitial blood flow and hypoxia causes the stimulation of the intrarenal RAAS which, in turn, contributes to the development of saltsensitive hypertension.
Several studies have shown the gene polymorphisms contributing to hypertension and the relationship between BP-related genes and disease progression in patients with IgAN. Men with AGT M235T TT were found to be at an increased risk of IgAN progression compared to those with the other genotypes16). CD14 -159CC and ACE DD were independently associated with hypertension17). There was a significant difference in the therapeutic response to ARB between the DD/ID genotype and the II genotype18).
New aspects of anti-hypertensive therapy in patients with chronic GN
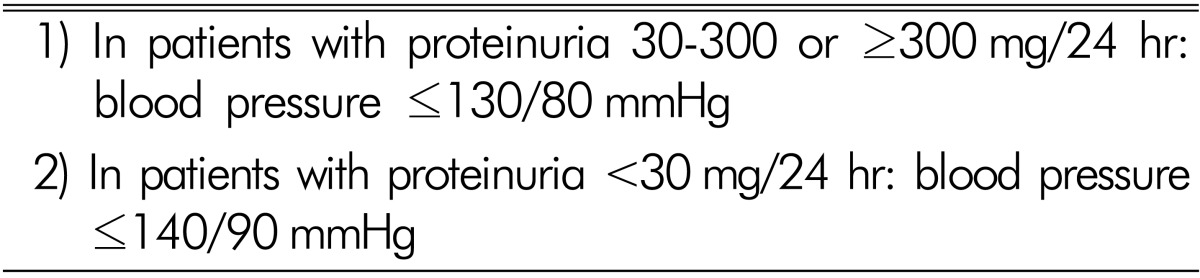
According to the KDIGO guideline19), the available evidence indicates that in chronic kidney disease patients without albuminuria the target BP should be ≤140mmHg systolic and ≤90mmHg diastolic. In most patients with an albumin excretion rate of ≥30mg /24 h (i.e., those with both micro- and macroalbuminuria), a lower target of ≤130mmHg systolic and ≤80mmHg diastolic is suggested (Table 3). In achieving BP control, the value of lifestyle changes and the need for multiple pharmacological agents is acknowledged. However, the JNC VIII and ESH/ESC guidelines recommend a systolic BP goal of <140mmHg in patients with chronic kidney disease20,21). Thus, the most appropriate targets for systolic BP to reduce cardiovascular morbidity and mortality among persons are still uncertain.
Table 3. The target blood pressure in the KDIGO clinical practice guidelines in non-diabetic chronic kidney disease.
Intensive BP treatment was examined in the several studies, such as ACCORD22), HALT-PKD23) and SPRINT24). It was recently reported in the SPRINT trial that among non-diabetic patients at high risk for cardiovascular events, targeting a systolic BP of <120mm Hg, as compared with <140mm Hg, resulted in lower rates of fatal and nonfatal major cardiovascular events and of death from any cause, although significantly higher rates of some adverse events were observed in the intensive-treatment group. Among patients with polycystic kidney disease in the HALT-PKD study, as compared with standard BP control, rigorous BP control was associated with a slower increase in total kidney volume, no overall change in the estimated GFR, a greater decline in the left-ventricularmass index, and greater reduction in urinary albumin excretion. The ACCORD study, on the contrary, showed in older patients with type 2 diabetes that intensive antihypertensive therapy did not significantly reduce the rate of a composite outcome of fatal and nonfatal major cardiovascular events. An intensive strategy reduced the new development of microalbuminuria by 16%, but had no significant effect on other microvascular end points or composites. So far, the evidence suggests that a lower target of ≤130mmHg systolic and ≤80mmHg diastolic may be appropriate for patients with chronic GN and both micro- and macroalbuminuria if they are not old and do not have high cardiovascular risk factors.
Which is the best anti-hypertensive drug? According to the KDOQI guideline, hypertensive people with diabetes and chronic kidney disease stages 1-4 should be treated with an ACE inhibitor or an angiotensin receptor blocker, usually in combination with a diuretic25). An approach to diabetic nephropathy based on RAAS blockade in type II diabetes is projected to result in a lower incidence of end-stage renal disease (66%) compared with placebo patients. It is also suggested that using an ACE inhibitor or an angiotensin receptor blocker in normotensive patients with diabetes and albuminuria levels ≥30mg/g is more beneficial. In the KDIGO guideline, the use of agents that block the RAAS system is recommended or suggested in all patients with an albumin excretion rate of ≥30 mg/24 h19). The ESH /ESC hypertension guideline announced that RAS blockers are more effective in reducing albuminuria than other antihypertensive agents21). The ONTARGET study showed that both angiotensin receptor blockers and ACE inhibitors were equally effective in improving outcome (dialysis, doubling of serum creatinine, and death), and the number of events for the composite outcome was similar for both26). Because of the reduction in renal function, the use of diuretics is mainly important in patients with chronic kidney disease who are usually volume overloaded.
The attainment of target BP in patients with chronic kidney disease typically requires multidrug therapy as seen in several clinical studies. It is commonly accepted that a combination of an ACE inhibitor with a calcium channel blocker is superior to placebo and equivalent or superior to ACE inhibitors alone for inhibiting the progression of diabetic kidney disease. The ACCOMPLISH study showed that the combination of an ACE inhibitor with a calcium channel blocker is superior to the combination of ACE inhibitors with a beta blocker27).
A number of trials and meta-analyses have shown that the combination of ACE inhibitor/angiotensin receptor blocker therapy has a greater anti-proteinuric effect than either agent alone. However, in the dual-therapy group of the Ontarget study, even though there was a significant reduction in proteinuria, there was an increase in adverse effects and worsening renal outcomes26). Whether the combination of ACE inhibitor/angiotensin receptor blocker therapy may be beneficial for the cardio-renal outcomes in non-elderly patients with chronic GN who do not have high cardiovascular risk remains unresolved.
In summary, the combination of an ACE inhibitor/angiotensin receptor blocker with a calcium channel blocker (long-acting dihydropyridine or non-dihydropyridine) and a diuretic may be effective to attain the target BP and to reduce the amount of urinary protein excretion in patients with chronic GN.
References
- 1.Nachman PH, Jennette JC, Falk RJ. Primary glomerular disease. In: Brenner BM, Rector FC Jr, editors. The kidney. 10th ed. Philadelphia: Saunders; 2011. pp. 1101–1191. [Google Scholar]
- 2.Orofino L, Quereda C, Lamas S, Orte L, Gonzalo A, Mampaso F, et al. Hypertension in primary chronic glomerulonephritis: analysis of 288 biopsied patients. Nephron. 1987;45:22–26. doi: 10.1159/000184065. [DOI] [PubMed] [Google Scholar]
- 3.Csiky B, Kovács T, Wágner L, Vass T, Nagy J. Ambulatory blood pressure monitoring and progression in patients with IgA nephropathy. Nephrol Dial Transplant. 1999;14:86–90. doi: 10.1093/ndt/14.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan NM. Clinical Hypertension. 7th ed. Baltimore: Williams & Wilkins; 1998. Renal parenchymal hypertension; pp. 281–299. [Google Scholar]
- 5.Joles JA, Koomans HA. Causes and consequences of increased sympathetic activity in renal disease. Hypertension. 2004;43:699–706. doi: 10.1161/01.HYP.0000121881.77212.b1. [DOI] [PubMed] [Google Scholar]
- 6.Valvo E, Gammaro L, Bedogna V, Giorgetti PG, Tonon M, Panzetta GO, et al. Hypertension in primary immunoglobulin A nephropathy (Berger’s disease): hemodynamic alterations and mechanisms. Nephron. 1987;45:219–223. doi: 10.1159/000184121. [DOI] [PubMed] [Google Scholar]
- 7.Ikee R, Kobayashi S, Saigusa T, Namikoshi T, Yamada M, Hemmi N, et al. Impact of hypertension and hypertension-related vascular lesions in IgA nephropathy. Hypertens Res. 2006;29:15–22. doi: 10.1291/hypres.29.15. [DOI] [PubMed] [Google Scholar]
- 8.Bazzi C, Stivali G, Rachele G, Rizza V, Casellato D, Nangaku M. Arteriolar hyalinosis and arterial hypertension as possible surrogate markers of reduced interstitial blood flow and hypoxia in glomerulonephritis. Nephrology. 2015;20:11–17. doi: 10.1111/nep.12339. [DOI] [PubMed] [Google Scholar]
- 9.Konishi Y, Okada N, Okamura M, Morikawa T, Okumura M, Yoshioka K, et al. Sodium sensitivity of blood pressure appearing before hypertension and related to histological damage in immunoglobulin a nephropathy. Hypertension. 2001;38:81–85. doi: 10.1161/01.hyp.38.1.81. [DOI] [PubMed] [Google Scholar]
- 10.Jang WS, Jeong KH, Moon JY, Lee SH, Cho JH, Lee TW, et al. Relationship between glomerulomegaly and clinicopathologic findings in IgA nephropathy. Clin Nephrol. 2012;78:470–477. doi: 10.5414/CN107614. [DOI] [PubMed] [Google Scholar]
- 11.Kim YG, Song SB, Lee SH, Moon JY, Jeong KH, Lee TW, et al. Urinary angiotensinogen as a predictive marker in patients with immunoglobulin A nephropathy. Clin Exp Nephrol. 2011;15:720–726. doi: 10.1007/s10157-011-0475-4. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Choi SY, Jeong KH, Sung JY, Moon SK, Moon JY, et al. Association of C1q deposition with renal outcomes in IgA nephropathy. Clin Nephrol. 2013;80:98–104. doi: 10.5414/CN107854. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, et al. Urnary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–177. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, et al. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, et al. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension. 2011;58:205–211. doi: 10.1161/HYPERTENSIONAHA.110.166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SM, Chin HJ, Oh YK, Kim YS, Kim S, Lim CS. Blood pressure-related genes and the progression of IgA nephropathy. Nephron Clin Pract. 2009;113:c301–c308. doi: 10.1159/000235948. [DOI] [PubMed] [Google Scholar]
- 17.Shinzawa M, Yamamoto R, Nagasawa Y, Shoji T, Obi Y, Namba T, et al. Gene polymorphisms contributing to hypertension in immunoglobulin A nephropathy. Clin Exp Nephrol. 2012;16:250–258. doi: 10.1007/s10157-011-0553-7. [DOI] [PubMed] [Google Scholar]
- 18.Park M, Jeong K, Moon J, Lee T, Ihm C, Lee S. The Treatment Response to Angiotensin Receptor Blocker According to ACE Gene Polymorphism in Patients with IgA Nephropathy. Korean J Nephrol. 2008;27:13–19. [Google Scholar]
- 19.Stevens PE, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 20.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 21.ESH/ESC Task Force for the Management of Arterial Hypertension: 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 22.ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson RG, Tuttle KR. The new KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and CKD. Blood Purif. 2007;25:112–114. doi: 10.1159/000096407. [DOI] [PubMed] [Google Scholar]
- 26.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, doubleblind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 27.Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, et al. Renal outcomes with different fixeddose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375:1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]