Abstract
Deregulation of angiogenesis – the growth of new blood vessels from an existing vasculature – is a main driving force in many severe human diseases including cancer. As such, tumor angiogenesis is important for delivering oxygen and nutrients to growing tumors, and therefore considered an essential pathologic feature of cancer, while also playing a key role in enabling other aspects of tumor pathology such as metabolic deregulation and tumor dissemination/metastasis. Recently, inhibition of tumor angiogenesis has become a clinical anti-cancer strategy in line with chemotherapy, radiotherapy and surgery, which underscore the critical importance of the angiogenic switch during early tumor development. Unfortunately the clinically approved anti-angiogenic drugs in use today are only effective in a subset of the patients, and many who initially respond develop resistance over time. Also, some of the anti-angiogenic drugs are toxic and it would be of great importance to identify alternative compounds, which could overcome these drawbacks and limitations of the currently available therapy. Finding “the most important target” may, however, prove a very challenging approach as the tumor environment is highly diverse, consisting of many different cell types, all of which may contribute to tumor angiogenesis. Furthermore, the tumor cells themselves are genetically unstable, leading to a progressive increase in the number of different angiogenic factors produced as the cancer progresses to advanced stages. As an alternative approach to targeted therapy, options to broadly interfere with angiogenic signals by a mixture of non-toxic natural compound with pleiotropic actions were viewed by this team as an opportunity to develop a complementary anti-angiogenesis treatment option. As a part of the “Halifax Project” within the “Getting to know cancer” framework, we have here, based on a thorough review of the literature, identified 10 important aspects of tumor angiogenesis and the pathological tumor vasculature which would be well suited as targets for anti-angiogenic therapy: (1) endothelial cell migration/tip cell formation, (2) structural abnormalities of tumor vessels, (3) hypoxia, (4) lymphangiogenesis, (5) elevated interstitial fluid pressure, (6) poor perfusion, (7) disrupted circadian rhythms, (8) tumor promoting inflammation, (9) tumor promoting fibroblasts and (10) tumor cell metabolism/acidosis. Following this analysis, we scrutinized the available literature on broadly acting anti-angiogenic natural products, with a focus on finding qualitative information on phytochemicals which could inhibit these targets and came up with 10 prototypical phytochemical compounds: (1) oleanolic acid, (2) tripterine, (3) silibinin, (4) curcumin, (5) epigallocatechin-gallate, (6) kaempferol, (7) melatonin, (8) enterolactone, (9) withaferin A and (10) resveratrol. We suggest that these plant-derived compounds could be combined to constitute a broader acting and more effective inhibitory cocktail at doses that would not be likely to cause excessive toxicity. All the targets and phytochemical approaches were further cross-validated against their effects on other essential tumorigenic pathways (based on the “hallmarks” of cancer) in order to discover possible synergies or potentially harmful interactions, and were found to generally also have positive involvement in/effects on these other aspects of tumor biology. The aim is that this discussion could lead to the selection of combinations of such anti-angiogenic compounds which could be used in potent anti-tumor cocktails, for enhanced therapeutic efficacy, reduced toxicity and circumvention of single-agent anti-angiogenic resistance, as well as for possible use in primary or secondary cancer prevention strategies.
Keywords: Angiogenesis, Cancer, Phytochemicals, Treatment, Anti-angiogenic
1. Introduction to tumor angiogenesis
Vessel formation in both health and disease occur through either vasculogenesis – i.e. the recruitment of bone marrow-derived endothelial progenitor cells to form new vessels, angiogenesis – i.e. the sprouting and growth of new vessels from an existing vasculature or intussusception – i.e. the division or splitting of a blood vessel into two or more new vessels [1]. The most common pathway for neo-vessel growth in malignancy is angiogenesis (reviewed in [2]) and the process is therefore called tumor angiogenesis.
In 1971, Judah Folkman first advanced the hypothesis that tumor growth depends on angiogenesis [3]. According to this hypothesis, endothelial cells may be switched from a resting state to a rapid growth phase by a diffusible chemical signal emanating from the tumor cells. The switch depends on increased production of one or more positive regulators of angiogenesis, such as vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), interleukin-8 (IL-8), placental growth factor (PlGF), transforming growth factor-beta (TGFbeta), platelet derived growth factor (PDGF), angiopoietins (Angs) and others (reviewed in [4]). These can be exported from tumor cells, mobilized from the extracellular matrix, or released from host cells recruited to the tumor. The switch may also involve down-regulation of endogenous inhibitors of angiogenesis such as endostatin, angiostatin or thrombospondin (reviewed in [5]) and has thus been regarded as the result of tipping the net balance between positive and negative regulators. Mature microRNAs (miRNAs) can furthermore regulate the levels of pro- or anti-angiogenic gene expression at the posttranscriptional level (reviewed in [6]).
Angiogenic signals lead to the preferential differentiation of certain endothelial cells into so-called tip cells, which start to migrate and exist at the leading front of the growing vessels. A number of factors including VEGF receptor (VEGFR)-3 (for lymphatic endothelial cells), VEGFR-1 and–2 (for blood endothelial cells), PDGF-B, and the Notch ligand delta-like ligand (Dll)-4 have been shown to contribute to the endothelial tip cell phenotype [7], [8]. In healthy angiogenesis during development for example, the number of tip-cells are limited leading to an orderly and organized expansion of the vasculature. Endothelial cells located behind the tip cell, so-called stalk cells, express other factors such as VEGFR-1 and Notch-1 and -4 which are important for inducing a quiescent state of these cells [9], [10], maturation of the vascular wall, lumen formation and to support perfusion. However, in pathological angiogenesis including tumor angiogenesis this process is usually disrupted by either excess production of pro-angiogenic signals, lack of angiogenesis inhibitors, path-finding signals or maturation factors, thus leading to excessive tip-cell formation and migration of endothelial cells [11], [12], which do not assume a quiescent phenotype associated with a healthy vasculature.
1.1. Structural and dysfunctional features of tumor blood vessels
As a result of the imbalance of angiogenic activators and inhibitors, tumor blood vessels display many structural and functional abnormalities including unusual leakiness (reviewed in [13]), potential for rapid growth and remodeling [14], high tortuosity and sinusoidal appearance (reviewed in [13]), poor coverage by vascular supportive cells including pericytes and smooth muscle cells [15], lack of arterial or venous identity leading to chaotic blood flow, poor functionality and perfusion [16], incorporation of tumor cells into the endothelial wall, alternatively differentiation of tumor stem-like cells to endothelial cells which contribute to the tumor vasculature – a process known as vascular mimicry [17]. These phenotypes, which can be considered “hallmarks of the tumor vasculature”, mediate the dissemination of tumor cells in the bloodstream and maintain the pathological characteristics of the tumor microenvironment.
Tumor vessel density is furthermore very heterogeneous: the highest values are found in the invading tumor edge, where the density is 4–10 times greater than inside the tumor and the arrangement of vessels in the center of a tumor is much more chaotic than at its edges (reviewed in [18]). Importantly, mechanical stress generated by proliferating tumor cells also compress vessels in tumors, with some vessels being oversized, whereas others are more immature and smaller. These structural abnormalities result in disturbed blood flow, hypoxia, hyperpermeability, and elevated interstitial pressure in many solid tumors (reviewed in [19]), responsible, in turn, for impaired delivery of anti-cancer drugs as well as oxygen, the former being critical for the success of chemo- and the latter for radiation therapy.
1.2. Tumor hypoxia – an emerging target
There is a complex interrelationship between tumor hypoxia and tumor angiogenesis. The production of several angiogenic cytokines and growth factors is regulated by hypoxia, but as mentioned, tumor angiogenesis also further elevate tumor hypoxia. This vicious circle is critical for driving many of the most pathogenic features of cancer including poor treatment outcome and progression to severe and metastatic disease.
Much of the dynamic regulation of this process involves transcriptionally mediated changes that promote the enhanced production of ligands and receptors, signaling aspects of both mitogenic growth and directing the organization of an endothelial network. While hypoxia and hypoxia signaling play major roles, they are only two means by which angiogenesis can be triggered. Other processes involving proteomic signaling, including those effects directly attributed to the tumor biology [20], can also be activated in support of the angiogenic response to various tissue conditions.
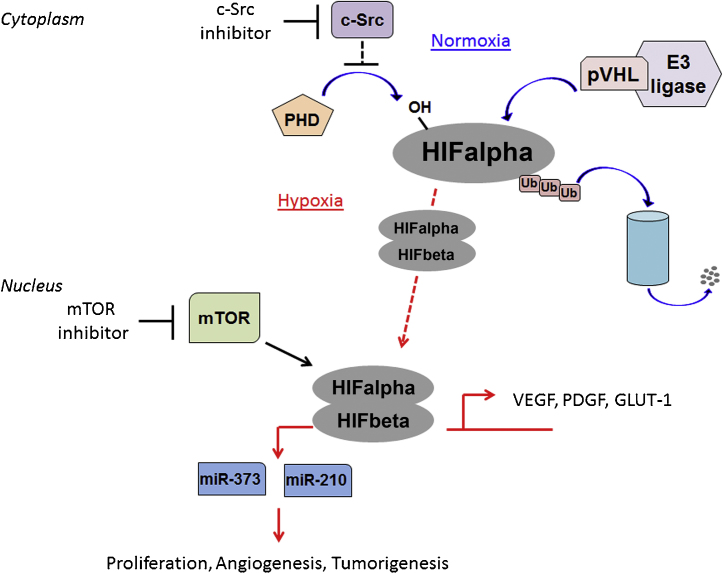
Hypoxia in tumors develops in the form of chronic hypoxia, resulting from long diffusion distances between (perfused) tumor vessels, and/or of acute hypoxia, resulting from a transient collapse of tumor vessels. One essential pathway activated in this series of events is the activation of hypoxia inducible factors (HIFs), heterodimeric transcription factors composed from alpha and beta subunits, which can be rapidly stabilized to fluidly adapt to and overcome the effects of a hypoxic environment. There are three HIFalpha subunits, HIF1alpha, HIF2alpha, HIF3alpha, which are regulated in an oxygen dependent manner. HIFalpha subunits are hydroxylated by prolyl hydroxylases (PHDs) [21] allowing HIFalpha subunits to be recognized by the von Hippel–Lindau (VHL) ubiquitin-ligase complex [22]. VHL poly-ubiquitinates HIFalpha subunits, leading to their subsequent proteasome-mediated degradation. Under low oxygen conditions PHDs have reduced activity, allowing for HIFalpha subunits to escape VHL-mediated degradation. HIFalpha subunits accumulate in the cytoplasm where they bind HIFbeta to form a heterodimer that subsequently translocates to the nucleus to activate transcription of target genes, including genes important for various processes such as metabolism (glucose transporter (GLUT)-1, hexokinase (HK)-1), cell growth (cyclin (CCN)-D1 [23]) and also angiogenesis, such as erythropoietin, VEGF and PDGF [24] (summarized in Fig. 1). In some cancers, mutations of the machinery (such as VHL or other components) regulating HIF stability can result in oxygen-independent constitutive stabilization of the HIFalpha factors, and as a result these tumors are notoriously highly vascularized [25].
Fig. 1.
Molecular mechanisms behind HIF regulation and responses in cells. The cellular oxygen sensing response is tightly regulated by a family of prolyl hydroxylases (PHD) which under normal oxygen conditions (normoxia; blue arrows) are responsible for hydroxylating proline residues on hypoxia inducible factor (HIF) alpha subunits. These hydroxylated residues are recognized by a pVHL-E3 ubiquitin ligase complex, whereby HIFalpha subunits are marked for polyubiquitination and subsequent proteosomal degradation. When oxygen levels are low (hypoxia; red arrow) PHDs cannot hydroxylate HIFalphas thereby allowing them to escape pVHL-mediated degradation. HIFalpha subunits accumulate and bind to their heterodimeric partner, HIFbeta, translocate into the nucleus and activate a cascade of hypoxic signaling first by the transcription of various target genes including microRNAs that are important for tumor promoting pathways. Alternatively, c-Src is also capable of activating HIFs by indirectly inhibiting PHD activity via the NADPH oxidase/Rac pathway. mTOR can also promote stabilization and HIF transcriptional activity. Critical points for therapeutic intervention include the use of c-Src and mTOR inhibitors to prevent HIFalpha accumulation and activation.
Additional factors besides HIF-mediated VEGF transcriptional activation have also been identified as promoting VEGF expression under hypoxic conditions. Environmental stress as a result of low oxygen and proper nutrient deprivation, such as glucose deprivation, are capable of inducing VEGF mRNA stabilization resulting in increased levels of the secreted ligand and angiogenic growth [26]. Hypoxic stress has also been described as inducing changes in miRNAs which can further influence the host microenvironment with effects on angiogenesis [27].
Targeting hypoxia signaling is a promising approach to provide more options for intervention in this critical pathway of tumor/host biology. Additionally, HIFalpha factors can be regulated by mammalian target of rapamycin (mTOR) family translational signals (Fig. 1), which provides a rich alternate source of targeting (reviewed in [28]), and mTOR drugs are already in the market or emerging.
1.3. Tumor lymphangiogenesis and lymphatic metastasis
Metastatic spread of tumor cells, via either blood or lymphatic vascular systems, accounts for the majority of morbidity and mortality in cancer patients. The presence of tumor cells within sentinel lymph nodes (LN) that accept afferent lymphatic vessels draining lymph from the primary tumor often indicates initial metastasis that precedes (or predicts) distant metastasis to other organs. It is also one of the most important markers for predicting patient prognosis and deciding on therapeutic options [29], [30].
Lymphangiogenesis, is often enhanced in malignant tumors, and associated with positive LN metastasis as well as poor survival of cancer patients [31], [32], [33], [34], [35]. Tumor-associated lymphangiogenesis may occur either at the immediate tumor periphery (peri-tumoral lymphatics) or within the tumor mass (intra-tumoral lymphatics), the former having been demonstrated to be functionally responsible for tumor cell dissemination [36], [37]. Tumor lymphangiogenesis is – as tumor blood- (or hem)angiogenesis – also regulated by a balance of pro- and anti-lymphangiogenesis factors. The most frequently studied tumor lymphangiogenic factors are members of the VEGF family, most predominantly VEGF-C and -D, through interactions with in particular VEGFR-3, with some additional evidence for VEGF-A interacting with VEGFR-2 (reviewed in [38]). These factors were found to increase LN metastasis and their expression correlated with poor prognosis in both animal models and human cancers [39], [40], [41], [42], [43], [44]. Other important lymphangiogenic factors include FGF-2 and the key receptor FGF receptor (FGFR)-1 [45], [46], [47], hepatocyte growth factor (HGF) and the cognate receptor c-met [48], insulin-like growth factors (IGF)-1, -2 and IGF receptor (IGFR) [49], [50], EphrinB-2 and Eph receptor tyrosine kinase [51], Ang-1, -2 and Tie2 [52], PDGF-BB and PDGF receptor (PDGFR)alpha and -beta [53], growth hormone and the growth hormone receptor [54], among others.
Tumor cells not only stimulate lymphangiogenesis within or around the primary tumor site, but also have the capability to induce neo-lymphangiogenesis in the LN itself, so as to prepare a “pro-metastatic niche” for the spread of tumor cells [55], [56], [57]. Lymphangiogenesis at the sentinel LN appears to occur beforehand and is further enhanced upon the arrival of metastatic cancer cells [55], [56], suggesting that the LN (possibly conditioned by certain tumor effectors, such as VEGF-A or VEGF-C) helps to provide a favorable environment for tumor metastasis (reviewed in [58]).
As lymphangiogenesis is associated with increased LN metastases (reviewed in [59]), blocking the process (or possibly inducing lymphatic endothelial apoptosis/regression) may serve as a favorable strategy to prevent lymph node metastasis. However, even if the strategy may result in fewer lymph-borne metastases over time, there's still the possibility that other biophysical functions are affected by the alteration in lymphatic flow within and surrounding the tumor. For example, a reduction in lymphatic drainage from the tumor may result in increased interstitial fluid pressure (IFP) within the tumor (reviewed in [60]). This in turn may increase tumor necrosis, hypoxia, and progression, while (at least temporarily) reducing the ability to deliver chemotherapy or other agents via the compressed tumor vasculature.
Adding to the complexity of the regulation and functions of tumor lymphatics, a variety of factors have been found to play key roles in the separation of lymphatic vasculature from blood vessels during development. While these pathways may also contribute to neolymphatic outgrowth in tumors [61], the application of inhibitory strategies to block the relevant pathways/effectors (which include podoplanin, spleen tyrosine kinase (SYK)/SH2-domain containing leukocyte protein of 76 kDa (SLP-76), Rac1/Ras homology gene family member (Rho), and sprout-related EVH1-domain containing (Spred)-1, -2 molecules) and examine the effects on tumor lymphatic investment as well as tumor vascular progression/remodeling has not been examined till date. Whether this might disrupt (or complement the inhibition of) tumor lymphangiogenesis while maintaining the delivery of chemotherapy to tumors during the relevant phase of treatment in the wider cancer treatment program remains to be examined.
1.4. Disrupted circadian rhythms in cancer
Social and occupational jetlag is a consequence of the disruption of our internal time-keeping system known as the circadian clock. Social jetlag arise in people who are often rotating between day and night shifts – often seen in healthcare workers, but a feature that is becoming increasingly prevalent in people employed in other types of jobs as well [62]. Occupational jetlag arises from traveling across several time zones and thus exposing oneself to a prolonged, unnatural day/light period, which is often experienced by airline pilots, cabin personnel and globally acting businessmen. It is becoming increasingly clear that such disruptions in the circadian rhythm are associated with higher risk of various diseases, most prominently sleep, metabolic, cardiovascular disorders and cancer [63], [64]. In a large epidemiological study following more than 100,000 American nurses over 10 years, it was found that nurses who worked rotating day and night shifts more than five times per month were at significantly increased risk of various types of cancer [65], [66].
Recently several research groups have found that the circadian rhythm is intimately involved in regulation of angiogenesis both during development [64], [67] as well as in disease [68], [69], [70]. As such, circadian transcription factors were found to directly regulate VEGF levels and were responsible for the elevated night-time spikes in VEGF which are very important for physiological, developmental angiogenesis [64], [71]. Due to the wide-range of cancers that have been associated with disrupted circadian rhythms, and the profound role of angiogenesis in the development of malignancy, it is tempting to speculate that circadian disruption may be an important player in pathological tumor angiogenesis.
2. Angiogenesis enables essential tumorigenic pathways
During tumor progression the amount and complexity of deregulated pathways which are essential for full blown malignancy, increase. Whereas pathological deregulation of cell cycle control in (often epithelial) cells is the first step toward tumor development, it is becoming increasingly clear that most of the essential tumorigenic pathways that lead to cancer are dependent on pathological deregulation of non-malignant host cells, and in particular angiogenesis and tumor vascular functions. As such, the tumor vasculature enables pathological tumor metabolism, genetic instability, inflammation, microenvironmental disruption and tumor cell invasion/metastasis.
Tumors are often hypoxic in spite of high vascularization, due to the poor structure and functionality of tumor blood vessels [11], [12]. Intratumoral hypoxia is, somewhat paradoxically, a main cause of high reactive oxygen species (ROS) formation within the tumor cells (reviewed in [72]), and also coupled to pathological tumor cell metabolism and acidosis (reviewed in [73], [74]). Thus, improving the quality of the tumor vasculature has been considered a way to improve perfusion, reduce the pathological leakiness of the tumor vessels and reduce tumor hypoxia [16], [19], which would also result in a more stable tumor genome.
Tumor angiogenesis and pathological activation of the endothelium, tumor vessel leakiness and hypoxia-induced apoptosis/necrosis in the tumor core, are resulting in a massive recruitment and activation of inflammatory cells, such as lymphocytes, neutrophils, macrophages and mast cells. These cells communicate by means of a complex network of intercellular signaling pathways mediated by surface adhesion molecules, cytokines and their receptors. These infiltrating immune cells, generate an environment abundant in growth and angiogenic factors and are implicated in enhancing cancer growth and subsequent resistance to therapy [4], [75]. The inflammatory cytokines interleukin (IL)-1alpha and IL-1beta as well as a wide panel of other signaling molecules produced by infiltrated inflammatory cells including VEGF and matrix-metalloproteinases (MMPs) may contribute to angiogenesis, tumor proliferation, and local invasion of cancer [76], [77].
The deregulated tumor vasculature not only affects the recruitment and activation of inflammatory cells, also cancer-associated fibroblasts (CAFs), myofibroblasts and a number of other cell types, which contribute to tumor progression and resistance to treatment [14], [78], is activated by endothelial cell- or hypoxia-derived factors such as PDGF-BB. On the other hand, CAFs are also rich sources of tumor angiogenic growth factors and cytokines, and thereby play an active role in sustaining tumor angiogenesis and providing resistance to anti-angiogenic therapy.
Tumors may disseminate both by local invasion as well as via blood or lymph vessels (hematologous or lymphatic dissemination). Clinically the latter two processes are the most problematic in most tumor types as they lead to multifocal metastases. Also in tumors that mostly disseminate locally (i.e. neurological cancers for example), this dissemination often occurs via the vasculature as tumor cells coopt the blood vessels and invade the tissue by crawling along the endothelium [79]. Thus in all cases blood and lymphatic vessels in or around the tumor are prerequisite for tumor invasion and metastasis. Metastasis is further enabled due to the poor structural integrity of the tumor blood vessels and pathological angiogenesis-associated tumor hypoxia. Recently, anti-angiogenic therapy has been reported to cause an increased metastatic phenotype, possibly via elevated tumor hypoxia and hypoxia-induced epithelial to mesenchymal transition (EMT) [80], [81], [82]. Anti-angiogenic therapy may however also increase the metastatic potential of tumor cells through adaptive resistance pathways not associated with hypoxia [83], indicating that anti-angiogenic therapy-induced changes in the tumor phenotype may lead to a more aggressive disease through a number of different mechanisms. It is therefore not clear if targeting the tumor vessels would be beneficial or detrimental from a tumor metastasis point of view. However, it may be possible to merely reduce tumor vascularization, improve the structure of the tumor blood vessels and perfusion in the tumor and thus reduce the pathological characteristics of the vessels, for example via sub-maximal dosing of anti-angiogenic drugs [19]. There are still not much clinical data supporting a beneficial role for promoting formation of less pathological vessels in tumors, and it is not known how to best achieve this in patients.
3. Targets for anti-angiogenic therapy
The complexity in the angiogenic system provides many targets for therapeutic intervention. On the other hand redundancy in the angiogenic pathways raises the possibility of resistance to selective therapeutic agents (reviewed in [84], [85]). Examples of agents that target circulating angiogenic factors include monoclonal antibodies targeted against VEGF (bevacizumab) [86] or fusion proteins that trap angiogenic factors (aflibercept or AMG386) [87]. Agents that target synthesis of angiogenic factors include inhibitors of mTOR, cyclo-oxygenase (COX) or heat shock protein 90 (HSP90) [88], [89]. These groups of agents in addition to inhibiting the synthesis of angiogenic factors can inhibit several other aspects of cancer biology such as growth, resistance to apoptosis or metastasis. Agents that target the angiogenic receptors are mainly tyrosine kinase inhibitors (sorafenib, sunitinib, pazopanib, regorafenib or axitinib) with multiple targets [90], [91], [92]. These agents are currently being used in the treatment of several malignant diseases ranging from breast, lung, gastric, colorectal, hepatocellular, glioblastoma, and neuroendocrine tumors.
Similarly, there are several agents in clinical trials aimed at blocking lymphangiogenesis/metastasis, mostly via neutralizing VEGF-A, -C or -D-induced receptor activation. For example, a major approach involves application of a variety of tyrosine kinase inhibitors such as Ki23057, used to block gastric cancer spread in mice through blockade of VEGFR3 autophosphorylation [93]. Other agents in clinical testing include PTK787/ZK222584 (Phase III – colorectal cancer); BAY43-9006 (Phase II, multiple carcinomas); CEP7055 (Phase I – various malignancies); or JNJ-26483327 (Phase I for multiple advanced solid tumors). These, among other anti-lymphangiogenic agents under study are reviewed in Ref. [59].
It is worthwhile mentioning that hormone- and chemotherapy could also have anti-angiogenic activity, particularly metronomic therapy which refers to the frequent, even daily, administration of chemotherapy (e.g. cyclophosphamide, methotrexate or capecitabine) in doses below the maximum tolerated dose, for long periods of time, with no prolonged drug-free breaks [94], [95]. Other chemotherapeutics, routinely used in clinic, may also have anti-angiogenic activity in vitro or in vivo [96] as: (1) paclitaxel [97], doxorubicin and thalidomide [98] which seems to be mediated via inhibition of VEGF and bFGF [99]; (2) celecoxib, which may cause a time-dependent reduction in circulating angiogenic markers; (3) bisphosphonates may have anti-angiogenic effects [100] via reduction of VEGF and PDGF serum levels [101]; (4) PI3K inhibitors (including rapamycin analogues as temsirolimus (CCI-779) and everolimus (RAD001)) decrease tumor angiogenesis [102], [103], [104] via the inhibition of HIF-1alpha caused by the blockade of mTOR activity.
Trials that have combined monoclonal antibodies and tyrosine kinase inhibitors have given rise to an increase in the side effects profile. A more rational approach would be to consider combinations of agents that block production of angiogenic factors with such that target angiogenic factors or receptors. The rationale behind such combinations include the fact that anti-angiogenic agents can improve the delivery of cytotoxic agents to the tumor site, may alter hypoxia in the tumor and sensitize it to chemotherapy or may impede the ability of the tumor to recover from cytotoxic effects of chemotherapeutic agents [105]. As tumors express more than one angiogenic cytokine and the fact that during tumor progression the palette of tumor-derived angiogenic factors grows more and more complex, any single inhibitor would not be sufficient for achieving sustained anti-tumor responses [74]. We hypothesize that simultaneously hitting multiple important aspects of tumor angiogenesis, each outlined in detail in the sections above, with a cocktail of compounds might create a more effective treatment. This could particularly be the case for indications such as cancer prevention in high risk settings, or maintenance therapies.
To facilitate the use of plant-derived compounds in cancer treatment, we have selected 10 key mechanisms that lead to pathological growth and functions of the tumor vasculature, such as EC migration/tip cell formation, phenotypic changes in the tumor microenvironment or pathogenic activation of stromal cells (macrophages or fibroblasts). These pathways (top row in Table 1) were selected as candidate targets for anti-angiogenic natural compound-based therapy development.
Table 1.
Effects of the selected targets for anti-angiogenic cancer therapy on other cancer “hallmarks”.
| Other cancer hallmarks | Angiogenesis targets |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Inhibit) endothelial cell migra-tion/tip cell formation | (Reduce) structural abnormalities of tumor vessels | (Reduce) hypoxia | (Inhibit) lymphangiogenesis | (Reduce) elevated interstitial fluid pressure | (Reverse) poor perfusion | (Norma-ize) disrupted circadian rhythms | (Suppress) tumor promoting inflammation | (De-activate) tumor promoting fibroblasts | (Normalize) tumor cell metabolism/acidosis | |
| Genetic instability | 0 | 0 | + [239] | 0 | 0 | 0 | + [240] | 0 | 0 | + [241] |
| Sustained proliferative signaling | 0 | 0 | + [242] | +/− [243], [244] | + [245], [246] | 0 | + [247], [248] | 0 | + [249], [250] | 0 |
| Tumor-promoting inflammation | + [251] | + [252] | + [253], [254] | 0 | + [255] | + [252], [256] | + [257] | NA | + [258] | + [259] |
| Evasion of anti-growth signaling | + [260] | 0 | +/− [261], [262] | 0 | + [246] | +/− [263], [264], [265] | +/− [266], [267] | + [268] | + [269], [270] | + [271], [272] |
| Resistance to apoptosis | 0 | + [273] | + [274] | 0 | + [275] | + [276] | + [277] | + [278] | + [279] | + [280] |
| Replicative immortality | 0 | 0 | + [281], [282], [283] | 0 | 0 | 0 | +/− [284], [285] | 0 | 0 | 0 |
| Dysregulated metabolism | + [286] | + [287], [288] | + [289], [290], [291] | 0 | + [292] | + [293], [294] | + [295] | 0 | + [296] | + [297] |
| Immune system evasion | + [298] | + [299], [300] | + [301], [302] | + [303] | + [304], [305] | + [306] | + [307] | + [308], [309] | + [310], [311] | + [312], [313] |
| Invasion and metastasis | + [287] | + [15], [314] | + [315] | + [316] | + [317], [318], [319] | + [320] | + [321], [322], [323] | + [324] | + [323] | + [325], [326] |
| Interactions in the tumor micro-environment | + [260] | + [327] | + [328] | + [329] | + [330] | + [327] | + [331] | + [332] | + [333], [334] | + [335] |
Our 10 identified targets of anti-angiogenesis therapy are presented in the top row. 10 other cancer “hallmarks” are listed in the column to the left. Positive interactions (i.e. if the anti-angiogenesis target could also be a target for the indicated “hallmark”) are denoted “+”, controversial interactions (i.e. if the anti-angiogenesis target could both promote and inhibit the indicated “hallmark”) are denoted “+/−” and no interaction (i.e. if we have not been able to find any interaction between the anti-angiogenesis target and the indicated “hallmark”) is denoted “0”.
4. Therapeutic potential of plant-derived compounds
During the last decades, phytochemicals have gained significant recognition for their potential therapeutic uses in cancer [106], [107], [108]. Extensive research has revealed enormous potential and exciting pharmacological properties of plant-based medicinal compounds, and demonstrated synergistic effects in combination with other agents to inhibit tumor angiogenesis, although the use of phytochemicals alone is still a limited option for cancer treatment. Some phytochemicals used in cancer therapies demonstrate relatively low side-effects, and some even limit the side-effects of chemotherapeutics or anti-angiogenic drugs. Fruits, vegetables, cereals, pulses, legumes, herbs, spices and medicinal plants – such as Artemisia annua (Chinese wormwood), Viscum album (European mistletoe), Curcuma longa (turmeric), Scutellaria baicalensis (Chinese skullcap), Vitis vinifera (grape seed extract), Magnolia officinalis (Chinese magnolia tree), Camellia sinensis (green tea), Ginkgo biloba (ginkgo), Poria cocos (tuckahoe), Zingiber officinalis (ginger), Panax ginseng (ginseng), Rabdosia rubescens hora (rabdosia), and Chinese destagnation herbs – are all considered to be good sources of phytochemicals exhibiting anti-cancer, and in particular anti-angiogenesis activities. The active ingredients in these plants are sometimes extracted and given in doses higher than what can be achieved from consuming the plants of which they are derived in order to give stronger therapeutic effect.
Many medicinal herbs and purified phytochemicals have recently been evaluated for anti-lymphangiogenic and anti-angiogenic properties in cancer (reviewed in [109], [110], [111]). The potential mechanisms underlying their anti-lymphangiogenic features involve (1) the control on cell proliferation, tube formation and cell cycle progression of lymphatic endothelial cells, as exhibited by multiple compounds fractionated from Korean and Japanese Saussureae radix, Psoraleae semen and Aurantti fructus immaturus [112]; (2) the inhibition of COX-2 expression and IL-1beta production and the subsequent reduction in VEGF-C-induced VEGFR-3 phosphorylation, as observed with wogonin [113]; and (3) the down-regulation of VEGFR3 and small GTPases, as well as the inhibition of VEGFR3-mediated extracellular-signal regulated kinase (ERK)-1 and -2 phosphorylation by cryptotanshinone [114]. The mechanisms behind their anti-angiogenic effects include: (1) inhibition of MMPs, (2) prevention of capillary sprout formation, endothelial cell proliferation and migration, and (3) modulation of angiogenic ligand/receptor mediated signaling pathways.
MMP inhibition blocks the degradation of endothelial basement membrane proteins, a process that otherwise leads to increased permeability and poor structure and stability of the tumor vasculature as well as release of sequestered angiogenic factors. However, caution is warranted regarding the effects on MMP/tissue inhibitor of metalloproteinases (TIMP) system as MMP inhibitors in clinical trials have failed and even induced worse survival rates compared to placebo treated patients [115]. In addition, several experimental studies have shown potent anti-tumorigenic activities of many MMPs including MMP-9 [116], [117], [118], [119], [120], [121]. In the case of TIMPs both tumor protective and tumor enhancing properties have been reported [122], [123], [124], [125]. For example, in breast cancer patients, high tumor and serum levels of TIMP-1 have been associated with decreased response to chemotherapy and consequently reduced survival [126], [127], [128].
Targeting capillary sprout formation and directional endothelial cell migration may depend on diverse inhibitors of the Notch signaling pathway, for which commercial drugs are in development. There are also phytochemicals which exhibit such effects.
Finally, angiogenic signaling inhibitors include the modulation of mitogen-activated protein kinase (MAPK) and Akt signaling, inhibition of activator protein (AP)-1 activation, and the down-regulation of VEGF, transforming growth factor (TGF)-beta, MMP-9, as well as the upregulation of TIMP-1 which lead to reduction of tumor cell invasion and blood vessel growth [129], [130], [131], [132].
4.1. Tea polyphenols
Tea is one of the most highly consumed beverages in the world and is rich in compounds exhibiting multiple health benefits (reviewed in [108]). The pharmacological action of tea is mainly attributed to large quantities of polyphenolic compounds known as catechins, which include epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epigallocatechin-3-gallate (EGCG) particularly in green tea and thearubigin and theaflavins in black tea. Tea polyphenols have been shown to act on angiogenesis through different signaling pathways. For instance, EGCG has been found to directly inhibit capillary endothelial cell proliferation at low concentrations, which illustrates the importance of this molecule as an important tumor angiogenesis inhibitor [133]. EGCG also inhibits VEGF production in MDA-MB231 breast cancer cells and human umbilical vein endothelial cells which correlate with the inhibition of protein kinase C, c-fos and c-jun RNA transcripts, suggesting that AP-1 responsive regions present in the human VEGF promoter may be involved [134]. In neuroblastoma, fibrosarcoma, glioblastoma, prostate cancer, and human gastric cancer cells, EGCG inhibited MMP-2 and MMP-9 while inducing the activity of their inhibitors TIMP-1 and TIMP-2 [135], [136], [137], [138]. In human breast cancer cells, EGCG treatment reduced MMP-2 activity and the expression of focal adhesion kinase (FAK), membrane type-1-MMP (MT1-MMP), nuclear factor (NF)-kappaB, VEGF, and the adhesion of cells to the extracellular matrix (ECM) [139]. Similar signaling pathways have also been demonstrated in animal studies. EGCG also targets urokinase plasminogen activator (u-PA), leading to a down-regulation of VEGF production in tumor cells and subsequent repression of AP-1, NF-kappaB and signal transducer and activator of transcription (STAT)-1 transcription factor pathway [140], [141]. EGCG furthermore inhibits aryl hydrocarbon receptor (AhR)-AhR-mediated transcription by binding to HSP90. Furthermore, a range of semi-synthetic and synthetic derivatives are more potent than EGCG in a luciferase refolding assay for HSP90 activity [106]. In transgenic adenocarcinoma of the mouse prostate (TRAMP) mice, green tea polyphenol infusion resulted in marked inhibition of effectors of angiogenesis and metastasis, notably VEGF, uPA, MMP-2, and MMP-9 [142]. In a dimethylaminoazobenzene (DAB) induced hepatoma model, administration of black tea polyphenols not only reduced the incidence of invasion, but also inhibited tumor hypoxia and angiogenesis [143]. In addition, EGCG targets tissue plasminogen activator (t-PA), which is one of the critical proteases that enable tumors to metastasize [144]. Several studies based on cell culture and animal models further demonstrated the cancer preventive function of tea, which is associated not only with the inhibition of VEGF, NF-kappaB, c-fos and cyclin D1 promoter activity, but also the decrease of Bcl-XL and the stabilization of p53 [145], [146].
4.2. Curcumin
Curcumin is a polyphenol isolated from Curcuma longa. As a yellow dye, curcumin has been used widely for centuries not only in cooking, but also in traditional therapies for various diseases, especially inflammatory diseases. Curcumin and tetrahydro-curcumin, one of its metabolites have been extensively investigated as anti-inflammatory and anti-cancer molecules [147], [148]. Curcumin inhibits the expression of epidermal growth factor receptor (EGFR), VEGFR-1, VEGFR-2 and VEGFR-3, and the kinase activity of Src and FAK, which are responsible for the induction of angiogenic genes as well as endothelial cell polarity and migration [149]. Curcumin also reduces the MMP-2 and MMP-9 expression, along with the suppression of growth and invasion potential of tumor cells in culture and xenograft experiments [150]. Oral administration of curcumin in nude mice xenografted with hepatocarcinoma cells led to significant lowering of tumor neocapillary density. The expression of angiogenic biomarkers COX-2 and serum levels of VEGF were significantly reduced in the curcumin-treated group [151]. This inhibition of both VEGF and VEGFR in different cancers, has fueled the interest in this compound for further investigations as a potential anti-angiogenesis agent [152].
4.3. Resveratrol
Resveratrol (3,4′,5-trihydroxy-trans-stilbene), a dietary polyphenol derived from grapes, berries, peanuts and other plant sources has been shown to affect several steps in the tumorigenic process including tumor angiogenesis [153], [154], [155]. Resveratrol inhibits capillary endothelial cell growth and new blood vessel growth in animals [130]. It also prevents diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis in rats through interrupting cell proliferation, inducing apoptosis [155] and impeding angiogenesis by suppressing VEGF expression through down-regulation of HIF-1alpha [156]. In in vitro studies, resveratrol was reported to inhibit cell proliferation of human ovarian cancer cells and human osteosarcoma cells by attenuating HIF-1alpha [157], [158]. Through abrogation of VEGF-mediated tyrosine phosphorylation of vascular endothelial cadherins and beta-catenin, resveratrol further prevents cytokine-induced vascular leakage and tumor metastasis [130]. The underlying molecular mechanisms include: blocking VEGF- and FGF-receptor-mediated MAPK activation, inhibiting Akt- and MAPK-driven HIF-1alpha basal expression and its induction by IGF-1, stimulating the proteasomal degradation of HIF-1alpha, inhibiting phosphatidyl inositol (PI)-3K/Akt and Ras/mitogen/extracellular signal-regulated kinase (MEK)/ERK pathways, and activation of forkhead box (FOX)O transcription factors [130], [157], [158], [159].
Furthermore, the synthetic stilbene derivatives of resveratrol have stronger inhibitory effect on angiogenesis than resveratrol, as measured by cell proliferation and tube formation in bovine aorta endothelial cells [160]. For instance, the stilbene derivative rhapoantigenin inhibits angiogenesis-induction by prostate cancer cells through HIF-1alpha degradation [161]. Likewise, resveratrol and a series of natural or synthetic stilbenes inhibit the growth of colon cancer-xenografts in mice through attenuation of VEGF and pro-MMP-9 [162]. While pre-clinical studies have been done broadly and for many different indications, resveratrol is yet to be clinically assessed for safety and prevention of cancer as well as in cancer therapeutic regimens.
4.4. Flavonoids
In the family of polyphenols, flavonoids have been found to suppress tumorigenesis via anti-angiogenesis, anti-oxidant as well as anti-proliferation effects on tumor- as well as tumor-associated stromal cells including endothelial cells [163], [164]. Flavonoids, including flavones (apigenin, luteolin), flavanols (quercetin, kaempferol), flavanones (hesperetin, naringenin), anthocyanins (cyanidin, delphinidin) and isoflavones (geneistein, daidzein) function as scavengers of free radicals and thus inhibit ROS formation and hypoxia-signaling cascades, modulate COX-2, and inhibit EGFR, IGFR-1 and NF-kappaB signaling pathways [165]. For instance, kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) has been reported to reduce ovarian cancer risk [166], [167]. Kaempferol exhibits anti-inflammatory effects through inhibition of IL-4 [168] and COX-2 expression by suppressing Src kinase [169], and further down-regulates the NF-kappaB pathway [170]. Flavonoids furthermore inhibit angiogenesis through multiple mechanisms, including interaction with the COX-2 and lipoxygenase-5 enzymes, EGFR and the human epidermal growth factor receptor (HER)-2 intracellular signaling pathway [171].
Silymarin is the standardized extract of the seeds of Silibum marianum (milk thistle). Silibinin, the major active constituent was initially developed as a hepatoprotective product. Recently, however, it has been reported that silibinin inhibit MMP-2 expression and suppresses capillary formation of human umbilical cord venous endothelial cells (HUVECs) on matrigel [172], [173], [174]. Other naturally occurring flavonoids have been showing anti-angiogenesis and anti-oxidant effects via interference with diverse signaling pathways. For example, myricetin has been shown to inhibit inhibitor of kappaB kinase (IKK) kinase activity and prevent degradation of I-kappaBalpha and I-kappakBbeta in tumor necrosis factor (TNF)-activated endothelial cells [175]. Sulforaphane has been demonstrated to inhibit VEGFR2 at the transcriptional level [176].
Studies also showed that licochalcone A (LicA), a major constituent of Glycyrrhiza inflata, significantly inhibits proliferation, migration, and tube formation of HUVECs as well as microvessel growth from rat aortic rings. Furthermore, LicA significantly inhibits the growth of CT-26 colon cancer implants in BALB/c mice, with fewer CD31- and Ki-67-positive cells but more apoptotic cells [177]. Isoliquiritigenin, another flavonoid found in G. inflata inhibits cell migration and invasion of human prostate cancer cells (DU145 and LNCaP) mediated by decreased c-Jun N-terminal kinase (JNK)/AP-1 signaling and reduced production of proangiogenic factors [178]. Taken together, these findings provide evidence that flavonoids inhibit angiogenesis in vitro and in vivo, via antioxidant, anti-inflammatory and anti-angiogenic signaling pathways.
4.5. Terpenoids
Terpenoids are the most diverse constituents in many plant species. They form a group of natural substances which includes steroids and sterols, exhibiting anti-inflammatory and anti-carcinogenic properties [20], [179], [180], [181], [182].
The bioactive terpenoid, tripterine, also known as celastrol, a quinine methide triterpenoid is the most abundant bioactive compound derived from the root of Trypterigium wilfordii. Tripterine modulates the expression of proteins with angiogenic and metastatic activities (MMP-9, intercellular adhesion molecule (ICAM)-1, VEGF) and those involved in cell survival (inhibitor of apoptosis protein (IAP)-1, X-linked (X)-IAP, B-cell lymphoma (Bcl)-2, Bcl-xL, flice inhibitory protein (cFLIP), survivin) or cell proliferation (cyclin D1, COX-2) in tumor cells [183], [184]. These findings provide evidence for its potential anti-angiogenic and anti-tumor activities.
Escin is a pentacyclic triterpenoid which is isolated from the seeds of Aesculus hippocastanum (horse chestnuts) [185]. Escin sodium has been shown to inhibit endothelial cell migration and motility. This anti-angiogenic activity was mediated partly by inhibiting ERK and p38 MAPK pathways, which are involved in cell proliferation, motility and apoptosis [186].
Withaferin A is a major steroidal lactone constituent of the medicinal plant Withania somnifera, consumed as a dietary supplement around the world and used in the treatment of tumors and inflammation in several Asian countries [187]. Withaferin A exerts potent anti-angiogenic activity in vivo even at 500-times lower dose than that exerting direct anti-tumor activity [188], [189]. Similar trends toward a more potent anti-angiogenic rather than anti-tumorigenic effects are also observed for other phytochemicals (such as tubulysin A as discussed below) suggesting that the vasculature may be a good target for treatments with phytochemicals.
In addition, carotenoids have anti-cancer activity in breast cancer animal models. The carotenoid group includes alpha-carotene, beta-carotene, lycopene, lutein, astaxanthin, cryptoxanthin and zeaxanthin [190], [191]. The anti-oxidant action is one of the presumed mechanisms for the anti-angiogenic effects of the carotenoids.
4.6. Phytoestrogens
Phytoestrogens are plant compounds which are structurally similar to estrogen. Thereby, these compounds may have beneficial effects in prevention of steroid hormone-dependent cancers such as breast and prostate cancer. There are two major classes of phytoestrogens, isoflavones, found in soy at high concentrations, and lignans, found in flaxseed in high amounts. Lignans are ubiquitous compounds in plant material and are present in seeds, whole grains, vegetables, and berries [192], [193], [194]. Genistein (GEN), the representative of isoflavone, and enterolactone (ENL) is the main active and most potent metabolite of lignin, both have agonistic and antagonistic actions in cancer, depending on their dose and the tumor-type. In Eastern societies, the diet contains large amounts of phytoestrogens, and accordingly, the incidence of cancers such as breast cancer and prostate cancer is low [195], [196]. In the diet, phytoestrogens are consumed in combination and various phytoestrogens may have different effects on tumor biology. GEN has been shown to reduce the angiogenic and metastatic potential of various cancer cell types in vitro [197]. However, the pro-estrogenic effects of GEN in breast cancer [198] suggest that caution with this particular phytoestrogen in hormone-dependent cancers is warranted. In addition, as the major circulating lignan in the human body, the majority of dietary lignans are converted into ENL. ENL inhibits cancer growth in vivo by enhancing apoptosis, inhibiting angiogenesis, and reducing inflammation without severe side-effects [199], [200], [201].
5. Prophylaxis, pharmacodynamics and safety issues
As outlined above, there is convincing evidence in support of beneficial effects of phytochemicals in cancer-related pathways, particularly with regard to anti-angiogenesis. Furthermore, many natural molecules exhibit potent anti-angiogenic activity similar to the synthetically produced drugs currently in clinical use. For example curcumin, EGCG, finasteride and barrigtozenol demonstrated comparable effects on VEGF receptor inactivation as pazopanib, the reference drug [202]. 10 μM curcumin significantly inhibited VEGF-induced HUVEC migration to a higher degree (52%) than the same concentration of a synthetic anti-angiogenic agent – the selective PDE4 inhibitor rolipram (41%) [203]. Furthermore, the EC50 dose–responses are usually lower for anti-angiogenic effects vs. cytotoxic effects on cancer cells both for synthetic and natural products. For example tubulysin A, a natural compound of myxobacterial origin, which inhibits tubulin polymerization has an EC50 for endothelial cell proliferation of 1.2 nM and up to 3.0 nM for cancer cell proliferation [204], [205]. The synthetic derivates of tubulysin A, AU816 and JB337, exhibit similar differences; EC50 of AU816 for endothelial cell proliferation is 4.4 nM, whereas inhibition of cancer cell proliferation is 10 nM and for JB337 the EC50 levels are 14 nM compared to 100 nM for endothelial and cancer cell proliferation, respectively [204], [206].
Since most phytochemicals have poor water solubility and low bioavailability, the use of nano-carriers allows the preparation of these compounds in solid or liquid formulations with highly improved pharmacodynamics properties. Such encapsulation, however, render these natural compounds semi-synthetic, but the active ingredients in such preparations are still all-natural. Often, delivery mechanisms based on nano-carriers may in addition to improved pharmacokinetics also lead to improve targeting of the active compounds to the appropriate – i.e. tumor – site. For example in the case of anti-lymphatic drugs, nano-carriers, which are not able to cross the endothelium by themselves, but extravasate in tumors due to the high leakiness of tumor blood vessels, accumulate in draining intra-tumoral lymphatics and thus serve as a means to target the drugs to the leaky tumor environment. Nanoparticles made of biodegradable polymers have been utilized recently for the delivery of anti-cancer phytochemicals [207], [208]. Siddiqui et al. [209] reported that nano-encapsulated EGCG retains its biological effectiveness, with over 10-fold dose advantage compared to nonencapsulated EGCG for exerting its pro-apoptotic, and anti-angiogenic effects. Encapsulation of kaempferol with nanoparticles significantly reduces cancer cell viability, as does coating it onto poly(dl-lactic acid-coglycolic acid) (PLGA) nanoparticles [210]. Furthermore, nano-encapsulated curcumin has increased in vivo tumor anti-angiogenic activity relative to its non-encapsulated form [211]. Solid lipid nano-encapsulation of berbarine also increased inhibition of cancer cell proliferation, cell cycle arrest, and apoptosis [212]. Nano-encapsulation therefore seems like a promising strategy to overcome pharmacodynamics issues associated with many phytochemicals.
The bioavailability, efficacy and stability of phytochemicals may also be increased by synthetic derivation – a procedure in which functional groups are added to or removed from the natural phytochemical, leading to improved pharmacokinetics of the resulting semi-synthetic phytochemical drug, while retaining or even improving its biological effects. Such an approach has proven particularly useful in rendering oleanolic acid attractive for therapeutic applications. Oleanolic acid is an oleanane-type triterpenoid that is widely found in dietary and medicinal plants [213]. Studies demonstrated that oleanolic acid has anti-angiogenic effects on bovine aortic endothelial cells and in the chick embryo chorioallantoic membrane assay [214]. However, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), the synthetic derivative of oleanolic acid, its C-28 methyl ester (CDDO-Me) and C-28 imidazole (CDDO-Im), are all shown to be potent inhibitors of angiogenesis in the Matrigel sponge assay and in tumors established from immortalized Kaposi's sarcoma cells. These compounds prevent endothelial cell tubulogenesis on Matrigel and inhibit the VEGF-induced ERK-1 and -2 pathway in HUVECs [214], [215]. It has been suggested that COX-2 overexpression may lead to increased tumor angiogenesis [216], [217]. Therefore, inhibition of the COX-2 pathway might prevent carcinogenesis and angiogenesis via anti-inflammatory signaling. For example, CDDO-Me has been shown as an effective inhibitor of COX-2 and tumor growth in breast cancer models in mice.
These studies underscore the importance of phytochemicals and semi-synthetic derivatives or formulations of phytochemicals, as a novel anti-tumor treatment option in the future. Moreover, their anti-angiogenic properties could help to optimize the effectiveness of existing chemotherapeutic drugs. The use of phytochemicals as adjuvants in combination with anti-angiogenic drugs is promising. For example, tumor hypoxia can be a deleterious result of anti-angiogenic cancer treatment leading to changes in cellular metabolism [73], [74], [218], elevated intracellular ROS-levels [72], de-differentiation of tumor cells and increased metastatic propensity [218], [219]. These hypoxia-induced pathways can be treated by various phytochemical antioxidants including EGCG, melatonin, resveratrol and silibinin [143], [157], [220], [221], [222], [223]. While this could provide a strong argument for synergy by combining such agents, it was traditionally believed that hypoxia should be the very goal of anti-angiogenic treatment and an important inducer of tumor cell apoptosis [224], [225]. Therefore addition of such antioxidants may reduce the hypoxia-induced cell death, as it does for non-malignant cells [226], [227], potentially leading to a poorer outcome from the anti-angiogenic treatment. Also in the interaction between tumor inflammation and angiogenesis the combination of anti-inflammatory phytochemicals with anti-angiogenic drugs may provide a potential for synergy as inflammatory cells are considered a source of alternative angiogenic factors and thus important for evasive resistance [4]. However, some inflammatory cells are thought to be anti-tumorigenic and important for tumor clearance [228], [229], and inhibiting them could lead to antagonistic interactions in such combinations. Thus each combination of phytochemicals and other treatment options should be thoroughly tested in pre-clinical and clinical trials before adopted by medical practitioners.
Tumor angiogenesis is not only required for late-stage progressive disease, but is crucial even for initial growth of the tumor to clinically detectable masses. Therefore, the inhibition of angiogenesis has been proposed as an important preventive strategy [230]. This idea, also known as angioprevention, is highly efficacious in preventing tumor growth in animal models [156], [231]. Angioprevention should be achieved using non-toxic agents such as phytochemicals, as they are often consumed on a daily basis. Ideally, we should however also aim to achieve anti-angiogenic treatment efficacy using phytochemical agents at sufficiently low doses to keep toxic side-effects are kept to a minimum. Thus safety concerns should weigh in heavily when deciding on the most appropriate drugs for anti-angiogenic treatment in cancer. Here, we intend to identify 10 suitable, prototypical anti-angiogenic therapeutic approaches based on phytochemicals that have proven to be effective against our previously defined targets. We aimed at finding compounds that are free from intellectual property constraints, readily available and cheap such that everyone, all over the world, could use them as an alternative or complementary treatment option. A modern approach to chemotherapy may be to use lower doses of combinations of chemicals, which work in complementary ways. Thus, while low-doses of a single compound may not achieve the full anti-angiogenic function, a mixture of compounds, or extracts from medical plants, may be able to specifically synergize in preventing angiogenesis while avoiding significant side-effects. Such an approach, combining phytochemicals in tea and soy extracts has been successfully applied to the anti-angiogenic treatment of prostate and breast cancer in animal models [47], [49]. Therefore we aimed at selecting phytochemicals from as many different classes of natural compounds listed in Section 4 as possible.
In line with this rationale, we have identified 10 natural compounds as potential therapeutic approaches (top row of Table 2) against the previously identified targets (top row of Table 1). These compounds are safer than the synthetic anti-angiogenesis inhibitors in clinical use today [232], [233]–and may even be considered as prophylactic (angioprevention) agents. These molecules were selected because they exhibit pleiotropic functions, i.e. disrupting both inflammation and vascularization during tumor onset and progression and while some of them share molecular targets, combined they will inhibit a broad spectrum of pro-angiogenic signaling pathways lowering the risk of adaptive resistance. As tumors often progressively turn on pro-angiogenic pathways during their development to more advanced stages (reviewed in [234]), which is one possible reason why many patient do not respond to single-agent therapies such as anti-VEGF drugs, the cocktail of 10 phytochemical-based therapeutic approaches mentioned here would be a more broad-acting treatment option, not least for such patients that do not respond to commercially available anti-VEGF drugs.
Table 2.
Effects of phytochemical approaches in anti-angiogenic therapy as effective also against other cancer “hallmarks”.
| Other cancer hallmarks | Phytochemical approach |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oleanolic acid | Tripterine | Silibinin | Curcumin | Epigallo-catechin-gallate (EGCG) | Kaempferol | Melatonin | Enterolactone | Withaferin A | Resveratrol | |
| Genetic instability | + [336] | 0 | + [337] | + [338] | +/− [339], [340] | 0 | + [341] | 0 | 0 | + [342] |
| Sustained proliferative signaling | 0 | 0 | +/− [343] | 0 | + [245], [246] | 0 | + [344], [345] | + [346] | + [249], [250] | + [347], [348] |
| Tumor-promoting inflammation | + [20] | 0 | + [349] | + [350], [351] | + [352], [353] | + [169] | 0 | + [354] | 0 | + [355], [356], [357] |
| Evasion of anti-growth signaling | + [341], [358], [359] | + [360], [361], [362] | + [363], [364], [365], [366] | + [367], [368], [369], [370] | + [371], [372], [373], [374] | + [375], [376], [377], [378] | + [379] | + [380] | + [381], [382], [383] | + [384], [385], [386], [387] |
| Resistance to apoptosis | + [388], [389] | + | + [390] | + [391] | + [374], [392], [393] | + [394] | + [395] | + [396] | + [397] | + [398] |
| Replicative immortality | + [399], [400] | 0 | + [401], [402], [403] | + [401], [404] | + [405], [406], [407] | 0 | + [408] | 0 | 0 | + [409] |
| Dysregulated metabolism | + [358], [410] | 0 | + [411], [412] | + [391], [392], [413] | + [414], [415], [416] | + [375], [417], [418] | + [419], [420], [421] | 0 | + [422], [423], [424] | + [425], [426], [427] |
| Immune system evasion | + [428], [429] | 0 | + [430] | + [431] | + [432], [433] | 0 | + [434] | 0 | + [435] | +/− [436], [437], [438] |
| Invasion and metastasis | + [439], [440] | + [441] | + [442] | + [443] | + [444], [445], [446] | + [447], [448] | + [449], [450] | + [451] | + [452] | +/− [453], [454], [455] |
| Interactions in the tumor micro-environment | + [456] | + [457] | + [458] | + [459] | + [433], [460] | + [461], [462] | + [331], [463] | + [199] | + [464] | + [465] |
Our 20 identified therapeutic approaches for anti-angiogenesis therapy are presented in the top row. 10 other cancer “hallmarks” are listed in the column to the left. Positive interactions (i.e. if the compound could also exhibit therapeutic potential against the indicated “hallmark”) are denoted “+”, controversial interactions (i.e. if the compound could both promote and inhibit the indicated “hallmark”) are denoted “+/−” and no interaction (i.e. if we have not been able to find any therapeutic activity of the compound against the indicated “hallmark”) is denoted “0”.
6. Validation of targets and approaches
Anti-angiogenic agents have demonstrated activity in a broad spectrum of malignancies. The questions of finding the best drug, or combination of drugs, for each patient and how to best evaluate the response to treatment are still not adequately answered. An alternative to current efforts on targeted therapy development could be to inhibit an array of pathological targets of tumor blood vessels such as the 10 targets identified here (Table 1, top row). We envision that such a broad approach, simultaneously hitting multiple or all of these targets of the tumor vasculature at the same time, would produce an improved therapeutic outcome compared to single-target therapy. The current strategy we endorse for further investigation considers a combination of phytochemicals, identified to act on more than one or a few of these targets (Table 2, top row). We believe that this approach circumvents the numerous avenues of emergent resistance, and has the potential to be an active therapeutic in a broader range of tumors. We encourage researchers and clinicians alike to test formulations based on this natural compound cocktail-principle and in this way further establish the paradigm of phytochemicals as anti-angiogenic treatment options in cancer.
Given the extensive cross-talk between mechanisms regulating angiogenesis and other aspects of cell biology as well as the broad effects of phytochemicals, we believe that it is important to be able to anticipate synergies that might be achieved through acting not only on aspects of tumor angiogenesis but also on other essential aspects of tumor biology such as the “hallmarks” of cancer. Accordingly, the identified targets for anti-angiogenic therapy and the therapeutic phytochemical-based approaches were cross-validated against their effects on other “hallmark” processes, by undertaking a thorough, qualitative background literature research. An independent team of scientists consisting of specialists in various “hallmark” areas specifically sought to determine the relevance of our identified targets and the nominated phytochemical approaches across these other important areas of cancer biology. The result of this elaborate cross-validation effort is shown in Table 1, Table 2, for the targets and suggested phytochemical-based approaches respectively. We found that many of our identified targets and approaches were also relevant for other aspects of cancer biology. In such cases they were noted as having “complementary” effects (denoted with a + in Table 1, Table 2). In instances where reports on relevant actions in other aspects of cancer biology were mixed (i.e. reports showing both pro- and anti-potential), the effects are considered “controversial” and denoted +/− in the tables. Finally, in instances where no literature was found to support the relevance of a target or approach in other aspects of cancer biology, we consider that there is “no known relationship” and denote this with a 0 in the tables. We did not find any instances in which our targets or approaches acted to promote other pathological aspects of tumor biology. As an example, reducing tumor hypoxia would also lead to reduced tumor-promoting inflammation and therefore we mark this with a + in the cell intersecting the “(reduce) hypoxia” column with the “tumor promoting inflammation” row in Table 1. Conversely, our suggested approach resveratrol was found to both inhibit and induce metastasis and we therefore denote this as +/− in the cell intersecting the “resveratrol” column with the “Invasion and metastasis” row in Table 2.
As it can be determined from closely inspecting these tables, most of our targets and approaches are found to also inhibit other essential aspects or hallmarks of tumor pathology. As such, we believe that the use of the suggested phytochemical approaches – or similarly acting derivatives or analogues – would be effective as alternative anti-angiogenic agents while also inhibiting multiple other aspects of tumor pathology at the same time. Especially oleanolic acid, silibinin, curcumin, EGCG, melatonin and resveratrol, have strong synergistic actions, i.e. anti-tumorigenic effects in almost all other hallmark areas in addition to being effective anti-tumor-angiogenic agents. It would be highly important to experimentally test the effects of such a cocktail of phytochemicals in pre-clinical and clinical studies in the future.
7. Future directions
The VEGF pathway holds the key in regulating angiogenesis and targeted therapies against this pathway have been established in the clinic. However, after initial positive results several studies have failed to show increased survival rates for many groups of patients treated with anti-VEGF drugs. It is clear that multiple factors contribute to enabling the angiogenic switch in tumors. However, till date an overwhelming focus has been placed on blocking the VEGF-VEGFR2 pathway. Using small synthetic molecules with a broader target profile, it has been possible to achieve better anti-angiogenic effects in tumors compared to using specific antibody based therapeutics. However, the former are more toxic, still not sufficiently effective and therefore other strategies need to be considered.
It is becoming increasingly clear that plant-derived medicinal compounds may in many cases be at least as effective in blocking angiogenesis as the currently used synthetic drugs, while exhibiting only a fraction of the negative side-effects. Some of these compounds may, however, be more effective in treatment of some tumors, such as the potential for using phytoestrogens in treatment of ER-positive breast cancer or that of melatonin in treating cancer in people with disrupted circadian rhythms. Identification of suitable biomarkers for selection of patients to different types of therapeutic regimens as well as response to treatment has however so far not been achieved but remain a very important avenue for future research.
The desired optimal effect of anti-angiogenic treatment is another issue, which requires further study. Is complete vascular regression what we aim for? Such an outcome would be expected to result in extensive tumor hypoxia, radically reduced perfusion and thus reduced delivery of not only oxygen and nutrients but also drugs to the tumor tissue. Some studies argue that such a result of anti-angiogenic treatment may in fact lead to a more invasive tumor phenotype and worse prognosis compared to the non-anti-angiogenesis treated group. Perhaps it would be a better option to merely reduce the vascular density, improve the structure and function of tumor blood vessels such that they lose their pathogenic characteristics and more closely resemble blood vessels in healthy tissues. Such a phenotype would be expected, however, to lead to improved perfusion, oxygenation and delivery of nutrients to the tumor, thereby potentially increasing tumor growth rate. On the other hand, this would improve the effects of co-administered chemotherapy, which would also be delivered more effectively to the tumor, and which would more effectively kill the tumor cells if these were well nourished and actively proliferating. Perhaps it should even be considered to withdraw anti-angiogenic treatment if it is found to result in dramatically reduced perfusion and oxygenation of the tumor, at least if such treatment is used in combination with chemo- or radiotherapy. The phytochemical approaches to inhibit tumor angiogenesis identified in Table 2 can, however, in addition to pruning non-perfused and pathological blood vessels in the tumor, also reduce the detrimental effects of chemotherapy, leading to improved tolerance and therefore improved health and potentially extended survival when used in combination with classical cytostatic therapies. It should therefore be considered whether such compounds could be beneficial for tumor patients receiving chemotherapy, even if they are also receiving anti-VEGF drugs as the ones in clinical use today do not reduce the side-effects associated with chemotherapy.
While many natural compounds have general beneficial effects, it is clear that some compounds are more effective in cancer treatment than others. For example, a recent meta-analysis of randomized placebo-controlled trials testing the effect of dietary supplementation of multi-vitamins and multi-minerals, failed to show any effect on cancer mortality suggesting that this is not a route to prioritize in future efforts [235]. Vitamin E and high doses of beta-carotene have been shown to increase prostate and lung cancer risk suggesting that caution is warranted with these powerful biological components [236], [237]. Recently it has become evident that the function of healthy tissues also relies on the activity of angiogenic factors. For example, currently used anti-VEGF drugs cause a marked reduction in vascular density, with a subsequent decline in the function of healthy organs such as the thymus and other endocrine glands, intestine and other parts of the digestive tract, gonads and the kidney [238]. As such, the effects that we are seeking from the treatment – potent and broad-acting anti-angiogenic activity – may end up leading to side-effects. Possible ways to overcome this issue is by targeted delivery such as via nanoparticle carriers as discussed above, or by titrating the amount of phytochemicals given such that it is effective in reducing/preventing pathological angiogenesis but not high enough to impair healthy vascular functions. Such an approach could logically be considered together with the combination of for example the 10 phytochemicals identified here in one cocktail where each compound is selected to complement the other hopefully leading to synergistic anti-angiogenic effects while maintaining negligible toxicity. This concept, as well as the identification of which tumor types are more susceptible to such treatment and identification of readouts for efficacy and toxicity, are important areas for future research.
There are, however, also potential drawbacks associated with the use of phytochemicals, which needs to be resolved before effective formulations of such compounds can be achieved. Due to poor absorption of many phytochemicals in their natural form, it may be necessary to chemically modify some of the compounds suggested here, or use them in semi-synthetic formulations such as nanoparticle encapsulations, which would lower their “natural” status but could promote their therapeutic and pharmacokinetic profiles. How best to take advantage of such derivations or modifications is still an open question.
8. Final conclusion
The complex and multi-factorial nature of tumor angiogenesis, especially in advanced tumors, necessitate the use of therapeutic compounds which act broadly in order to reduce problems with developing resistance. Cancer is a chronic disorder, which in cases where no curative treatment exists, has to be treated medically for a long period of time. Therefore treatment options with very low if any toxicity are preferred. Cancer is furthermore a growing medical issue also in developing countries and in societies in which the high costs associated with cutting edge treatment at modern medical institutions is prohibitive to the majority of the patients. Therefore low-cost treatment options have great potential. Phytochemicals constitute a class of broadly acting and very cheap natural drugs with very low toxicity especially if given at relatively low doses in combination. The list of 10 prototypical phytochemical approaches to inhibit 10 important pro-angiogenic targets in cancer presented here, constitute a framework for further studies on how to mix a potentially effective, non-toxic cocktail of natural compounds as a complement or alternative to other types of anti-cancer drugs available today. We hope that this review could serve as a primer for such investigations, which hopefully could lead to cheaper, safer and more effective anti-cancer treatment in the future.
Conflict of interest statement
The authors declare no conflict of interest.
Author contributions
Z.W., C.D., X.Y., M.F., A.A., W.K.R., D.G., G.R.N., B.E.R., D.R., Y.C.C. and L.D.J. wrote the manuscript, K.H., H.F., A.G.G., S.H., A.A., E.N., A.A., S.S.A., B.H., X.Y., G.G., D.B., M.R.C., K.A., S.C., D.H., S.I.M., A.A., A.B. and N.K. provided validation of the prioritized targets and approaches listed in Table 1, the result of which is shown in Table 1, Table 2.
Acknowledgments
We would like to thank Mr. Leroy Lowe for his vision and initiative in the GettingToKnowCancer organization. Dr. Jensen was funded by the Swedish Society for Medical Research, the Goesta Fraenkel Foundation, Åke Wiberg's Foundation, Ollie och Elof Ericsson's Foundation, the Karolinska Institute and Linköping University. Drs Keith and Bilsland were funded by the University of Glasgow, Beatson Oncology Center Fund and Cancer Research UK grant C301/A14762. Dr. Arreola was funded by NIH NRSA grant F31CA154080. Dr. Georgakilas was funded by EU grant MC-CIG-303514, the National Strategic Reference Framework grant MIS-379346 and COST action CM1201. Dr. Amedei was funded by the University of Florence and the Italian Ministry of Research. Dr. Amin was partially funded by the Terry Fox Foundation, 2013 and the UAEU Program for Advanced Research 2013. Dr. Yi Charlie Chen was funded by the West Virginia Experimental Program to Stimulate Competitive Research and NIH grants 5P20RR016477 and 8P20GM104434. Dr. Dabrosin was funded by the Swedish Research Council and the Swedish Research Society. Dr. Generali was funded by the EU Seventh Framework Program grant 278570. Dr. Halicka was funded by the NIH. Dr. Niccolai was funded by the Italian Ministry of Research and the University of Italy. Dr. Honoki was funded by the Japanese Ministry of Science grant 24590493. Dr. Rathmell was funded by Galaxo Smith Kline for an investigator-initiated clinical trial. Dr. Ciriolo was funded by the AIRC. Dr. Fuster was funded by the NIH/NHLBI grant R01HL107652 and an Eisai Healthcare Investigator grant. Dr. Sophie Chen was funded by the Ovarian and Prostate Cancer Research Trust. Dr. Yin was funded by NIH/NHLBI grant T32HL098062.
Contributor Information
Zongwei Wang, Email: zwang0@partners.org.
Lasse D. Jensen, Email: lasse.jensen@liu.se.
References
- 1.Ribatti D., Djonov V. Intussusceptive microvascular growth in tumors. Cancer Lett. 2012;316:126–131. doi: 10.1016/j.canlet.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y., Arbiser J., D’Amato R.J., D’Amore P.A., Ingber D.E., Kerbel R. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3:114rv113. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Bergers G., Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribatti D. Endogenous inhibitors of angiogenesis: a historical review. Leuk Res. 2009;33:638–644. doi: 10.1016/j.leukres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Landskroner-Eiger S., Moneke I., Sessa W.C. miRNAs as modulators of angiogenesis. Cold Spring Harb Perspect Med. 2013;3:a006643. doi: 10.1101/cshperspect.a006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellstrom M., Phng L.K., Hofmann J.J., Wallgard E., Coultas L., Lindblom P. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 8.Siekmann A.F., Lawson N.D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson L., Franco C.A., Bentley K., Collins R.T., Ponsioen B., Aspalter I.M. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 10.Krueger J., Liu D., Scholz K., Zimmer A., Shi Y., Klein C. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 2011;138:2111–2120. doi: 10.1242/dev.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridgway J., Zhang G., Wu Y., Stawicki S., Liang W.C., Chanthery Y. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 12.Noguera-Troise I., Daly C., Papadopoulos N.J., Coetzee S., Boland P., Gale N.W. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 13.Nagy J.A., Dvorak A.M., Dvorak H.F. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue Y., Lim S., Yang Y., Wang Z., Jensen L.D., Hedlund E.M. PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing erythropoietin production in stromal cells. Nat Med. 2012;18:100–110. doi: 10.1038/nm.2575. [DOI] [PubMed] [Google Scholar]
- 15.Hosaka K., Yang Y., Seki T., Nakamura M., Andersson P., Rouhi P. Tumour PDGF-BB expression levels determine dual effects of anti-PDGF drugs on vascular remodelling and metastasis. Nat Commun. 2013;4:2129. doi: 10.1038/ncomms3129. [DOI] [PubMed] [Google Scholar]
- 16.Hedlund E.M., Yang X., Zhang Y., Yang Y., Shibuya M., Zhong W. Tumor cell-derived placental growth factor sensitizes antiangiogenic and antitumor effects of anti-VEGF drugs. Proc Natl Acad Sci U S A. 2013;110:654–659. doi: 10.1073/pnas.1209310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Schaft D.W., Seftor R.E., Seftor E.A., Hess A.R., Gruman L.M., Kirschmann D.A. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst. 2004;96:1473–1477. doi: 10.1093/jnci/djh267. [DOI] [PubMed] [Google Scholar]
- 18.Nagy J.A., Dvorak H.F. Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin Exp Metastasis. 2012;29:657–662. doi: 10.1007/s10585-012-9500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 20.Shanmugam M.K., Dai X., Kumar A.P., Tan B.K., Sethi G., Bishayee A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: preclinical and clinical evidence. Cancer Lett. 2014;346:206–216. doi: 10.1016/j.canlet.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruick R.K., McKnight S.L. A conserved family of prolyl-4-hydroxylases that modify HIF. Sci Signal. 2001;294:1337. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell P., Wiesener M., Chang G., Clifford S., Vaux E., Cockman M. The tumour suppressor protein VHL targets hypoxia inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 23.Raval R.R., Lau K.W., Tran M.G.B., Sowter H.M., Mandriota S.J., Li J.L. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel–Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu C.J., Wang L.Y., Chodosh L.A., Keith B., Simon M.C. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliopoulos O., Levy A.P., Jiang C., Kaelin W.G., Goldberg M.A. Negative regulation of hypoxia-inducible genes by the von Hippel–Lindau protein. Proc Natl Acad Sci U S A. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosetti F., Ferrari N., De Flora S., Albini A. Angioprevention: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16:2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 27.Ivan M., Harris A.L., Martelli F., Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaelin W.G. The von Hippel–Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res. 2004;10:6290S–6295S. doi: 10.1158/1078-0432.CCR-sup-040025. [DOI] [PubMed] [Google Scholar]
- 29.Leong S.P., Cady B., Jablons D.M., Garcia-Aguilar J., Reintgen D., Jakub J. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25:221–232. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]
- 30.Sleeman J.P. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res. 2000;157:55–81. doi: 10.1007/978-3-642-57151-0_6. [DOI] [PubMed] [Google Scholar]
- 31.Bono P., Wasenius V.M., Heikkila P., Lundin J., Jackson D.G., Joensuu H. High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res. 2004;10:7144–7149. doi: 10.1158/1078-0432.CCR-03-0826. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura Y., Yasuoka H., Tsujimoto M., Imabun S., Nakahara M., Nakao K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;91:125–132. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- 33.Gombos Z., Xu X., Chu C.S., Zhang P.J., Acs G. Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early-stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res. 2005;11:8364–8371. doi: 10.1158/1078-0432.CCR-05-1238. [DOI] [PubMed] [Google Scholar]
- 34.Chen W., Shen W., Chen M., Cai G., Liu X. Study on the relationship between lymphatic vessel density and distal intramural spread of rectal cancer. Eur Surg Res. 2007;39:332–339. doi: 10.1159/000104837. [DOI] [PubMed] [Google Scholar]
- 35.Renyi-Vamos F., Tovari J., Fillinger J., Timar J., Paku S., Kenessey I. Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non-small cell lung cancer. Clin Cancer Res. 2005;11:7344–7353. doi: 10.1158/1078-0432.CCR-05-1077. [DOI] [PubMed] [Google Scholar]
- 36.Padera T.P., Kadambi A., di Tomaso E., Carreira C.M., Brown E.B., Boucher Y. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 37.Wang X.L., Fang J.P., Tang R.Y., Chen X.M. Different significance between intratumoral and peritumoral lymphatic vessel density in gastric cancer: a retrospective study of 123 cases. BMC Cancer. 2010;10:299. doi: 10.1186/1471-2407-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravindranath M.H., Muthugounder S., Presser N., Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- 39.Mattila M.M., Ruohola J.K., Karpanen T., Jackson D.G., Alitalo K., Harkonen P.L. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946–951. doi: 10.1002/ijc.10283. [DOI] [PubMed] [Google Scholar]
- 40.Karpanen T., Egeblad M., Karkkainen M.J., Kubo H., Yla-Herttuala S., Jaattela M. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 41.Ochi N., Matsuo Y., Sawai H., Yasuda A., Takahashi H., Sato M. Vascular endothelial growth factor-C secreted by pancreatic cancer cell line promotes lymphatic endothelial cell migration in an in vitro model of tumor lymphangiogenesis. Pancreas. 2007;34:444–451. doi: 10.1097/mpa.0b13e31803dd307. [DOI] [PubMed] [Google Scholar]
- 42.Skobe M., Hawighorst T., Jackson D.G., Prevo R., Janes L., Velasco P. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 43.Mumprecht V., Detmar M. Lymphangiogenesis and cancer metastasis. J Cell Mol Med. 2009;13:1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ran S., Volk L., Hall K., Flister M.J. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17:229–251. doi: 10.1016/j.pathophys.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang L.K., Garcia-Cardena G., Farnebo F., Fannon M., Chen E.J., Butterfield C. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101:11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubo H., Cao R., Brakenhielm E., Makinen T., Cao Y., Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J.R., Yu L., Zhong Y., Blackburn G.L. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133:516–521. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kajiya K., Hirakawa S., Ma B., Drinnenberg I., Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J.R., Yu L., Mai Z., Blackburn G.L. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int J Cancer. 2004;108:8–14. doi: 10.1002/ijc.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H., Adachi Y., Yamamoto H., Min Y., Ohashi H., Ii M. Insulin-like growth factor-I receptor blockade reduces tumor angiogenesis and enhances the effects of bevacizumab for a human gastric cancer cell line, MKN45. Cancer. 2011;117:3135–3147. doi: 10.1002/cncr.25893. [DOI] [PubMed] [Google Scholar]
- 51.Makinen T., Adams R.H., Bailey J., Lu Q., Ziemiecki A., Alitalo K. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helfrich I., Edler L., Sucker A., Thomas M., Christian S., Schadendorf D. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res. 2009;15:1384–1392. doi: 10.1158/1078-0432.CCR-08-1615. [DOI] [PubMed] [Google Scholar]
- 53.Cao R., Bjorndahl M.A., Religa P., Clasper S., Garvin S., Galter D. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 54.Banziger-Tobler N.E., Halin C., Kajiya K., Detmar M. Growth hormone promotes lymphangiogenesis. Am J Pathol. 2008;173:586–597. doi: 10.2353/ajpath.2008.080060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirakawa S., Kodama S., Kunstfeld R., Kajiya K., Brown L.F., Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirakawa S., Brown L.F., Kodama S., Paavonen K., Alitalo K., Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishii H., Chikamatsu K., Sakakura K., Miyata M., Furuya N., Masuyama K. Primary tumor induces sentinel lymph node lymphangiogenesis in oral squamous cell carcinoma. Oral Oncol. 2010;46:373–378. doi: 10.1016/j.oraloncology.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Christiansen A., Detmar M. Lymphangiogenesis and cancer. Genes Cancer. 2011;2:1146–1158. doi: 10.1177/1947601911423028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 60.Fukumura D., Jain R.K. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 61.Duong T., Koopman P., Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J Oncol. 2012:204946. doi: 10.1155/2012/204946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roenneberg T. Chronobiology: the human sleep project. Nature. 2013;498:427–428. doi: 10.1038/498427a. [DOI] [PubMed] [Google Scholar]
- 63.Jensen L.D., Cao Y.H. Clock controls angiogenesis. Cell Cycle. 2013;12:405–408. doi: 10.4161/cc.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen L.D., Cao Z., Nakamura M., Yang Y., Brautigam L., Andersson P. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep. 2012;2:231–241. doi: 10.1016/j.celrep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Schernhammer E.S., Laden F., Speizer F.E., Willett W.C., Hunter D.J., Kawachi I. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 66.Schernhammer E.S., Laden F., Speizer F.E., Willett W.C., Hunter D.J., Kawachi I. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 67.Rao S., Chun C., Fan J., Kofron J.M., Yang M.B., Hegde R.S. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busik J.V., Tikhonenko M., Bhatwadekar A., Opreanu M., Yakubova N., Caballero S. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anea C.B., Cheng B., Sharma S., Kumar S., Caldwell R.W., Yao L. Increased superoxide and endothelial NO synthase uncoupling in blood vessels of Bmal1-knockout mice. Circ Res. 2012;111:1157–1165. doi: 10.1161/CIRCRESAHA.111.261750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang C.Y., Wen M.S., Wang H.W., Hsieh I.C., Li Y., Liu P.Y. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118:2166–2173. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koyanagi S., Kuramoto Y., Nakagawa H., Aramaki H., Ohdo S., Soeda S. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. 2003;63:7277–7283. [PubMed] [Google Scholar]
- 72.Guzy R.D., Schumacker P.T. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 73.Denko N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 74.Gillies R.J., Verduzco D., Gatenby R.A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferrara N. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr Opin Hematol. 2010;17:219–224. doi: 10.1097/MOH.0b013e3283386660. [DOI] [PubMed] [Google Scholar]
- 76.Apte R.N., Krelin Y., Song X., Dotan S., Recih E., Elkabets M. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour–host interactions. Eur J Cancer. 2006;42:751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Nicolini A., Carpi A., Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Crawford Y., Kasman I., Yu L., Zhong C., Wu X., Modrusan Z. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Ricci-Vitiani L., Pallini R., Biffoni M., Todaro M., Invernici G., Cenci T. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 80.Maione F., Capano S., Regano D., Zentilin L., Giacca M., Casanovas O. Semaphorin 3A overcomes cancer hypoxia and metastatic dissemination induced by antiangiogenic treatment in mice. J Clin Invest. 2012;122:1832–1848. doi: 10.1172/JCI58976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Vinals F. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ebos J.M., Lee C.R., Cruz-Munoz W., Bjarnason G.A., Christensen J.G., Kerbel R.S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu K.V., Chang J.P., Parachoniak C.A., Pandika M.M., Aghi M.K., Meyronet D. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jensen L.D., Cao R., Cao Y. In vivo angiogenesis and lymphangiogenesis models. Curr Mol Med. 2009;9:982–991. doi: 10.2174/156652409789712738. [DOI] [PubMed] [Google Scholar]
- 85.Jensen L.D., Rouhi P., Cao Z., Lanne T., Wahlberg E., Cao Y. Zebrafish models to study hypoxia-induced pathological angiogenesis in malignant and nonmalignant diseases. Birth Defects Res C Embryo Today. 2011;93:182–193. doi: 10.1002/bdrc.20203. [DOI] [PubMed] [Google Scholar]
- 86.Willett C.G., Boucher Y., di Tomaso E., Duda D.G., Munn L.L., Tong R.T. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lockhart A.C., Rothenberg M.L., Dupont J., Cooper W., Chevalier P., Sternas L. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong H., Chiles K., Feldser D., Laughner E., Hanrahan C., Georgescu M.M. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 89.Mabjeesh N.J., Post D.E., Willard M.T., Kaur B., Van Meir E.G., Simons J.W. Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Res. 2002;62:2478–2482. [PubMed] [Google Scholar]
- 90.Gotink K.J., Verheul H.M. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13:1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mihaly Z., Sztupinszki Z., Surowiak P., Gyorffy B. A comprehensive overview of targeted therapy in metastatic renal cell carcinoma. Curr Cancer Drug Targets. 2012;12:857–872. doi: 10.2174/156800912802429265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 93.Yashiro M., Shinto O., Nakamura K., Tendo M., Matsuoka T., Matsuzaki T. Effects of VEGFR-3 phosphorylation inhibitor on lymph node metastasis in an orthotopic diffuse-type gastric carcinoma model. Br J Cancer. 2009;101:1100–1106. doi: 10.1038/sj.bjc.6605296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Penel N., Adenis A., Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 95.Kerbel R.S. Reappraising antiangiogenic therapy for breast cancer. Breast. 2011;20(Suppl. 3):S56–S60. doi: 10.1016/S0960-9776(11)70295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller K.D., Sweeney C.J., Sledge G.W., Jr. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol. 2001;19:1195–1206. doi: 10.1200/JCO.2001.19.4.1195. [DOI] [PubMed] [Google Scholar]
- 97.Klauber N., Parangi S., Flynn E., Hamel E., D’Amato R.J. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res. 1997;57:81–86. [PubMed] [Google Scholar]
- 98.Browder T., Butterfield C.E., Kraling B.M., Shi B., Marshall B., O’Reilly M.S. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 99.Singhal S., Mehta J., Desikan R., Ayers D., Roberson P., Eddlemon P. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 100.Wood J., Bonjean K., Ruetz S., Bellahcene A., Devy L., Foidart J.M. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 101.Santini D., Vincenzi B., Dicuonzo G., Avvisati G., Massacesi C., Battistoni F. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9:2893–2897. [PubMed] [Google Scholar]
- 102.Land S.C., Tee A.R. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 103.Hudson C.C., Liu M., Chiang G.G., Otterness D.M., Loomis D.C., Kaper F. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guba M., von Breitenbuch P., Steinbauer M., Koehl G., Flegel S., Hornung M. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 105.Zhang D., Hedlund E.M., Lim S., Chen F., Zhang Y., Sun B. Antiangiogenic agents significantly improve survival in tumor-bearing mice by increasing tolerance to chemotherapy-induced toxicity. Proc Natl Acad Sci U S A. 2011;108:4117–4122. doi: 10.1073/pnas.1016220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh B.N., Singh B.R., Sarma B.K., Singh H.B. Potential chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark. Chem Biol Interact. 2009;181:20–28. doi: 10.1016/j.cbi.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Singh B.N., Singh H.B., Singh A., Naqvi A.H., Singh B.R. Dietary phytochemicals alter epigenetic events and signaling pathways for inhibition of metastasis cascade: phytoblockers of metastasis cascade. Cancer Metastasis Rev. 2014;33(1):41–85. doi: 10.1007/s10555-013-9457-1. [DOI] [PubMed] [Google Scholar]
- 108.Singh B.N., Shankar S., Srivastava R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gullett N.P., Ruhul Amin A.R., Bayraktar S., Pezzuto J.M., Shin D.M., Khuri F.R. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 110.Singh M., Singh P., Shukla Y. New strategies in cancer chemoprevention by phytochemicals. Front Biosci (Elite Ed) 2012;4:426–452. doi: 10.2741/389. [DOI] [PubMed] [Google Scholar]
- 111.Bishayee A. Editorial: current advances in cancer prevention and treatment by natural products. Curr Pharm Biotechnol. 2012;13:115–116. doi: 10.2174/138920112798868629. [DOI] [PubMed] [Google Scholar]
- 112.Jeong D., Watari K., Shirouzu T., Ono M., Koizumi K., Saiki I. Studies on lymphangiogenesis inhibitors from Korean and Japanese crude drugs. Biol Pharm Bull. 2013;36:152–157. doi: 10.1248/bpb.b12-00871. [DOI] [PubMed] [Google Scholar]
- 113.Kimura Y., Sumiyoshi M. Anti-tumor and anti-metastatic actions of wogonin isolated from Scutellaria baicalensis roots through anti-lymphangiogenesis. Phytomedicine. 2013;20:328–336. doi: 10.1016/j.phymed.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 114.Luo Y., Chen W., Zhou H., Liu L., Shen T., Alexander J.S. Cryptotanshinone inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3/ERK and small GTPase pathways. Cancer Prev Res (Phila) 2011;4:2083–2091. doi: 10.1158/1940-6207.CAPR-11-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coussens L.M., Fingleton B., Matrisian L.M. Cancer therapy – matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 116.Bendrik C., Robertson J., Gauldie J., Dabrosin C. Gene transfer of matrix metalloproteinase-9 induces tumor regression of breast cancer in vivo. Cancer Res. 2008;68:3405–3412. doi: 10.1158/0008-5472.CAN-08-0295. [DOI] [PubMed] [Google Scholar]
- 117.Hamano Y., Zeisberg M., Sugimoto H., Lively J.C., Maeshima Y., Yang C.Q. Physiological levels of tumstatin, a fragment of collagen IV alpha 3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alpha V beta 3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nilsson U.W., Dabrosin C. Estradiol and tamoxifen regulate endostatin generation via matrix metalloproteinase activity in breast cancer in vivo. Cancer Res. 2006;66:4789–4794. doi: 10.1158/0008-5472.CAN-05-4012. [DOI] [PubMed] [Google Scholar]
- 119.Pozzi A., LeVine W.F., Gardner H.A. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene. 2002;21:272–281. doi: 10.1038/sj.onc.1205045. [DOI] [PubMed] [Google Scholar]
- 120.Decock J., Thirkettle S., Wagstaff L., Edwards D.R. Matrix metalloproteinases: protective roles in cancer. J Cell Mol Med. 2011;15:1254–1265. doi: 10.1111/j.1582-4934.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leifler K.S., Svensson S., Abrahamsson A., Bendrik C., Robertson J., Gauldie J. Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. J Immunol. 2013;190:4420–4430. doi: 10.4049/jimmunol.1202610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Partridge J.J., Madsen M.A., Ardi V.C., Papagiannakopoulos T., Kupriyanova T.A., Quigley J.P. Functional analysis of matrix metalloproteinases and tissue inhibitors of metalloproteinases differentially expressed by variants of human HT-1080 fibrosarcoma exhibiting high and low levels of intravasation and metastasis. J Biol Chem. 2007;282:35964–35977. doi: 10.1074/jbc.M705993200. [DOI] [PubMed] [Google Scholar]
- 123.Kopitz C., Gerg M., Bandapalli O.R., Ister D., Pennington C.J., Hauser S. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67:8615–8623. doi: 10.1158/0008-5472.CAN-07-0232. [DOI] [PubMed] [Google Scholar]
- 124.Lipton A., Ali S.M., Leitzel K., Demers L., Evans D.B., Hamer P. Elevated plasma tissue inhibitor of metalloproteinase-1 level predicts decreased response and survival in metastatic breast cancer. Cancer. 2007;109:1933–1939. doi: 10.1002/cncr.22637. [DOI] [PubMed] [Google Scholar]
- 125.Miyagi M., Aoyagi K., Kato S., Shirouzu K. The TIMP-1 gene transferred through adenovirus mediation shows a suppressive effect on peritoneal metastases from gastric cancer. Int J Clin Oncol. 2007;12:17–24. doi: 10.1007/s10147-006-0616-z. [DOI] [PubMed] [Google Scholar]
- 126.Klintman M., Wurtz S.O., Christensen I.J., Hertel P.B., Ferno M., Malmberg M. Association between tumor tissue TIMP-1 levels and objective response to first-line chemotherapy in metastatic breast cancer. Breast Cancer Res Treat. 2010;121:365–371. doi: 10.1007/s10549-009-0483-1. [DOI] [PubMed] [Google Scholar]
- 127.Schrohl A.S., Christensen I.J., Pedersen A.N., Jensen V., Mouridsen H., Murphy G. Tumor tissue concentrations of the proteinase inhibitors tissue inhibitor of metalloproteinases-1 (TIMP-1) and plasminogen activator inhibitor type 1 (PAI-1) are complementary in determining prognosis in primary breast cancer. Mol Cell Proteomics. 2003;2:164–172. doi: 10.1074/mcp.M300019-MCP200. [DOI] [PubMed] [Google Scholar]
- 128.Schrohl A.S., Look M.P., Meijer-van Gelder M.E., Foekens J.A., Brunner N. Tumor tissue levels of Tissue Inhibitor of Metalloproteinases-1 (TIMP-1) and outcome following adjuvant chemotherapy in premenopausal lymph node-positive breast cancer patients: a retrospective study. BMC Cancer. 2009;9 doi: 10.1186/1471-2407-9-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stanley G., Harvey K., Slivova V., Jiang J., Sliva D. Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-beta1 from prostate cancer cells. Biochem Biophys Res Commun. 2005;330:46–52. doi: 10.1016/j.bbrc.2005.02.116. [DOI] [PubMed] [Google Scholar]
- 130.Brakenhielm E., Cao R., Cao Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001;15:1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- 131.Huang Y.T., Hwang J.J., Lee P.P., Ke F.C., Huang J.H., Huang C.J. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999;128:999–1010. doi: 10.1038/sj.bjp.0702879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tosetti F., Benelli R., Albini A. The angiogenic switch in solid tumors: clinical implications. Suppl Tumori. 2002;1:S9–S11. doi: 10.1177/03008916020016s103. [DOI] [PubMed] [Google Scholar]
- 133.Cao Y., Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 134.Sartippour M.R., Shao Z.M., Heber D., Beatty P., Zhang L., Liu C. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307–2311. doi: 10.1093/jn/132.8.2307. [DOI] [PubMed] [Google Scholar]
- 135.Maeda-Yamamoto M., Kawahara H., Tahara N., Tsuji K., Hara Y., Isemura M. Effects of tea polyphenols on the invasion and matrix metalloproteinases activities of human fibrosarcoma HT1080 cells. J Agric Food Chem. 1999;47:2350–2354. doi: 10.1021/jf9811525. [DOI] [PubMed] [Google Scholar]
- 136.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 137.Yang H., Sun D.K., Chen D., Cui Q.C., Gu Y.Y., Jiang T. Antitumor activity of novel fluoro-substituted (−)-epigallocatechin-3-gallate analogs. Cancer Lett. 2010;292:48–53. doi: 10.1016/j.canlet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Slivova V., Zaloga G., DeMichele S.J., Mukerji P., Huang Y.S., Siddiqui R. Green tea polyphenols modulate secretion of urokinase plasminogen activator (uPA) and inhibit invasive behavior of breast cancer cells. Nutr Cancer. 2005;52:66–73. doi: 10.1207/s15327914nc5201_9. [DOI] [PubMed] [Google Scholar]
- 139.Sen T., Moulik S., Dutta A., Choudhury P.R., Banerji A., Das S. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009;84:194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 140.Lin J.K., Liang Y.C., Lin-Shiau S.Y. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999;58:911–915. doi: 10.1016/s0006-2952(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 141.Jung Y.D., Kim M.S., Shin B.A., Chay K.O., Ahn B.W., Liu W. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844–850. doi: 10.1054/bjoc.2000.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khan N., Adhami V.M., Mukhtar H. Review: green tea polyphenols in chemoprevention of prostate cancer: preclinical and clinical studies. Nutr Cancer. 2009;61:836–841. doi: 10.1080/01635580903285056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang C.S., Wang X., Lu G., Picinich S.C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jankun J., Selman S.H., Swiercz R., Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 145.Hastak K., Gupta S., Ahmad N., Agarwal M.K., Agarwal M.L., Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 146.Masuda M., Suzui M., Lim J.T., Deguchi A., Soh J.W., Weinstein I.B. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 147.Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 148.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arbiser J.L., Klauber N., Rohan R., van Leeuwen R., Huang M.T., Fisher C. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 150.Hong J.H., Ahn K.S., Bae E., Jeon S.S., Choi H.Y. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006;9:147–152. doi: 10.1038/sj.pcan.4500856. [DOI] [PubMed] [Google Scholar]
- 151.Yoysungnoen P., Wirachwong P., Bhattarakosol P., Niimi H., Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006;34:109–115. [PubMed] [Google Scholar]
- 152.Hasima N., Aggarwal B.B. Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol. 2012;3:328–351. [PMC free article] [PubMed] [Google Scholar]
- 153.Shukla Y., Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 154.Garvin S., Ollinger K., Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006;231:113–122. doi: 10.1016/j.canlet.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 155.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 156.Bishayee A., Petit D.M., Samtani K. Angioprevention is implicated in resveratrol chemoprevention of experimental hepatocarcinogenesis. J Carcinog Mutagen. 2010:1. [Google Scholar]
- 157.Cao Z., Fang J., Xia C., Shi X., Jiang B.H. trans-3,4,5′-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10:5253–5263. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- 158.Liu Z., Li Y., Yang R. Effects of resveratrol on vascular endothelial growth factor expression in osteosarcoma cells and cell proliferation. Oncol Lett. 2012;4:837–839. doi: 10.3892/ol.2012.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Srivastava R.K., Unterman T.G., Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem. 2010;337:201–212. doi: 10.1007/s11010-009-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marti-Centelles R., Cejudo-Marin R., Falomir E., Murga J., Carda M., Marco J.A. Inhibition of VEGF expression in cancer cells and endothelial cell differentiation by synthetic stilbene derivatives. Bioorg Med Chem. 2013;21:3010–3015. doi: 10.1016/j.bmc.2013.03.072. [DOI] [PubMed] [Google Scholar]
- 161.Jung D.B., Lee H.J., Jeong S.J., Lee E.O., Kim Y.C., Ahn K.S. Rhapontigenin inhibited hypoxia inducible factor 1 alpha accumulation and angiogenesis in hypoxic PC-3 prostate cancer cells. Biol Pharm Bull. 2011;34:850–855. doi: 10.1248/bpb.34.850. [DOI] [PubMed] [Google Scholar]
- 162.Kimura Y., Sumiyoshi M., Baba K. Antitumor activities of synthetic and natural stilbenes through antiangiogenic action. Cancer Sci. 2008;99:2083–2096. doi: 10.1111/j.1349-7006.2008.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fotsis T., Pepper M.S., Aktas E., Breit S., Rasku S., Adlercreutz H. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 164.Fotsis T., Pepper M., Adlercreutz H., Hase T., Montesano R., Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125:790S–797S. doi: 10.1093/jn/125.suppl_3.790S. [DOI] [PubMed] [Google Scholar]
- 165.van Meeteren M.E., Hendriks J.J., Dijkstra C.D., van Tol E.A. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem Pharmacol. 2004;67:967–975. doi: 10.1016/j.bcp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 166.Chen A.Y., Chen Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duthie G., Crozier A. Plant-derived phenolic antioxidants. Curr Opin Clin Nutr Metab Care. 2000;3:447–451. doi: 10.1097/00075197-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 168.Cortes J.R., Perez G.M., Rivas M.D., Zamorano J. Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK3. J Immunol. 2007;179:3881–3887. doi: 10.4049/jimmunol.179.6.3881. [DOI] [PubMed] [Google Scholar]
- 169.Lee K.M., Lee K.W., Jung S.K., Lee E.J., Heo Y.S., Bode A.M. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem Pharmacol. 2010;80:2042–2049. doi: 10.1016/j.bcp.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcia-Mediavilla V., Crespo I., Collado P.S., Esteller A., Sanchez-Campos S., Tunon M.J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur J Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 171.Suh Y., Afaq F., Johnson J.J., Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Singh R.P., Sharma G., Dhanalakshmi S., Agarwal C., Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev. 2003;12:933–939. [PubMed] [Google Scholar]
- 173.Singh R.P., Dhanalakshmi S., Agarwal C., Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 174.Jiang C., Agarwal R., Lu J. Anti-angiogenic potential of a cancer chemopreventive flavonoid antioxidant, silymarin: inhibition of key attributes of vascular endothelial cells and angiogenic cytokine secretion by cancer epithelial cells. Biochem Biophys Res Commun. 2000;276:371–378. doi: 10.1006/bbrc.2000.3474. [DOI] [PubMed] [Google Scholar]
- 175.Tsai S.H., Liang Y.C., Lin-Shiau S.Y., Lin J.K. Suppression of TNFalpha-mediated NFkappaB activity by myricetin and other flavonoids through downregulating the activity of IKK in ECV304 cells. J Cell Biochem. 1999;74:606–615. [PubMed] [Google Scholar]
- 176.Bertl E., Bartsch H., Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 177.Kim Y.H., Shin E.K., Kim D.H., Lee H.H., Park J.H., Kim J.K. Antiangiogenic effect of licochalcone A. Biochem Pharmacol. 2010;80:1152–1159. doi: 10.1016/j.bcp.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 178.Kwon G.T., Cho H.J., Chung W.Y., Park K.K., Moon A., Park J.H. Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: possible mediation by decreased JNK/AP-1 signaling. J Nutr Biochem. 2009;20:663–676. doi: 10.1016/j.jnutbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 179.Hasmeda M., Kweifio-Okai G., Macrides T., Polya G.M. Selective inhibition of eukaryote protein kinases by anti-inflammatory triterpenoids. Planta Med. 1999;65:14–18. doi: 10.1055/s-1999-13954. [DOI] [PubMed] [Google Scholar]
- 180.Beveridge T.H., Li T.S., Drover J.C. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 181.Thoppil R.J., Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J Hepatol. 2011;3:228–249. doi: 10.4254/wjh.v3.i9.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bishayee A., Ahmed S., Brankov N., Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci (Landmark Ed) 2011;16:980–996. doi: 10.2741/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sethi G., Ahn K.S., Pandey M.K., Aggarwal B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 184.Huang Y., Zhou Y., Fan Y., Zhou D. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 2008;264:101–106. doi: 10.1016/j.canlet.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 185.Matsuda H., Li Y., Murakami T., Ninomiya K., Yamahara J., Yoshikawa M. Effects of escins Ia, Ib, IIa, and IIb from horse chestnut, the seeds of Aesculus hippocastanum L., on acute inflammation in animals. Biol Pharm Bull. 1997;20:1092–1095. doi: 10.1248/bpb.20.1092. [DOI] [PubMed] [Google Scholar]
- 186.Wang X.H., Xu B., Liu J.T., Cui J.R. Effect of beta-escin sodium on endothelial cells proliferation, migration and apoptosis. Vasc Pharmacol. 2008;49:158–165. doi: 10.1016/j.vph.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 187.Yang H., Shi G., Dou Q.P. The tumor proteasome is a primary target for the natural anticancer compound Withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71:426–437. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 188.Mohan R., Hammers H.J., Bargagna-Mohan P., Zhan X.H., Herbstritt C.J., Ruiz A. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 189.Jayaprakasam B., Zhang Y., Seeram N.P., Nair M.G. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003;74:125–132. doi: 10.1016/j.lfs.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 190.Park J.S., Chew B.P., Wong T.S. Dietary lutein from marigold extract inhibits mammary tumor development in BALB/c mice. J Nutr. 1998;128:1650–1656. doi: 10.1093/jn/128.10.1650. [DOI] [PubMed] [Google Scholar]
- 191.Krinsky N.I., Johnson E.J. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 192.Milder I.E., Arts I.C., van de Putte B., Venema D.P., Hollman P.C. Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr. 2005;93:393–402. doi: 10.1079/bjn20051371. [DOI] [PubMed] [Google Scholar]
- 193.Thompson L.U., Boucher B.A., Liu Z., Cotterchio M., Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54:184–201. doi: 10.1207/s15327914nc5402_5. [DOI] [PubMed] [Google Scholar]
- 194.Mazur W., Fotsis T., Wahala K., Ojala S., Salakka A., Adlercreutz H. Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal Biochem. 1996;233:169–180. doi: 10.1006/abio.1996.0025. [DOI] [PubMed] [Google Scholar]
- 195.Touillaud M.S., Thiebaut A.C., Fournier A., Niravong M., Boutron-Ruault M.C., Clavel-Chapelon F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst. 2007;99:475–486. doi: 10.1093/jnci/djk096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Trock B.J., Hilakivi-Clarke L., Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 197.Banerjee S., Li Y., Wang Z., Sarkar F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Helferich W.G., Andrade J.E., Hoagland M.S. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–226. doi: 10.1007/s10787-008-8020-0. [DOI] [PubMed] [Google Scholar]
- 199.Lindahl G., Saarinen N., Abrahamsson A., Dabrosin C. Tamoxifen, flaxseed, and the lignan enterolactone increase stroma- and cancer cell-derived IL-1Ra and decrease tumor angiogenesis in estrogen-dependent breast cancer. Cancer Res. 2011;71:51–60. doi: 10.1158/0008-5472.CAN-10-2289. [DOI] [PubMed] [Google Scholar]
- 200.Bergman Jungestrom M., Thompson L.U., Dabrosin C. Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res. 2007;13:1061–1067. doi: 10.1158/1078-0432.CCR-06-1651. [DOI] [PubMed] [Google Scholar]
- 201.Saarinen N.M., Abrahamsson A., Dabrosin C. Estrogen-induced angiogenic factors derived from stromal and cancer cells are differently regulated by enterolactone and genistein in human breast cancer in vivo. Int J Cancer. 2010;127:737–745. doi: 10.1002/ijc.25052. [DOI] [PubMed] [Google Scholar]
- 202.Chatterjee S., Bhattacharjee B. Use of natural molecules as anti-angiogenic inhibitors for vascular endothelial growth factor receptor. Bioinformation. 2012;8:1249–1254. doi: 10.6026/97320630081249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Abusnina A., Keravis T., Zhou Q., Justiniano H., Lobstein A., Lugnier C. Tumour growth inhibition and anti-angiogenic effects using curcumin correspond to combined PDE2 and PDE4 inhibition. Thromb Haemost. 2015;113:319–328. doi: 10.1160/TH14-05-0454. [DOI] [PubMed] [Google Scholar]
- 204.Rath S., Liebl J., Furst R., Ullrich A., Burkhart J.L., Kazmaier U. Anti-angiogenic effects of the tubulysin precursor pretubulysin and of simplified pretubulysin derivatives. Br J Pharmacol. 2012;167:1048–1061. doi: 10.1111/j.1476-5381.2012.02037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schluep T., Gunawan P., Ma L., Jensen G.S., Duringer J., Hinton S. Polymeric tubulysin-peptide nanoparticles with potent antitumor activity. Clin Cancer Res. 2009;15:181–189. doi: 10.1158/1078-0432.CCR-08-1848. [DOI] [PubMed] [Google Scholar]
- 206.Kubisch R., von Gamm M., Braig S., Ullrich A., Burkhart J.L., Colling L. Simplified pretubulysin derivatives and their biological effects on cancer cells. J Nat Prod. 2014;77:536–542. doi: 10.1021/np4008014. [DOI] [PubMed] [Google Scholar]
- 207.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 208.Gref R., Minamitake Y., Peracchia M.T., Trubetskoy V., Torchilin V., Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 209.Siddiqui I.A., Adhami V.M., Bharali D.J., Hafeez B.B., Asim M., Khwaja S.I. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–1716. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Luo H., Jiang B., Li B., Li Z., Jiang B.H., Chen Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int J Nanomed. 2012;7:3951–3959. doi: 10.2147/IJN.S33670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gou M., Men K., Shi H., Xiang M., Zhang J., Song J. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3:1558–1567. doi: 10.1039/c0nr00758g. [DOI] [PubMed] [Google Scholar]
- 212.Wang L., Li H., Wang S., Liu R., Wu Z., Wang C. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech. 2014;15:834–844. doi: 10.1208/s12249-014-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Parikh N.R., Mandal A., Bhatia D., Siveen K.S., Sethi G., Bishayee A. Oleanane triterpenoids in the prevention and therapy of breast cancer: current evidence and future perspectives. Phytochem Rev. 2014;13:793–810. doi: 10.1007/s11101-014-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sohn K.H., Lee H.Y., Chung H.Y., Young H.S., Yi S.Y., Kim K.W. Anti-angiogenic activity of triterpene acids. Cancer Lett. 1995;94:213–218. doi: 10.1016/0304-3835(95)03856-r. [DOI] [PubMed] [Google Scholar]
- 215.Sogno I., Vannini N., Lorusso G., Cammarota R., Noonan D.M., Generoso L. Anti-angiogenic activity of a novel class of chemopreventive compounds: oleanic acid terpenoids. Recent Results Cancer Res. 2009;181:209–212. doi: 10.1007/978-3-540-69297-3_19. [DOI] [PubMed] [Google Scholar]
- 216.Masferrer J.L., Leahy K.M., Koki A.T., Zweifel B.S., Settle S.L., Woerner B.M. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 217.Parrett M., Harris R., Joarder F., Ross M., Clausen K., Robertson F. Cyclooxygenase-2 gene expression in human breast cancer. Int J Oncol. 1997;10:503–507. doi: 10.3892/ijo.10.3.503. [DOI] [PubMed] [Google Scholar]
- 218.Voss M.J., Niggemann B., Zanker K.S., Entschladen F. Tumour reactions to hypoxia. Curr Mol Med. 2010;10:381–386. doi: 10.2174/156652410791317020. [DOI] [PubMed] [Google Scholar]
- 219.Lofstedt T., Jogi A., Sigvardsson M., Gradin K., Poellinger L., Pahlman S. Induction of ID2 expression by hypoxia-inducible factor-1: a role in dedifferentiation of hypoxic neuroblastoma cells. J Biol Chem. 2004;279:39223–39231. doi: 10.1074/jbc.M402904200. [DOI] [PubMed] [Google Scholar]
- 220.Garcia-Maceira P., Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy. Oncogene. 2009;28:313–324. doi: 10.1038/onc.2008.398. [DOI] [PubMed] [Google Scholar]
- 221.Otalora B.B., Madrid J.A., Alvarez N., Vicente V., Rol M.A. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J Pineal Res. 2008;44:307–315. doi: 10.1111/j.1600-079X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 222.Trapp V., Parmakhtiar B., Papazian V., Willmott L., Fruehauf J.P. Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis. 2010;13:305–315. doi: 10.1007/s10456-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Park S.Y., Jeong K.J., Lee J., Yoon D.S., Choi W.S., Kim Y.K. Hypoxia enhances LPA-induced HIF-1alpha and VEGF expression: their inhibition by resveratrol. Cancer Lett. 2007;258:63–69. doi: 10.1016/j.canlet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 224.Li J.L., Sainson R.C., Shi W., Leek R., Harrington L.S., Preusser M. Delta-like 4 notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 225.Lickliter J.D., Francesconi A.B., Smith G., Burge M., Coulthard A., Rose S. Phase I trial of CYT997, a novel cytotoxic and vascular-disrupting agent. Br J Cancer. 2010;103:597–606. doi: 10.1038/sj.bjc.6605841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lekli I., Ray D., Mukherjee S., Gurusamy N., Ahsan M.K., Juhasz B. Co-ordinated autophagy with resveratrol and gamma-tocotrienol confers synergetic cardioprotection. J Cell Mol Med. 2010;14:2506–2518. doi: 10.1111/j.1582-4934.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kuang X., Yao Y., Du J.R., Liu Y.X., Wang C.Y., Qian Z.M. Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res. 2006;1102:145–153. doi: 10.1016/j.brainres.2006.04.110. [DOI] [PubMed] [Google Scholar]
- 228.Forssell J., Oberg A., Henriksson M.L., Stenling R., Jung A., Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 229.Garaude J., Kent A., van Rooijen N., Blander J.M. Simultaneous targeting of toll- and nod-like receptors induces effective tumor-specific immune responses. Sci Transl Med. 2012;4:120ra116. doi: 10.1126/scitranslmed.3002868. [DOI] [PubMed] [Google Scholar]
- 230.Tosetti F., Noonan D.M., Albini A. Metabolic regulation and redox activity as mechanisms for angioprevention by dietary phytochemicals. Int J Cancer. 2009;125:1997–2003. doi: 10.1002/ijc.24677. [DOI] [PubMed] [Google Scholar]
- 231.Deep G., Gangar S.C., Rajamanickam S., Raina K., Gu M., Agarwal C. Angiopreventive efficacy of pure flavonolignans from milk thistle extract against prostate cancer: targeting VEGF–VEGFR signaling. PLoS ONE. 2012;7:e34630. doi: 10.1371/journal.pone.0034630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 233.Thomasset S.C., Berry D.P., Garcea G., Marczylo T., Steward W.P., Gescher A.J. Dietary polyphenolic phytochemicals – promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer. 2007;120:451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 234.Bisacchi D., Benelli R., Vanzetto C., Ferrari N., Tosetti F., Albini A. Anti-angiogenesis and angioprevention: mechanisms, problems and perspectives. Cancer Detect Prev. 2003;27:229–238. doi: 10.1016/s0361-090x(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 235.Macpherson H., Pipingas A., Pase M.P. Multivitamin-multimineral supplementation and mortality: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97:437–444. doi: 10.3945/ajcn.112.049304. [DOI] [PubMed] [Google Scholar]
- 236.Klein E.A., Thompson I.M., Jr., Tangen C.M., Crowley J.J., Lucia M.S., Goodman P.J. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sommer A., Vyas K.S. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012;96:1204S–1206S. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 238.Yang Y., Zhang Y., Cao Z., Ji H., Yang X., Iwamoto H. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci U S A. 2013;110:12018–12023. doi: 10.1073/pnas.1301331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kumareswaran R., Ludkovski O., Meng A., Sykes J., Pintilie M., Bristow R.G. Chronic hypoxia compromises repair of DNA double-strand breaks to drive genetic instability. J Cell Sci. 2012;125:189–199. doi: 10.1242/jcs.092262. [DOI] [PubMed] [Google Scholar]
- 240.Gaddameedhi S., Reardon J.T., Ye R., Ozturk N., Sancar A. Effect of circadian clock mutations on DNA damage response in mammalian cells. Cell Cycle. 2012;11:3481–3491. doi: 10.4161/cc.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dai C., Sun F., Zhu C., Hu X. Tumor environmental factors glucose deprivation and lactic acidosis induce mitotic chromosomal instability – an implication in aneuploid human tumors. PLOS ONE. 2013;8:e63054. doi: 10.1371/journal.pone.0063054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xiang L., Gilkes D.M., Chaturvedi P., Luo W., Hu H., Takano N. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J Mol Med (Berl) 2014;92:151–164. doi: 10.1007/s00109-013-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Leelahavanichkul K., Amornphimoltham P., Molinolo A.A., Basile J.R., Koontongkaew S., Gutkind J.S. A role for p38 MAPK in head and neck cancer cell growth and tumor-induced angiogenesis and lymphangiogenesis. Mol Oncol. 2014;8:105–118. doi: 10.1016/j.molonc.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Thiele W., Rothley M., Teller N., Jung N., Bulat B., Plaumann D. Delphinidin is a novel inhibitor of lymphangiogenesis but promotes mammary tumor growth and metastasis formation in syngeneic experimental rats. Carcinogenesis. 2013;34:2804–2813. doi: 10.1093/carcin/bgt291. [DOI] [PubMed] [Google Scholar]
- 245.Yu T., Liu K., Wu Y., Fan J., Chen J., Li C. High interstitial fluid pressure promotes tumor cell proliferation and invasion in oral squamous cell carcinoma. Int J Mol Med. 2013;32:1093–1100. doi: 10.3892/ijmm.2013.1496. [DOI] [PubMed] [Google Scholar]
- 246.Hofmann M., Guschel M., Bernd A., Bereiter-Hahn J., Kaufmann R., Tandi C. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rana S., Munawar M., Shahid A., Malik M., Ullah H., Fatima W. Deregulated expression of circadian clock and clock-controlled cell cycle genes in chronic lymphocytic leukemia. Mol Biol Rep. 2014;41:95–103. doi: 10.1007/s11033-013-2841-7. [DOI] [PubMed] [Google Scholar]
- 248.Sotak M., Polidarova L., Ergang P., Sumova A., Pacha J. An association between clock genes and clock-controlled cell cycle genes in murine colorectal tumors. Int J Cancer. 2013;132:1032–1041. doi: 10.1002/ijc.27760. [DOI] [PubMed] [Google Scholar]
- 249.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 250.Jia C.C., Wang T.T., Liu W., Fu B.S., Hua X., Wang G.Y. Cancer-associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLOS ONE. 2013;8:e63243. doi: 10.1371/journal.pone.0063243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang Y.H., Dong Y.Y., Wang W.M., Xie X.Y., Wang Z.M., Chen R.X. Vascular endothelial cells facilitated HCC invasion and metastasis through the Akt and NF-kappaB pathways induced by paracrine cytokines. J Exp Clin Cancer Res. 2013;32:51. doi: 10.1186/1756-9966-32-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shi S., Chen L., Huang G. Antiangiogenic therapy improves the antitumor effect of adoptive cell immunotherapy by normalizing tumor vasculature. Med Oncol. 2013;30:698. doi: 10.1007/s12032-013-0698-1. [DOI] [PubMed] [Google Scholar]
- 253.Casazza A., Laoui D., Wenes M., Rizzolio S., Bassani N., Mambretti M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 254.Tripathi C., Tewari B.N., Kanchan R.K., Baghel K.S., Nautiyal N., Shrivastava R. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget. 2014;5:5350–5368. doi: 10.18632/oncotarget.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tomei A.A., Siegert S., Britschgi M.R., Luther S.A., Swartz M.A. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol. 2009;183:4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 256.Mamlouk S., Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer. 2013;132:2721–2729. doi: 10.1002/ijc.27901. [DOI] [PubMed] [Google Scholar]
- 257.Lee J.H., Sancar A. Regulation of apoptosis by the circadian clock through NF-kappaB signaling. Proc Natl Acad Sci U S A. 2011;108:12036–12041. doi: 10.1073/pnas.1108125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Erez N., Truitt M., Olson P., Arron S.T., Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 259.Ohashi T., Akazawa T., Aoki M., Kuze B., Mizuta K., Ito Y. Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. Int J Cancer. 2013;133:1107–1118. doi: 10.1002/ijc.28114. [DOI] [PubMed] [Google Scholar]
- 260.Ghajar C.M., Peinado H., Mori H., Matei I.R., Evason K.J., Brazier H. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gardner L.B., Li Q., Park M.S., Flanagan W.M., Semenza G.L., Dang C.V. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–7926. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 262.Goda N., Ryan H.E., Khadivi B., McNulty W., Rickert R.C., Johnson R.S. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23:359–369. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yamanda S., Ebihara S., Asada M., Okazaki T., Niu K., Ebihara T. Role of ephrinB2 in nonproductive angiogenesis induced by Delta-like 4 blockade. Blood. 2009;113:3631–3639. doi: 10.1182/blood-2008-07-170381. [DOI] [PubMed] [Google Scholar]
- 264.Bussink J., Kaanders J.H., Rijken P.F., Martindale C.A., van der Kogel A.J. Multiparameter analysis of vasculature, perfusion and proliferation in human tumour xenografts. Br J Cancer. 1998;77:57–64. doi: 10.1038/bjc.1998.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rupertus K., Dahlem C., Menger M.D., Schilling M.K., Kollmar O. Rapamycin inhibits hepatectomy-induced stimulation of metastatic tumor growth by reduction of angiogenesis, microvascular blood perfusion, and tumor cell proliferation. Ann Surg Oncol. 2009;16:2629–2637. doi: 10.1245/s10434-009-0564-8. [DOI] [PubMed] [Google Scholar]
- 266.Li X.M., Delaunay F., Dulong S., Claustrat B., Zampera S., Fujii Y. Cancer inhibition through circadian reprogramming of tumor transcriptome with meal timing. Cancer Res. 2010;70:3351–3360. doi: 10.1158/0008-5472.CAN-09-4235. [DOI] [PubMed] [Google Scholar]
- 267.Yang W.S., Stockwell B.R. Inhibition of casein kinase 1-epsilon induces cancer-cell-selective, PERIOD2-dependent growth arrest. Genome Biol. 2008;9:R92. doi: 10.1186/gb-2008-9-6-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lin H., Yan J., Wang Z., Hua F., Yu J., Sun W. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology. 2013;57:171–182. doi: 10.1002/hep.25991. [DOI] [PubMed] [Google Scholar]
- 269.Wang W., Li Q., Yamada T., Matsumoto K., Matsumoto I., Oda M. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15:6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 270.Pontiggia O., Sampayo R., Raffo D., Motter A., Xu R., Bissell M.J. The tumor microenvironment modulates tamoxifen resistance in breast cancer: a role for soluble stromal factors and fibronectin through beta1 integrin. Breast Cancer Res Treat. 2012;133:459–471. doi: 10.1007/s10549-011-1766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Madhok B.M., Yeluri S., Perry S.L., Hughes T.A., Jayne D.G. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. Br J Cancer. 2010;102:1746–1752. doi: 10.1038/sj.bjc.6605701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Riemann A., Schneider B., Ihling A., Nowak M., Sauvant C., Thews O. Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLoS ONE. 2011;6:e22445. doi: 10.1371/journal.pone.0022445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Vangestel C., Van de Wiele C., Van Damme N., Staelens S., Pauwels P., Reutelingsperger C.P. (99)mTc-(CO)(3) His-annexin A5 micro-SPECT demonstrates increased cell death by irinotecan during the vascular normalization window caused by bevacizumab. J Nucl Med. 2011;52:1786–1794. doi: 10.2967/jnumed.111.092650. [DOI] [PubMed] [Google Scholar]
- 274.Hernandez-Luna M.A., Rocha-Zavaleta L., Vega M.I., Huerta-Yepez S. Hypoxia inducible factor-1alpha induces chemoresistance phenotype in non-Hodgkin lymphoma cell line via up-regulation of Bcl-xL. Leuk Lymphoma. 2013;54:1048–1055. doi: 10.3109/10428194.2012.733874. [DOI] [PubMed] [Google Scholar]
- 275.Islam N., Haqqi T.M., Jepsen K.J., Kraay M., Welter J.F., Goldberg V.M. Hydrostatic pressure induces apoptosis in human chondrocytes from osteoarthritic cartilage through up-regulation of tumor necrosis factor-alpha, inducible nitric oxide synthase, p53, c-myc, and bax-alpha, and suppression of bcl-2. J Cell Biochem. 2002;87:266–278. doi: 10.1002/jcb.10317. [DOI] [PubMed] [Google Scholar]
- 276.Campbell N.E., Greenaway J., Henkin J., Moorehead R.A., Petrik J. The thrombospondin-1 mimetic ABT-510 increases the uptake and effectiveness of cisplatin and paclitaxel in a mouse model of epithelial ovarian cancer. Neoplasia. 2010;12:275–283. doi: 10.1593/neo.91880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hua H., Wang Y., Wan C., Liu Y., Zhu B., Yang C. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006;97:589–596. doi: 10.1111/j.1349-7006.2006.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Schafer H., Struck B., Feldmann E.M., Bergmann F., Grage-Griebenow E., Geismann C. TGF-beta1-dependent L1CAM expression has an essential role in macrophage-induced apoptosis resistance and cell migration of human intestinal epithelial cells. Oncogene. 2013;32:180–189. doi: 10.1038/onc.2012.44. [DOI] [PubMed] [Google Scholar]
- 279.Kinugasa Y., Matsui T., Takakura N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells. 2014;32:145–156. doi: 10.1002/stem.1556. [DOI] [PubMed] [Google Scholar]
- 280.Golding J.P., Wardhaugh T., Patrick L., Turner M., Phillips J.B., Bruce J.I. Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br J Cancer. 2013;109:976–982. doi: 10.1038/bjc.2013.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nishi H., Nakada T., Kyo S., Inoue M., Shay J.W., Isaka K. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT) Mol Cell Biol. 2004;24:6076–6083. doi: 10.1128/MCB.24.13.6076-6083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Heeg S., Hirt N., Queisser A., Schmieg H., Thaler M., Kunert H. EGFR overexpression induces activation of telomerase via PI3K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesis. Cancer Sci. 2011;102:351–360. doi: 10.1111/j.1349-7006.2010.01796.x. [DOI] [PubMed] [Google Scholar]
- 283.Anderson C.J., Hoare S.F., Ashcroft M., Bilsland A.E., Keith W.N. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene. 2006;25:61–69. doi: 10.1038/sj.onc.1209011. [DOI] [PubMed] [Google Scholar]
- 284.Qu Y., Wang Z., Huang X., Wan C., Yang C.L., Liu B. Circadian telomerase activity and DNA synthesis for timing peptide administration. Peptides. 2003;24:363–369. doi: 10.1016/s0196-9781(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 285.Qu Y., Mao M., Li X., Liu Y., Ding J., Jiang Z. Telomerase reconstitution contributes to resetting of circadian rhythm in fibroblasts. Mol Cell Biochem. 2008;313:11–18. doi: 10.1007/s11010-008-9736-2. [DOI] [PubMed] [Google Scholar]
- 286.Martinive P., Defresne F., Bouzin C., Saliez J., Lair F., Gregoire V. Preconditioning of the tumor vasculature and tumor cells by intermittent hypoxia: implications for anticancer therapies. Cancer Res. 2006;66:11736–11744. doi: 10.1158/0008-5472.CAN-06-2056. [DOI] [PubMed] [Google Scholar]
- 287.Mazzone M., Dettori D., Leite de Oliveira R., Loges S., Schmidt T., Jonckx B. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kieda C., El Hafny-Rahbi B., Collet G., Lamerant-Fayel N., Grillon C., Guichard A. Stable tumor vessel normalization with pO(2) increase and endothelial PTEN activation by inositol trispyrophosphate brings novel tumor treatment. J Mol Med (Berl) 2013;91:883–899. doi: 10.1007/s00109-013-0992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Sun R.C., Denko N.C. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 2014;19:285–292. doi: 10.1016/j.cmet.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Liao J., Qian F., Tchabo N., Mhawech-Fauceglia P., Beck A., Qian Z. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLOS ONE. 2014;9:e84941. doi: 10.1371/journal.pone.0084941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Lamonte G., Tang X., Chen J.L., Wu J., Ding C.K., Keenan M.M. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 2013;1:23. doi: 10.1186/2049-3002-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Matsubara T., Diresta G.R., Kakunaga S., Li D., Healey J.H. Additive influence of extracellular pH, oxygen tension, and pressure on invasiveness and survival of human osteosarcoma cells. Front Oncol. 2013;3:199. doi: 10.3389/fonc.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kelly C.J., Hussien K., Fokas E., Kannan P., Shipley R.J., Ashton T.M. Regulation of O2 consumption by the PI3K and mTOR pathways contributes to tumor hypoxia. Radiother Oncol. 2014;111:72–80. doi: 10.1016/j.radonc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Seront E., Boidot R., Bouzin C., Karroum O., Jordan B.F., Gallez B. Tumour hypoxia determines the potential of combining mTOR and autophagy inhibitors to treat mammary tumours. Br J Cancer. 2013;109:2597–2606. doi: 10.1038/bjc.2013.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Okazaki H., Matsunaga N., Fujioka T., Okazaki F., Akagawa Y., Tsurudome Y. Circadian regulation of mTOR by the ubiquitin pathway in renal cell carcinoma. Cancer Res. 2014;74:543–551. doi: 10.1158/0008-5472.CAN-12-3241. [DOI] [PubMed] [Google Scholar]
- 296.Rattigan Y.I., Patel B.B., Ackerstaff E., Sukenick G., Koutcher J.A., Glod J.W. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp Cell Res. 2012;318:326–335. doi: 10.1016/j.yexcr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wojtkowiak J.W., Rothberg J.M., Kumar V., Schramm K.J., Haller E., Proemsey J.B. Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Res. 2012;72:3938–3947. doi: 10.1158/0008-5472.CAN-11-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Belkin D.A., Mitsui H., Wang C.Q., Gonzalez J., Zhang S., Shah K.R. CD200 upregulation in vascular endothelium surrounding cutaneous squamous cell carcinoma. JAMA Dermatol. 2013;149:178–186. doi: 10.1001/jamadermatol.2013.1609. [DOI] [PubMed] [Google Scholar]
- 299.Hamzah J., Jugold M., Kiessling F., Rigby P., Manzur M., Marti H.H. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 300.Chi Sabins N., Taylor J.L., Fabian K.P., Appleman L.J., Maranchie J.K., Stolz D.B. DLK1: a novel target for immunotherapeutic remodeling of the tumor blood vasculature. Mol Ther. 2013;21:1958–1968. doi: 10.1038/mt.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Garufi A., Pistritto G., Ceci C., Di Renzo L., Santarelli R., Faggioni A. Targeting COX-2/PGE(2) pathway in HIPK2 knockdown cancer cells: impact on dendritic cell maturation. PLoS ONE. 2012;7:e48342. doi: 10.1371/journal.pone.0048342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Deng B., Zhu J.M., Wang Y., Liu T.T., Ding Y.B., Xiao W.M. Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-beta1 in gastric cancer. PLOS ONE. 2013;8:e63777. doi: 10.1371/journal.pone.0063777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lund A.W., Duraes F.V., Hirosue S., Raghavan V.R., Nembrini C., Thomas S.N. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 304.Craig D.H., Schaubert K.L., Shiratsuchi H., Kan-Mitchell J., Basson M.D. Increased pressure stimulates aberrant dendritic cell maturation. Cell Mol Biol Lett. 2008;13:260–270. doi: 10.2478/s11658-007-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Baronzio G., Schwartz L., Kiselevsky M., Guais A., Sanders E., Milanesi G. Tumor interstitial fluid as modulator of cancer inflammation, thrombosis, immunity and angiogenesis. Anticancer Res. 2012;32:405–414. [PubMed] [Google Scholar]
- 306.Preise D., Scherz A., Salomon Y. Antitumor immunity promoted by vascular occluding therapy: lessons from vascular-targeted photodynamic therapy (VTP) Photochem Photobiol Sci. 2011;10:681–688. doi: 10.1039/c0pp00315h. [DOI] [PubMed] [Google Scholar]
- 307.Miyazaki K., Wakabayashi M., Hara Y., Ishida N. Tumor growth suppression in vivo by overexpression of the circadian component, PER2. Genes Cells. 2010;15:351–358. doi: 10.1111/j.1365-2443.2010.01384.x. [DOI] [PubMed] [Google Scholar]
- 308.Hsiao Y.W., Li C.F., Chi J.Y., Tseng J.T., Chang Y., Hsu L.J. CCAAT/enhancer binding protein delta in macrophages contributes to immunosuppression and inhibits phagocytosis in nasopharyngeal carcinoma. Sci Signal. 2013;6:ra59. doi: 10.1126/scisignal.2003648. [DOI] [PubMed] [Google Scholar]
- 309.Daurkin I., Eruslanov E., Stoffs T., Perrin G.Q., Algood C., Gilbert S.M. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res. 2011;71:6400–6409. doi: 10.1158/0008-5472.CAN-11-1261. [DOI] [PubMed] [Google Scholar]
- 310.Mace T.A., Ameen Z., Collins A., Wojcik S., Mair M., Young G.S. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–3018. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Villalba M., Rathore M.G., Lopez-Royuela N., Krzywinska E., Garaude J., Allende-Vega N. From tumor cell metabolism to tumor immune escape. Int J Biochem Cell Biol. 2013;45:106–113. doi: 10.1016/j.biocel.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 312.Chirasani S.R., Leukel P., Gottfried E., Hochrein J., Stadler K., Neumann B. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int J Cancer. 2013;132:843–853. doi: 10.1002/ijc.27712. [DOI] [PubMed] [Google Scholar]
- 313.Calcinotto A., Filipazzi P., Grioni M., Iero M., De Milito A., Ricupito A. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 314.James S.E., Salsbury A.J. Effect of (plus or minus)-1,2-bis(3,5-dioxopiperazin-1-yl)propane on tumor blood vessels and its relationship to the antimetastatic effect in the Lewis lung carcinoma. Cancer Res. 1974;34:839–842. [PubMed] [Google Scholar]
- 315.Lee S.L., Rouhi P., Dahl Jensen L., Zhang D., Ji H., Hauptmann G. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc Natl Acad Sci U S A. 2009;106:19485–19490. doi: 10.1073/pnas.0909228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5:735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 317.Lunt S.J., Kalliomaki T.M., Brown A., Yang V.X., Milosevic M., Hill R.P. Interstitial fluid pressure, vascularity and metastasis in ectopic, orthotopic and spontaneous tumours. BMC Cancer. 2008;8:2. doi: 10.1186/1471-2407-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rofstad E.K., Tunheim S.H., Mathiesen B., Graff B.A., Halsor E.F., Nilsen K. Pulmonary and lymph node metastasis is associated with primary tumor interstitial fluid pressure in human melanoma xenografts. Cancer Res. 2002;62:661–664. [PubMed] [Google Scholar]
- 319.Yu T., Wang Z., Liu K., Wu Y., Fan J., Chen J. High interstitial fluid pressure promotes tumor progression through inducing lymphatic metastasis-related protein expressions in oral squamous cell carcinoma. Clin Transl Oncol. 2014;16:539–547. doi: 10.1007/s12094-013-1115-0. [DOI] [PubMed] [Google Scholar]
- 320.Rofstad E.K., Gaustad J.V., Egeland T.A., Mathiesen B., Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer. 2010;127:1535–1546. doi: 10.1002/ijc.25176. [DOI] [PubMed] [Google Scholar]
- 321.Oshima T., Takenoshita S., Akaike M., Kunisaki C., Fujii S., Nozaki A. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep. 2011;25:1439–1446. doi: 10.3892/or.2011.1207. [DOI] [PubMed] [Google Scholar]
- 322.Wu M., Zeng J., Chen Y., Zeng Z., Zhang J., Cai Y. Experimental chronic jet lag promotes growth and lung metastasis of Lewis lung carcinoma in C57BL/6 mice. Oncol Rep. 2012;27:1417–1428. doi: 10.3892/or.2012.1688. [DOI] [PubMed] [Google Scholar]
- 323.Zhang Y., Tang H., Cai J., Zhang T., Guo J., Feng D. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303:47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 324.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rofstad E.K., Mathiesen B., Kindem K., Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 326.Peppicelli S., Bianchini F., Torre E., Calorini L. Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clin Exp Metastasis. 2014;31(4):423–433. doi: 10.1007/s10585-014-9637-6. [DOI] [PubMed] [Google Scholar]
- 327.Huang Y., Yuan J., Righi E., Kamoun W.S., Ancukiewicz M., Nezivar J. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Mo J., Sun B., Zhao X., Gu Q., Dong X., Liu Z. Hypoxia-induced senescence contributes to the regulation of microenvironment in melanomas. Pathol Res Pract. 2013;209:640–647. doi: 10.1016/j.prp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 329.Shin J.Y., Yoon I.H., Kim J.S., Kim B., Park C.G. Vascular endothelial growth factor-induced chemotaxis and IL-10 from T cells. Cell Immunol. 2009;256:72–78. doi: 10.1016/j.cellimm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 330.Aung K.Z., Pereira B.P., Tan P.H., Han H.C., Nathan S.S. Interstitial fluid pressure as an alternate regulator of angiogenesis independent of hypoxia driven HIF-1alpha in solid tumors. J Orthop Res. 2012;30:2038–2045. doi: 10.1002/jor.22154. [DOI] [PubMed] [Google Scholar]
- 331.Mao L., Dauchy R.T., Blask D.E., Slakey L.M., Xiang S., Yuan L. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3beta. Mol Endocrinol. 2012;26:1808–1820. doi: 10.1210/me.2012-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Kulbe H., Chakravarty P., Leinster D.A., Charles K.A., Kwong J., Thompson R.G. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Dayan D., Salo T., Salo S., Nyberg P., Nurmenniemi S., Costea D.E. Molecular crosstalk between cancer cells and tumor microenvironment components suggests potential targets for new therapeutic approaches in mobile tongue cancer. Cancer Med. 2012;1:128–140. doi: 10.1002/cam4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Karagiannis G.S., Poutahidis T., Erdman S.E., Kirsch R., Riddell R.H., Diamandis E.P. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Estrella V., Chen T., Lloyd M., Wojtkowiak J., Cornnell H.H., Ibrahim-Hashim A. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73:1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pratheeshkumar P., Kuttan G. Oleanolic acid induces apoptosis by modulating p53, Bax, Bcl-2 and caspase-3 gene expression and regulates the activation of transcription factors and cytokine profile in B16F. J Environ Pathol Toxicol Oncol. 2011;30:21–31. doi: 10.1615/jenvironpatholtoxicoloncol.v30.i1.30. [DOI] [PubMed] [Google Scholar]
- 337.Roy S., Deep G., Agarwal C., Agarwal R. Silibinin prevents ultraviolet B radiation-induced epidermal damages in JB6 cells and mouse skin in a p53-GADD45alpha-dependent manner. Carcinogenesis. 2012;33:629–636. doi: 10.1093/carcin/bgr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Thomas P., Wang Y.J., Zhong J.H., Kosaraju S., O’Callaghan N.J., Zhou X.F. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer's disease. Mutat Res. 2009;661:25–34. doi: 10.1016/j.mrfmmm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 339.Marden A., Walmsley R.M., Schweizer L.M., Schweizer M. Yeast-based assay for the measurement of positive and negative influences on microsatellite stability. FEMS Yeast Res. 2006;6:716–725. doi: 10.1111/j.1567-1364.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 340.Lu L.Y., Ou N., Lu Q.B. Antioxidant induces DNA damage, cell death and mutagenecity in human lung and skin normal cells. Sci Rep. 2013;3:3169. doi: 10.1038/srep03169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Liu R., Fu A., Hoffman A.E., Zheng T., Zhu Y. Melatonin enhances DNA repair capacity possibly by affecting genes involved in DNA damage responsive pathways. BMC Cell Biol. 2013;14:1. doi: 10.1186/1471-2121-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Denissova N.G., Nasello C.M., Yeung P.L., Tischfield J.A., Brenneman M.A. Resveratrol protects mouse embryonic stem cells from ionizing radiation by accelerating recovery from DNA strand breakage. Carcinogenesis. 2012;33:149–155. doi: 10.1093/carcin/bgr236. [DOI] [PubMed] [Google Scholar]
- 343.Mamede A.C., Abrantes A.M., Pedrosa L., Casalta-Lopes J.E., Pires A.S., Teixo R.J. Beyond the limits of oxygen: effects of hypoxia in a hormone-independent prostate cancer cell line. ISRN Oncol. 2013;2013:918207. doi: 10.1155/2013/918207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wu J., Dauchy R.T., Tirrell P.C., Wu S.S., Lynch D.T., Jitawatanarat P. Light at night activates IGF-1R/PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res. 2011;71:2622–2631. doi: 10.1158/0008-5472.CAN-10-3837. [DOI] [PubMed] [Google Scholar]
- 345.Elshazley M., Sato M., Hase T., Yamashita R., Yoshida K., Toyokuni S. The circadian clock gene BMAL1 is a novel therapeutic target for malignant pleural mesothelioma. Int J Cancer. 2012;131:2820–2831. doi: 10.1002/ijc.27598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ryder M., Gild M., Hohl T.M., Pamer E., Knauf J., Ghossein R. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLOS ONE. 2013;8:e54302. doi: 10.1371/journal.pone.0054302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Gershon T.R., Crowther A.J., Tikunov A., Garcia I., Annis R., Yuan H. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 2013;1 doi: 10.1186/2049-3002-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Onodera Y., Nam J.M., Bissell M.J. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest. 2014;124:367–384. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Tyagi A., Singh R.P., Ramasamy K., Raina K., Redente E.F., Dwyer-Nield L.D. Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev Res (Phila) 2009;2:74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lin Y.G., Kunnumakkara A.B., Nair A., Merritt W.M., Han L.Y., Armaiz-Pena G.N. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 351.Moghaddam S.J., Barta P., Mirabolfathinejad S.G., Ammar-Aouchiche Z., Garza N.T., Vo T.T. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30:1949–1956. doi: 10.1093/carcin/bgp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Mineva N.D., Paulson K.E., Naber S.P., Yee A.S., Sonenshein G.E. Epigallocatechin-3-gallate inhibits stem-like inflammatory breast cancer cells. PLOS ONE. 2013;8:e73464. doi: 10.1371/journal.pone.0073464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Surh Y.J., Chun K.S., Cha H.H., Han S.S., Keum Y.S., Park K.K. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 354.Corsini E., Dell’Agli M., Facchi A., De Fabiani E., Lucchi L., Boraso M.S. Enterodiol and enterolactone modulate the immune response by acting on nuclear factor-kappaB (NF-kappaB) signaling. J Agric Food Chem. 2010;58:6678–6684. doi: 10.1021/jf100471n. [DOI] [PubMed] [Google Scholar]
- 355.Jeong S.K., Yang K., Park Y.S., Choi Y.J., Oh S.J., Lee C.W. Interferon gamma induced by resveratrol analog, HS-1793, reverses the properties of tumor associated macrophages. Int Immunopharmacol. 2014;22:303–310. doi: 10.1016/j.intimp.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 356.Salado C., Olaso E., Gallot N., Valcarcel M., Egilegor E., Mendoza L. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J Transl Med. 2011;9:59. doi: 10.1186/1479-5876-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 358.Wang X., Bai H., Zhang X., Liu J., Cao P., Liao N. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis. 2013;34:1323–1330. doi: 10.1093/carcin/bgt058. [DOI] [PubMed] [Google Scholar]
- 359.Tae N., Seo J., Min B.S., Ryoo S., Lee J.H. 3-Oxoolean-12-en-27-oic acid inhibits the proliferation of non-small cell lung carcinoma cells by inducing cell-cycle arrest at G0/G1 phase. Anticancer Res. 2011;31:2179–2185. [PubMed] [Google Scholar]
- 360.Jiang H.L., Jin J.Z., Wu D., Xu D., Lin G.F., Yu H. Celastrol exerts synergistic effects with PHA-665752 and inhibits tumor growth of c-Met-deficient hepatocellular carcinoma in vivo. Mol Biol Rep. 2013;40:4203–4209. doi: 10.1007/s11033-013-2501-y. [DOI] [PubMed] [Google Scholar]
- 361.Wang W.B., Feng L.X., Yue Q.X., Wu W.Y., Guan S.H., Jiang B.H. Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J Cell Physiol. 2012;227:2196–2206. doi: 10.1002/jcp.22956. [DOI] [PubMed] [Google Scholar]
- 362.Ge P., Ji X., Ding Y., Wang X., Fu S., Meng F. Celastrol causes apoptosis and cell cycle arrest in rat glioma cells. Neurol Res. 2010;32:94–100. doi: 10.1179/016164109X12518779082273. [DOI] [PubMed] [Google Scholar]
- 363.Tyagi A., Agarwal C., Agarwal R. Inhibition of retinoblastoma protein (Rb) phosphorylation at serine sites and an increase in Rb-E2F complex formation by silibinin in androgen-dependent human prostate carcinoma LNCaP cells: role in prostate cancer prevention. Mol Cancer Ther. 2002;1:525–532. [PubMed] [Google Scholar]
- 364.Tyagi A., Agarwal C., Harrison G., Glode L.M., Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25:1711–1720. doi: 10.1093/carcin/bgh180. [DOI] [PubMed] [Google Scholar]
- 365.Kaur M., Velmurugan B., Tyagi A., Deep G., Katiyar S., Agarwal C. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther. 2009;8:2366–2374. doi: 10.1158/1535-7163.MCT-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Agarwal C., Singh R.P., Dhanalakshmi S., Tyagi A.K., Tecklenburg M., Sclafani R.A. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 367.Kang N., Wang M.M., Wang Y.H., Zhang Z.N., Cao H.R., Lv Y.H. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem Toxicol. 2014;67:193–200. doi: 10.1016/j.fct.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 368.Lim T.G., Lee S.Y., Huang Z., Lim do Y., Chen H., Jung S.K. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev Res (Phila) 2014;7:466–474. doi: 10.1158/1940-6207.CAPR-13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Guo H., Xu Y.M., Ye Z.Q., Yu J.H., Hu X.Y. Curcumin induces cell cycle arrest and apoptosis of prostate cancer cells by regulating the expression of IkappaBalpha, c-Jun and androgen receptor. Pharmazie. 2013;68:431–434. [PubMed] [Google Scholar]
- 370.Mukhopadhyay A., Banerjee S., Stafford L.J., Xia C., Liu M., Aggarwal B.B. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 371.Ahmad N., Adhami V.M., Gupta S., Cheng P., Mukhtar H. Role of the retinoblastoma (pRb)-E2F/DP pathway in cancer chemopreventive effects of green tea polyphenol epigallocatechin-3-gallate. Arch Biochem Biophys. 2002;398:125–131. doi: 10.1006/abbi.2001.2704. [DOI] [PubMed] [Google Scholar]
- 372.Huh S.W., Bae S.M., Kim Y.W., Lee J.M., Namkoong S.E., Lee I.P. Anticancer effects of (−)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol Oncol. 2004;94:760–768. doi: 10.1016/j.ygyno.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 373.Saldanha S.N., Kala R., Tollefsbol T.O. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp Cell Res. 2014;324:40–53. doi: 10.1016/j.yexcr.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Shen X., Zhang Y., Feng Y., Zhang L., Li J., Xie Y.A. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the AKT pathway. Int J Oncol. 2014;44:791–796. doi: 10.3892/ijo.2014.2251. [DOI] [PubMed] [Google Scholar]
- 375.Huang W.W., Tsai S.C., Peng S.F., Lin M.W., Chiang J.H., Chiu Y.J. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol. 2013;42:2069–2077. doi: 10.3892/ijo.2013.1909. [DOI] [PubMed] [Google Scholar]
- 376.Xu W., Liu J., Li C., Wu H.Z., Liu Y.W. Kaempferol-7-O-beta-d-glucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. 2008;264:229–240. doi: 10.1016/j.canlet.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 377.Choi E.J., Ahn W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr Res Pract. 2008;2:322–325. doi: 10.4162/nrp.2008.2.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Casagrande F., Darbon J.M. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem Pharmacol. 2001;61:1205–1215. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 379.Hill S.M., Blask D.E., Xiang S., Yuan L., Mao L., Dauchy R.T. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia. 2011;16:235–245. doi: 10.1007/s10911-011-9222-4. [DOI] [PubMed] [Google Scholar]
- 380.McCann M.J., Gill C.I., Linton T., Berrar D., McGlynn H., Rowland I.R. Enterolactone restricts the proliferation of the LNCaP human prostate cancer cell line in vitro. Mol Nutr Food Res. 2008;52:567–580. doi: 10.1002/mnfr.200700052. [DOI] [PubMed] [Google Scholar]
- 381.Stan S.D., Zeng Y., Singh S.V. Ayurvedic medicine constituent withaferin A causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60(Suppl. 1):51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Roy R.V., Suman S., Das T.P., Luevano J.E., Damodaran C. Withaferin A, a steroidal lactone from Withania somnifera, induces mitotic catastrophe and growth arrest in prostate cancer cells. J Nat Prod. 2013;76:1909–1915. doi: 10.1021/np400441f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Grogan P.T., Sleder K.D., Samadi A.K., Zhang H., Timmermann B.N., Cohen M.S. Cytotoxicity of withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Invest New Drugs. 2013;31:545–557. doi: 10.1007/s10637-012-9888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Adhami V.M., Afaq F., Ahmad N. Involvement of the retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) cells. Biochem Biophys Res Commun. 2001;288:579–585. doi: 10.1006/bbrc.2001.5819. [DOI] [PubMed] [Google Scholar]
- 385.Wolter F., Akoglu B., Clausnitzer A., Stein J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutr. 2001;131:2197–2203. doi: 10.1093/jn/131.8.2197. [DOI] [PubMed] [Google Scholar]
- 386.Kim A.L., Zhu Y., Zhu H., Han L., Kopelovich L., Bickers D.R. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp Dermatol. 2006;15:538–546. doi: 10.1111/j.1600-0625.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 387.Yang Q., Wang B., Zang W., Wang X., Liu Z., Li W. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLOS ONE. 2013;8:e70627. doi: 10.1371/journal.pone.0070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Tang S., Gao D., Zhao T., Zhou J., Zhao X. An evaluation of the anti-tumor efficacy of oleanolic acid-loaded PEGylated liposomes. Nanotechnology. 2013;24:235102. doi: 10.1088/0957-4484/24/23/235102. [DOI] [PubMed] [Google Scholar]
- 389.Li W., Ding Y., Sun Y.N., Yan X.T., Yang S.Y., Choi C.W. Oleanane-type triterpenoid saponins from the roots of Pulsatilla koreana and their apoptosis-inducing effects on HL-60 human promyelocytic leukemia cells. Arch Pharm Res. 2013;36:768–774. doi: 10.1007/s12272-013-0042-5. [DOI] [PubMed] [Google Scholar]
- 390.Dizaji M.Z., Malehmir M., Ghavamzadeh A., Alimoghaddam K., Ghaffari S.H. Synergistic effects of arsenic trioxide and silibinin on apoptosis and invasion in human glioblastoma U87MG cell line. Neurochem Res. 2012;37:370–380. doi: 10.1007/s11064-011-0620-1. [DOI] [PubMed] [Google Scholar]
- 391.Fang H.Y., Chen S.B., Guo D.J., Pan S.Y., Yu Z.L. Proteomic identification of differentially expressed proteins in curcumin-treated MCF-7 cells. Phytomedicine. 2011;18:697–703. doi: 10.1016/j.phymed.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 392.Lee W.Y., Chen Y.C., Shih C.M., Lin C.M., Cheng C.H., Chen K.C. The induction of heme oxygenase-1 suppresses heat shock protein 90 and the proliferation of human breast cancer cells through its byproduct carbon monoxide. Toxicol Appl Pharmacol. 2014;274:55–62. doi: 10.1016/j.taap.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 393.Martin S., Lamb H.K., Brady C., Lefkove B., Bonner M.Y., Thompson P. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78. Br J Cancer. 2013;109:433–443. doi: 10.1038/bjc.2013.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Song W., Dang Q., Xu D., Chen Y., Zhu G., Wu K. Kaempferol induces cell cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38 signaling. Oncol Rep. 2014;31:1350–1356. doi: 10.3892/or.2014.2965. [DOI] [PubMed] [Google Scholar]
- 395.Kim C.H., Yoo Y.M. Melatonin induces apoptotic cell death via p53 in LNCaP cells. Korean J Physiol Pharmacol. 2010;14:365–369. doi: 10.4196/kjpp.2010.14.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Danbara N., Yuri T., Tsujita-Kyutoku M., Tsukamoto R., Uehara N., Tsubura A. Enterolactone induces apoptosis and inhibits growth of Colo 201 human colon cancer cells both in vitro and in vivo. Anticancer Res. 2005;25:2269–2276. [PubMed] [Google Scholar]
- 397.Hahm E.R., Lee J., Singh S.V. Role of mitogen-activated protein kinases and Mcl-1 in apoptosis induction by withaferin A in human breast cancer cells. Mol Carcinog. 2013;53(11):907–916. doi: 10.1002/mc.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Wesolowska O., Wisniewski J., Bielawska-Pohl A., Paprocka M., Duarte N., Ferreira M.J. Stilbenes as multidrug resistance modulators and apoptosis inducers in human adenocarcinoma cells. Anticancer Res. 2010;30:4587–4593. [PubMed] [Google Scholar]
- 399.Deeb D., Gao X., Liu Y., Kim S.H., Pindolia K.R., Arbab A.S. Inhibition of cell proliferation and induction of apoptosis by oleanane triterpenoid (CDDO-Me) in pancreatic cancer cells is associated with the suppression of hTERT gene expression and its telomerase activity. Biochem Biophys Res Commun. 2012;422:561–567. doi: 10.1016/j.bbrc.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Deeb D., Gao X., Liu Y., Varma N.R., Arbab A.S., Gautam S.C. Inhibition of telomerase activity by oleanane triterpenoid CDDO-Me in pancreatic cancer cells is ROS-dependent. Molecules. 2013;18:3250–3265. doi: 10.3390/molecules18033250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Nasiri M., Zarghami N., Koshki K.N., Mollazadeh M., Moghaddam M.P., Yamchi M.R. Curcumin and silibinin inhibit telomerase expression in T47D human breast cancer cells. Asian Pac J Cancer Prev. 2013;14:3449–3453. doi: 10.7314/apjcp.2013.14.6.3449. [DOI] [PubMed] [Google Scholar]
- 402.Ebrahimnezhad Z., Zarghami N., Keyhani M., Amirsaadat S., Akbarzadeh A., Rahmati M. Inhibition of hTERT gene expression by silibinin-loaded PLGA-PEG-Fe3O4 in T47D breast cancer cell line. Bioimpacts. 2013;3:67–74. doi: 10.5681/bi.2013.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 403.Thelen P., Wuttke W., Jarry H., Grzmil M., Ringert R.H. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J Urol. 2004;171:1934–1938. doi: 10.1097/01.ju.0000121329.37206.1b. [DOI] [PubMed] [Google Scholar]
- 404.Khaw A.K., Hande M.P., Kalthur G. Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumour cells. J Cell Biochem. 2013;114:1257–1270. doi: 10.1002/jcb.24466. [DOI] [PubMed] [Google Scholar]
- 405.Wang X., Hao M.W., Dong K., Lin F., Ren J.H., Zhang H.Z. Apoptosis induction effects of EGCG in laryngeal squamous cell carcinoma cells through telomerase repression. Arch Pharm Res. 2009;32:1263–1269. doi: 10.1007/s12272-009-1912-8. [DOI] [PubMed] [Google Scholar]
- 406.Sadava D., Whitlock E., Kane S.E. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun. 2007;360:233–237. doi: 10.1016/j.bbrc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 407.Berletch J.B., Liu C., Love W.K., Andrews L.G., Katiyar S.K., Tollefsbol T.O. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103:509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Martinez-Campa C.M., Alonso-Gonzalez C., Mediavilla M.D., Cos S., Gonzalez A., Sanchez-Barcelo E.J. Melatonin down-regulates hTERT expression induced by either natural estrogens (17beta-estradiol) or metalloestrogens (cadmium) in MCF-7 human breast cancer cells. Cancer Lett. 2008;268:272–277. doi: 10.1016/j.canlet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 409.Lanzilli G., Fuggetta M.P., Tricarico M., Cottarelli A., Serafino A., Falchetti R. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int J Oncol. 2006;28:641–648. [PubMed] [Google Scholar]
- 410.Liu J., Wu N., Ma L., Liu M., Liu G., Zhang Y. Oleanolic acid suppresses aerobic glycolysis in cancer cells by switching pyruvate kinase type M isoforms. PLOS ONE. 2014;9:e91606. doi: 10.1371/journal.pone.0091606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Raina K., Agarwal C., Wadhwa R., Serkova N.J., Agarwal R. Energy deprivation by silibinin in colorectal cancer cells: a double-edged sword targeting both apoptotic and autophagic machineries. Autophagy. 2013;9:697–713. doi: 10.4161/auto.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Yousefi M., Ghaffari S.H., Soltani B.M., Nafissi S., Momeny M., Zekri A. Therapeutic efficacy of silibinin on human neuroblastoma cells: Akt and NF-kappaB expressions may play an important role in silibinin-induced response. Neurochem Res. 2012;37:2053–2063. doi: 10.1007/s11064-012-0827-9. [DOI] [PubMed] [Google Scholar]
- 413.Vaughan R.A., Garcia-Smith R., Dorsey J., Griffith J.K., Bisoffi M., Trujillo K.A. Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int J Cancer. 2013;133:2504–2510. doi: 10.1002/ijc.28264. [DOI] [PubMed] [Google Scholar]
- 414.Moreira L., Araujo I., Costa T., Correia-Branco A., Faria A., Martel F. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp Cell Res. 2013;319:1784–1795. doi: 10.1016/j.yexcr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 415.Valenti D., de Bari L., Manente G.A., Rossi L., Mutti L., Moro L. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim Biophys Acta. 2013;1832:2085–2096. doi: 10.1016/j.bbadis.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 416.Huang C.H., Tsai S.J., Wang Y.J., Pan M.H., Kao J.Y., Way T.D. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53:1156–1165. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 417.Filomeni G., Desideri E., Cardaci S., Graziani I., Piccirillo S., Rotilio G. Carcinoma cells activate AMP-activated protein kinase-dependent autophagy as survival response to kaempferol-mediated energetic impairment. Autophagy. 2010;6:202–216. doi: 10.4161/auto.6.2.10971. [DOI] [PubMed] [Google Scholar]
- 418.Thors L., Belghiti M., Fowler C.J. Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. Br J Pharmacol. 2008;155:244–252. doi: 10.1038/bjp.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Wang B.Q., Yang Q.H., Xu R.K., Xu J.N. Elevated levels of mitochonrial respiratory complexes activities and ATP production in 17-beta-estradiol-induced prolactin-secretory tumor cells in male rats are inhibited by melatonin in vivo and in vitro. Chin Med J (Engl) 2013;126:4724–4730. [PubMed] [Google Scholar]
- 420.Sanchez-Sanchez A.M., Martin V., Garcia-Santos G., Rodriguez-Blanco J., Casado-Zapico S., Suarez-Garnacho S. Intracellular redox state as determinant for melatonin antiproliferative vs cytotoxic effects in cancer cells. Free Radic Res. 2011;45:1333–1341. doi: 10.3109/10715762.2011.623700. [DOI] [PubMed] [Google Scholar]
- 421.Gonzalez A., del Castillo-Vaquero A., Miro-Moran A., Tapia J.A., Salido G.M. Melatonin reduces pancreatic tumor cell viability by altering mitochondrial physiology. J Pineal Res. 2011;50:250–260. doi: 10.1111/j.1600-079X.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 422.Hahm E.R., Lee J., Kim S.H., Sehrawat A., Arlotti J.A., Shiva S.S. Metabolic alterations in mammary cancer prevention by withaferin A in a clinically relevant mouse model. J Natl Cancer Inst. 2013;105:1111–1122. doi: 10.1093/jnci/djt153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Hahm E.R., Moura M.B., Kelley E.E., Van Houten B., Shiva S., Singh S.V. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE. 2011;6:e23354. doi: 10.1371/journal.pone.0023354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Malik F., Kumar A., Bhushan S., Khan S., Bhatia A., Suri K.A. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 425.Gomez L.S., Zancan P., Marcondes M.C., Ramos-Santos L., Meyer-Fernandes J.R., Sola-Penna M. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie. 2013;95:1336–1343. doi: 10.1016/j.biochi.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 426.Fouad M.A., Agha A.M., Merzabani M.M., Shouman S.A. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: calorie restriction is the force to the cytotoxicity. Hum Exp Toxicol. 2013;32:1067–1080. doi: 10.1177/0960327113475679. [DOI] [PubMed] [Google Scholar]
- 427.Filomeni G., Graziani I., Rotilio G., Ciriolo M.R. trans-Resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007;2:295–305. doi: 10.1007/s12263-007-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Nagaraj S., Youn J.I., Weber H., Iclozan C., Lu L., Cotter M.J. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Deng W., Sun H.X., Chen F.Y., Yao M.L. Immunomodulatory activity of 3beta, 6beta-dihydroxyolean-12-en-27-oic acid in tumor-bearing mice. Chem Biodivers. 2009;6:1243–1253. doi: 10.1002/cbdv.200800187. [DOI] [PubMed] [Google Scholar]
- 430.Forghani P., Khorramizadeh M.R., Waller E.K. Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 2014;3:215–224. doi: 10.1002/cam4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Bhattacharyya S., Md Sakib Hossain D., Mohanty S., Sankar Sen G., Chattopadhyay S., Banerjee S. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 2010;7:306–315. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Meeran S.M., Mantena S.K., Katiyar S.K. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clin Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 433.Jang J.Y., Lee J.K., Jeon Y.K., Kim C.W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Lissoni P., Ardizzoia A., Barni S., Tancini G., Muttini M. Immunotherapy with subcutaneous low dose interleukin-2 plus melatonin as salvage therapy of heavily chemotherapy-pretreated ovarian cancer. Oncol Rep. 1996;3:947–949. doi: 10.3892/or.3.5.947. [DOI] [PubMed] [Google Scholar]
- 435.Sinha P., Ostrand-Rosenberg S. Myeloid-derived suppressor cell function is reduced by Withaferin A, a potent and abundant component of Withania somnifera root extract. Cancer Immunol Immunother. 2013;62:1663–1673. doi: 10.1007/s00262-013-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Hushmendy S., Jayakumar L., Hahn A.B., Bhoiwala D., Bhoiwala D.L., Crawford D.R. Select phytochemicals suppress human T-lymphocytes and mouse splenocytes suggesting their use in autoimmunity and transplantation. Nutr Res. 2009;29:568–578. doi: 10.1016/j.nutres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Wang B., Sun J., Li X., Zhou Q., Bai J., Shi Y. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr Res. 2013;33:971–981. doi: 10.1016/j.nutres.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 438.Yang Y., Paik J.H., Cho D., Cho J.A., Kim C.W. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8:542–547. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 439.Deeb D., Gao X., Liu Y., Jiang D., Divine G.W., Arbab A.S. Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival signaling. Carcinogenesis. 2011;32:757–764. doi: 10.1093/carcin/bgr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Guo G., Yao W., Zhang Q., Bo Y. Oleanolic acid suppresses migration and invasion of malignant glioma cells by inactivating MAPK/ERK signaling pathway. PLOS ONE. 2013;8:e72079. doi: 10.1371/journal.pone.0072079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Huang L., Zhang Z., Zhang S., Ren J., Zhang R., Zeng H. Inhibitory action of Celastrol on hypoxia-mediated angiogenesis and metastasis via the HIF-1alpha pathway. Int J Mol Med. 2011;27:407–415. doi: 10.3892/ijmm.2011.600. [DOI] [PubMed] [Google Scholar]
- 442.Singh R.P., Raina K., Sharma G., Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial–mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14:7773–7780. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Yang C.L., Liu Y.Y., Ma Y.G., Xue Y.X., Liu D.G., Ren Y. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS ONE. 2012;7:e37960. doi: 10.1371/journal.pone.0037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Maruyama T., Murata S., Nakayama K., Sano N., Ogawa K., Nowatari T. (−)-Epigallocatechin-3-gallate suppresses liver metastasis of human colorectal cancer. Oncol Rep. 2014;31:625–633. doi: 10.3892/or.2013.2925. [DOI] [PubMed] [Google Scholar]
- 445.Sharma C., Nusri Qel A., Begum S., Javed E., Rizvi T.A., Hussain A. (−)-Epigallocatechin-3-gallate induces apoptosis and inhibits invasion and migration of human cervical cancer cells. Asian Pac J Cancer Prev. 2012;13:4815–4822. doi: 10.7314/apjcp.2012.13.9.4815. [DOI] [PubMed] [Google Scholar]
- 446.Park J.H., Yoon J.H., Kim S.A., Ahn S.G. (−)-Epigallocatechin-3-gallate inhibits invasion and migration of salivary gland adenocarcinoma cells. Oncol Rep. 2010;23:585–590. [PubMed] [Google Scholar]
- 447.Astin J.W., Jamieson S.M., Eng T.C., Flores M.V., Misa J.P., Chien A. An in vivo antilymphatic screen in zebrafish identifies novel inhibitors of mammalian lymphangiogenesis and lymphatic-mediated metastasis. Mol Cancer Ther. 2014;13:2450–2462. doi: 10.1158/1535-7163.MCT-14-0469-T. [DOI] [PubMed] [Google Scholar]
- 448.Chen H.J., Lin C.M., Lee C.Y., Shih N.C., Peng S.F., Tsuzuki M. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep. 2013;30:925–932. doi: 10.3892/or.2013.2490. [DOI] [PubMed] [Google Scholar]
- 449.Rapozzi V., Perissin L., Zorzet S., Giraldi T. Effects of melatonin administration on tumor spread in mice bearing Lewis lung carcinoma. Pharmacol Res. 1992;25(Suppl. 1):71–72. doi: 10.1016/1043-6618(92)90545-m. [DOI] [PubMed] [Google Scholar]
- 450.Cos S., Fernandez R., Guezmes A., Sanchez-Barcelo E.J. Influence of melatonin on invasive and metastatic properties of MCF-7 human breast cancer cells. Cancer Res. 1998;58:4383–4390. [PubMed] [Google Scholar]
- 451.Miura D., Saarinen N.M., Miura Y., Santti R., Yagasaki K. Hydroxymatairesinol and its mammalian metabolite enterolactone reduce the growth and metastasis of subcutaneous AH109A hepatomas in rats. Nutr Cancer. 2007;58:49–59. doi: 10.1080/01635580701308133. [DOI] [PubMed] [Google Scholar]
- 452.Thaiparambil J.T., Bender L., Ganesh T., Kline E., Patel P., Liu Y. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–2755. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 453.Ji Q., Liu X., Fu X., Zhang L., Sui H., Zhou L. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLOS ONE. 2013;8:e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 454.Sheth S., Jajoo S., Kaur T., Mukherjea D., Sheehan K., Rybak L.P. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Yeh C.B., Hsieh M.J., Lin C.W., Chiou H.L., Lin P.Y., Chen T.Y. The antimetastatic effects of resveratrol on hepatocellular carcinoma through the downregulation of a metastasis-associated protease by SP-1 modulation. PLOS ONE. 2013;8:e56661. doi: 10.1371/journal.pone.0056661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Wu P.K., Chi Shing Tai W., Liang Z.T., Zhao Z.Z., Hsiao W.L. Oleanolic acid isolated from Oldenlandia diffusa exhibits a unique growth inhibitory effect against ras-transformed fibroblasts. Life Sci. 2009;85:113–121. doi: 10.1016/j.lfs.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 457.Ni H., Zhao W., Kong X., Li H., Ouyang J. Celastrol inhibits lipopolysaccharide-induced angiogenesis by suppressing TLR4-triggered nuclear factor-kappa B activation. Acta Haematol. 2014;131:102–111. doi: 10.1159/000354770. [DOI] [PubMed] [Google Scholar]
- 458.Xu P., Yin Q., Shen J., Chen L., Yu H., Zhang Z. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int J Pharm. 2013;454:21–30. doi: 10.1016/j.ijpharm.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 459.Xu Y., Zhang J., Han J., Pan X., Cao Y., Guo H. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of alpha1-antitrypsin in lung cancer. Mol Oncol. 2012;6:405–417. doi: 10.1016/j.molonc.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Zgheib A., Lamy S., Annabi B. Epigallocatechin gallate targeting of membrane type 1 matrix metalloproteinase-mediated Src and Janus kinase/signal transducers and activators of transcription 3 signaling inhibits transcription of colony-stimulating factors 2 and 3 in mesenchymal stromal cells. J Biol Chem. 2013;288:13378–13386. doi: 10.1074/jbc.M113.456533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Luo H., Jiang B.H., King S.M., Chen Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 462.Bandyopadhyay S., Romero J.R., Chattopadhyay N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol Cell Endocrinol. 2008;287:57–64. doi: 10.1016/j.mce.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 463.Alvarez-Garcia V., Gonzalez A., Alonso-Gonzalez C., Martinez-Campa C., Cos S. Antiangiogenic effects of melatonin in endothelial cell cultures. Microvasc Res. 2013;87:25–33. doi: 10.1016/j.mvr.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 464.Szarc vel Szic K., Op de Beeck K., Ratman D., Wouters A., Beck I.M., Declerck K. Pharmacological levels of Withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specific to triple negative breast cancer cells. PLOS ONE. 2014;9:e87850. doi: 10.1371/journal.pone.0087850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Shamim U., Hanif S., Albanyan A., Beck F.W., Bao B., Wang Z. Resveratrol-induced apoptosis is enhanced in low pH environments associated with cancer. J Cell Physiol. 2012;227:1493–1500. doi: 10.1002/jcp.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]